Abstract
We previously reported that patients with schizophrenia failed to demonstrate normal sleep-dependent improvement in motor procedural learning. Here, we tested whether this failure was associated with the duration of Stage 2 sleep in the last quartile of the night (S2q4) and with spindle activity during this epoch. Fourteen patients with schizophrenia and 15 demographically matched controls performed a motor sequence task (MST) before and after a night of polysomnographically monitored sleep. Patients showed no significant overnight task improvement and significantly less than controls, who did show significant improvement. While there were no group differences in overall sleep architecture, patients showed significant reductions in fast sigma frequency power (45%) and in spindle density (43%) during S2q4 sleep at the electrode proximal to the motor cortex controlling the hand that performed the MST. Although spindle activity did not correlate with overnight improvement in either group, S2q4 sleep duration in patients significantly correlated with the plateau level of overnight improvement seen at the end of the morning testing session, and slow wave sleep (SWS) duration correlated with the delay in reaching this plateau. SWS and S2q4 sleep each predicted the initial level of overnight improvement in schizophrenia, and their product explained 77% of the variance, suggesting that both sleep stages are necessary for consolidation. These findings replicate our prior observation of reduced sleep-dependent consolidation of motor procedural learning in schizophrenia and link this deficit to specific sleep stages. They provide further evidence that sleep is an important contributor to cognitive deficits in schizophrenia.
Keywords: sleep, schizophrenia, procedural learning, motor skill, memory, consolidation
Sleep disturbances in schizophrenia have been described since Kraepelin (Kraepelin 1919) and are associated with poor coping skills and diminished quality of life (Goldman et al 1996; Hofstetter et al 2005). Accumulating evidence suggests that abnormal sleep also contributes to cognitive deficits in schizophrenia (e.g., Forest et al 2007; Goder et al 2004; Goder et al 2008; Yang and Winkelman 2006). In a prior study, we reported that chronic medicated patients with schizophrenia failed to demonstrate normal improvements in procedural learning after a night of sleep, in spite of showing intact practice-dependent learning during training the previous day (Manoach et al 2004). The goal of the present study was to determine whether this reduced overnight consolidation of procedural learning in schizophrenia is associated with alterations in specific sleep stages or their characteristics, which could provide insight into the mechanisms underlying this cognitive deficit.
Subjective sleep disturbance is common in patients with schizophrenia and often presages psychotic decompensation (Benson 2006; Lieberman et al 2005). The presence of sleep abnormalities in antipsychotic-naïve and unmedicated patients indicates that abnormal sleep is not merely a side-effect of medications (for meta-analysis see Chouinard et al 2004). While there are reports of diverse abnormalities of sleep architecture in schizophrenia, reduced slow wave sleep (SWS) is the most consistent (e.g., Keshavan et al 1998; Monti and Monti 2004; Yang and Winkelman 2006), but not universal (e.g., Chouinard et al 2004; Lauer et al 1997), finding. In spite of its ubiquity, abnormal sleep has generally been overlooked as a potential contributor to cognitive deficits in schizophrenia. This neglect may stem from a tendency to regard disturbed sleep as secondary to other factors and from difficulty specifying the exact nature of the disturbance. There is now overwhelming evidence that sleep plays a critical role in memory consolidation (e.g., Stickgold 2005) and recent studies of schizophrenia report associations between sleep and cognitive performance in medicated (Goder et al 2004; Goder et al 2008) and antipsychotic-naïve (Forest et al 2007) patients. These findings support the hypothesis that abnormal sleep contributes to cognitive deficits in schizophrenia and highlight the need for further study.
In the present study, we employed the same simple, well-characterized test of motor skill learning, the finger tapping motor sequence test (MST) (Karni et al 1998; Walker et al 2002) that we used in our previous study of schizophrenia (Manoach et al 2004). When healthy young participants are trained on this task, they show significant improvements in speed after a night of sleep, but not after an equivalent period of daytime wake (Walker et al 2002). Additional nights of sleep lead to more improvement, even with no additional practice (Walker et al 2003b), but sleep deprivation the first night after training blocks all subsequent non-practice related improvement (Fischer et al 2002). These findings demonstrate that overnight improvement on this task depends on sleep rather than the mere passage of time. Sleep following MST training also leads to increased functional MRI activation in right primary motor cortex, contralateral to the hand performing the task, and to decreased activation in regions that mediate the conscious monitoring of performance (Walker et al 2005). These and other findings suggest that sleep-dependent consolidation leads to task automation, resulting in performance that is faster, less variable, and less dependent on voluntary attention (Atienza et al 2004; Kuriyama et al 2004; Walker et al 2005).
Overnight improvement on the MST and other simple motor skill tasks specifically correlates with the amount of Stage 2 sleep in the last quartile of the night (S2q4, Fogel et al 2007; Smith and MacNeill 1994; Walker et al 2002). MST improvement also correlates with the number and density of fast spindles (Rasch et al 2008), and since the MST is performed with the left hand, it is interesting to note that it is associated with right > left asymmetry of spindle density and power at central electrodes proximal to primary motor cortex (Nishida and Walker 2007). Sleep spindles are brief, powerful bursts of synchronous neural firing that reach peak density late in the night (De Gennaro et al 2000) and are hypothesized to mediate the consolidation of procedural memory on the MST (Nishida and Walker 2007; Rasch et al 2008; Walker et al 2002) and other motor tasks (Fogel and Smith 2006; Tamaki et al 2008). Studies of schizophrenia show reduced spindle activity (Ferrarelli et al 2007), and positive relations between Stage 2 spindle density and verbal declarative memory performance (Goder et al 2008). Here, we expected to replicate our finding of reduced overnight improvement of motor procedural learning in schizophrenia and to correlate it with the duration of S2q4 sleep (Walker et al 2002), reduced sigma frequency power, which corresponds to sleep spindles, during S2q4 sleep, specifically at the right central (C4) electrode, and reduced right > left sigma asymmetry at central electrodes (C4-C3) during S2q4 sleep (Nishida and Walker 2007; Rasch et al 2008; Walker et al 2005).
Methods
Participants
All participants were screened to exclude substance abuse or dependence within the past six months, diagnosed sleep disorders, or any independent conditions that might affect brain function. Outpatients with schizophrenia (n=16) were recruited from an urban mental health center. Two patients were excluded for failing to type a single correct sequence during training. The remaining 14 patients had all been maintained on stable doses of antipsychotic medications for at least six weeks, 12 on atypicals, one on typicals, and one on both. No patients took anticholinergic medications and ten took diverse adjunctive medications for anxiety, agitation, and/or concurrent mood disturbance. Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV (First et al 1997). Clinical status was characterized with the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham 1962), the Positive and Negative Syndrome Scale (PANSS) (Kay et al 1987) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen 1983).
Healthy control participants (n=16) without a history of psychiatric illness were recruited from the community. One control did not complete the study. The 14 schizophrenia and 15 control participants did not differ in age, sex, handedness (modified Edinburgh Handedness Inventory (White and Ashton 1976)), or mean parental education (Table 1). Participants gave written informed consent and the study was approved by the Institutional Review Boards of Massachusetts General Hospital, the Massachusetts Department of Mental Health, and Beth Israel Deaconess Medical Center. Remuneration included a bonus based on the number of correct sequences typed on each MST administration.
Table 1.
Means, standard deviations, and group comparisons of demographic data and rating scale scores. The Phi value is the result of a Fisher’s Exact Test.
| Participant Characteristics | Healthy (n=15) | Schizophrenia (n=14) | t | p |
|---|---|---|---|---|
| Age | 42 ± 6 | 41 ± 7 | 0.6 | 0.54 |
| Sex | 11M / 4F | 11M / 3F | Phi = 0.06 | 0.99 |
| Laterality Score (Handedness) | 65 ± 46 | 60 ± 59 | .27 | 0.79 |
| Parental Education (years) † | 13 ± 3 | 13 ± 3 | 0.25 | 0.80 |
| Age of Onset | 24 ± 6 | Average Level of Severity | ||
| Length of Illness (years) | 16 ± 8 | |||
| BPRS | 18 ± 12 | minimal | ||
| PANSS positive | 15 ± 7 | mild | ||
| PANSS negative | 15 ± 5 | mild | ||
| SANS | 39 ± 17 | mild | ||
One healthy and two schizophrenia participants were unable to provide this information.
Procedures
Experimental design
In the week prior to their stay in the Mallinckrodt General Clinical Research Center (GCRC) at Massachusetts General Hospital, participants met with study staff to complete informed consent, tour the GCRC, and receive a wrist actigraph to wear. Following admission to the GCRC, participants were Trained on the MST and Tested nine hours later (Figure 1). They were then Trained on a second MST sequence and Tested on this sequence after an additional 9 hours. Finally, they were retested on the first sequence. For one sequence, Training and Testing occurred across the day (Wake interval), and for the second sequence, Training and Testing occurred across a night (Sleep interval). The order of the Wake and Sleep intervals and of the two MST sequences were counterbalanced within each group. Participants were monitored to ensure that they did not nap during the day.
Figure 1.
Experimental protocols. Participants were pseudorandomly assigned to (A) “Wake First” or (B) “Sleep First” protocols. MST sequence order was also counterbalanced within protocol order. Arrows indicate times of admission and discharge from the GCRC. (A) Wake first: Participants arrived at the GCRC at approximately 10am. Ten hours prior to their habitual bedtime (approximately 1 PM), they Trained on the first MST Sequence (MST Seq 1). Nine hours later, they were Tested on this sequence, and 10 minutes later, they trained on MST Seq 2. They were subsequently wired for polysomnography and allowed to go to sleep. Seq 2 Test occurred in the morning, approximately nine hours after Training, and after electrodes had been removed and breakfast eaten. Ten minutes later, they were retested on MST Seq 1. (B) Sleep first: Participants arrived at the GCRC 2.5 hours prior to their habitual bedtime (approximately 8:30 PM), and were Trained on MST Seq 1 90 minutes later. Prior to going to sleep, they were wired for polysomnography. Testing of MST Seq 1 occurred the next morning after electrodes had been removed and breakfast eaten, approximately nine hours after Training. Ten minutes later, they trained on MST Seq 2. Testing of MST Seq 2 occurred nine hours later, during the afternoon, followed 10 minutes later by the retest of MST Seq 1. (C) Measurements of Overnight Change in Performance: Initial improvement = percent increase from the last three Training trials to the first three Test trials; Plateau improvement = percent increase from the last six Training trials to the last six Test trials; Initial lag = plateau improvement – initial improvement.
Finger Tapping Motor Sequence Test (MST)
The MST is described in detail elsewhere (Manoach et al 2004). In brief, participants pressed four numerically labeled keys on a standard computer keyboard with the fingers of their left hand, repeating a five element tapping sequence (e.g., 4–1–3–2–4) “as quickly and accurately as possible” for 30 s. Throughout the finger tapping trials, the numeric sequence was displayed at the top of the screen. Each session consisted of 12 trials separated by 30-second rest periods. Each of the 12 trials was scored for the number of correct sequences and the number of errors. The primary outcome measure was the number of correct sequences typed, which reflects both the speed and accuracy of performance. We also examined error rates, but no significant changes, across time, conditions, or groups, were observed.
Subjective Alertness
Prior to each MST session, participants completed the Stanford Sleepiness Scale (SSS), a standard measure of subjective alertness (Hoddes et al 1973).
Actigraphy
Participants wore a Mini-Mitter Actiwatch®-64 actigraph (Mini-Mitter Company, Inc., Bend OR) on their wrist from enrollment to study completion, including at least three nights prior to their stay in the GCRC. The actigraph monitors and records wrist movement in 15-sec epochs, and provides an estimates of sleep and nap time based on periods of wrist immobility.
Polysomnography
Participants were wired for standard polysomnographic (PSG) sleep recordings prior to retiring. EEG (C3, C4, Cz, O1, 02), EOG, and EMG were recorded on an Embla A10 ambulatory monitor (Medcare Systems, Buffalo NY). EEG data were sampled at 200Hz and band-pass filtered between .3 and 35Hz for analysis. The spectral data analyses were conducted with a resolution of .25Hz.
Data Analysis
MST Performance
Schizophrenia and control groups were compared on MST performance using t-tests. Practice-dependent improvement was calculated as the increase in correct sequences from the first trial to the average of the last three trials of Training. Initial improvement was calculated as the percent increase from the last three Training trials to the first three Test trials. Plateau improvement was calculated as the percent improvement from the last six Training trials of to the last six Test trials. Initial lag, which reflects the delay in expressing the plateau level of improvement, was calculated as plateau improvement minus initial improvement. We examined the relations between measures of overnight improvement and sleep with linear regression models.
Polysomnography
Data were manually scored for sleep stages for each 30 s epoch according to standard criteria (Rechtschaffen and Kales 1968) by an experienced PSG technician who was blind to MST results. Time spent in Stage 1, Stage 2, S2q4, SWS, and rapid eye movement (REM) sleep were quantified as both the number of minutes and the fraction of the total night’s sleep (percent). Sleep efficiency was calculated as total sleep time divided by time spent in bed. Number of awakenings, awakenings per hour, awakenings greater than 60s, and awakenings greater than 60s per hour were calculated.
Further analyses were conducted using MATLAB and the EEGLAB signal processing toolbox (MathWorks, Natick MA) (Delorme and Makeig 2004). Following manual artifact rejection, relative spectral power was examined in the low (12–13.5 Hz) and high (13.5–15 Hz) frequency sigma bands. We also conducted exploratory analyses of power in the delta (1–4Hz), theta (4–7Hz), alpha (8–12Hz), sigma (12–15Hz), and beta (15–35Hz) bands via Welch’s method, using a Hanning window with 50% overlap. Spindle density during S2q4 was also analyzed using the automated algorithm of Ferrarelli et al (2007), and calculated as spindles per minute.
Results
MST Performance (Figure 2, Table 2)
Figure 2.
MST performance. Motor skill learning across Training and Test trials for healthy control (n=15, open circles) and schizophrenia (n=14, filled squares) groups. The data point for each trial represents the group average ± SE. The y-axis represents the number of correct sequences typed in each 30 s trial. The shaded bar represents a night of sleep in the GCRC in between Training and Test trials. The solid lines fit through the data points for Training and Test were derived using an exponential model of motor learning (see Manoach et al., 2004). While patients and controls did not differ in the absolute amount of learning during Training trials (Table 2), only controls showed significant overnight improvement, which was realized at the plateau of Test.
Table 2.
Practice- and sleep-dependent changes in MST performance. Practice-dependent improvement is given in sequences per 30 sec trial; intial, plateau, and initial lag values are percent change compared to pre-sleep levels. Within-group t-tests are compared to no improvement; between group t-tests compare healthy control (HC) and schizophrenia (Sz) participants.
| Practice-Dependent improvement | Initial % improvement | Plateau % improvement | Initial % Lag | |
|---|---|---|---|---|
| Healthy controls t(14), p | 4.6 ± 2.8 | 2.8 ± 13.9 | 15.2 ± 13.5 | 12.4 ± 11.4 |
| 6.32, <.0001 | 0.74, .47 | 5.31, <.0001 | 4.16, .001 | |
| Schizophrenia t(13), p | 5.2 ± 3.1 | 4.7 ± 18.1 | 5.0 ± 10.7 | 0.3 ± 15.3 |
| 6.28, <.0001 | 0.23, .82 | 1.05, .31 | 0.66, .95 | |
| HC vs. Sz t(27), p | 0.56, .58 | 0.33, .74 | 2.23, .03 | 2.4, .02 |
Practice-dependent improvement
Both groups showed significant improvement across Training. While the groups did not differ in absolute improvement, patients showed greater proportional improvement (45 vs. 115%, t(27)=2.27, p=.03).
Overnight Improvement
While controls showed significant plateau improvement overnight, patients did not, and improvement was significantly greater in controls (15.2% vs. 5.0%). This is similar to our previous study (Manoach et al 2004) in which only controls showed significant plateau improvement (16.6% vs. 7.6%). Neither group showed significant initial overnight improvement, and the group difference was not significant. In controls, the absence of significant initial improvement reflects the presence of a significant initial lag. Initial improvement was 12.4% less than plateau improvement. No significant initial lag was seen in patients (0.3%) since they did not improve at plateau, and initial lag was significantly greater in controls.
Polysomnography (Table 3)
Table 3.
Sleep stage durations expressed in minutes and percentages of total sleep time and other sleep parameters in healthy control (HC) and schizophrenia (Sz) participants.
| HC (n=15) | Sz (n=14) | t | p | ||
|---|---|---|---|---|---|
| Stage 1 | min | 25± 13 | 34± 20 | −1.47 | .15 |
| % | 6 ± 3 | 8 ± 6 | −1.30 | .20 | |
| Stage 2 | min | 247± 69 | 282± 70 | −1.35 | .19 |
| % | 62± 12 | 67± 11 | −1.14 | .26 | |
| Slow wave sleep | min | 38± 23 | 41 ± 37 | −0.26 | .80 |
| % | 10 ± 7 | 9 ± 8 | 0.37 | .71 | |
| REM | min | 86± 40 | 66± 36 | 1.39 | .18 |
| % | 22± 10 | 16 ± 8 | 1.77 | .08 | |
| Stage 2: 4th Quarter (S2q4) | min | 56± 18 | 79± 23 | −1.95 | .06 |
| % | 15 ± 6 | 17± 32 | −0.93 | .25 | |
| S2q4 fast sigma power C4 | % | 2.91 ± 2.06 | 1.61 ± 1.22 | 2.05 | .05 |
| S2q4 fast sigma power C4-C3 | % | .49 ± 2.19 | −.02 ± .29 | 0.87 | .39 |
| S2q4 spindle density C4 | min−1 | .60 ± .36 | .34 ± .31 | 2.05 | .05 |
| Time in Bed | min | 492± 53 | 551± 72 | −2.57 | .02 |
| Sleep Onset Latency | min | 35± 33 | 72± 60 | 2.04 | .05 |
| Sleep Efficiency | % | 80 ± 9 | 77± 11 | 0.90 | .38 |
| Total Sleep Time | min | 396± 74 | 423± 85 | −0.91 | .37 |
Although patients spent significantly more time in bed in the GCRC than controls and took twice as long to initiate sleep, they did not show significant differences in either sleep efficiency or total sleep time. Nor were there significant group differences in either the amount or percent of time spent in any sleep stage, although there were trends for increased time in S2q4 and decreased percent time in REM for patients.
As predicted, fast sigma power, which corresponds to sleep spindles, was significantly lower in patients than controls at C4 during S2q4 (45% decrease; Table 3). There were also trends to reduced fast sigma power during S2q4 at the right occipital lead (43% decrease; t(28)=1.90, p=.07), and averaged over all leads (40% decrease; t(28)=1.76, p=.09). Decreased fast sigma power during S2q4 was accompanied by a shift in the average spindle frequency, which was significantly lower in patients (13.67 vs. 14.04 Hz, t(27)=3.62, p=.001) at all leads (all p’s <.01). Spindle density during S2q4 also showed a significant 43% reduction in schizophrenia patients at C4 (Table 3), but not at other electrodes (all p’s > .19). No group differences were seen in the slow sigma band (all p’s >.50). Asymmetry of fast sigma power at central electrodes (C4-C3) during S2q4 did not differ between groups.
Exploratory analyses of spectral power at alpha, beta, delta, theta, and slow oscillation frequencies across all NREM sleep revealed significant reductions in patients in delta power at the left occipital lead (47% reduction, t(26)=2.33, p=.03) and in theta power at both occipital leads (left/right 31%/32% reduction, t(26)=2.11, p=.05, t(26)=2.27, 32%, p=.03), although these differences did not survive correction for multiple comparisons.
Correlations of Overnight Improvement with sleep parameters (Table 4, Figure 3, Figure 4)
Table 4.
Relations of minutes in specific sleep stages to overnight improvement in healthy control (HC) and schizophrenia (Sz) participants.
| Initial improvement | Plateau improvement | Initial Lag | |||||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| Stage 1 NREM | HC | −.04 | .88 | −.10 | .73 | −.06 | .83 |
| Sz | .31 | .28 | .02 | .95 | −.35 | .21 | |
| Stage 2 NREM | HC | −.16 − | .57 | −.50 − | .05* | −.40 | .15 |
| Sz | −.17 | .56 | −.03 | .92 | .18 | .53 | |
| Slow wave sleep (SWS) | HC | .17 | .54 | .23 | .41 | .06 | .83 |
| Sz | .71 | .003 | .14 | .63 | −.74 | .002 | |
| REM | HC | .20 | .48 | .52 | .05* | .37 | .18 |
| Sz | .00 | .99 | −.12 | .69 | −.09 | .77 | |
| Stage 2: 4th Quarter(S2q4) | HC | −.31 | .27 | −.58 | .02* | −.31 | .28 |
| Sz | .58 | .03 | .77 − | .001 | −.15 | .61 | |
| SWS x S2q4 | HC | −.11 | .71 | −.25 | .37 | −.17 | .55 |
| Sz | .89 | <.0001 | .44 | .12 | −.74 | .002 | |
The significant correlations in controls between plateau improvement and Stage 2, S2q4, and REM sleep were due to a single outlier whose plateau improvement was more than 2 standard deviations from the mean. When this individual was removed from the analyses, the correlations were no longer significant (Stage 2: r=−.09, p=.76; S2q4: r=−.11, p=.71; REM: r=.05, p=.88).
Figure 3.
Correlation of initial overnight improvement with minutes spent in SWS and S2q4 in schizophrenia patients. (A) Correlation with S2-4; (B) Correlation with SWS; (C) Correlation with SWS × S2q4
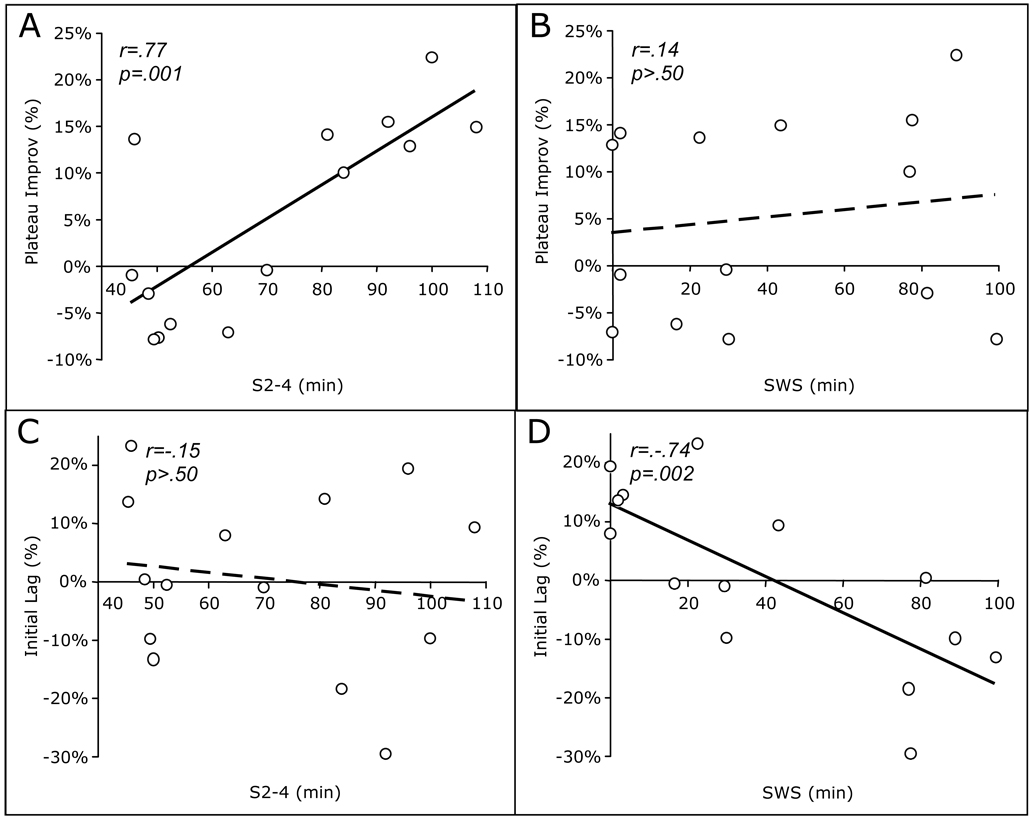
Figure 4.
Correlations of sleep stages with measures of overnight improvement in schizophrenia patients. A) Plateau Improvement vs. S2-4; B) Plateau Improvement vs. SWS; C) Initial Lag vs. S2-4; D) Initial Lag vs. SWS.
Initial improvement in schizophrenia, which ranged from 15% deterioration to 45% improvement, was significantly predicted by the amount of S2q4 sleep (Fig. 3A), as well as by the amount of SWS (Fig. 3B). Furthermore, the product of SWS and S2q4 provided an even better fit for initial improvement, explaining 77% of the variance (Fig. 3C). SWS and S2q4 were not themselves correlated (r=-.15, p=.61), and linear regressions showed that while SWS and S2q4 contributed independently to initial improvement (SWS t(13)=4.01, p=.002; S2q4 t(13)=3.08, p=.01), when the product SWS x S2q4 was added to the model, only the product contributed significantly (adjusted R2=.78; SWS t(13)=0.89, p=.39; S2q4 t(13)=0.27, p=.79; SWS x S2q4 t(13)=2.38, p=.04). When age was added to the regression models, the effects of S2q4, SWS, and their product remained significant.
Since the product of S2q4 and SWS strongly predicted initial improvement, and initial improvement is the difference between plateau improvement and initial lag, we investigated whether SWS and S2q4 had separate effects on these parameters. When we did so, a double dissociation emerged. Plateau improvement correlated positively with S2q4 (Fig. 4A), but not SWS (Fig. 4B), while initial lag correlated negatively with SWS (Fig. 4D), but not S2q4 (FIg. 4C). Thus, SWS strongly predicts initial lag and S2q4 strongly predicts plateau improvement, and together they even more strongly predict initial improvement in schizophrenia.
In controls, plateau improvement was negatively correlated with stage 2 and S2q4 sleep, and positively correlated with REM sleep, but these relations were entirely driven by an outlier whose plateau improvement was more than two standard deviations higher than the group mean. Removing this individual from the analyses rendered these correlations non-significant (all p’s > .70; see Table 4). Surprisingly, no other correlations between overnight improvement and the amount of S2q4 sleep or any other sleep stage were observed. Nor were either fast or slow sigma power, or asymmetry of fast or slow sigma power during S2q4 sleep significantly correlated with any measure of overnight improvement in either group. In controls, a higher spindle density at C4 during S2q4 sleep was associated with a smaller initial lag (r=-.68, p=.004). This relation was not expected, and when age, which correlated with initial lag (r=.52, p=.05), but not with initial (r=-.31, p=.27) or plateau (r=.13, p=.65) improvement, was entered as a covariate in the regression model, it remained significant, but the effect of spindle density was no longer significant (p=.90). Age did not correlate with any measure of overnight change in performance in the schizophrenia group (all p’s > .60).
Supplemental Analyses
Subjective alertness
There were no significant group differences in SSS scores at any MST session (all p’s ≥ .17), nor were there any significant differences in alertness in either group between Training and Test (all p’s ≥ .10). On the seven point scale (1 being most alert), mean values were as follows: Wake: controls 2.1 ± 0.7, patients 2.2 ± 0.9; Sleep: controls 2.6 ± 1.0, patients 2.2 ± 1.0).
Actigraphy
In controls, simultaneous PSG and actigraphy measurements during the GCRC night showed good correspondence for total sleep time (r=.83, p=.001), sleep onset latency (r=.61, p=.02), and sleep efficiency (r=.54, p=.04). A comparison of actigraphy during home nights with the GCRC night suggests that sleep was similar in both settings. Controls did not differ significantly on measures of time in bed, sleep onset latency, sleep efficiency, or total sleep time. For patients, in contrast, simultaneous actigraphy and PSG measurements during the GCRC night showed poor correlation (total sleep time: r=.44, p=.12; sleep onset latency: r=.08, p=.79; sleep efficiency, r=–.28, p=.33). This largely reflected that patients lay still in bed for long periods prior to falling asleep leading actigraphy to underestimate sleep onset latency, on which calculations of sleep efficiency and total sleep time are also based. As a result, wrist actigraphy was not a reliable measure of sleep in the patient group, preventing meaningful comparison of measures of sleep in the GCRC and home.
PSG Awakenings
Patients and controls did not differ on any index of awakenings (all p’s >.85).
Medication effects
In patients, antipsychotic medication dose as measured by chlorpromazine equivalents (Woods 2003) was not significantly correlated with any measure of overnight improvement or with SWS, S2q4, or their product. In addition, the five patients taking benzodiazepines did not significantly differ from the rest of the group on any performance measure.
Order effects
Although the order of the Wake and Sleep intervals was counterbalanced, it is possible that learning two different sequences in close temporal proximity affected the results. This is unlikely as a prior study showed that test performance on the sequences learned first and second did not significantly differ if Training for the second sequence occurred after Testing on the first sequence, as was the case in the present study (Walker et al 2003a). In addition, ANOVAs showed no significant main effects of order or group by order interaction for any of the outcome measures.
Wake State MST Improvement
To exclude the possibility that reduced overnight improvement in schizophrenia actually reflected deterioration of learning during wake periods rather than a failure to improve overnight, we also Trained and Tested participants across an equivalent period of wake. Patients showed no change in performance across the Wake interval (initial improvement, t(13)=0.23, p=.83; plateau improvement, t(13)=1.67, p=.12). While controls showed no initial improvement across Wake (0.0 ± 0.1), they did show significant plateau improvement (10.3 ± 17%, t(14)=2.4, p=.03), which did not differ significantly from plateau improvement over a night of sleep (15.2 ± 14%, t(14)=0.94, p=.36).
Discussion
Consistent with our previous report (Manoach et al 2004), in the context of intact practice-dependent learning, chronic medicated schizophrenia patients failed to demonstrate significant overnight improvement of motor procedural memory. In this respect, they differed significantly from healthy controls, who did show significant improvement. The present study extends these findings by demonstrating that in schizophrenia, overnight improvement is correlated with the amount of time spent in specific sleep stages. As predicted, based on findings in young healthy individuals (Smith and MacNeill 1994; Walker et al 2002), the amount of S2q4 sleep significantly predicted initial overnight improvement in schizophrenia. But unexpectedly, SWS did as well, and the product of SWS and S2q4 sleep accounted for 77% of the variance in overnight improvement. These findings demonstrate reduced sleep-dependent consolidation of procedural memory in schizophrenia and support the hypothesis that sleep makes an important contribution to cognitive deficits.
Although patients showed significantly reduced consolidation, there were no significant group differences in the amounts or distribution of time spent in specific sleep stages, or in any index of awakenings. This excludes the possibility that group differences in overnight improvement reflect sleep disturbances secondary to sleep disorders such as apnea or restless leg syndrome. Importantly, we cannot exclude the possibility that these group differences are consequences of medication. All of the patients took antipsychotic medications, which affect neurotransmitter systems that play an important role in sleep regulation and have diverse effects on sleep (Benson 2008; Krystal et al 2008; Monti and Monti 2004). Overall, however, antipsychotic and adjunctive medications tend to improve sleep maintenance and to normalize sleep architecture (e.g., Maixner et al 1998; Salin-Pascual et al 1999). While dosage as measured by CPZ equivalent was not correlated with any measure of overnight improvement and antipsychotic medications generally normalize sleep measures, we cannot exclude the possibility that they contributed to variability in improvement. Our sample was too small to allow any firm conclusions regarding the effect of the range of medications used on overnight improvement, although we note that the two subjects taking olanzapine were among the three participants in either group with the highest initial improvement (binomial test, p<.01). Olanzapine is known to increase Stage 2 and SWS (Monti and Monti 2004; Salin-Pascual et al 1999), although a recent study reported that it also decreased spindle density in schizophrenia (Goder et al 2008). Excluding the two patients on olanzapine, the correlation of initial improvement with the product of SWS and S2q4 remained highly significant (r=.89, p<.0001). The extent to which the deficits in sleep-dependent memory consolidation reported here reflect medication side-effects versus a disease process, thus remains unclear. Another limitation of the present study is that since participants were only studied for one night, we cannot exclude the possibility of first night effects, which may have differentially affected controls and patients. In controls, actigraphy suggested that sleep initiation and maintenance were similar at home and in the GCRC. In patients, however, actigraphy proved to be an unreliable index of sleep. Nevertheless, the fact that the current GCRC findings of a failure to show significant overnight improvement in schizophrenia replicate those seen in our previous home study (Manoach et al, 2004) argues that sleep in the laboratory was not a critical determinant of the failure of sleep-dependent memory consolidation in schizophrenia.
We previously proposed that it might not be the overall architecture of sleep that is culpable in schizophrenia; rather it may be specific memory consolidation processes that are normally activated during sleep (Manoach et al 2004). Regardless of whether these memory deficits reflect medication side-effects or disease process, the present findings implicate processes occurring during SWS and S2q4 sleep. As predicted, patients showed a significant reduction in fast spindle frequency power during S2q4 sleep compared to controls, specifically at the electrode proximal to the motor cortex that controls the hand that performed the MST. This replicates the finding of Ferrarelli and colleagues (2007) of a decrease in fast spindle frequency power in medicated patients with schizophrenia. Moreover, in the present study, sleep was recorded following training on a memory task, and the spindle power decrease only reached significance at C4, the lead overlying motor cortex contralateral to the hand that performed the task. Contrary to our predictions, however, spindle activity did not correlate with overnight improvement in either group. Recent findings suggest that it may be the change from baseline spindle activity levels that correlates with overnight improvement of motor procedural learning, rather than the absolute level of spindles (Peters et al 2008). Having only one night of PSG, we were not able to test that possibility here.
Although the duration of SWS and S2q4 both predicted initial overnight improvement in schizophrenia they were not themselves correlated, suggesting that their contributions were independent. Furthermore, when their product was added to a regression model of initial improvement, their individual contributions were no longer significant, only their product was. This suggests that both stages are necessary for consolidation and is consistent with the two-stage model of procedural learning proposed in our earlier studies (Stickgold et al 2000). We previously found that overnight improvement on a visuoperceptual procedural learning task correlated with both SWS early in the night and REM sleep in the last quarter, but even more strongly with their product (Stickgold et al 2000). A subsequent study of naps showed that while SWS prevented deterioration in visuoperceptual performance over the day, naps with both SWS and REM led to same-day improvement (Mednick et al 2002). These findings suggest that SWS, which is predominant early in the night, stabilizes visuoperceptual procedural memory, while REM sleep later in the night enhances it. A similar model fits our motor procedural memory findings. While both SWS and S2q4 correlated with initial improvement, when initial improvement was broken into its component parts, there was a striking double dissociation. SWS appeared to prevent the initial lag, while S2q4 appeared to facilitate the sleep-dependent enhancement seen at plateau.
Although the present control group did not show the significant initial improvement that we observed in our prior study (Manoach et al 2004), the difference in initial improvement between the two control samples did not significantly differ, nor were there significant differences in plateau improvement or initial lag. Nor did the patient samples from the two studies differ in any measure of overnight change in performance. Our present findings in controls also differ from prior MST studies of healthy college-aged participants in two important respects. First, studies of younger participants (Nishida and Walker 2007; Walker et al 2002; Walker et al 2003b) do not usually show the ramping up of performance over the first 2 or 3 Test trials that characterizes the initial lag seen in our healthy middle-aged sample. However, more recent studies of middle-aged (Manoach et al 2004), and healthy elderly participants (ages 60–79) (McKinley 2008) revealed similar initial lags. In addition, in the present study, initial lag increased with age (r=.52, p=.05). In addition to age, task difficulty may play a role in the initial lag, as healthy young college students do show an initial ramping up of test performance when a longer and more difficult finger tapping sequence is used (Kuriyama et al 2004). Thus, the finding that only older participants show an initial lag with the standard 5-digit sequence may simply reflect the fact that the task is more difficult for them.
A second, and possibly related novel finding is the correlation of SWS with initial improvement, and specifically the initial lag component of initial improvement. No correlations of MST improvement with SWS have been reported in studies of young healthy participants, but this may simply reflect the absence of an initial lag. Interestingly, there are reports of increased SWS following learning of motor procedural tasks, one in young adults (Huber et al 2004), and one in older, but not younger adults (Peters et al 2008). It is important to note, however, that the correlation with SWS in the present study was only observed in patients.
Although it is unclear why sleep stage dependencies were only seen in patients, the lack of correlations in controls is consistent with recent findings in an elderly cohort (60–79, McKinley 2008) and may reflect changes in sleep architecture (Ohayon et al 2004), reductions in Stage 2 sleep spindles (e.g., Peters et al 2008), and/or reliance of different procedural learning strategies in older individuals (Brown et al in press). As a result of these differences, consolidation may rely on both wake- and sleep-dependent processes in older cohorts (Robertson et al 2005). This could help explain our findings of significant improvement across wake as well as sleep in controls, a result not seen in younger individuals. This leaves unresolved the obvious question of why middle-aged schizophrenia patients, who as a group showed no significant overnight improvement, nonetheless showed significant sleep-stage dependencies.
In summary, we have replicated our prior observation of reduced sleep-dependent consolidation of motor procedural learning in schizophrenia (Manoach et al 2004) and now link variation in the expression of this deficit to specific sleep stages. These correlations with sleep stages (R2=.78 for SWS x S2q4), provides the first direct evidence of the sleep-dependency of this deficit in overnight improvement. Insofar as sleep-dependent consolidation of procedural learning reflects task automation (Atienza et al 2004; Kuriyama et al 2004; Walker et al 2005), our findings support the hypothesis of impaired automation in schizophrenia (Manoach 2003). Since all tasks have procedural components, a fundamental breakdown of sleep-dependent automation in schizophrenia could contribute to a generalized performance deficit (Chapman 1978; Dickinson and Harvey 2009). Greater allocation of limited-capacity attentional resources to task components that should have been automated would leave fewer available for other, higher-order, task demands. Taken as a whole, these findings provide further evidence that sleep is an important contributor to cognitive deficits in schizophrenia. Understanding this contribution, and clarifying the contribution of medications to this effect, may open new avenues for treatment.
Acknowledgments
We are grateful to the staff of the Mallinckrodt GCRC program, particularly Mary Sullivan for assistance in running participants.
Support: General Clinical Research Centers Program; NIMH (MH48832) and NIH T32 training grant HL07901-10 to the Harvard Division of Sleep Medicine. Study sponsors had no role in the acquisition, analysis, or presentation of study data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: This was not an industry-supported study. Dr. Manoach has received research funding and consulting fees from Sepracor Inc. Dr. Stickgold has received research funding from Merck & Co., Actelion Pharmaceuticals Ltd., and Sepracor Inc., as well as consulting fees from Actelion Pharmaceuticals Ltd. and Sepracor Inc, speaking fees from Epix Pharmaceuticals, and an educational grant from Takeda Inc. Ms. Stroynowski is presently employed by Alkermes. Dr. Goff has received honoraria or research support over the past year from Organon, Xytis, Wyeth, Forest Labs, Eli Lilly, Pfizer, and Ortho-McNeil-Janssen. None of the other authors have any conflicts of interest to disclose.
Contributors:
Dara S. Manoach: is an expert on cognition in schizophrenia and was responsible for all aspects of the present study including the design and execution of the study, data analysis, and manuscript preparation.
Katharine N. Thakkar: data acquisition and analysis of MST findings.
Eva Stroynowski: data acquisition and scoring and analysis of PSG data.
Alice Ely: data acquisition and scoring, analysis, and quality control of PSG data.
Sophia K. McKinley: analysis and interpretation of actigraphy data.
Erin Wamsley: analysis and interpretation of spectral PSG data.
Ina Djonlagic: consultant regarding medication effects on sleep parameters.
Mark G. Vangel: provided statistical consultation to all aspects of data analysis.
Donald C. Goff: responsible for patient recruitment and characterization. Contributed to interpretation of the findings.
Robert Stickgold: is an expert on sleep dependent cognition and participated with Dr. Manoach on all aspects of the present study including the design and execution of the study, data analysis, and manuscript preparation.
References
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Atienza M, Cantero JL, Stickgold R. Posttraining sleep enhances automaticity in perceptual discrimination. J Cogn Neurosci. 2004;16:53–64. doi: 10.1162/089892904322755557. [DOI] [PubMed] [Google Scholar]
- Benson KL. Sleep in schizophrenia: impairments, correlates, and treatment. Psychiatr Clin North Am. 2006;29:1033–1045. doi: 10.1016/j.psc.2006.08.002. abstract ix-x. [DOI] [PubMed] [Google Scholar]
- Benson KL. Sleep in schizophrenia. Sleep Med Clinics. 2008;3:251–260. [Google Scholar]
- Brown R, Robertson E, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. doi: 10.1371/journal.pone.0006683. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30:957–967. doi: 10.1093/oxfordjournals.schbul.a007145. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M. Topographical distribution of spindles: variations between and within nrem sleep cycles. Sleep Res Online. 2000;3:155–160. [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull. 2009;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–255. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Cote KA. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Forest G, Poulin J, Daoust AM, Lussier I, Stip E, Godbout R. Attention and non-REM sleep in neuroleptic-naive persons with schizophrenia and control participants. Psychiatry Res. 2007;149:33–40. doi: 10.1016/j.psychres.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Goder R, Boigs M, Braun S, Friege L, Fritzer G, Aldenhoff JB, Hinze-Selch D. Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res. 2004;38:591–599. doi: 10.1016/j.jpsychires.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, Serafin I, Aldenhoff JB. Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry. 2008;41:92–99. doi: 10.1055/s-2007-1004592. [DOI] [PubMed] [Google Scholar]
- Goldman M, Tandon R, DeQuardo JR, Taylor SF, Goodson J, McGrath M. Biological predictors of 1-year outcome in schizophrenia in males and females. Schizophr Res. 1996;21:65–73. doi: 10.1016/0920-9964(96)00021-7. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Philips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Lysaker PH, Mayeda AR. Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry. 2005;5:13. doi: 10.1186/1471-244X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Reynolds CF, 3rd, Miewald MJ, Montrose DM, Sweeney JA, Vasko RC, Jr, Kupfer DJ. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998;55:443–448. doi: 10.1001/archpsyc.55.5.443. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Edinburgh, Scotland: E.S. Livingston; 1919. [Google Scholar]
- Krystal AD, Goforth HW, Roth T. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol. 2008;23:150–160. doi: 10.1097/YIC.0b013e3282f39703. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer CJ, Schreiber W, Pollmacher T, Holsboer F, Krieg JC. Sleep in schizophrenia: a polysomnographic study on drug-naive patients. Neuropsychopharmacology. 1997;16:51–60. doi: 10.1016/S0893-133X(96)00159-5. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Maixner S, Tandon R, Eiser A, Taylor S, DeQuardo JR, Shipley J. Effects of antipsychotic treatment on polysomnographic measures in schizophrenia: a replication and extension. Am J Psychiatry. 1998;155:1600–1602. doi: 10.1176/ajp.155.11.1600. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- McKinley SK. Neurobiology. Cambridge: Harvard University; 2008. Sleep, Learning, and Memory in the Elderly. [Google Scholar]
- Mednick SC, Nakayama K, Cantero JL, Atienza M, Levin AA, Pathak N, Stickgold R. The restorative effect of naps on perceptual deterioration. Nat Neurosci. 2002;5:677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8:133–148. doi: 10.1016/S1087-0792(02)00158-2. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res. 2008;17:23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2008 doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: Health UDo editor; 1968. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin-Pascual RJ, Herrera-Estrella M, Galicia-Polo L, Laurrabaquio MR. Olanzapine acute administration in schizophrenic patients increases delta sleep and sleep efficiency. Biol Psychiatry. 1999;46:141–143. doi: 10.1016/s0006-3223(98)00372-2. [DOI] [PubMed] [Google Scholar]
- Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31:204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003a;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003b;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yang C, Winkelman JW. Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res. 2006;82:251–260. doi: 10.1016/j.schres.2005.10.021. [DOI] [PubMed] [Google Scholar]