Abstract
The more we learn about the intervertebral disc (IVD), the more we come to appreciate the intricacies involved in transmission of forces through the ECM to the cell, and in the biological determinants of its response to mechanical stress. This review highlights recent developments in our knowledge of IVD physiology and examines their impact on cellular mechanobiology. Discussion centers around the continually evolving cellular and microstructural anatomy of the nucleus pulposus (NP) and the annulus fibrosus (AF) in response to complex stresses generated in support of axial load and spinal motion. Particular attention has been given to cells from the immature NP and the interlamellar AF, and assessment of their potential mechanobiologic contributions to the health and function of the IVD. In addition, several innovative approaches that have been brought to bear on studying the interplay between disc cells and their micromechanical environment are discussed. Techniques for “engineering” cellular function and technologies for fabricating more structurally defined biomaterial scaffolds have recently been employed in disc research. Such tools can be used to elucidate the biological and physical mechanisms by which different IVD cell populations are regulated by mechanical stress, and contribute to advancement of preventative and therapeutic measures.
Keywords: intervertebral disc, nucleus pulposus, notochordal, annulus fibrosus, interlamellar
Introduction
The mechanobiology of intervertebral disc (IVD) cells has been an area of significant interest from the perspectives of growth, remodeling, degeneration, and repair. For the human IVD, however, there are several unique challenges that obscure our understanding of these areas. The first is that the reference state of the disc is a moving target. Cell phenotype and extracellular composition are constantly evolving, such that the nature of both the transduced mechanical stimulus on the resident cells and their consequent responses depend on the state of disc maturity and health. Secondly, spinal stresses are inextricably entwined with other intrinsic and extrinsic influences. This coupling of cumulative mechanical factors with these influences such as aging (Buckwalter, 1995, Nerlich, et al., 1997), genetics (Chan, et al., 2006, Videman, et al., 2006), electrokinetic phenomena (Gu, et al., 1999), and transport of nutrients and metabolic products (Jackson, et al., 2008, Soukane, et al., 2007), makes it particularly difficult to distinguish the precise role of mechanobiology in disc physiology and pathophysiology. Thirdly, the link between mechanical stress and clinical disorder is not understood. While there certainly is involvement of mechanical stress in governing extracellular matrix (ECM) alterations, the severity of such changes do not always coincide with the onset of pain associated with degenerative disc disease (DDD). The combination of these challenges have impeded a consensus from being formed as to a set of functional outcomes for reparative/regenerative strategies, which, in turn, has made it difficult to mount a unified effort toward a common endgoal of treatment.
As new knowledge in IVD biology, pathobiology, mechanobiology, and repair is generated, our views of these challenges are being reshaped. Over the past few years, numerous review articles have been published on these various aspects of the IVD, demonstrating the burgeoning efforts in this field. In particular, some very comprehensive reviews of disc mechanobiology serve as excellent reference material (Iatridis, et al., 2006, Lotz, et al., 2002, Setton and Chen, 2004, Setton and Chen, 2006). Rather than re-visit concepts adroitly covered by these other reviews, we will center our discussion on two relatively new focus areas in IVD research whose contributions to disc mechanobiology are not well understood, but are potentially significant: the immature nucleus pulposus (NP) and the interlamellar annulus fibrosus (AF). In the following sections, we briefly outline the structural, microstructural, and compositional elements of the disc and consider their roles in the mechanobiology of immature NP and interlamellar AF cells. We believe that an improved understanding of these cells could have profound impact on the perception of how mechanical stress regulates disc health. Space limitations and the specific nature of this review precluded comprehensively citing all work in IVD cell mechanobiology, and we sincerely apologize to those authors whose work was omitted due to these constraints.
Biomechanical function of the intervertebral disc
The IVD provides six degrees of freedom to a spinal motion segment and serves as a central axial structure for cushioning loads. Physiologically, the disc experiences intricate combinations of forces and deformations (Li, et al., 2009), determined by voluntary movements, external perturbations, and stabilizing forces in the spine. Range of motion is limited by known kinematic constraints of the facet joints and ligamentous structures (Onan, et al., 1998, Smith, 1991). Complexities arise because the spine is subjected to moments that generate coupled rotations (Li, et al., 2009, Little, et al., 2008, Panjabi, et al., 1989). Follower loads from stabilizing structures (Patwardhan, et al., 1999, Rohlmann, et al., 2001, Wilke, et al., 2003) and intra-abdominal pressure (Andersson, et al., 1977, Cholewicki, et al., 1999, Daggfeldt and Thorstensson, 1997, Grillner, et al., 1978) also influence the resultant loads. Further, intrinsic and extrinsic mechanical stresses are often dynamic and can occur over multiple time scales. Taken together, these various elements complicate the loading environment of the IVD, such that experimental replication of true physiologic loading conditions in the disc is problematic.
Despite these complexities, the disc appears optimally organized such that subregions can maintain load-bearing responsibilities for a majority of time during transient spinal motions. Thus, different physiologic loading modes are more likely to influence magnitude, rather than mode, of mechanical stress. Specifically, the NP is associated with hydrostatic pressurization, while the AF resists biaxial tensile stresses and shear, both within and between lamellae. The ability for each subregion to remain within physiologic ranges of stress/strain, however, changes with load history and health. Linking the biological and biomechanical principles identified from reductionist approaches to the physiology of the disc has been, and continues to be, a key hurdle in IVD mechanobiology.
Nucleus pulposus
Although immature NP cells have previously been thought to have little bearing on disc health, a review of the anatomic and microstructural changes of the NP during growth and aging suggests that they may possess characteristics necessary for IVD homeostasis. This has stimulated some recent work focused on the nature of immature NP cells (Chen, et al., 2006, Guehring, et al., 2009, Hunter, et al., 2007, Hunter, et al., 2004, Hunter, et al., 2004, Rastogi, et al., 2009). Interestingly, in vivo animal models of disc loading, intended to examine stresses experienced by adult discs, have provided a wealth of information upon which to build more mechanistic investigations into immature NP cell mechanobiology.
Anatomy and microstructure
An area of the IVD that is in a relatively high state of flux early in life is the NP. The immature NP contains both large, highly vacuolated chordocytes and small chordoblasts inherited from its precursor, the notochord (Bancroft and Bellairs, 1976, Parsons, et al., 2002, Pettway, et al., 1996, Vilovic, et al., 2001). While largely considered only as a transient signaling structure, the notochord also serves as a primitive axial support structure (Stemple, 2005), possibly by acting as a hydrostatic skeleton. There is evidence that the interior of the notochord is pressurized with its integrity maintained by the proteoglycan (PG)- and laminin-rich sheath (Platz, 2006). During embryonic morphogenesis, the notochord is pinched off by the formation of vertebral bodies (Aszodi, et al., 1998, Grotmol, et al., 2003). With growth, the resulting notochordal islands that constitute the NP develop even greater pressures. Resting intradiscal pressures have been measured in situ to range from 80–340 kPa in immature discs of rat, rabbit, and pig (Ekstrom, et al., 2004, Guehring, et al., 2005, Nesson, et al., 2008). Similarities between immature NP and the notochord in composition and function suggests that these resident cells and their protein products may be crucial.
In humans, the NP changes drastically by adulthood. The cell population becomes what has been described as predominantly chondrocytic. Although mature NP cells in humans are small and produce an aggrecan-rich matrix, their gene expression profile and metabolic activity are distinct from articular cartilage (Melrose, et al., 2003, Poiraudeau, et al., 1999, Steck, et al., 2005). It remains unclear what the mechanisms are that drive the change in cell population. Some evidence points to migration of cells from the inner annulus and cartilage endplate (Kim, et al., 2003), but one possibility not previously considered is that chordoblasts, which are responsible for maintaining the PG sheath, could proliferate and form an NP cell population unique to the adult disc. While the cell population has changed, the tissue retains a relatively gelatinous consistency early into adulthood (Humzah and Soames, 1988). Over time, however, the elastic properties of the NP begin to dominate (Iatridis, et al., 1997), and the swelling characteristics become compromised (Johnstone, et al., 1992, Urban and McMullin, 1988). Together, these changes reduce hydration and impair the NP’s ability to function as a hydrostatic element, leading to altered stress distributions in the disc (Adams, et al., 1996, Adams, et al., 1996, Gabai and Hsieh, 2007, Yerramalli, et al., 2007). Although it is generally assumed that changes are due entirely to dismantling of the ECM by adult NP cells, it is also possible that adult NP cells are intrinsically unable to produce appropriate matrix proteins to maintain tissue architecture.
In terms of the ECM, the collagen of immature NP is exclusively type II (Adams, et al., 1977, Nerlich, et al., 1998), but in only sparse amounts on the order of 5–10% of total dry weight (Ghosh, et al., 1976). More precisely, the expressed isoform is type IIA procollagen, which is also expressed by progenitor cells during chondrogenesis (Sandell, et al., 1991), but not by mature chondrocytes (Sandell, 1994, Sandell, et al., 1994). The NH2-propeptide of type IIA collagen has been hypothesized to play a signaling, rather than structural, role, because in the NP it does not co-localize with the triple helical region (Zhu, et al., 2001). Aggrecan levels are very high and have unique characteristics in the immature NP. In particular, PGs exist mostly as monomers that exhibit lesser tendency to aggregate via hyaluronic acid compared with articular cartilage (Buckwalter, et al., 1985, Buckwalter, et al., 1989, Stevens, et al., 1979). Their lengths are estimated to be 70% shorter than PGs from cartilage and 40% shorter than those from the AF (Buckwalter, et al., 1989), and have a relatively high keratan sulfate content (Melrose, et al., 2001, Stevens, et al., 1979). Together, these components of the ECM impart a fluidic nature to the NP that suggests an enhanced ability to generate and sustain hydrostatic pressure during disc loading.
With aging, alterations in tissue properties stem from microstructural reorganization due, in part, to changes in collagen composition, increases in collagen content, and decreases in PG content. Instead of consisting exclusively of types II and IX collagens, adult NP over 30 years of age possess relatively large amounts of types I and III collagens, with an increase in type X collagen expression in older discs (Nerlich, et al., 1998, Nerlich, et al., 1997). It is also not clear when the type IIA isoform gives way to type IIB, which comprises adult cartilage, but loss of the putative signaling NH2-propeptide may also contribute to the gradual change in cell and ECM composition. PG content and its ability to aggregate decreases with age (Buckwalter, et al., 1985, Cole, et al., 1986, Olczyk, 1994) due to the degradation of link protein (Donohue, et al., 1988, Pearce, et al., 1989) and proteolytic cleavage of PG monomers by matrix metalloproteinases and aggrecanases (Sztrolovics, et al., 1997). It is believed that as a consequence, PGs are less able to stay entrapped in the NP. Glycosaminoglycan (GAG) profiles of existing PGs shift further toward keratan sulfate (Adams, et al., 1977, Cole, et al., 1986, Olczyk, 1994). The increasing collagen-to-PG ratio of the aging ECM may induce higher shear stresses during compressive load-bearing in the NP, leading to a vicious cycle of stimulating greater amounts of fibrous tissue formation.
Cell Mechanobiology
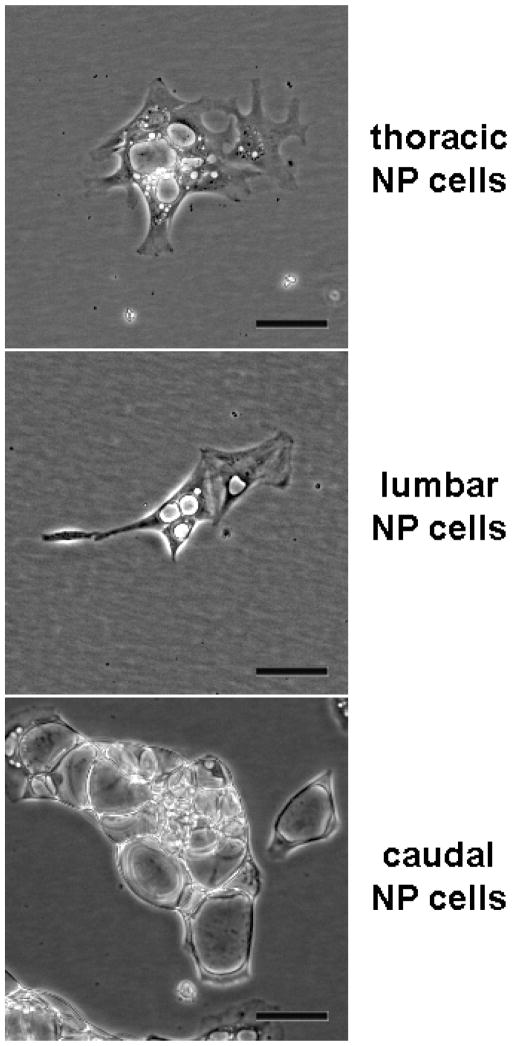
Evidence from our lab and others suggests that changes in immature NP cells may be very sensitive to mechanical environment. For example, our unpublished observations of morphologic distinctions among NP cells isolated from different spinal levels of juvenile Macaca fascicularis (Long-tailed or Crab-eating macaques) suggest an association with expected loading exposure. Specifically, NP cells from lumbar levels, those typically subjected to the greatest stress in upright species, possess smaller vacuoles and extend greater numbers of cell processes compared with those from caudal discs, which likely support much lower levels of compression (Figure 1).
Figure 1.
Morphology of immature NP cells isolated from juvenile Macaca fascicularis spines after 1 week in culture. Vacuolar appearance and cell shape depend on spinal level, suggesting subtle phenotypic differences associated with variation in disc mechanical exposures. Scale bars represent 50 μm.
In rodents, dynamic compression of caudal discs has been one way to study immature NP cell mechanobiology in situ, and have provided great insight into the regulation of gene expression in response to tissue level dynamic loading regimens. It has been shown that NP cells exhibit little alteration in expression of genes relevant to matrix remodeling under low loads comparable to normal activities, regardless of loading frequency (MacLean, et al., 2004). Supraphysiologic loads applied for short durations stimulate a remodeling response consistent with normal composition of the NP, whereas very low frequency or longer duration loading tends to generate increased type I collagen expression.
In long-term applications of cyclic 1 MPa compression, brief sessions of daily loading result in maintenance of NP gene expression and PG content, as long as stimulation frequencies remain around 1 Hz. With much lower or higher frequencies, and longer durations per loading session, genes associated with ECM remodeling are upregulated (MacLean, et al., 2004, MacLean, et al., 2005, Wuertz, et al., 2009). Others have demonstrated greater decreases in disc height and mechanical stability, even while PG content increases (Ching, et al., 2003, Ching, et al., 2004). These observations might be explained in part by apoptosis induction observed concurrently with increased PG areal density (Walsh and Lotz, 2004). The space created by cell death could be filled (ineffectively) by PGs, leading to compromised disc function and altered tissue stress distributions.
Prior studies using static compression to stimulate degenerative changes suggest spatio-temporal dependent compaction of NP cells and tissue in mouse tail discs (Hsieh, et al., 2005, Lotz, 2004) that appear related to apoptosis (Lotz and Chin, 2000) as well as other catabolic processes (Hsieh and Lotz, 2003). These cellular effects lead to changes in the mechanics of the disc, as observed in both mice (Lotz, et al., 1998) and rats (Iatridis, et al., 1999). Overall, the data from these animal models suggest a complex dependence of deformation on loading magnitude, frequency, and duration of loading regimen over time.
For the immature NP, the fluidic nature of the ECM allows effective hydrostatic pressure generation during loading. Although typically regarded as beneficial, hydrostatic pressure can also have undesired effects. In immature NP cells from rabbit and pig, hydrostatic pressures as low as 1 MPa can increase collagen degradation if applied at high frequencies (Kasra, et al., 2003, Kasra, et al., 2006). Based on our recent studies of load history effects on stresses in the immature NP, the ability for the NP to pressurize further appears to be tied to the disc’s hydration and stress state (Hwang, et al., 2009). Because immature NP cells appear to be very sensitive to distortion and pressure, improved characterization of age group-specific mechanical stress exposures on the disc and stress distribution through the disc would benefit our understanding of initial changes during growth and aging.
Annulus fibrosus
Functionally, the AF of the IVD is an intriguing tissue that bears resemblance to both ligaments and large arteries. While tethered to vertebrae and providing tensile integrity to multiaxial translations and rotations, it is also responsible for resisting circumferential elongation generated by internal pressure from the NP. It is tempting to simplify the mechanical environment within the AF to gross level deformations, but a closer look reveals microstructural complexities that belie such assumptions and represent important considerations in cellular mechanobiology.
Anatomy and microstructure
The AF surrounds the NP with layers of unidirectional collagen lamellae. Across successive lamellae, collagen fibers alternate between directions of approximately +30° and −30° with respect to the transverse plane, but exhibit both intra- and interlamellar variations in fiber angle (Cassidy, et al., 1989, Hickey and Hukins, 1980, Holzapfel, et al., 2005, Stokes and Greenapple, 1985). The AF has traditionally been divided into three regions, an inner, middle, and outer AF. This is both a morphologic and a developmental distinction, as the inner AF arises simultaneously with endochondral formation of the vertebral bodies (Rufai, et al., 1995). In contrast, the outer AF originates as a separate cell condensation with slower matrix formation kinetics. Accordingly, there is variation in the structural components from inner to outer AF. While lamellae of the inner AF consist mostly of type II collagen and fibrochondrocytes, those of the outer AF are composed predominantly of type I collagen and are populated by fibroblasts (Eyre and Muir, 1976). A population of interlamellar cells that exhibit a flattened “pancake” morphology has also been described (Bruehlmann, et al., 2002, Errington, et al., 1998, Hastreiter, et al., 2001), but their relationship to inner and outer AF cells are not yet known. The PG profile changes from mostly aggrecan in the inner AF to mostly decorin and fibromodulin in the outer AF (Hayes, et al., 2001, Melrose, et al., 2001). Elastin fibers have been detected within lamellae, in vertebral attachments, at the NP-AF interface, and abundantly in “bridges” across the interlamellar ECM. Although elastin exists in relatively low amounts (less than 5% of the IVD dry weight), this component has been hypothesized to play a significant role in the mechanical function of the AF (Buckwalter, et al., 1976, Johnson, et al., 1982, Yu, 2002, Yu, et al., 2007).
Microstructurally, the AF bears several similarities to ligaments and tendons, relying on collagen fiber bundles organized in parallel to resist tensile stresses (Broberg and von Essen, 1980, Cassidy, et al., 1989, Hickey and Hukins, 1980, Holzapfel, et al., 2005, Stokes and Greenapple, 1985). The staggered discontinuous nature of the fibrillar collagen microstructure (Holzapfel, et al., 2005, Marchand and Ahmed, 1990) results in complex transmission of force along lamellae, and has profound impact on our view of mechanotransduction. As observed in bovine AF (Bruehlmann, et al., 2004, Bruehlmann, et al., 2004), and similarly in rat tail tendon (Screen, et al., 2004), tissue stretch does not uniformly propagate across the levels of collagen. Rather, straightening of collagen crimp and fibril-fibril sliding leads to nonuniformly increasing fibril recruitment at different loads, and appears to account for the majority of tissue level deformations. These phenomena have been demonstrated both through intercellular measurements within and among collagen fibers (Bruehlmann, et al., 2004, Bruehlmann, et al., 2004, Screen, et al., 2004) and measuring the deformation of photobleached lines across labeled collagen fibers, themselves (Bruehlmann, et al., 2004). The mechanisms and kinetics of recovery, whether passive or actively cell-mediated, are not yet clear. Thus, not only is cell stretch a mechanism of stimulation during AF deformation, but shear stress likely also serves as a robust determinant of cell function.
Recent studies have improved our understanding of the interlamellar and translamellar space, both of which could play important roles in cellular mechanobiology. Evidence suggests that these regions arise from distinct origins but become compositionally similar during maturation. Developmentally, translamellar channels appear to be at least partially involved in tissue vascularization of the outer AF (Melrose, et al., 2008). With growth, vascular structures disappear, and the space becomes occupied with components similar to the interlamellar ECM (Melrose, et al., 2008, Yu, 2002, Yu, et al., 2007). Specifically, these regions consist of an elaborate age- and zonal-dependent network containing collagen microfibrils, elastin, and large PGs. The outer AF possesses higher elastin density and greater elastin co-localization with microfibrils compared with the inner AF (Yu, et al., 2007). Large PGs such as aggrecan and versican, as well as types I and VI collagen, permeate both inter- and translamellar ECM (Melrose, et al., 2008, Ortolani, et al., 1988).
Cell Mechanobiology
As with the NP, most of what is known about AF mechanobiology has come from in vivo animal studies and organ culture experiments. The advantage of such approaches is the ability to study cellular response in the context of the cells’ native biochemical and biophysical milieu. In the AF, this is particularly critical, given the complex three-dimensional microstructure, which can greatly influence cell deformations, pressurization, and convective forces. Thus, responses of AF cells to stresses experienced in situ are more likely to be physiologically relevant. On the other hand, the ability to resolve specific physical mechanisms of mechanotransduction is sacrificed, which is significant considering the varied physical microenvironments and cell phenotypes in the AF. Through these types of studies, the general trends in reported data are consistent with what has been theorized to be the primary load-bearing function of the AF. Specifically, loading regimens that tend to maintain the AF under tension minimize deleterious effects. For instance, cyclic loading at lower applied stresses and higher frequencies are associated with less apoptosis and lower levels of expression for certain MMPs and ADAM-TSs (MacLean, et al., 2004, Walsh and Lotz, 2004). Lower frequencies of loading results in a shift in the balance of gene expression toward a more catabolic profile (MacLean, et al., 2004). In extreme cases of static loading at high stress magnitudes, which has been computationally attributed to decreased annular tension (Lotz, et al., 1998), AF cells become apoptotic, and possess de-regulated gene expression and MMP activation (Hsieh and Lotz, 2003, Lotz and Chin, 2000, Lotz, et al., 1998). The importance of tensional stress in the AF has also been demonstrated by subjecting the disc to transient bending (Court, et al., 2007, Court, et al., 2001, Lotz, et al., 2008), and by impairing the disc’s ability to pressurize via puncture injury (Hsieh, et al., 2009).
As with most tissues that possess an organized hierarchical structure of collagen, the mechanobiology of cultured AF cells has generally been studied using monolayer stretch approaches, under the assumption that the primary stimulus of cells in tissue is elongation. Considering that within-fiber strains are estimated to be a fraction (12–20%) of tissue level strain for fascicular structures (Bruehlmann, et al., 2004, Screen, et al., 2004), subjecting AF cells to strains (~1–2%) corresponding to axial IVD compression or single degree of freedom rotations (Heuer, et al., 2008, Stokes, 1987) appear not to influence PG synthesis significantly (Rannou, et al., 2003). However, larger cell stretch magnitudes – such as those that would be encountered during multi-axial bending motions with compression – decrease PG synthesis and moderate the adverse effects of inflammatory stimuli (Rannou, et al., 2003, Sowa and Agarwal, 2008). Very high deformations induce apoptosis and production of inflammatory mediators (Miyamoto, et al., 2006, Rannou, et al., 2004). Cyclic hydrostatic pressure and unconfined alginate compression studies have also been conducted, with general trends indicating that relatively high levels of pressure or deformation (within limits) are required to induce a stimulatory response (Chen, et al., 2004, Handa, et al., 1997, Hutton, et al., 1999, Hutton, et al., 2001, Ishihara, et al., 1996, Kasra, et al., 2003, Kasra, et al., 2006, Korecki, et al., 2008, Reza and Nicoll, 2008).
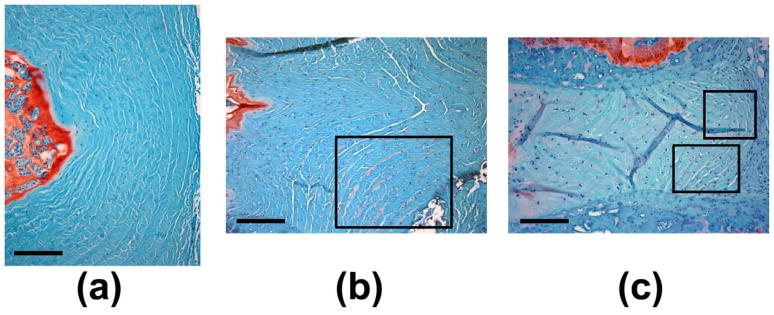
Considering the AF’s inhomogeneous cell population, non-uniform stress distribution, and spatially varying microstructure, our understanding of AF cell mechanobiology would benefit from improved characterization of cell phenotype-specific responses. Distinguishing between inner and outer AF cells has been a typical approach, but one unstudied area with potentially important implications in disc health is the mechanobiology of the interlamellar compartment. Changes observed in the interlamellar space suggest that these cells are mechanobiologically active and may contribute to age-related changes in disc mechanics and the progression of disease. Based on prior findings, we may be able to make some inferences regarding the mechanical environment and its role in disc health. In humans, AF elastin content as measured by a dye-binding assay increases with degeneration grade, with greater increases in the inner AF compared with outer AF (Cloyd and Elliott, 2007). Human scoliotic discs, however, exhibit a decrease in elastin immunostaining with less regularity and greater distortion (Yu, et al., 2005). In some of our own investigations (Hsieh, et al., 2009, Lotz, et al., 2008), degenerative changes induced by static 1.3 MPa mechanical compression or 18g hypodermic needle puncture in rodent caudal discs have been accompanied by increases in PG content within the interlamellar space, as demonstrated by greater Safranin-O staining together with rounded cell morphologies (Figure 2). The interlamellar composition also appears to change dramatically with growth. Specifically, in ovine discs aggrecan, versican, and type VI collagen are present in adult discs but not detectable in newborn discs (Melrose, et al., 2008).
Figure 2.
Images of annulus fibrosus regions from mid-sagittal paraffin sections stained with Safranin-O/Fast green. Normal rat caudal discs (a) possess very little detectable interlamellar Safranin-O staining. Boxed regions highlight substantial interlamellar proteoglycan staining with overall degenerative changes in (b) punctured rat caudal discs, and (c) compressed mouse caudal discs. Scale bars represent 200 μm.
Some recent studies using cryosectioned and fully hydrated bovine AF tissues have provided some insight into interlamellar matrix deformation during annular tension. When tension is applied along the direction of collagen bundles of alternating lamellae (and obliquely in intervening lamellae), significant shear appears to be generated in the interlamellar ECM (Pezowicz, et al., 2006). Although it is unclear how much these observations are accentuated by sample preparation and idealized uniaxial loading, induction of interlamellar shear deformations during IVD loading is plausible and probably important. One piece of supporting evidence is the localization of lubricin in the interlamellar space (Shine and Spector, 2008), suggesting that these regions facilitate relative sliding of adjacent lamellae during spinal motions and inhibit delamination of lamellae. In addition to shear, there is likely significant radial compression caused by bulging of successive lamellae, as well. Collagen crimp angle increases from outer to inner AF (Cassidy, et al., 1989), leading to depth-dependent decreases in both toe and linear region stiffness (Holzapfel, et al., 2005). Thus, during NP pressurization as one moves deeper from outer to inner AF, each lamella may exhibit successively greater tendency to bulge outward, generating compression between lamellae. The resultant compressive stress coupled with lamellar sliding could create a unique mechanical environment in the AF similar to that of the superficial zone of cartilage, and could explain the localization of lubricin and PGs in the interlamellar space in the outer AF. With excessive deformations and pressurization, the progressive shear failure of the interlamellar ECM together with changes in radial stresses may result in the observed changes in human and animal models of degenerate discs.
Novel approaches for IVD cellular mechanobiology research
Traditional approaches in cellular mechanobiology involve the application of various modes, magnitudes, and frequencies of mechanical stress in different experimental systems. These include loading tissues in vivo and in organ culture, or loading cells in monolayer and in scaffolds. Each of these general strategies possesses its own set of advantages and disadvantages, and allows specific aspects of mechanobiology to be addressed, as driven by the research question.
Various techniques in genetic manipulation and biomaterials fabrication have been used to augment the implementation of these traditional approaches in disc cell mechanobiology research. In the realm of molecular and cell biology, the ability to “engineer” cellular function has broadened our capabilities to address mechanistic questions. Previously, such studies were limited to investigating short-term changes in cellular processes using exogenously added inhibitors or stimulating factors. Alternatively, transgene technology has permitted studying how the presence or absence of a protein might influence tissue and disease development.
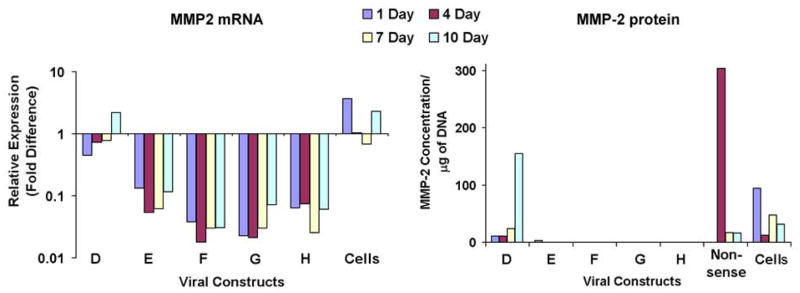
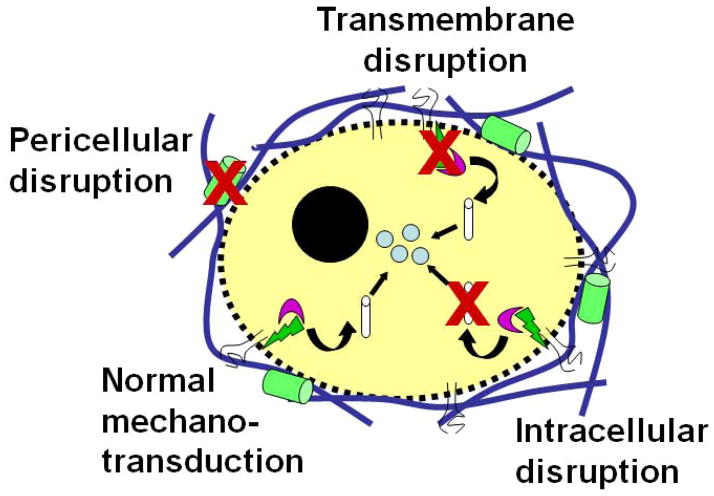
Relatively new recombinant DNA techniques for protein engineering as well as for stable endogenous expression and silencing of genes can be used to impart functionality to cells. Thus far, studies using these tactics in the IVD have focused on increasing endogenous growth factor expression (Cui, et al., 2008, Moon, et al., 2008, Wang, et al., 2004, Yoon, et al., 2004, Zhang, et al., 2006) and silencing matrix metalloproteinase (MMP) (Rastogi and Hsieh, 2009) and Fas ligand expression (Suzuki, et al., 2009), in order to understand factors involved in disc degeneration and regeneration. Using lentiviral transduction of primary AF cells, we have been able to demonstrate stable knockdowns > 95% of MMP-2 at both mRNA and protein levels using various shRNA constructs (Figure 3). These techniques can also be adopted in mechanobiology for elucidating the roles of specific molecules in cellular mechanosensation, mechanotransduction, and mechanoresponsiveness. One of the paradigms that we have begun exploring is the use of RNA interference (RNAi) for engineering the mechanobiology of mesenchymal stem cells at these different levels (Figure 4). Considering the adverse mechanical environment that is believed to exist in degenerate discs, such an approach could be used to engineer a desired response in cells injected into the disc space.
Figure 3.
Knockdown of MMP-2 gene expression in AF cells using RNA interference. Real-time RT-PCR (left) reveals greater than 95% knockdown of mRNA levels relative to a nonsense control for 2 out of the 5 shRNA constructs tested (F and G). An ELISA-based protein assay (right), however, shows effective sustained knockdown on the protein level for 4 out of the 5 constructs (E, F, G, and H).
Figure 4.
Schematic illustrating the potential implementation of RNA interference to modify mechanotransduction at the extracellular, transcellular, and intracellular levels.
In the area of chemical and biomolecular engineering, there has been tremendous growth in the development of scaffolds geared toward tissue replacement. Important limitations in the interpretation of prior mechanobiologic studies arose from the lack of structural influences on cultured cell deformations, and the inability to relate mechanobiology directly to cell deformations in native tissues. The capability to control the nanostructural and biochemical properties of such scaffolds offers the potential to study cellular mechanobiology with unprecedented resolution. Recently, several reports of the nanofibrous scaffolds using different polymers have demonstrated strong potential for use in tissue engineering applications (Nerurkar, et al., 2007, Nesti, et al., 2008, Yang, et al., 2008). It has been shown that scaffolds such as these are amenable to cell deformation measurements, which can then be quantitatively related to scaffold deformation as well as mechanobiologic response (Stella, et al., 2008). Native fibrillar collagen thin films have also been developed recently (Elliott, et al., 2003). Advantages of this platform include the ease with which live cell imaging experiments can be performed to examine how cells interact with and remodel fibrillar collagen, and the ability to modulate microstructural density by varying collagen concentration. Our preliminary studies have found that type II collagen possesses what appear to be straighter, thicker fibrils compared with type I collagen (Figure 5a). Moreover, primary AF cell function are better preserved on collagen films than on tissue culture plastic, particularly with short duration cultures (Morschauser, et al., 2009). Specifically, cell adopt a less flattened cell shape with greater extension of cell processes and fewer stress fibers (Figure 5b), along with smaller variations in cell area as assessed by quantitative microscopy (Figure 5c). Importantly, real-time RT-PCR demonstrates that expression of type II collagen is maintained and that of aggrecan is stimulated on CTFs, suggesting improved retention of cell phenotype (Figure 5d). These substrates and others can potentially be adapted further to study important interactions between microstructure and mechanobiology.
Figure 5.
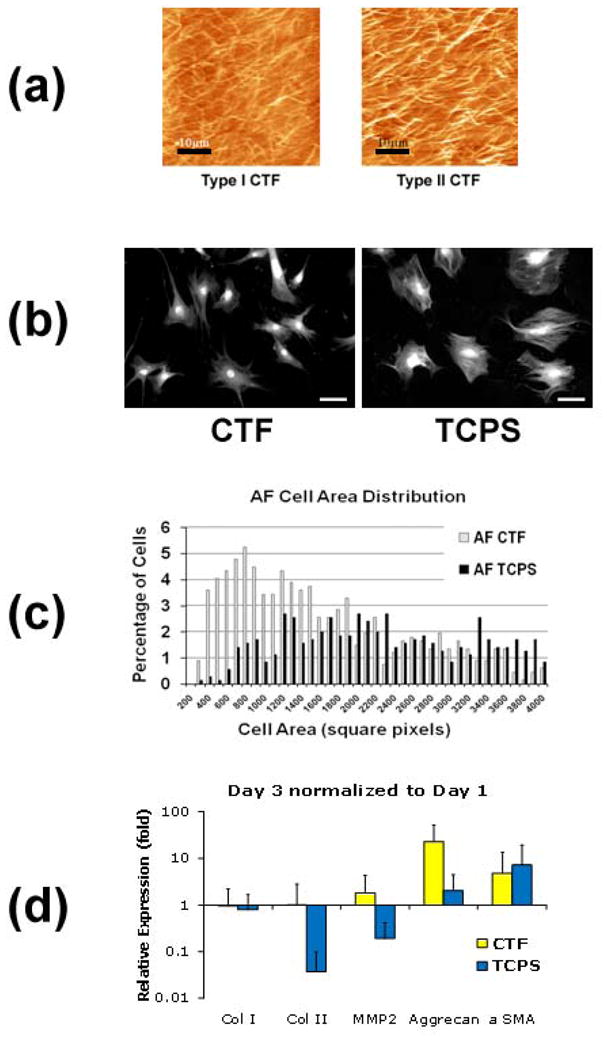
(a) Type I (left) and type II (right) collagen thin films consist of a bed of native collagen fibrils. Scale bars represent 10 μm. (b) Culturing AF cells on type I collagen films (CTF) stimulates more physiologic cell morphologies compared with tissue culture polystyrene (TCPS). Scale bars represent 20 μm. (c) Automated quantitative microscopy shows smaller variation in attachment area for cells cultured on CTFs. (d) CTFs promote the maintenance of cell phenotype as assessed by real-time RT-PCR.
Concluding Remarks
Our understanding of cellular mechanobiology in the IVD has grown by leaps and bounds during this past decade. Nonetheless, because of technological advances that have spurred the development of new methods to characterize, manipulate, and measure how cells interact with and respond to their micromechanical environment, there continue to be opportunities to improve current understanding and address unresolved research questions. Innovation will ensure that we continue to make progress toward our goal toward prevention and therapy of disc disorders.
Acknowledgments
The authors would like to thank the National Institutes of Health (AR054051), the National Science Foundation (CBET-0845754), the National Institute of Standards and Technology, and the University of Maryland, College Park for providing funding that supported some of the work mentioned as well as the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MA, McMillan DW, et al. Sustained loading generates stress concentrations in lumbar intervertebral discs. Spine. 1996;21:434–8. doi: 10.1097/00007632-199602150-00006. [DOI] [PubMed] [Google Scholar]
- Adams MA, McNally DS, et al. 'Stress' distributions inside intervertebral discs. The effects of age and degeneration. Journal of Bone and Joint Surgery. British Volume. 1996;78:965–72. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- Adams P, Eyre DR, et al. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehabil. 1977;16:22–9. doi: 10.1093/rheumatology/16.1.22. [DOI] [PubMed] [Google Scholar]
- Andersson GB, Ortengren R, et al. Intradiskal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop Relat Res. 1977:156–64. doi: 10.1097/00003086-197711000-00018. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Chan D, et al. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft M, Bellairs R. The development of the notochord in the chick embryo, studied by scanning and transmission electron microscopy. J Embryol Exp Morphol. 1976;35:383–401. [PubMed] [Google Scholar]
- Broberg KB, von Essen HO. Modeling of intervertebral discs. Spine. 1980;5:155–67. doi: 10.1097/00007632-198003000-00010. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Hulme PA, et al. In situ intercellular mechanics of the bovine outer annulus fibrosus subjected to biaxial strains. J Biomech. 2004;37:223–31. doi: 10.1016/s0021-9290(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Matyas JR, et al. ISSLS prize winner: Collagen fibril sliding governs cell mechanics in the anulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine. 2004;29:2612–20. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, et al. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–71. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Cooper RR, et al. Elastic fibers in human intervertebral discs. J Bone Joint Surg Am. 1976;58:73–6. [PubMed] [Google Scholar]
- Buckwalter JA, Pedrini-Mille A, et al. Proteoglycans of human infant intervertebral disc. Electron microscopic and biochemical studies. J Bone Joint Surg Am. 1985;67:284–94. [PubMed] [Google Scholar]
- Buckwalter JA, Smith KC, et al. Articular cartilage and intervertebral disc proteoglycans differ in structure: an electron microscopic study. J Orthop Res. 1989;7:146–51. doi: 10.1002/jor.1100070121. [DOI] [PubMed] [Google Scholar]
- Cassidy JJ, Hiltner A, et al. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- Chan D, Song Y, et al. Genetics of disc degeneration. Eur Spine J. 2006;15(Suppl 3):S317–25. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yan W, et al. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–83. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Yan W, et al. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 15):303–11. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching CT, Chow DH, et al. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech (Bristol, Avon) 2003;18:182–9. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Ching CT, Chow DH, et al. Changes in nuclear composition following cyclic compression of the intervertebral disc in an in vivo rat-tail model. Med Eng Phys. 2004;26:587–94. doi: 10.1016/j.medengphy.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Juluru K, et al. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32:13–7. doi: 10.1016/s0021-9290(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Cloyd JM, Elliott DM. Elastin content correlates with human disc degeneration in the anulus fibrosus and nucleus pulposus. Spine. 2007;32:1826–31. doi: 10.1097/BRS.0b013e3181132a9d. [DOI] [PubMed] [Google Scholar]
- Cole TC, Ghosh P, et al. Variations of the proteoglycans of the canine intervertebral disc with ageing. Biochim Biophys Acta. 1986;880:209–19. doi: 10.1016/0304-4165(86)90082-6. [DOI] [PubMed] [Google Scholar]
- Court C, Chin JR, et al. Biological and mechanical consequences of transient intervertebral disc bending. Eur Spine J. 2007;16:1899–906. doi: 10.1007/s00586-007-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court C, Colliou OK, et al. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1:239–45. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Cui M, Wan Y, et al. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–95. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Daggfeldt K, Thorstensson A. The role of intra-abdominal pressure in spinal unloading. J Biomech. 1997;30:1149–55. doi: 10.1016/s0021-9290(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Donohue PJ, Jahnke MR, et al. Characterization of link protein(s) from human intervertebral-disc tissues. Biochem J. 1988;251:739–47. doi: 10.1042/bj2510739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom L, Holm S, et al. In vivo porcine intradiscal pressure as a function of external loading. J Spinal Disord Tech. 2004;17:312–6. doi: 10.1097/01.bsd.0000092068.78152.00. [DOI] [PubMed] [Google Scholar]
- Elliott JT, Tona A, et al. Thin films of collagen affect smooth muscle cell morphology. Langmuir. 2003;19:1506–1514. [Google Scholar]
- Errington RJ, Puustjarvi K, et al. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192 ( Pt 3):369–78. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 1976;157:267–70. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai AS, Hsieh AH. Transient disc mechanical behavior during exertion is dependent on load history. International Society for the Study of the Lumbar Spine 34th Annual Meeting.2007. [Google Scholar]
- Ghosh P, Taylor TK, et al. The collagenous and non-collagenous protein of the canine intervertebral disc and their variation with age, spinal level and breed. Gerontology. 1976;22:124–34. doi: 10.1159/000212129. [DOI] [PubMed] [Google Scholar]
- Grillner S, Nilsson J, et al. Intra-abdominal pressure changes during natural movements in man. Acta Physiol Scand. 1978;103:275–83. doi: 10.1111/j.1748-1716.1978.tb06215.x. [DOI] [PubMed] [Google Scholar]
- Grotmol S, Kryvi H, et al. Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat Embryol (Berl) 2003;207:263–72. doi: 10.1007/s00429-003-0349-y. [DOI] [PubMed] [Google Scholar]
- Gu WY, Mao XG, et al. Streaming potential of human lumbar anulus fibrosus is anisotropic and affected by disc degeneration. J Biomech. 1999;32:1177–82. doi: 10.1016/s0021-9290(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Guehring T, Unglaub F, et al. Intradiscal pressure measurements in normal discs, compressed discs and compressed discs treated with axial posterior disc distraction: an experimental study on the rabbit lumbar spine model. Eur Spine J. 2005 doi: 10.1007/s00586-005-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehring T, Wilde G, et al. Notochordal intervertebral disc cells: Sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60:1026–34. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- Handa T, Ishihara H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–91. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- Hastreiter D, Ozuna RM, et al. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha-smooth muscle actin. J Orthop Res. 2001;19:597–604. doi: 10.1016/S0736-0266(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, et al. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–21. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- Heuer F, Schmidt H, et al. The relation between intervertebral disc bulging and annular fiber associated strains for simple and complex loading. J Biomech. 2008;41:1086–94. doi: 10.1016/j.jbiomech.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Hickey DS, Hukins DW. Relation between the structure of the annulus fibrosus and the function and failure of the intervertebral disc. Spine. 1980;5:106–16. doi: 10.1097/00007632-198003000-00004. [DOI] [PubMed] [Google Scholar]
- Hickey DS, Hukins DW. X-ray diffraction studies of the arrangement of collagenous fibres in human fetal intervertebral disc. J Anat. 1980;131:81–90. [PMC free article] [PubMed] [Google Scholar]
- Holzapfel GA, Schulze-Bauer CA, et al. Single lamellar mechanics of the human lumbar anulus fibrosus. Biomech Model Mechanobiol. 2005 doi: 10.1007/s10237-004-0053-8. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, Hwang D, et al. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine. 2009;34:998–1005. doi: 10.1097/BRS.0b013e31819c09c4. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine. 2003;28:1781–8. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- Hsieh AH, Wagner DR, et al. Dependence of mechanical behavior of the murine tail disc on regional material properties: a parametric finite element study. J Biomech Eng. 2005;127:1158–67. doi: 10.1115/1.2073467. [DOI] [PubMed] [Google Scholar]
- Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988;220:337–56. doi: 10.1002/ar.1092200402. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Bianchi S, et al. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc running title: an osmoregulatory vacuole. Mol Cell Biomech. 2007;4:227–37. [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, et al. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–62. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, et al. The functional significance of cell clusters in the notochordal nucleus pulposus: survival and signaling in the canine intervertebral disc. Spine. 2004;29:1099–104. doi: 10.1097/00007632-200405150-00010. [DOI] [PubMed] [Google Scholar]
- Hutton WC, Elmer WA, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine. 1999;24:1507–15. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- Hutton WC, Elmer WA, et al. Do the intervertebral disc cells respond to different levels of hydrostatic pressure? Clin Biomech (Bristol, Avon) 2001;16:728–34. doi: 10.1016/s0268-0033(01)00080-8. [DOI] [PubMed] [Google Scholar]
- Hwang D, Yu M, et al. Dependence of intervertebral disc pressure generation on load history. ASME Summer Bioengineering Conference; Lake Tahoe, CA. 2009. [Google Scholar]
- Iatridis JC, MacLean JJ, et al. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88(Suppl 2):41–6. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridis JC, Mente PL, et al. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Setton LA, et al. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15:318–22. doi: 10.1002/jor.1100150224. [DOI] [PubMed] [Google Scholar]
- Ishihara H, McNally DS, et al. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol. 1996;80:839–46. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- Jackson AR, Yuan TY, et al. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine. 2008;33:1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EF, Chetty K, et al. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J Anat. 1982;135:301–9. [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Urban JP, et al. The fluid content of the human intervertebral disc. Comparison between fluid content and swelling pressure profiles of discs removed at surgery and those taken postmortem. Spine. 1992;17:412–6. doi: 10.1097/00007632-199204000-00006. [DOI] [PubMed] [Google Scholar]
- Kasra M, Goel V, et al. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21:597–603. doi: 10.1016/S0736-0266(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Kasra M, Merryman WD, et al. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24:1967–73. doi: 10.1002/jor.20253. [DOI] [PubMed] [Google Scholar]
- Kim KW, Lim TH, et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine. 2003;28:982–90. doi: 10.1097/01.BRS.0000061986.03886.4F. [DOI] [PubMed] [Google Scholar]
- Korecki CL, Kuo CK, et al. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2008 doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang S, et al. Segmental in vivo vertebral motion during functional human lumbar spine activities. Eur Spine J. 2009 doi: 10.1007/s00586-009-0936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP, de Visser H, et al. Are coupled rotations in the lumbar spine largely due to the osseo-ligamentous anatomy?--a modeling study. Comput Methods Biomech Biomed Engin. 2008;11:95–103. doi: 10.1080/10255840701552143. [DOI] [PubMed] [Google Scholar]
- Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742–50. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–83. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Colliou OK, et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Hadi T, et al. Anulus fibrosus tension inhibits degenerative structural changes in lamellar collagen. Eur Spine J. 2008;17:1149–59. doi: 10.1007/s00586-008-0721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz JC, Hsieh AH, et al. Mechanobiology of the intervertebral disc. Biochem Soc Trans. 2002;30:853–858. doi: 10.1042/bst0300853. [DOI] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, et al. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–7. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–10. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Melrose J, Ghosh P, et al. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Smith S, et al. Assessment of the cellular heterogeneity of the ovine intervertebral disc: comparison with synovial fibroblasts and articular chondrocytes. Eur Spine J. 2003;12:57–65. doi: 10.1007/s00586-002-0434-6. [DOI] [PubMed] [Google Scholar]
- Melrose J, Smith SM, et al. Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. Eur Spine J. 2008;17:314–24. doi: 10.1007/s00586-007-0538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Doita M, et al. Effects of cyclic mechanical stress on the production of inflammatory agents by nucleus pulposus and anulus fibrosus derived cells in vitro. Spine. 2006;31:4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- Moon SH, Nishida K, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine. 2008;33:1850–5. doi: 10.1097/BRS.0b013e31817e1cd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschauser MA, Plant AL, et al. Intervertebral disc cell morphology on collagen thin films. Transactions of the 55th Annual Meeting of the Orthopaedic Research Society; 2009. p. 1656. [Google Scholar]
- Nerlich AG, Boos N, et al. Immunolocalization of major interstitial collagen types in human lumbar intervertebral discs of various ages. Virchows Arch. 1998;432:67–76. doi: 10.1007/s004280050136. [DOI] [PubMed] [Google Scholar]
- Nerlich AG, Schleicher ED, et al. Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22:2781–95. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Elliott DM, et al. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–28. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- Nesson SC, Yu M, et al. Transient pressure measurements in rat intervertebral discs during stress relaxation. Transactions of the 54th Annual Meeting of the Orthopaedic Research Society; 2008. p. 46. [Google Scholar]
- Nesti LJ, Li WJ, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14:1527–37. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olczyk K. Age-related changes in proteoglycans of human intervertebral discs. Z Rheumatol. 1994;53:19–25. [PubMed] [Google Scholar]
- Onan OA, Heggeness MH, et al. A motion analysis of the cervical facet joint. Spine. 1998;23:430–9. doi: 10.1097/00007632-199802150-00005. [DOI] [PubMed] [Google Scholar]
- Ortolani F, Raspanti M, et al. Localization of different alcian blue-proteoglycan particles in the intervertebral disc. Basic Appl Histochem. 1988;32:443–53. [PubMed] [Google Scholar]
- Panjabi M, Yamamoto I, et al. How does posture affect coupling in the lumbar spine? Spine. 1989;14:1002–11. doi: 10.1097/00007632-198909000-00015. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pollard SM, et al. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–46. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- Patwardhan AG, Havey RM, et al. A follower load increases the load-carrying capacity of the lumbar spine in compression. Spine. 1999;24:1003–9. doi: 10.1097/00007632-199905150-00014. [DOI] [PubMed] [Google Scholar]
- Pearce RH, Mathieson JM, et al. Effect of age on the abundance and fragmentation of link protein of the human intervertebral disc. J Orthop Res. 1989;7:861–7. doi: 10.1002/jor.1100070612. [DOI] [PubMed] [Google Scholar]
- Pettway Z, Domowicz M, et al. Age-dependent inhibition of neural crest migration by the notochord correlates with alterations in the S103L chondroitin sulfate proteoglycan. Exp Cell Res. 1996;225:195–206. doi: 10.1006/excr.1996.0170. [DOI] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, et al. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317–30. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz F. Structural and experimental investigations of the functional anatomy and the turgor of the notochord in the larval tail of anuran tadpoles. Ann Anat. 2006;188:289–302. doi: 10.1016/j.aanat.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Poiraudeau S, Monteiro I, et al. Phenotypic characteristics of rabbit intervertebral disc cells. Comparison with cartilage cells from the same animals. Spine (Phila Pa 1976) 1999;24:837–44. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- Rannou F, Lee TS, et al. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–24. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannou F, Richette P, et al. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J Cell Biochem. 2003;90:148–57. doi: 10.1002/jcb.10608. [DOI] [PubMed] [Google Scholar]
- Rastogi A, Hsieh AH. Engineering the matrix metabolism of intervertebral disc cells using RNA interference. Transactions of the 55th Annual Meeting of the Orthopaedic Research Society; Las Vegas, NV. 2009. p. 1788. [Google Scholar]
- Rastogi A, Thakore P, et al. Environmental regulation of notochordal gene expression in nucleus pulposus cells. J Cell Physiol. 2009;220:698–705. doi: 10.1002/jcp.21816. [DOI] [PubMed] [Google Scholar]
- Reza AT, Nicoll SB. Hydrostatic pressure differentially regulates outer and inner annulus fibrosus cell matrix production in 3D scaffolds. Ann Biomed Eng. 2008;36:204–13. doi: 10.1007/s10439-007-9407-6. [DOI] [PubMed] [Google Scholar]
- Rohlmann A, Neller S, et al. Influence of a follower load on intradiscal pressure and intersegmental rotation of the lumbar spine. Spine. 2001;26:E557–61. doi: 10.1097/00007632-200112150-00014. [DOI] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, et al. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- Sandell LJ. In situ expression of collagen and proteoglycan genes in notochord and during skeletal development and growth. Microsc Res Tech. 1994;28:470–82. doi: 10.1002/jemt.1070280603. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Morris N, et al. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991;114:1307–19. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LJ, Nalin AM, et al. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994;199:129–40. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- Screen HR, Lee DA, et al. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng [H] 2004;218:109–19. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29:2710–23. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am 88 Suppl. 2006;2:52–7. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- Shine KM, Spector M. The presence and distribution of lubricin in the caprine intervertebral disc. J Orthop Res. 2008;26:1398–406. doi: 10.1002/jor.20614. [DOI] [PubMed] [Google Scholar]
- Smith TJ. In vitro spinal biomechanics. Experimental methods and apparatus. Spine. 1991;16:1204–10. doi: 10.1097/00007632-199110000-00013. [DOI] [PubMed] [Google Scholar]
- Soukane DM, Shirazi-Adl A, et al. Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J Biomech. 2007;40:2645–54. doi: 10.1016/j.jbiomech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sowa G, Agarwal S. Cyclic tensile stress exerts a protective effect on intervertebral disc cells. Am J Phys Med Rehabil. 2008;87:537–44. doi: 10.1097/PHM.0b013e31816197ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck E, Bertram H, et al. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- Stella JA, Liao J, et al. Tissue-to-cellular level deformation coupling in cell micro-integrated elastomeric scaffolds. Biomaterials. 2008;29:3228–36. doi: 10.1016/j.biomaterials.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Stevens RL, Ewins RJ, et al. Proteoglycans of the intervertebral disc. Homology of structure with laryngeal proteoglycans. Biochem J. 1979;179:561–72. doi: 10.1042/bj1790561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes I, Greenapple DM. Measurement of surface deformation of soft tissue. J Biomech. 1985;18:1–7. doi: 10.1016/0021-9290(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Stokes IA. Surface strain on human intervertebral discs. J Orthop Res. 1987;5:348–55. doi: 10.1002/jor.1100050306. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nishida K, et al. Sustained long-term RNA interference in nucleus pulposus cells in vivo mediated by unmodified small interfering RNA. Eur Spine J. 2009;18:263–70. doi: 10.1007/s00586-008-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztrolovics R, Alini M, et al. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326 ( Pt 1):235–41. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–87. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Videman T, Battie MC, et al. Determinants of the progression in lumbar degeneration: a 5-year follow-up study of adult male monozygotic twins. Spine. 2006;31:671–8. doi: 10.1097/01.brs.0000202558.86309.ea. [DOI] [PubMed] [Google Scholar]
- Vilovic K, Sapunar D, et al. Morphological characteristics of dying cells in axial structures of developing human embryos. Cells Tissues Organs. 2001;169:347–54. doi: 10.1159/000047901. [DOI] [PubMed] [Google Scholar]
- Walsh AJ, Lotz JC. Biological response of the intervertebral disc to dynamic loading. J Biomech. 2004;37:329–37. doi: 10.1016/s0021-9290(03)00290-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Kroeber M, et al. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med. 2004;82:126–34. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- Wilke HJ, Rohlmann A, et al. ISSLS prize winner: A novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine. 2003;28:2585–93. doi: 10.1097/01.BRS.0000096673.16363.C7. [DOI] [PubMed] [Google Scholar]
- Wuertz K, Godburn K, et al. In vivo remodeling of intervertebral discs in response to short- and long-term dynamic compression. J Orthop Res. 2009 doi: 10.1002/jor.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kandel RA, et al. Polar surface chemistry of nanofibrous polyurethane scaffold affects annulus fibrosus cell attachment and early matrix accumulation. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32331. [DOI] [PubMed] [Google Scholar]
- Yerramalli CS, Chou AI, et al. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- Yoon ST, Park JS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603–11. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- Yu J. Elastic tissues of the intervertebral disc. Biochem Soc Trans. 2002;30:848–852. doi: 10.1042/bst0300848. [DOI] [PubMed] [Google Scholar]
- Yu J, Fairbank JC, et al. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine. 2005;30:1815–20. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]
- Yu J, Tirlapur U, et al. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–71. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, An HS, et al. Comparative effects of bone morphogenetic proteins and sox9 overexpression on extracellular matrix metabolism of bovine nucleus pulposus cells. Spine. 2006;31:2173–9. doi: 10.1097/01.brs.0000232792.66632.d8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, McAlinden A, et al. Type IIA procollagen in development of the human intervertebral disc: regulated expression of the NH(2)-propeptide by enzymic processing reveals a unique developmental pathway. Dev Dyn. 2001;220:350–62. doi: 10.1002/dvdy.1115. [DOI] [PubMed] [Google Scholar]