Abstract
The integrin lies at the center of our efforts to understand mechanotransduction in the human body. Over the past two decades, a wealth of information has yielded important insights into integrin structure and functioning in biochemical pathways; however, relatively little emphasis has been placed on mechanics. In this article, we review the current knowledge base of integrin mechanobiology by examining the role of integrins in stabilizing tissue structure, the mechanisms of integrin force transfer, the process of cell migration, and the pathology of cancer. In order to successfully address the gaps in cancer and other disease research going forward, future efforts of integrin mechanobiology must focus on examining cells in 3D environments and integrating our current understanding into computational models that predict the behavior of integrins in non-equilibrium interactions.
Introducing: The Integrin
A cell surface protein that first received its name over two decades ago (Tamkun et al., 1986) is now recognized as the central component of the most studied mechanotransduction circuit to date (Chen, 2008; Paszek and Weaver, 2004; Puklin-Faucher and Sheetz, 2009). This protein was called the “integrin,” a name that was intended to denote its physical structure as the integral membrane protein that connects the extracellular matrix (ECM) to the cytoskeleton (Hynes, 2004). Since this time, the fields of cell and molecular biology have yielded a wealth of information regarding the structure of integrins and their role in several important biochemical pathways (Katz and Yamada, 1997). In fact, the physical composition of the integrin is now well-characterized; these transmembrane heterodimers consist of α and β subunits that are non-covalently bound (Alberts et al., 2008). The extracellular domain of the integrin is comprised of a ligand-binding head that is connected to two legs, and each leg is linked to the intracellular domain of the integrin via a single-pass transmembrane helix. A total of 18 α and 8 β subunits have been identified to date, whose combinations give rise to a family of over 24 distinct integrins (Alberts et al., 2008). The extracellular domain heads bind with specificity to several ECM components (Plow et al., 2000; van der Flier and Sonnenberg, 2001), the most notable including collagen, fibronectin, and laminin. In some instances, integrins also mediate cell-cell attachments (Russell et al., 2003). While an equally thorough understanding of integrin biophysical mechanisms has lagged behind, the evolution of mechanobiology as a discipline (Wang and Thampatty, 2006) has promoted an increasing interest in the role of integrins in stabilizing tissue architecture (Ingber, 2003; Maniotis et al., 1997), the forces transduced by integrins during cell migration (Lauffenburger and Horwitz, 1996; Wolf et al., 2003; Zaman et al., 2006), and the consequences of perturbed integrin mechanics in human pathophysiology and disease (Clark and Brugge, 1995; Weaver et al., 2002). As we shall discuss later, an improved knowledge of integrin mechanics and mechanisms stands to serve as a key component of potentially ground-breaking developments in addressing cancer (Bosserhoff, 2006), which accounts for greater than 10% of all human mortality worldwide (WHO, 2009).
Integrins Stabilize Tissue Structure and Architecture
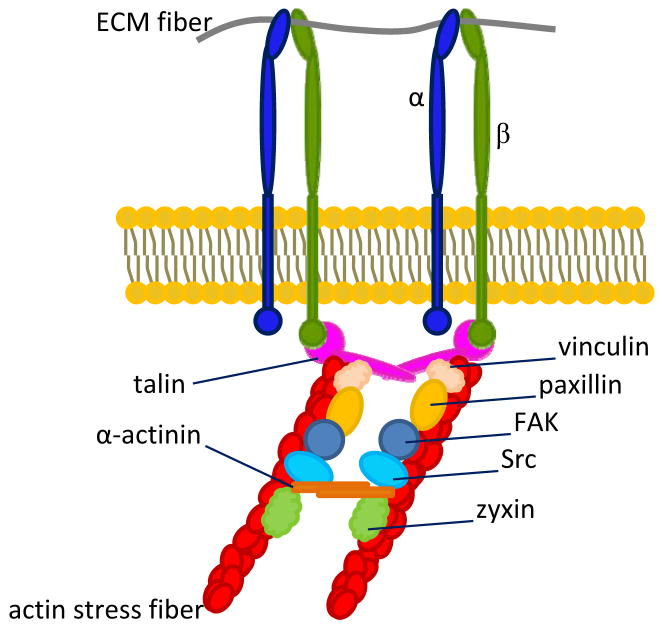
The human body is organized as a hierarchy of structural units: at the macroscale, organs are comprised of tissues, and at the microscale, tissues are comprised of cells surrounded by ECMs. Both continuum and discrete element models have been proposed to describe the mechanical state of tissue structure and architecture (Mofrad, 2009). One such discrete model is the tensegrity (or, tensional integrity) model (Ingber, 2006), which describes the body as a set of prestressed entities whose resting tensions are borne by filamentous structures (Ingber, 2003; Wang et al., 2002) (e.g. cytoskeletal and ECM fibers (Wang et al., 1993)) that stabilize entire tissues. In this context, integrins serve as the nanoscale mechanoreceptors that transmit forces between the cytoskeleton and the ECM (Ingber, 2006) to maintain structural integrity of the tissue. The majority of integrin-mediated attachments between ECM fibers and resting cells exist at sites where the intracellular domain of an integrin attaches to a bundle of actin filaments, also known as a stress fiber (Figure 1). This specialized attachment site is called a focal adhesion (FA) (Petit and Thiery, 2000; Puklin-Faucher and Sheetz) and ranges from approximately 1 – 10 μm in length (Balaban et al., 2001; Beningo et al., 2001; Kato and Mrksich, 2004). FAs consist not only of integrins, but also include several proteins (Figure 1) that stabilize the tissue, anchor the actin filaments, and relay important biochemical signals (Wiesner et al., 2005).
Figure 1.
Illustration of a focal adhesion (FA). β subunits of activated integrins bind to ECM ligands on the extracellular side of the cell membrane, as well as both directly and indirectly to various mechanical anchorage and signaling proteins on the cytoplasmic side of the membrane. While determining FA composition comprises an active area of research, several of the most well understood FA constituent proteins are depicted. They include anchorage proteins talin, vinculin, paxillin, and α-actinin, as well as signaling proteins FAK, Src, and zyxin, which is a major component of a mature FA.
Recent investigations have modeled the actin cytoskeleton of adherent cells as a cable network of variable lattice geometry to investigate the intracellular propagation of mechanical stress and stress distribution at FAs (Paul et al., 2008). The studies incorporated both theoretical analyses and experimental data to examine the effect of cytoskeletal prestrain and externally applied cellular forces. Results suggested that forces transduced by FAs depend more on FA spatial distribution than on cytoskeletal network geometry (Paul et al., 2008) and thus have provided insight into additional factors that govern tensional homeostasis in tissues (Tomasek et al., 2002). Other models of tissue structure and architecture (Ko and McCulloch, 2001) describe the tissue mechanical environment in terms of cellular circuits that consist of various intercellular mechanotransduction elements, including but not limited to integrins, gap junctions, adherens junctions, as well as cytokines. The circuits comprise a complex multicellular sensor/effector system that dictates tissue remodeling by globally coordinating cellular responses to mechanical stimuli (Ko and McCulloch, 2001).
In contrast to discrete element models of the cytoplasm, continuum models (Deshpande et al., 2007; Mofrad, 2009) treat the cell as a homogeneous elastic or viscoelastic medium that uniformly dissipates cell surface stresses over relatively short distances (< 10 μm) from the cell membrane (Wang et al., 2009), thus ignoring the explicit role of integrins as force-channeling conduits (Rosenbluth et al., 2008) that stabilize tissue structure. Alternative models describe cells and tissues as soft glassy materials or poroelastic mediums. The former model considers the cell as a soft elastic solid that exhibits stress-induced relaxation according to characteristic time-scales (Bursac et al., 2005; Deng et al., 2006), whereas the latter treats the cell as a two-phase material that consists of a porous elastic cytoskeletal network and a fluidic cytosol that suffuses the network in response to pressure gradients generated by stresses applied to the network (Mitchison et al., 2008; Rosenbluth et al., 2008). Given that continuum and poroelastic models do not resolve cell-matrix interactions at the level of the integrin, an improved understanding of integrin-mediated tissue homeostasis necessitates that future models and experiments integrate aspects of discrete element theories, as well as the continual flow of experimental findings regarding integrin mechanics. Overall, integrin adhesion sites can serve not only as very static interactions that stabilize the microstructure of a tissue via focal adhesions (Petit and Thiery, 2000) or other sites such as hemidesmosomes that anchor epithelial cells to underlying basement membrane (Jones et al., 1998), but they can also participate in interactions that regulate the tissue mechanical state via mechanisms such as activation of ion channels (Waitkus-Edwards et al., 2002). Deviation of normal integrin-mediated tissue mechanical homeostasis can lead to several pathologies and is a major feature of cancer (Paszek et al., 2005).
Integrins Bear Stress and Transmit Force
Several fluorescence and other imaging modalities have been utilized in correlating integrin- mediated forces with integrin visualization (Worth and Parsons, 2008). The primary insights into the nature and magnitude of forces transmitted by integrins have arisen from studies of cell traction force (CTF) and single-molecule level experiments. Traction force is the cytoskeletal tension exerted on the ECM via FAs (Figure 2), and has been measured in both stationary (Balaban et al., 2001; Tan et al., 2003) and motile cells (Dembo and Wang, 1999; Lee et al., 1994). In both motile and non-motile cells, this tension is born primarily by actin stress fibers; CTFs exerted by a motile cell are additionally driven by actin polymerization at the leading edge of the cell. Briefly, CTFs are determined using CTF microscopy techniques, where cells are allowed to attach to well designed substrates that exhibit specific chemical and mechanical properties (Burton and Taylor, 1997; du Roure et al., 2005; Reinhart-King et al., 2003); the forces transmitted via FAs induce substrate displacements (Harris et al., 1980) that are captured via microscopy imaging before and after substrate deformation. Substrate displacements are then used to inversely calculate CTFs using one of several mathematical formulations (Butler et al., 2002; Dembo and Wang, 1999; Yang et al., 2006). These techniques yield CTF “maps” across the entire surface of the cell that is in contact with the substrate and can then be compared with fluorescence microscopy visualization of integrins and additional FA proteins (Worth and Parsons, 2008). CTFs have been measured in the range of 10 – 103 nN (Burton et al., 1999; Tan et al., 2003) and in general depend on FA maturation and FA involvement in cell spreading and migration (Beningo et al., 2001). Tractions measured at individual locations can be integrated over the entire cell surface to compute a total traction force exerted by a cell on a substrate. While these methods do not provide direct measure of force transmitted by an individual integrin, they do provide a wealth of insight into the integrin mediated mechanical response of FAs to alterations of the extracellular environment. For example, the magnitude of CTFs in stationary cells has been shown to increase proportionally to FA size (Balaban et al., 2001; Tan et al., 2003) and depends on substrate stiffness (Lo et al., 2000; Paszek et al., 2005). A more thorough review of CTF methods has been detailed by Wang and Lin (Wang and Lin, 2007). Integrin mechanics have also been probed indirectly via studies of cell detachment force. For this method, cells are seeded onto a ligand-coated coverslip, which is then mounted upon a spinning disk that imparts acceleration-dependent shear stress to the cells. An accounting of adherent cells remaining upon completion of the spin cycle provides a measure of the detachment force required to break the integrin-ligand bonds (Garcia et al., 1998). Typical shear detachment stresses have been measured in the range of 1 – 10 Pa and have exhibited correlation with integrin and ligand densities (Garcia et al., 1998; Shi and Boettiger, 2003) and integrin clustering (Paszek et al., 2005).
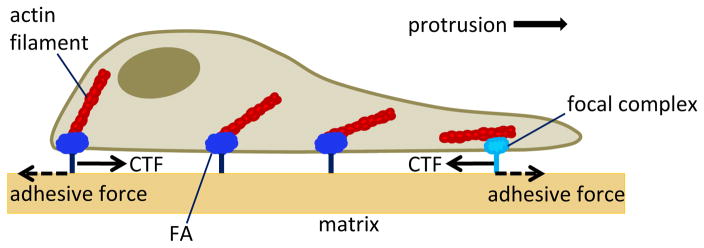
Figure 2.
Illustration of cell traction forces (CTFs) generated during cell migration. Actin polymerization facilitates protrusion of the leading edge that is accompanied by traction forces exerted across nascent focal complexes and mature focal adhesions (Wiesner et al.) (Wiesner et al.). Forward protrusion in concert with contraction of the rear cell body overcomes ECM resistive adhesive tractions to allow forward cell migration.
Another approach that has been used to investigate integrin-mediated force transmission across the cell surface analyzes single integrin-ligand bonds to probe the local viscoelasticity of the cell membrane region that anchors the integrin (Benoit and Gaub, 2002; Schmitz et al., 2008). In this type of study, the integrin is viewed as a cell membrane “tether” that can be stretched in tension and modeled as a viscoelastic Kelvin Body (Barakat, 2001; Bausch et al., 1998). Here, atomic force microscopy is employed to pull a cell from its adhesive surface until a single integrin-ligand bond is ruptured. The resulting force versus distance curve is fit to a Kelvin Body model which yields an estimate of the local membrane stiffness and viscosity and the stiffness of the integrin-mediated attachment (tether stiffness). Previous studies have estimated the tether stiffness to be on the order of 10−6 N/m (Schmitz et al., 2008). Upon analytical manipulation of the model, these parameters can further be used to compute compliance of the integrin anchorage, which may contribute significantly to cell adhesiveness (Schmitz et al., 2008), irrespective of intrinsic integrin-ligand conformational affinities (Deshpande et al., 2008; Xiao et al., 2004; Zhao et al., 2009). Other single-molecule level experiments examining rupture forces have revealed distinct activation barriers of the integrin-ECM bond (Kokkoli et al., 2004; Li et al., 2003) by employing atomic force microscopy in a manner similar to that of the aforementioned experiment. For example, pulling a cell from the its ECM coated substrate at a loading rate of > 104 pN/s was found to increase the maximum rupture force (on the order 100 pN) by approximately 50%, as compared to cells that were pulled at a rate of < 104 pN/s (Li et al., 2003). Theoretical models (Bell et al., 1984; Evans and Ritchie, 1997; Merkel et al., 1999) were then used to calculate energies from the force measurements and indicated the presence of separate, force-dependent activation barriers of integrin-ECM linkages.
Given the numerous findings borne from CTF and single-molecule level studies, it is well established that cells sense external tension. Within a native cellular environment, this tension is often manifested in the form of ECM stiffness and is detected by integrins. Transfer of this force from the ECM to the inside of the cell via integrins induces remodeling of cytoskeletal filaments, which can structurally protect the cell from detrimental external mechanical stimuli (Maniotis et al., 1997; Wang et al., 1993). The notion of force transfer via integrins is further supported by the fact that forces applied at non-adhesion cell membrane receptors merely produce local effects that dissipate within the actin cortex along the inner surface of the membrane (Wang et al.,2001). However, several additional studies have probed the forces exerted by cells on two-dimensional substrata and estimate that FAs in fact transmit forces on the order of 10−9 N (Burton et al., 1999; Lee et al., 1994; Tan et al., 2003). Moreover, theoretical models suggest that FA dynamics are linked to the thickness and elasticity of the ECM (Nicolas and Safran, 2006). New experimental findings have also shown that blocking integrins on cancer cells in three-dimensional (3D) matrices results in a reduction of intracellular stiffness (Baker et al., 2009), further strengthening the paradigm that integrins relay force between the cytoskeleton and the ECM.
Integrins Facilitate Cell Migration
In addition to imparting structural integrity to tissue architecture and transmitting force, integrins play a significant mechanical role in cell migration, which constitutes a key aspect of morphogenesis (Shih and Keller, 1992), wound healing (Chien et al., 2005), and cancer (Zaman et al., 2006), among several other biological phenomena. In order for a cell to migrate within its environment, integrins must rapidly undergo conformational changes that enable the cell to rapidly attach to and detach from the ECM in a coordinated fashion (Lauffenburger and Horwitz, 1996; Lock et al., 2008; Puklin-Faucher and Sheetz, 2009). Allosteric regulation, governed by both intracellular and extracellular signaling cues, permits an integrin to switch between its active state, wherein the integrin binds to both intracellular and extracellular ligands, as well as an inactive state, for which ligands are released (Hynes, 2002a). We now take a closer look at the integrin-dependent mechanical cycle (Puklin-Faucher and Sheetz, 2009) that underlies cell migration.
In response to both matrix mechanical cues (Harley et al., 2008; Lo et al., 2000; Pelham and Wang, 1997; Peyton and Putnam, 2005; Raeber et al., 2008; Zaman et al., 2006) and local chemical stimuli (Doerr and Jones, 1996; Russell et al., 2003), a migrating cell develops directional polarity that manifests as protrusion of the cell’s leading edge. Membrane protrusions, in the form of either pseudopodia, filopodia, or lamellipodia (the most extensively studied (Alberts et al., 2008)), are the result of actin polymerization in the direction of cell movement and are regulated by the Rho GTPases Rac and Cdc42 (Goldfinger et al., 2008; Jaffe and Hall, 2005). Protrusion transports integrins to the leading edge, where their β subunits first bind to ECM proteins (Worth and Parsons, 2008). Engagement of the integrin with the ECM ligand commences integrin activation, inducing a conformational change that permits rapid binding of the cytoplasmic β tail to the actin cytoskeleton via the talin anchorage protein (del Rio et al., 2009; Wegener et al., 2007), as well as actin stabilizing proteins vinculin and paxillin (Clark and Brugge, 1995). Binding of these stabilizing proteins exposes their own additional binding sites which in turn recruit signaling proteins FAK and Src (Worth and Parsons, 2008). Incremental recruitment of these anchorage and signaling proteins signals sequestration of additional integrins, which yields a relatively small, transient cluster of integrins (10–100 nm in length), termed a focal complex (Puklin-Faucher and Sheetz, 2009; Worth and Parsons, 2008).
The growing end of an actin stress fiber generates a traction force across the nascent focal complex, resulting in the recruitment of zyxin (Zaidel-Bar et al., 2003), additional signaling proteins, and integrin clustering along the leading edge to form a FA. Surprisingly, focal complexes at the leading edge of motile cells have been found to generate relatively high traction stresses (on the order of 103 Pa) before aggregating into larger, mature FAs (Beningo et al., 2001) that anchor the actin stress fibers to the matrix as the cell crawls over these sites. Forward migration in concert with contraction of the rear cell body to overcome ECM resistive adhesive traction forces results in disassembly of spent FAs, whose integrins and remaining protein components are then recycled to the leading edge of the cell to participate in new FAs (Lauffenburger and Horwitz, 1996; Puklin-Faucher and Sheetz, 2009; Vogel and Sheetz, 2006; Zaidel-Bar et al., 2003). Insight into the role of integrins in cell migration has increased rapidly in recent years, and determining the numerous proteins and mechanisms involved in FA assembly is an active area of research (Ridley et al., 2003) that stands to illuminate the mechanisms of cancer metastasis (Caswell et al., 2007) and other disease processes.
Integrins Influence Key Cellular Processes
A hallmark of integrin functionality is the ability of these receptors to exhibit bi-directional signaling (Arnaout et al., 2007; Hynes, 2002a). For example, intracellular signaling can cause the cytoskeleton to effect changes in the affinity of integrin-ligand binding, constituting inside-out signaling; in the case of cell motility, this binding results in remodeling of the ECM. On the other hand, the same integrin-ECM binding, in part depending on matrix rigidity (Pelham and Wang, 1997), can also induce structural changes in the linked cytoskeleton (Geiger et al., 2001), thereby utilizing an outside-in signaling cascade to inversely regulate motility (Vogel and Sheetz, 2006). Beyond its role in cell motility, integrin signaling influences a wide variety of cellular processes and cellular features. These include, but are not limited to, proliferation (Mainiero et al., 1997), apoptosis (Schwartz and Ingber, 1994), development (Katz and Yamada, 1997), angiogenesis (Hynes, 2002b), gene expression (Ritzenthaler et al., 2008), and morphology (Chen et al., 1994). The major classes of proteins involved in these integrin-mediated signal transduction processes consist of kinases, SH2-SH3 related molecules, GTPases, and phospholipid mediators; detailed pathways involving the myriad of individual protein contributors have been reviewed elsewhere (Clark and Brugge, 1995; Yamada and Miyamoto, 1995).
Integrins Contribute to Disease
Given the extensive involvement of integrins in key cellular processes, it follows that perturbed integrin mechanics contributes significantly to several pathophysiological and disease states (Wehrle-Haller and Imhof, 2003), such as deleterious embryonic development, autoimmune diseases, cardiovascular diseases, and cancer (Schwartz and Ginsberg, 2002). For example, cardiovascular cells produce several ECM components and continually remodel the ECM in response to both static and cyclic mechanical strains (Gupta and Grande-Allen, 2006) sensed by activated integrins and other cell-surface receptors. Hypertension, atherosclerosis, and cardiac hypertrophy are just a few of the cardiovascular pathologies that can develop over time as a result of disturbed integrin-mediated mechanics and loss of native cellular contractility (Li and Xu, 2000; Ross and Borg, 2001). The major platelet integrin (αVβ3) has become the target of clinical therapies aimed at treating arterial thrombosis (Shattil and Newman, 2004), and numerous cardiovascular studies are now focusing on the role of integrin mechanics in angiogenesis (Ingber, 2002; Serini et al., 2006). Studies illuminating angiogenesis are advancing the fields of both cardiovascular science (Hynes, 2007) and cancer, owing to the critical involvement of angiogenesis in metastasis (Hanahan and Weinberg, 2000).
Integrins Facilitate Cancer Progression
Indeed, several recent investigations have shown that integrin mechanics are altered in cancer (Guo and Giancotti, 2004; Nikolopoulos et al., 2004), facilitate metastasis, and drive expression of the malignant phenotype (White et al., 2004). A significant finding examining tumorigenesis revealed that matrix stiffness and cytoskeletal stress are functionally linked through key signaling proteins that cooperate to drive FA assembly, contributing to a rigid tumor microenvironment (Paszek et al., 2005). Additional studies indicate that in some instances, integrin-induced tissue polarity plays a role in conferring resistance to apoptosis, a hallmark of cancer, in both nonmalignant and transformed breast tissues (Weaver et al., 2002). The reduced requirement of integrin-mediated cell anchorage is another trait that is unique to cancer cells and is particularly evident in in vitro culture systems (Zahir et al., 2003). While normal cellular processes such as survival and proliferation necessitate anchorage-dependent mechanotransduction (Assoian, 1997), cancer cells instead receive analogous signals from oncogenes or indirectly via the loss of tumor suppressor genes (Hanahan and Weinberg, 2000), therefore relieving their dependence on matrix adhesion. This is consistent with findings that show cancer cell growth insensitivity to substrate stiffness (Wang et al., 2000) and internal cancer cell stiffness insensitivity to two-dimensional matrix stiffness (Baker et al., 2009 Baker et al., in press) as compared to non-transformed cells (Discher et al., 2005).
Integrin Mechanobiology: The Next Frontier
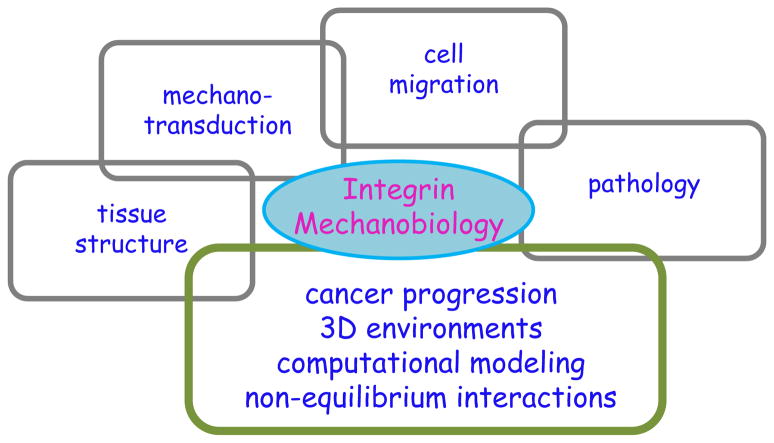
While there has been significant progress in understanding the role of integrins in mechanobiology, the overall systems-level picture is, at best, incomplete (Figure 3). In particular, direct quantitative measurements of integrin-relayed forces in healthy and diseased cells within native (or native-like) environments are lacking. This lack of specific context-dependent information has particularly limited our ability to understand how integrins on specific cells interact with dynamic changes in their extracellular environments in order to regulate adhesion, migration, and inside-out signaling. The behavior of extracellular mechano-chemical properties is also critical to comprehending the physical dynamics of integrins on membranes in 3D tissue environments. The random or persistent diffusion of integrins within membranes is not only a function of integrin structure but is also dependent on the mechanical properties of the lipid bilayer and the surrounding environment (Ballestrem et al., 2001; Palecek et al., 1996; Pankov et al., 2000). Given the dearth of definitive evidence regarding the nature of integrin clustering in vitro and within 3D in vivo environments, the exact role of matrix regulation of this phenomenon remains elusive. Current experiments have also failed to provide a direct measure of integrin-relayed force, cluster formation kinetics, and adhesion strength in native environments, and our ability to make connections among the wealth of integrin mechanics findings will remain largely qualitative unless we are able to measure these forces directly in 3D.
Figure 3.
Systems-level view of integrin mechanobiology. Over the past two decades, the study of integrins has largely been associated with structural and biochemical information. Although relatively little emphasis has been placed on integrin mechanobiology, recent strides have been made in elucidating the role of integrins in tissue structural maintenance, mechanotransduction, cell migration, and pathophysiology. In order to successfully navigate the complex landscape of diseases such as cancer, future efforts of integrin mechanobiology must focus on examining cells in 3D environments, accurately integrating the current knowledge base into computational models, and understanding the behavior of integrins in non-equilibrium interactions.
Efforts in the computational realm have focused largely on continuum level models and have not incorporated molecular level information. Thus, these approaches have had some success at the bulk level but have not been able to capture the detailed mechano-chemical interactions between integrins and the ECM. In particular, the effects of deleterious mutations that lead to various diseases have been beyond the scope of most computational or mathematical modeling techniques thus far. The success of mathematical models relies not only on accurately capturing and quantifying what is already known about integrin mechanics, but also rests on predicting what is unknown. To date, we have not developed sophisticated computational models that allow us to make quantitative predictions with relevance to molecular interactions of integrins in normal and diseased systems. Two major challenges persist and must be overcome before mathematical models and computational approaches can provide useful and quantitative disease-relevant information regarding integrin mechanobiology at the molecular level. The first challenge is the scarcity of reliable, high resolution integrin crystal structures, which are needed to form the basis of equilibrium and non-equilibrium molecular dynamics; this is perhaps the single, largest obstacle to obtaining in silico predictive data about the effect of mutations on integrin structure, conformations, clustering, and binding. Membrane proteins are particularly difficult to crystallize, and consequently, only a handful of these crystal structures have been obtained. The second major hindrance is the lack of reliable multi-scale computational methods that can translate information at the amino acid level to forces and mechano-chemical interactions at the macromolecular and cellular level. In this regard, there have been some developments in the last few years that have employed polymer statistical physics to extend alterations in integrin molecular structure to adhesion at the cellular level (Yang and Zaman, 2007a; Yang and Zaman, 2007b; Yang and Zaman, 2008a; Yang and Zaman, 2008b). However, these tools have largely focused on equilibrium systems and have given little attention to the non-equilibrium scenarios that are often encountered in vivo. Extension of these models to non-equilibrium scenarios and complex native environments would be of tremendous value to fundamental and applied biologists engaged in understanding integrin mechanobiology with respect to cell biology and molecular medicine.
Recent efforts at the interface of mechanics and biology has yielded an improved understanding of many biological phenomena, such as cardiovascular mechanisms (Hynes, 2007; Ingber, 2002; Ross and Borg, 2001; Shyy and Chien, 1997) and stem cell differentiation (Dobson, 2008; Docheva et al., 2007; Fox et al., 2007; Janes et al., 2002; Papayannopoulou et al., 2001; Suzuki et al., 2003; Voermans et al., 2000). Unfortunately, similar inroads have not been made in cancer, despite the wealth of knowledge implicating integrin structure and function in nearly all stages of tumor progression and metastasis. Due to its complexity, the mechanobiology of cancer remains largely unclear. A systems-level understanding of cancer should include not only the genetic and signaling components, but must also incorporate the underlying mechanical machinery that allows for tumor growth, angiogenesis, invasion, and metastasis.
Since the discovery of integrins over two decades ago, the study of integrins with respect to fundamental cell biology and disease has largely been associated with structural and biochemical information. Relatively little emphasis has been placed on integrin mechanobiology, even though many processes regulated by integrins in healthy and diseased cells are highly mechano-sensitive. Hence, the role of integrin mechanobiology in disease regulation is still underappreciated. While there have been significant improvements in both experimental and computational tools to quantify integrin-matrix mechanical interactions, there is a critical need for applying these tools to robust, well-characterized, and relevant disease models in vitro and in vivo. These connections are essential not only to developing an accurate comprehension of integrin mechanobiology in physiological systems, but also to navigating the complex landscape of disease, which will ultimately lead to efficient, patient-specific therapeutic targets.
Acknowledgments
The authors gratefully acknowledge support from the NIH (1R01CA132633 to MHZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts B, et al. Molecular Biology of the Cell. Garland Science, Taylor & Francis Group; New York: 2008. [Google Scholar]
- Arnaout MA, et al. Structure and mechanics of integrin-based cell adhesion. Current Opinion in Cell Biology. 2007;19:495–507. doi: 10.1016/j.ceb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. Journal of Cell Biology. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EL, et al. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophysical Journal. 2009;97:1–9. doi: 10.1016/j.bpj.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, et al. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319–1332. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat AI. A model for shear stress-induced deformation of a flow sensor on the surface of vascular endothelial cells. Journal of Theoretical Biology. 2001;210:221–236. doi: 10.1006/jtbi.2001.2290. [DOI] [PubMed] [Google Scholar]
- Bausch AR, et al. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophysical Journal. 1998;75:2038–2049. doi: 10.1016/S0006-3495(98)77646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GI, et al. CELL-ADHESION - COMPETITION BETWEEN NONSPECIFIC REPULSION AND SPECIFIC BONDING. Biophysical Journal. 1984;45:1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, et al. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. Journal of Cell Biology. 2001;153:881–887. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Gaub HE. Measuring cell adhesion forces with the atomic force microscope at the molecular level. Karger. 2002:174–189. doi: 10.1159/000066964. [DOI] [PubMed] [Google Scholar]
- Bosserhoff AK. Integrins as targets in therapy. Expert Opinion on Therapeutic Patents. 2006;16:963–975. [Google Scholar]
- Bursac P, et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nature Materials. 2005;4:557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- Burton K, et al. Keratocytes generate traction forces in two phases. Molecular Biology of the Cell. 1999;10:3745–3769. doi: 10.1091/mbc.10.11.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K, Taylor DL. Traction forces of cytokinesis measured with optically modified elastic substrata. Nature. 1997;385:450–454. doi: 10.1038/385450a0. [DOI] [PubMed] [Google Scholar]
- Butler JP, et al. Traction fields, moments, and strain energy that cells exert on their surroundings. American Journal of Physiology-Cell Physiology. 2002;282:C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- Caswell PT, et al. Rab25 associates with alpha 5 beta 1 integrin to promote invasive migration in 3D microenvironments. Developmental Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Chen CS. Mechanotransduction - a field pulling together? Journal of Cell Science. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- Chen QM, et al. INTEGRIN-MEDIATED CELL-ADHESION ACTIVATES MITOGEN-ACTIVATED PROTEIN-KINASES. Journal of Biological Chemistry. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chien S, et al. Molecular basis of mechanical modulation of endothelial cell migration. Frontiers in Bioscience. 2005;10:1985–2000. doi: 10.2741/1673. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. INTEGRINS AND SIGNAL-TRANSDUCTION PATHWAYS - THE ROAD TAKEN. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- del Rio A, et al. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LH, et al. Fast and slow dynamics of the cytoskeleton. Nature Materials. 2006;5:636–640. doi: 10.1038/nmat1685. [DOI] [PubMed] [Google Scholar]
- Deshpande VS, et al. A model for the contractility of the cytoskeleton including the effects of stress-fibre formation and dissociation. Proceedings of the Royal Society a-Mathematical Physical and Engineering Sciences. 2007;463:787–815. [Google Scholar]
- Deshpande VS, et al. A bio-mechanical model for coupling cell contractility with focal adhesion formation. Journal of the Mechanics and Physics of Solids. 2008;56:1484–1510. [Google Scholar]
- Discher DE, et al. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dobson J. Remote control of cellular behaviour with magnetic nanoparticles. Nature Nanotechnology. 2008;3:139–143. doi: 10.1038/nnano.2008.39. [DOI] [PubMed] [Google Scholar]
- Docheva D, et al. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. Journal of Cellular and Molecular Medicine. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr ME, Jones JI. The roles of integrins and extracellular matrix proteins in the insulin-like growth factor I-stimulated chemotaxis of human breast cancer cells. Journal of Biological Chemistry. 1996;271:2443–2447. doi: 10.1074/jbc.271.5.2443. [DOI] [PubMed] [Google Scholar]
- du Roure O, et al. Force mapping in epithelial cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2390–2395. doi: 10.1073/pnas.0408482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophysical Journal. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JM, et al. Recent advances into the understanding of mesenchymal stem cell trafficking. British Journal of Haematology. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, et al. Force required to break alpha(5)beta(1) integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. Journal of Biological Chemistry. 1998;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- Geiger B, et al. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nature Reviews Molecular Cell Biology. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, et al. Localized alpha 4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circulation Research. 2008;103:177–185. doi: 10.1161/CIRCRESAHA.108.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WJ, Giancotti FG. Integrin signalling during tumour progression. Nature Reviews Molecular Cell Biology. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovascular Research. 2006;72:375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harley BAC, et al. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophysical Journal. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, et al. SILICONE-RUBBER SUBSTRATA - NEW WRINKLE IN THE STUDY OF CELL LOCOMOTION. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002a;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nature Medicine. 2002b;8:918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biology. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Cell-matrix adhesion in vascular development. Blackwell Publishing; 2007. pp. 32–40. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanical signalling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circulation Research. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. Journal of Cell Science. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb Journal. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annual Review of Cell and Developmental Biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Janes SM, et al. Epidermal stem cells. Journal of Pathology. 2002;197:479–491. doi: 10.1002/path.1156. [DOI] [PubMed] [Google Scholar]
- Jones JCR, et al. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kato M, Mrksich M. Using model substrates to study the dependence of focal adhesion formation on the affinity of integrin-ligand complexes. Biochemistry. 2004;43:2699–2707. doi: 10.1021/bi0352670. [DOI] [PubMed] [Google Scholar]
- Katz BZ, Yamada KM. Integrins in morphogenesis and signaling. Biochimie. 1997;79:467–476. doi: 10.1016/s0300-9084(97)82738-1. [DOI] [PubMed] [Google Scholar]
- Ko KS, McCulloch CAG. Intercellular mechanotransduction: Cellular circuits that coordinate tissue responses to mechanical loading. Biochemical and Biophysical Research Communications. 2001;285:1077–1083. doi: 10.1006/bbrc.2001.5177. [DOI] [PubMed] [Google Scholar]
- Kokkoli E, et al. Collective and single-molecule interactions of alpha(5)beta(1) integrins. Langmuir. 2004;20:2397–2404. doi: 10.1021/la035597l. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee J, et al. TRACTION FORCES GENERATED BY LOCOMOTING KERATOCYTES. Journal of Cell Biology. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Xu QB. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cellular Signalling. 2000;12:435–445. doi: 10.1016/s0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- Li FY, et al. Force measurements of the alpha(5)beta(1) integrin-fibronectin interaction. Biophysical Journal. 2003;84:1252–1262. doi: 10.1016/S0006-3495(03)74940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, et al. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, et al. Cell-matrix adhesion complexes: Master control machinery of cell migration. Seminars in Cancer Biology. 2008;18:65–76. doi: 10.1016/j.semcancer.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Mainiero F, et al. The coupling of alpha(6)beta(4) integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. Embo Journal. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, et al. Demonstration of mechanical connections between integrins cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel R, et al. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397:50–53. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, et al. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Seminars in Cell & Developmental Biology. 2008;19:215–223. doi: 10.1016/j.semcdb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofrad MRK. Rheology of the Cytoskeleton. Annual Review of Fluid Mechanics. 2009;41:433–453. [Google Scholar]
- Nicolas A, Safran SA. Limitation of cell adhesion by the elasticity of the extracellular matrix. Biophysical Journal. 2006;91:61–73. doi: 10.1529/biophysj.105.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, et al. Integrin beta 4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–483. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Palecek SP, et al. Integrin dynamics on the tail region of migrating fibroblasts. J Cell Sci. 1996;109(Pt 5):941–52. doi: 10.1242/jcs.109.5.941. [DOI] [PubMed] [Google Scholar]
- Pankov R, et al. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–90. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, et al. Molecular pathways in bone marrow homing: dominant role of alpha 4 beta 1 over beta 2-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–42. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Paul R, et al. Propagation of mechanical stress through the actin cytoskeleton toward focal adhesions: Model and experiment. Biophysical Journal. 2008;94:1470–1482. doi: 10.1529/biophysj.107.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V, Thiery JP. Focal adhesions: structure and dynamics. Biology of the Cell. 2000;92:477–494. doi: 10.1016/s0248-4900(00)01101-1. [DOI] [PubMed] [Google Scholar]
- Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cellular Physiology. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- Plow EF, et al. Ligand binding to integrins. Journal of Biological Chemistry. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. Journal of Cell Science. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber GP, et al. Part II: Fibroblasts preferentially migrate in the direction of principal strain. Biomechanics and Modeling in Mechanobiology. 2008;7:215–225. doi: 10.1007/s10237-007-0090-1. [DOI] [PubMed] [Google Scholar]
- Reinhart-King CA, et al. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19:1573–1579. [Google Scholar]
- Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler JD, et al. Stimulation of lung carcinoma cell growth by fibronectin-integrin signalling. Molecular Biosystems. 2008;4:1160–1169. doi: 10.1039/b800533h. [DOI] [PubMed] [Google Scholar]
- Rosenbluth MJ, et al. Slow Stress Propagation in Adherent Cells. Biophysical Journal. 2008;95:6052–6059. doi: 10.1529/biophysj.108.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circulation Research. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Russell AJ, et al. alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. Journal of Cell Science. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- Schmitz J, et al. The viscoelasticity of membrane tethers and its importance for cell adhesion. Biophysical Journal. 2008;95:1448–1459. doi: 10.1529/biophysj.107.124289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nature Cell Biology. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ingber DE. INTEGRATING WITH INTEGRINS. Molecular Biology of the Cell. 1994;5:389–393. doi: 10.1091/mbc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, et al. Integrins and angiogenesis: A sticky business. Experimental Cell Research. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- Shi Q, Boettiger D. A novel mode for integrin-mediated signaling: Tethering is required for phosphorylation of FAK Y397. Molecular Biology of the Cell. 2003;14:4306–4315. doi: 10.1091/mbc.E03-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Keller R. PATTERNS OF CELL MOTILITY IN THE ORGANIZER AND DORSAL MESODERM OF XENOPUS-LAEVIS. Development. 1992;116:915. doi: 10.1242/dev.116.4.915. [DOI] [PubMed] [Google Scholar]
- Shyy JYJ, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Current Opinion in Cell Biology. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K, et al. Cell-matrix, and cell-cell interactions during corneal epithelial wound healing. Progress in Retinal and Eye Research. 2003;22:113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, et al. STRUCTURE OF INTEGRIN, A GLYCOPROTEIN INVOLVED IN THE TRANSMEMBRANE LINKAGE BETWEEN FIBRONECTIN AND ACTIN. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Tan JL, et al. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews Molecular Cell Biology. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell and Tissue Research. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- Voermans C, et al. Adhesion molecules involved in transendothelial migration of human hematopoietic progenitor cells. Stem Cells. 2000;18:435–443. doi: 10.1634/stemcells.18-6-435. [DOI] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Waitkus-Edwards KR, et al. alpha(4)beta(1) integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circulation Research. 2002;90:473–480. doi: 10.1161/hh0402.105899. [DOI] [PubMed] [Google Scholar]
- Wang HB, et al. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. American Journal of Physiology-Cell Physiology. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Lin JS. Cell traction force and measurement methods. Biomechanics and Modeling in Mechanobiology. 2007;6:361–371. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- Wang JHC, Thampatty BP. An introductory review of cell mechanobiology. Biomechanics and Modeling in Mechanobiology. 2006;5:1–16. doi: 10.1007/s10237-005-0012-z. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. MECHANOTRANSDUCTION ACROSS THE CELL-SURFACE AND THROUGH THE CYTOSKELETON. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American Journal of Physiology-Cell Physiology. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Wang N, et al. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Weaver VM, et al. beta 4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener KL, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, Imhof BA. Integrin-dependent pathologies. Journal of Pathology. 2003;200:481–487. doi: 10.1002/path.1399. [DOI] [PubMed] [Google Scholar]
- White DE, et al. Targeted disruption of beta 1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- WHO. Cancer Fact Sheet No 297. World Health Organization; 2009. [Google Scholar]
- Wiesner S, et al. Integrin-actin interactions. Cmls-Cellular and Molecular Life Sciences. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. Journal of Cell Biology. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth DC, Parsons M. Adhesion dynamics: Mechanisms and measurements. International Journal of Biochemistry & Cell Biology. 2008;40:2397–2409. doi: 10.1016/j.biocel.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Xiao T, et al. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. INTEGRIN TRANSMEMBRANE SIGNALING AND CYTOSKELETAL CONTROL. Current Opinion in Cell Biology. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Yang T, Zaman MH. Free Energy Landscape of Receptor Mediated Cell Adhesion. Journal of Chemical Physics. 2007a doi: 10.1063/1.2424985. [DOI] [PubMed] [Google Scholar]
- Yang T, Zaman MH. Regulation of cell adhesion free energy by external sliding velocities. Experimental Mechanics. 2007b In press. [Google Scholar]
- Yang T, Zaman MH. Cell adhesion to nanoligands: effects of ligand size and concentration in solution. Langmuir. 2008a;24:11819–27. doi: 10.1021/la801885c. [DOI] [PubMed] [Google Scholar]
- Yang T, Zaman MH. Thermodynamics of clustered and unclustered receptor systems in cell adhesion. Chem Phys Lett. 2008b In Press. [Google Scholar]
- Yang ZC, et al. Determining substrate displacement and cell traction fields - a new approach. Journal of Theoretical Biology. 2006;242:607–616. doi: 10.1016/j.jtbi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zahir N, et al. Autocrine laminin-5 ligates alpha 6 beta 4 integrin and activates RAC and NF kappa B to mediate anchorage-independent survival of mammary tumors. Journal of Cell Biology. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, et al. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. Journal of Cell Science. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaman MH, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, et al. How Focal Adhesion Size Depends on Integrin Affinity. Langmuir. 2009;25:1540–1546. doi: 10.1021/la8026804. [DOI] [PubMed] [Google Scholar]