Abstract
Background: The reasons for variation in survival in breast cancer are multifactorial.
Methods: From 1999 to 2003, the vital status of 9051 cases of invasive breast cancer was identified in the Eastern Region of England. Survival analysis was by Cox proportional hazards regression. Data were analysed separately for patients aged <70 years and those older due to differences in treatment policies.
Results: Overall 5-year survival was 78%. In patients aged <70 years, significant differences in survival lost their formal significance after adjustment for detection mode and node status, although this remained close to statistical significance with some residual differences between relative hazards. There was significant negative ecological correlation between proportion with nodes positive or not examined and 9-year survival rates. Patients with estrogen receptor (ER) status unknown were at significantly higher risk of dying than ER-positive patients. There was a clear trend of increasing hazard of dying with increasing deprivation. Survival differences in women aged ≥70 years were related to whether surgery was included as part of treatment.
Conclusion: This variation in treatment and survival may be attributed to lack of information, in particular nodal and ER status, thereby impacting on staging and prescription of adjuvant therapy.
Keywords: breast cancer, survival, treatment, variation
introduction
The recent decrease in breast cancer deaths in the UK [1] has been attributed to early diagnosis and improvement in breast cancer treatment. Data, however, indicate discrepancies in breast cancer survival between regions and countries in Europe [2]. The major determinants of survival in breast cancer are pathological tumour size, lymph node status and histological grade [3]. Randomised trials have shown reduced fatality with hormonal treatment [in estrogen receptor (ER)-positive tumours] and cytotoxic and other systemic therapies [4, 5]. Trials of mammographic screening have shown reduced mortality in association with early detection [6]. In terms of results by treatment centre, higher survival has been observed in association with increased specialisation and a high-volume caseload [7–9]. In addition, lower survival tends to be observed in groups of low socio-economic status [10, 11]. It is not clear however whether the poorer survival in low socio-economic status groups is due to later stage at presentation, innate disease severity, co-morbidity or disparities in treatment.
Standardisation of care has been attempted in England by the introduction of Regional Cancer Networks and in Scotland by the Clinical Standards Board. Both strategies were adopted to ensure maximal adoption of national guidelines and reduce any geographical variation in outcome.
In this study, we examine survival with up to 109 months follow-up in 10 National Health Service (NHS) hospitals in the Eastern Region of England, all reporting to the Eastern Cancer Registration and Information Centre (ECRIC). We describe differences in outcomes between hospitals and seek to identify factors which explain these differences. We examine host factors (age and socio-economic status), tumour attributes (pathological size, node status and histological grade), hospital volume of breast cancer patients and treatment factors (type of surgery, radiotherapy, chemotherapy and hormone therapy) in relation to patient outcome.
methods
Female patients diagnosed from 1999 to 2003 with invasive breast cancer (International Classification of Diseases10 site code C50*) were identified by ECRIC. During this period, ECRIC covered a population of ∼2.75 million people in the counties of Bedfordshire, Cambridgeshire, Norfolk and Suffolk. After exclusion of stage 4 cases, and unstageable cases, we identified 9051 (6106 under age 70) cases of female breast cancer, of which >97% were confirmed histologically. The vital status of patients was determined at the end of September 2008 and censored to 31 March 2008, 6 months earlier, to allow for any delay in reporting of vital status. ECRIC actively followed up the vital status of each individual patient in this study in late 2008 by querying the National Health Service Strategic Tracing Service, so it is expected that these data are substantially complete and reliable. Data elements recorded by ECRIC include hospital of diagnosis, age at diagnosis, pathological tumour size, number of nodes excised and status, ER status, treatment type (wide local excision, mastectomy, axillary surgery, radiotherapy, chemotherapy, hormone therapy), mode of detection (screen detected or symptomatic) and Index of Multiple Deprivation (IMD) based on patients’ electoral ward of residence. The primary sources of registration and treatment data are reports from all pathology laboratories and hospital patient notes which are viewed by registry staff who are either based at all major NHS hospitals in the region or visit them on at least a monthly basis. Both electronic and paper-based reports are received by the registry, so a high level of completeness of registration is also expected.
From the tumour size, lymph node status and histological grade, we calculated the Nottingham Prognostic Index (NPI) [12] for each case. The NPI has been validated in other breast cancer populations and allows assessment of the effect of different treatments in each of the five different prognostic groups, excellent (NPI ≤ 2.4), very good (2.4 < NPI ≤ 3.4), moderate 1 (3.4 < NPI ≤ 4.4), moderate 2 (4.4 < NPI ≤ 5.4) and poor (NPI > 5.4) [13]. Each tumour in the study was also staged by the fourth author (CHB) using the condensed tumour–node–metastasis system [14]. Analyses of outcomes were restricted to the 10 main NHS hospitals in the area studied; between 448 and 2051 cases were diagnosed at each of these hospitals during the 6-year period, while no other hospital in the area diagnosed >200 cases.
statistical analysis
We analysed the data for patients aged <70 years and patients aged ≥70 separately due to perceived differences in treatment policies in these age groups during the study period. Survival analysis used overall survival (OS) as the end point and was carried out using Cox proportional hazards regression, accompanied by life table estimates of survival probabilities by time [15]. Thereafter, analysis was aimed at identifying and describing differences in survival between institutions, attempting to attribute these to patient volume by hospital, case mix and treatment factors. The latter was carried out by adjusting for volume, tumour, host and treatment factors to ascertain whether adjustment for particular factors accounted for inter-hospital differences (i.e. after adjustment for which factors do the inter-hospital differences lose their statistical significance) and by subgroup analyses, identifying the predictors of survival in different groups of patients, such as node-negative and node-positive patients separately.
results
patients aged <70 years
Table 1 shows the number of patients and deaths by hospital and by patient and tumour attributes. Data were available on a total of 6106 patients aged <70 at diagnosis. Significant differences in survival were observed among the 10 hospitals (P = 0.03).
Table 1.
Breast cancer cases and deaths by hospital, and by patient and tumour attributes, in patients under age 70 at diagnosis
| Factor | Category | Cases (%) | Deaths | 5-year overall % survival | 9-year overall % survival |
| Age (years) | <50 | 1570 (26) | 282 | 85 | 79 |
| 50–69 | 4536 (74) | 705 | 88 | 80 | |
| IMD | Below median | 3055 (50) | 448 | 89 | 81 |
| Median or above | 3051 (50) | 539 | 86 | 78 | |
| Hospital | 1 | 921 (15) | 120 | 89 | 84 |
| 2 | 235 (4) | 43 | 87 | 75 | |
| 3 | 644 (10) | 100 | 87 | 81 | |
| 4 | 1219 (20) | 199 | 88 | 77 | |
| 5 | 465 (8) | 74 | 87 | 81 | |
| 6 | 580 (9) | 84 | 88 | 82 | |
| 7 | 716 (12) | 124 | 85 | 79 | |
| 8 | 470 (8) | 84 | 86 | 77 | |
| 9 | 324 (5) | 71 | 82 | 71 | |
| 10 | 532 (9) | 88 | 87 | 79 | |
| Tumour size (mm) | ≤20 | 3548 (64) | 356 | 92 | 87 |
| 21–50 | 1832 (33) | 409 | 82 | 72 | |
| >50 | 195 (3) | 94 | 58 | 42 | |
| Not known | 531 | 128 | 80 | 73 | |
| Node status | Negative | 3397 (62) | 293 | 93 | 88 |
| 1–3 positive | 1409 (26) | 270 | 84 | 77 | |
| 4+ positive | 657 (12) | 284 | 65 | 47 | |
| Not examined | 643 | 140 | 83 | 75 | |
| Grade | 1 | 1148 (20) | 49 | 97 | 94 |
| 2 | 2860 (50) | 368 | 90 | 83 | |
| 3 | 1751 (30) | 507 | 75 | 65 | |
| Not known | 347 | 63 | 75 | 76 | |
| ER status | Negative | 824 (17) | 255 | 72 | 66 |
| Positive | 3893 (83) | 424 | 92 | 85 | |
| Not known | 1389 | 308 | 82 | 73 |
IMD, Index of Multiple Deprivation; ER, estrogen receptor.
Table 2 shows the relative hazards by hospital from the Cox regression analysis for hospital, node status and detection mode, unadjusted in univariate analyses and mutually adjusted in a multivariate analysis. From the survival rates in Table 1, and the unadjusted relative hazards of death in Table 2, hospitals 2 and 7–10 have poorer survival than the others. The differences lost their formal significance after adjustment for detection mode and node status, although the result remained close to statistical significance (P = 0.07) with some residual differences between the relative hazards, notably increased risks for hospitals 7–10, as can be seen in the adjusted relative hazards in Table 2.
Table 2.
Results of univariate and multivariate Cox regression analysis for hospital, node status and detection mode in patients aged <70
| Factor | Category | Relative hazard and 95% CI |
|
| Univariate, unadjusted | Multivariate, mutually adjusted | ||
| Node status | Negative | 1.00 (–) | 1.00 (–) |
| 1–3 positive | 2.36 (1.99–2.79) | 2.18 (1.84–2.58) | |
| 4+ positive | 6.22 (5.37–7.32) | 5.55 (4.70–6.56) | |
| Not examined | 2.50 (2.04–3.06) | 2.31 (1.88–2.84) | |
| Detection mode | Symptomatic | 1.00 (–) | 1.00 (–) |
| Screening | 0.43 (0.36–0.51) | 0.56 (0.47–0.66) | |
| Hospital | 1 | 1.00 (–) | 1.00 (–) |
| 2 | 1.44 (1.01–2.04) | 1.12 (0.79–1.61) | |
| 3 | 1.21 (0.92–1.58) | 1.26 (0.96–1.65) | |
| 4 | 1.26 (1.00–1.58) | 1.21 (0.96–1.53) | |
| 5 | 1.24 (0.93–1.67) | 1.15 (0.86–1.54) | |
| 6 | 1.11 (0.84–1.48) | 1.10 (0.83–1.46) | |
| 7 | 1.36 (1.06–1.76) | 1.37 (1.06–1.77) | |
| 8 | 1.40 (1.06–1.86) | 1.38 (1.04–1.82) | |
| 9 | 1.77 (1.31–2.38) | 1.59 (1.18–2.15) | |
| 10 | 1.29 (0.97–1.70) | 1.45 (1.09–1.91) | |
| Significance of hospital differences | P = 0.03 | P = 0.07 | |
CI, confidence interval.
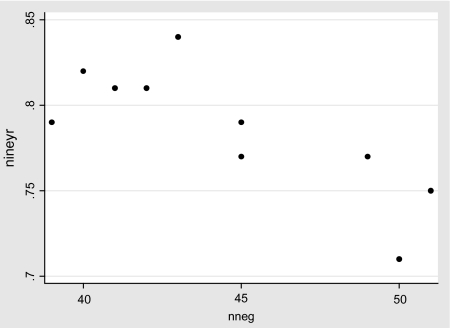
The observation that the inter-hospital survival differences are partially attributable to node status, including nodes not examined, is consistent with a highly significant (P = 0.008) negative ecological correlation between the proportion with nodes positive or not examined and the 9-year survival rates. The institutions with larger proportions node positive or nodes not examined have considerably lower survival (see Figure 1).
Figure 1.
Nine-year survival by percentage of cases node positive or with nodes not examined in the 10 hospitals, patients aged <70 years.
The inter-hospital survival differences were manifested in both early and late survival. Significant differences were observed both for survival up to 2 years after diagnosis and after 2 years (P = 0.02 in both cases), although the magnitude of the differences was greater after 2 years (data available from the authors). From Table 1, it can be seen that the hospitals with better survival at 5 years also had better survival at 9 years.
The inter-hospital survival differences were complicated by significant heterogeneity of the differences by node status categories (P = 0.01). Table 3 shows the relative hazards by hospital for the four node status categories separately. The inter-hospital differences in survival were not significant for node-negative cases but were significant for those with 1–3 nodes positive and those with nodes not examined. For cases with four or more nodes positive, the differences were of borderline significance. For 1–3 node-positive cases, the poorest survival was observed in hospitals 2, 8 and 9. For four or more nodes positive, the poorest survival was observed in hospitals 7 and 9, and in those with nodes not examined, the poorest survival was noted in hospitals 3 and 10.
Table 3.
Relative hazards by hospital, stratified by node status in patients aged <70 years
| Hospital | Relative hazard by node status |
|||
| Node negative | 1–3 positive | 4+ positive | Not examined | |
| 1 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.26 | 2.09 | 1.25 | –a |
| 3 | 1.27 | 1.21 | 1.03 | 2.58 |
| 4 | 1.27 | 1.09 | 1.26 | 1.45 |
| 5 | 1.26 | 1.28 | 1.16 | 1.39 |
| 6 | 0.78 | 1.53 | 1.20 | 1.45 |
| 7 | 1.24 | 1.66 | 1.51 | 1.05 |
| 8 | 1.45 | 2.22 | 1.32 | 1.08 |
| 9 | 1.33 | 2.05 | 2.47 | 0.97 |
| 10 | 1.18 | 1.64 | 1.00 | 2.46 |
| Significance | P = 0.6 | P = 0.03 | P = 0.09 | P = 0.004 |
Only 20 patients and no observed deaths.
Further multivariate survival analysis indicated that the differences between hospitals for those with 1–3 nodes positive were mainly due to differences between institutions in ER status and socio-economic status as measured by the IMD. Table 4 shows the inter-hospital relative hazards in this group, unadjusted and adjusted for ER status and IMD. In addition to the attenuation of the inter-hospital differences after adjustment for ER status and IMD, it is also worth noting that those with ER status unknown are at significantly higher risk of dying than the ER-positive subjects and that there is a clear trend of increasing hazard of dying with increasing deprivation.
Table 4.
Cox regression analysis for hospital, with and without adjustment for ER status and IMD for those with 1–3 nodes positive in patients aged <70 years
| Factor | Category | Relative hazard and 95% CI |
|
| Univariate, unadjusted | Multivariate, mutually adjusted | ||
| ER status | Negative | 1.00 (–) | 1.00 (–) |
| Positive | 0.30 (0.22–0.40) | 0.31 (0.23–0.41) | |
| Not known | 0.77 (0.55–1.08) | 0.73 (0.51–1.03) | |
| IMD score | Trend per 10% | 1.10 (1.03–1.28) | 1.10 (0.99–1.22) |
| Hospital | 1 | 1.00 (–) | 1.00 (–) |
| 2 | 2.09 (1.15–3.78) | 1.76 (0.97–3.20) | |
| 3 | 1.21 (0.72–2.02) | 1.07 (0.63–1.80) | |
| 4 | 1.09 (0.69–1.73) | 1.01 (0.63–1.61) | |
| 5 | 1.28 (0.71–2.29) | 1.10 (0.60–2.00) | |
| 6 | 1.53 (0.91–2.56) | 1.38 (0.82–2.31) | |
| 7 | 1.66 (1.02–2.71) | 1.47 (0.89–2.40) | |
| 8 | 2.22 (1.27–3.88) | 1.75 (0.96–3.18) | |
| 9 | 2.05 (1.20–3.49) | 1.61 (0.94–2.77) | |
| 10 | 1.64 (0.96–2.79) | 1.46 (0.84–2.52) | |
| Significance of hospital differences | P = 0.03 | P = 0.8 | |
ER, estrogen receptor; IMD, Index of Multiple Deprivation; CI, confidence interval.
For those with four or more nodes positive, the differences were not formally significant, but such differences in survival as were observed between hospitals were greater in women under age 50, i.e. below the age limit for screening. These did not seem to be attributable to hospital patient volume, host, tumour or treatment factors. Within the screening age range, survival was very similar for all hospitals. For those with nodes not examined, no host, tumour or treatment factors could be found which accounted for the inter-hospital differences.
In relation to the above it should be noted that for all node status groups, the survival differences between hospitals were larger for patients aged <50 years than for those with patients aged 50–69 years. Table 5 shows the significance of inter-hospital differences and the range of 5-year survival rates by node status, for the ages <50 and 50–69 separately. For all node status groups, the variation in survival between hospitals is considerably wider in the younger age group.
Table 5.
Range and significance of inter-hospital differences in survival for ages <50 and 50–69 separately, by Node status
| Node status | Age group | Significance of inter-hospital differences | Range of 5-year survival rates among hospitals |
| Negative | <50 | P = 0.4 | 88–98 |
| 50–69 | P = 0.7 | 92–95 | |
| 1–3 positive | <50 | P = 0.1 | 69–91 |
| 50–69 | P = 0.3 | 72–88 | |
| 4+ positive | <50 | P = 0.07 | 36–80 |
| 50–69 | P = 0.5 | 52–76 | |
| Not examined | <50 | P < 0.005 | 33–92 |
| 50–69 | P = 0.1 | 72–88 | |
| All cases | <50 | P = 0.4 | 76–92 |
| 50–69 | P = 0.7 | 84–90 |
patients aged ≥70 years
Data were available on 2945 patients aged ≥70 years. Numbers of patients and survival rates by hospital, IMD and tumour attributes are given in Table 6. The variation in survival rates between hospitals was of significance (P = 0.05) and was further complicated by significant heterogeneity of the inter-hospital differences between those 2156 patients who had primary surgery and the 789 who did not (P < 0.001). Table 7 shows the numbers having primary surgery and 5-year survival rates by hospital for the two treatment groups. Significant differences in survival were observed among hospitals for both those who received surgery (P = 0.03) and those who did not (P < 0.001).
Table 6.
Breast cancer cases and deaths by hospital, and by patient and tumour attributes, in patients aged ≥70 at diagnosis
| Factor | Category | Cases (%) | Deaths | 5-year overall % survival | 9-year overall % survival |
| IMD | Below median | 1476 (50) | 699 | 61 | 41 |
| Median or above | 1469 (50) | 777 | 57 | 35 | |
| Hospital | 1 | 381 (13) | 182 | 66 | 49 |
| 2 | 126 (4) | 72 | 52 | 36 | |
| 3 | 254 (9) | 122 | 62 | 38 | |
| 4 | 971 (19) | 315 | 55 | 33 | |
| 5 | 276 (9) | 117 | 63 | 50 | |
| 6 | 240 (8) | 126 | 54 | 39 | |
| 7 | 396 (14) | 192 | 62 | 43 | |
| 8 | 300 (10) | 141 | 62 | 44 | |
| 9 | 192 (7) | 97 | 62 | 36 | |
| 10 | 209 (7) | 112 | 57 | 31 | |
| Tumour size (mm) | ≤20 | 1077 (44) | 393 | 72 | 53 |
| 21–50 | 1222 (50) | 606 | 59 | 39 | |
| >50 | 157 (6) | 115 | 32 | 18 | |
| Not known | 489 | 362 | 37 | 19 | |
| Node status | Negative | 1049 (60) | 280 | 81 | 60 |
| 1–3 positive | 463 (27) | 181 | 67 | 54 | |
| 4+ positive | 234 (13) | 149 | 45 | 25 | |
| Not examined | 1199 | 866 | 38 | 20 | |
| Grade | 1 | 419 (17) | 172 | 69 | 45 |
| 2 | 1339 (56) | 562 | 67 | 47 | |
| 3 | 650 (27) | 352 | 51 | 32 | |
| Not known | 537 | 390 | 39 | 19 | |
| ER status | Negative | 181 (16) | 97 | 53 | 29 |
| Positive | 981 (84) | 293 | 76 | 61 | |
| Not known | 1783 | 1086 | 50 | 30 |
IMD, Index of Multiple Deprivation; ER, estrogen receptor.
Table 7.
Surgery and survival rates, by hospital in patients aged ≥70
| Hospital | Total patients aged 70+ | Patients having surgery (% of total) | 5-year % survival (no surgery) | 5-year % survival (surgery) | 5-year % survival, all patients |
| 1 | 381 | 268 (70) | 28 | 72 | 66 |
| 2 | 126 | 82 (65) | 20 | 69 | 52 |
| 3 | 254 | 189 (74) | 44 | 68 | 62 |
| 4 | 571 | 355 (62) | 32 | 69 | 55 |
| 5 | 276 | 193 (70) | 29 | 77 | 63 |
| 6 | 240 | 186 (78) | 20 | 64 | 54 |
| 7 | 396 | 350 (88) | 19 | 67 | 62 |
| 8 | 300 | 254 (85) | 11 | 72 | 62 |
| 9 | 192 | 160 (83) | 16 | 71 | 62 |
| 10 | 209 | 119 (57) | 36 | 73 | 57 |
| Significance of hospital differences | P < 0.001 | P < 0.001 | P = 0.03 | P = 0.05 |
Among those receiving surgery, the inter-hospital differences in survival were partly attributable to age, ER status and hormonal therapy but not to any other factors. Table 8 shows the Cox regression relative hazards unadjusted and adjusted for age, ER status and hormonal therapy. After adjustment, the inter-hospital differences in survival were no longer significant.
Table 8.
Results of univariate and multivariate Cox regression analysis for hospital, age, ER status and hormonal therapy in patients aged 70 years
| Factor | Category | Relative hazard and 95% CI |
|
| Univariate, unadjusted | Multivariate, mutually adjusted | ||
| Age | Trend per year | 1.09 (1.07–1.10) | 1.08 (1.06–1.10) |
| ER status | Negative | 1.00 (–) | 1.00 (–) |
| Positive | 0.43 (0.33–0.54) | 0.52 (0.40–0.68) | |
| Not known | 0.65 (0.52–0.82) | 0.65 (0.51–0.84) | |
| Hormone therapy | No | 1.00 (–) | 1.00 (–) |
| Yes | 0.60 (0.50–0.71) | 0.76 (0.62–0.91) | |
| Hospital | 1 | 1.00 (–) | 1.00 (–) |
| 2 | 1.35 (0.91–1.99) | 1.38 (0.93–2.04) | |
| 3 | 1.24 (0.91–1.69) | 1.26 (0.92–1.71) | |
| 4 | 1.22 (0.94–1.60) | 1.26 (0.96–1.65) | |
| 5 | 0.79 (0.56–1.12) | 0.81 (0.57–1.14) | |
| 6 | 1.38 (1.02–1.87) | 1.26 (0.92–1.71) | |
| 7 | 1.35 (1.04–1.76) | 1.16 (0.88–1.52) | |
| 8 | 1.15 (0.86–1.54) | 1.05 (0.78–1.41) | |
| 9 | 1.30 (0.95–1.79) | 1.24 (0.90–1.70) | |
| 10 | 1.10 (0.76–1.60) | 1.05 (0.72–1.52) | |
| Significance of hospital differences | P = 0.03 | P = 0.1 | |
ER, estrogen receptor; CI, confidence interval.
Among those not receiving surgery, pathological factors were not available. Of the patient, hospital and treatment attributes, the only factor which partly accounted for the differences among hospitals was the proportion receiving hormone therapy. After adjustment for the proportion of patients aged ≥70 treated with hormone therapy, the survival differences were of borderline significance (P = 0.07).
discussion
Despite the overall 5-year survival rate of 78% for the whole group being good when compared with the rest of Europe, the above results show significant differences between 10 major treatment centres in the Eastern Region of England with respect to OS of breast cancer patients. These differences in women <70 years at diagnosis just lost significance when adjusted for node status and detection mode (screen detected or symptomatic) and revealed four hospitals with increased risks (relative hazards 1.37–1.59, P = 0.07). We have recently shown that the mode of detection does impact on breast cancer survival [16] and should therefore be adjusted for in any inter-hospital comparison. Furthermore, not only is node positivity in this group associated with worse 9-year survival, the same negative correlation exists for nodes not examined. Survival differences in women aged ≥70 years were related to whether surgery was included as part of the treatment regimen in this age group. Overall, women in this age group who underwent surgery did better than those who did not. In addition, the hospitals in which a higher proportion of these older patients had surgery were characterised by better OS in patients aged >70 as a whole.
Significant inter-hospital survival differences were found both for survival up to 2 years after diagnosis and after 2 years. At 9 years follow-up, survival rates ranged from 71% to 84%, an absolute difference of 13%. The inter-hospital differences in survival were significant for two node status groups: those with 1–3 nodes positive and those with nodes not examined. It is likely that differences in selection of systemic therapy in the 1–3 node-positive group (between hospitals) may partly explain these differences and that understaging of axillary nodes leads to inadequate systemic therapy.
In our data, other variables such as tumour size, histological grade and NPI were also associated with survival, as one would expect. We have not reported on these as our focus was to identify factors which explained the survival difference between institutions. In addition to accurate tumour staging, including assessment of lymph node status, a number of additional factors have previously been shown to influence selection of appropriate adjuvant therapy, including specialisation, caseload and a teaching hospital setting [7, 8, 17]. These factors however should now be much less relevant with the introduction of breast surgery as a surgical speciality and multidisciplinary team decision making.
The availability of ER status also significantly influences the choice of adjuvant systemic therapy [18] and a postal survey published in 1998 found that only 84% of breast units in the UK had access to ER measurement, with only 60% on site [19]. An updated survey, published near the end of the study period for this paper, showed that although access to measurement had improved, there was considerable variability in both the specific technique of ER measurement and the absolute cut-off point for positivity (5%–80%) [20]. Multivariate survival analysis indicates that the differences between hospitals for women with 1–3 nodes positive in this study is mainly explained by differences between units in ER status as well as socio-economic status, measured by IMD. It is therefore noteworthy that this study has identified a group with ER status unknown during this time period (1999–2003) and that this group has a significantly worse survival than ER-positive patients. As previously described, increased deprivation in this study is associated with increased hazard of dying from breast cancer [10].
The fact that inter-hospital survival differences were significant for patients with 1–3 positive nodes indicates inter-hospital differences in the selection of appropriate adjuvant systemic therapy for this group. Quantitative decision support tools such as ADJUVANT! (www.adjuvantonline.com) [21] have been developed to estimate prognosis and absolute treatment benefits for an individual patient for both chemotherapy and hormone therapy. Although this more quantitative approach should allow greater consistency in prescription of chemotherapy, there is currently no international consensus on what should be the threshold benefit for recommending chemotherapy.
The importance of lymph node status as a powerful prognostic indicator in invasive breast cancer has been highlighted in professional guidelines in the UK since 1995 [22]. Despite this, a recent report by the NHS Breast Screening Programme (BSP) (presented to Association of Breast Surgeons at BASO, unpublished data) documented marked regional variation (1%–8%) in the percentage of screen-detected, invasive cancers in England with unknown nodal status. The figures from this study concur with these data from the NHS BSP and highlight that axillary staging, for some patients with invasive breast cancer in the Eastern Region of England, was omitted during the years of this study (1999–2003).
Although survival was generally good in all the hospitals studied, hospitals with a higher proportion of patients with node status unknown had a poorer OS. Therefore, in order to improve survival in breast cancer patients, an important strategy would be to ensure adequate axillary nodal staging in women with primary operable invasive breast cancer to ensure the most appropriate selection of adjuvant therapies. Furthermore, it is hoped that with the introduction of sentinel node biopsy, and widespread training of UK breast surgeons in this technique [23], the proportion of patients with unknown nodal status will reduce to a minimum.
In conclusion, the variation in treatment and survival observed in this study may be attributed to lack of information from surgical intervention, in particular assessment of nodal status and also ER status, thereby impacting on accurate staging of disease and prescription of appropriate adjuvant therapy. These factors can and should be easily addressed to improve both the treatment and survival for women with early breast cancer.
funding
National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre to GCW; NIHR Comprehensive Biomedical Research Centre at Guy's and St Thomas’ NHS Foundation Trust/King's College London to ADP.
Acknowledgments
Authors’ contribution—GCW: concept, design, compilation and scrutiny of data, interpretation of data and writing the paper; DCG: concept, design, collection, compilation and scrutiny of data, interpretation of data and writing the paper; PC: analysis and interpretation of data; SD: concept, design, scrutiny, analysis and interpretation of data and writing the paper; CHB: concept, design, collection, compilation and scrutiny of data and interpretation of data; ADP: concept, design, compilation and scrutiny of data, interpretation of data and writing the paper.
Conflict of interest statement: none.
Statement of originality: the work presented in this manuscript is entirely original and has not been submitted for publication elsewhere. The work has not been presented to date.
References
- 1.Peto R, Boreham J, Clarke M, et al. UK and USA breast-cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet. 2000;355:1822. doi: 10.1016/S0140-6736(00)02277-7. [DOI] [PubMed] [Google Scholar]
- 2.Quinn MJ, Martinez-Garcia C, Berrino F. EUROCARE Working Group. Variations in survival from breast cancer in Europe by age and country, 1978–89. Int J Cancer. 1998;34:2204–2211. doi: 10.1016/s0959-8049(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 3.Tabar L, Fagerberg G, Day NE, et al. The Swedish two-county trial of mammographic screening for breast cancer: recent results on mortality and tumour characteristics. Pathol Biol. 1992;39:846. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith RA, Duffy SW, Gabe R, et al. The randomized trials of breast cancer screening: what have we learned? [review] Radiol Clin North Am. 2004;42(5):793–806. doi: 10.1016/j.rcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Sainsbury R, Haward B, Rider L, et al. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet. 1995;345:1265–1270. doi: 10.1016/s0140-6736(95)90924-9. [DOI] [PubMed] [Google Scholar]
- 8.Gillis CR, Hole DJ. Survival outcome of care by specialist surgeons in breast cancer: a study of 3786 patients in the West of Scotland. BMJ. 1996;312:145–148. doi: 10.1136/bmj.312.7024.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsmore D, Hole D, Gillis C. Why does specialist treatment of breast cancer improve survival? The role of surgical management. Br J Cancer. 2004;90:1920–1925. doi: 10.1038/sj.bjc.6601846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnon AG, Ssemwogerere A, Lamont DW, et al. Relation between socio-economic deprivation and pathological prognostic factors in women with breast cancer. BMJ. 1994;309:1054–1057. doi: 10.1136/bmj.309.6961.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaffashian F, Godward S, Davies T, et al. Socioeconomic effects on breast cancer survival: proportion attributable to stage and morphology. Br J Cancer. 2003;89:1693–1696. doi: 10.1038/sj.bjc.6601339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haybittle JL, Blamey RW, Elston CW, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundquist M, Thorstenson S, Brudin L, et al. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Group. Breast Cancer Res Treat. 1999;53:1–8. doi: 10.1023/a:1006052115874. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 5th edition. New York: Wiley-Liss; 1997. [Google Scholar]
- 15.Machin D, Cheung YB, Parmar MKB. Survival Analysis: A Practical Approach. Chichester: Wiley; 2006. [Google Scholar]
- 16.Wishart GC, Greenberg DC, Britton PD, et al. Screen-detected versus symptomatic breast cancer—is improved survival due to stage migration alone? Br J Cancer. 2008;98:1741–1744. doi: 10.1038/sj.bjc.6604368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basnett I, Gill M, Tobias JS. Variations in breast cancer management between a teaching and non-teaching district. Eur J Cancer. 1992;28:1945–1950. doi: 10.1016/0959-8049(92)90233-r. [DOI] [PubMed] [Google Scholar]
- 18.Wishart GC, Harnett AN, Purushotham AD. Oestrogen receptor status: no longer an optional extra. Breast. 1998;7:154–155. [Google Scholar]
- 19.Mander BJ, Heal K, Purushotham AD, et al. The importance, availability, and measurement of oestrogen receptor (ER) status in the management of breast cancer in the UK: results of a nationwide survey. Lancet. 1998;352:36–37. doi: 10.1016/s0140-6736(05)79517-9. [DOI] [PubMed] [Google Scholar]
- 20.Wishart GC, Gaston M, Poultsidis AA, Purushotham AD. Hormone receptor status in primary breast cancer—time for a consensus? Eur J Cancer. 2002;38:1201–1203. doi: 10.1016/s0959-8049(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 21.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 22.British Association of Surgical Oncologists (BASO) Guidelines for the management of symptomatic breast disease. Eur J Surg Oncol. 2005;31(Suppl):1–21. doi: 10.1016/j.ejso.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.MacNeill F NEW START Steering Group. NEW START: the UK SLNB training programme—a progress report. Ann R Coll Surg Engl. 2007;89(Suppl):60–61. [Google Scholar]