Abstract
Background
Mutations in multiple genes have been implicated in familial atrial fibrillation (AF), but the underlying mechanisms, and thus implications for therapy, remain ill-defined.
Methods and Results
Among 231 participants in the Vanderbilt AF Registry, we found a mutation in KCNQ1 (encoding the α-subunit of slow delayed rectifier potassium current [IKs]) and separately a mutation in natriuretic peptide precursor A (NPPA) gene (encoding atrial natriuretic peptide, ANP), both segregating with early-onset lone AF in different kindreds. The functional effects of these mutations yielded strikingly similar IKs “gain of function.” In Chinese Hamster Ovary (CHO) cells, coexpression of mutant KCNQ1 with its ancillary subunit KCNE1 generated ~3-fold larger currents that activated much faster than wild-type (WT)-IKs. Application of the WT NPPA peptide fragment produced similar changes in WT-IKs, and these were exaggerated with the mutant NPPA S64R peptide fragment. Anantin, a competitive ANP receptor antagonist, completely inhibited the changes in IKs gating observed with NPPA-S64R. Computational simulations identified accelerated transitions into open states as the mechanism for variant IKs gating. Incorporating these IKs changes into computed human atrial action potentials (AP) resulted in 37% shortening (120 vs. 192 ms at 300 ms cycle length), reflecting loss of the phase II dome which is dependent on L-type calcium channel current.
Conclusions
We found striking functional similarities due to mutations in KCNQ1 and NPPA genes which led to IKs “gain of function”, atrial AP shortening, and consequent altered calcium current as a common mechanism between diverse familial AF syndromes.
Keywords: atrial fibrillation, ion channels, atrial natriuretic peptide, genetics, action potentials
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting ~2% of the US population and resulting in substantial morbidity and mortality [1-4]. While most AF is associated with other cardiac or systemic disorders, 10-30% of individuals with AF have no evidence of structural heart disease (so called idiopathic or `lone' AF) [5, 6]. There is now accumulating evidence that genetic factors play a role in the pathogenesis of lone AF [7, 8]. Identification of specific electrophysiologic abnormalities in genetically-defined AF holds the promise of moving therapy from the current empiric approach to one that is mechanism-based.
The role of gene variants in the pathogenesis of AF has only recently begun to be appreciated [7, 9]. KCNQ1, the first disease gene identified for familial AF [10], encodes the pore-forming α-subunit of the potassium channel that conducts the slow component of the delayed rectifier potassium current (IKs) in the atrium and ventricle. Functional analysis of the S140G mutant revealed a gain-of-function, which contrasts with the dominant negative or loss-of-function effects of KCNQ1 mutations previously associated with the congenital long QT syndrome [11]. Similarly, small kindreds with AF and mutations in other potassium channel genes, including KCNE2 [12], KCNJ2 [13], and KCNA5 [14], and in genes encoding sodium channel α- and β-subunits have been reported [15, 16].
To date, familial AF has been linked to mutations in potassium and sodium channels that are predicted to either shorten or lengthen the duration of the cardiac action potential (AP). However, a recent report has identified a novel molecular genetic basis for AF with the identification of a mutation in the natriuretic peptide precursor A gene (NPPA), which encodes atrial natriuretic peptide (ANP) [17]. This mutation segregated with familial AF, thereby uncovering an unexpected association between a defect in a circulating hormone and susceptibility to AF. Although the precise mechanism through which the mutant ANP leads to the development AF is not completely clear, a shortening of the atrial AP duration was demonstrated in an isolated rat heart model.
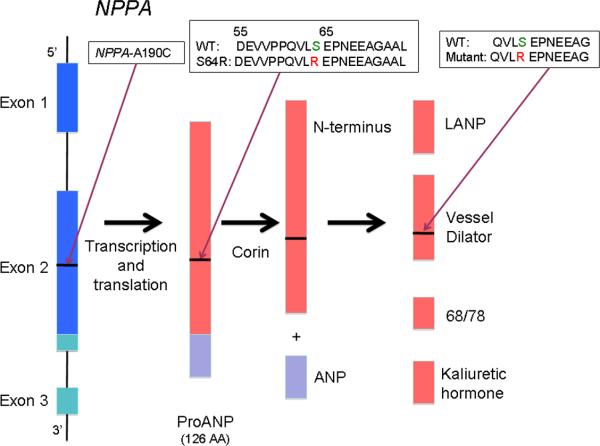
The three coding exons of NPPA transcribe a 151 amino acid peptide called preproANP (Figure 1) [18, 20]. This peptide then undergoes dual modification involving a signal peptidase and the enzyme corin to produce ANP, the mature 28 amino acid carboxy terminal end and a 98 amino acid N-terminus [21, 22]. The N-terminal peptide undergoes further degradation to produce a 16 amino acid peptide called long acting natriuretic peptide (LANP), a vessel dilator, and kaliuretic hormone [23, 24]. These additional peptides generated from preproANP have been shown to have biological activity similar to that of the mature ANP [23, 25].
Figure 1.
NPPA post-transcriptional processing. The initial 151-peptide product, PreproANP, is further processed into ANP and a collection of other protein fragments, including the N-terminal proANP `vessel dilator' peptide fragment. The NPPA-A190C mutation leading to PreproANPS64R propagates into this vessel dilator peptide fragment. The amino acid sequences of the WT and mutant fragments are shown (inset). (Figure modified from Veseley, 2001 [19].)
Here we report genetic analyses of KCNQ1 and NPPA in a large cohort of individuals with lone AF or AF associated with heart disease, as well as in population controls. We identified mutations in both genes that cosegregated with AF in separate kindreds. In vitro studies demonstrated strikingly similar gain-of-function defects associated with the mutant forms. Computational simulations of atrial APs incorporating wild-type (WT) or mutant IKs demonstrated a mutation-dependent change in AP configuration - AP shortening due to loss of the AP “dome” with premature stimuli - known to increase AF susceptibility [26, 27].
Materials and Methods
Study subjects
Subjects prospectively enrolled between November 2002 and October 2005 in the Vanderbilt AF Registry, which comprises clinical and genetic databases, were studied. Individuals enrolled in the Registry were greater than 18 years old with an ECG-confirmed diagnosis of AF. Subjects were excluded if AF was diagnosed in the setting of recent cardiac surgery or if they were unable to give informed consent or report for follow-up. The study protocol was approved by the Institutional Review Board of Vanderbilt University, and participants were enrolled following informed written consent. Controls were a cohort of anonymous population controls obtained from the Coriell repository, representing four different ethnic groups (Caucasian, African-American, Hispanic, Asian, n = 47 for each group).
Probands and their relatives were clinically classified by a consistently applied set of definitions. For the purposes of our study, AF was defined as replacement of sinus P waves by rapid oscillations or fibrillatory waves that varied in size, shape, and timing and were associated with an irregular ventricular response when atrioventricular conduction was intact. Documentation of AF on an ECG, rhythm strip, event recorder, or Holter monitor recording was necessary. Lone AF was defined as AF occurring in individuals less than 65 years of age without hypertension or overt structural heart disease as determined by clinical examination, ECG and echocardiography. An echocardiogram was obtained on all patients at time of enrollment into the registry.
Paroxysmal AF was defined as AF lasting more than 30 seconds that terminated spontaneously. Persistent AF was defined as AF lasting more than seven days and requiring either pharmacologic therapy or electrical cardioversion for termination. AF that was refractory to cardioversion or that was allowed to continue was classified as permanent.
Familial AF was defined as the presence of lone AF in one or more first-degree relatives of the index case. Family history information was initially obtained from the medical record and was supplemented by a questionnaire detailing past medical history, family history, and clinical symptoms. For individuals with a positive family history, a more detailed pedigree was generated by history and review of medical records of relatives.
Mutation screening
Whole blood was collected for genomic DNA extraction and analysis from all participating subjects. The coding and flanking intronic regions of KCNQ1 and NPPA were amplified by PCR using primers designed to obtain fragments of appropriate size (see online supplement for details). PCR-amplified DNA fragments were analyzed using the Reveal® Discovery System (based on temperature gradient capillary electrophoresis) to identify aberrant conformers, which were then directly sequenced. Plasma ANP levels were measured in participating subjects using the Phoenix radioimmunoassay (Phoenix Pharmaceuticals, Inc., Burlingame, CA).
Electrophysiology, FuGENE6-mediated channel expression and cell transfection
cDNAs for WT human KCNQ1 and KCNE1 were provided by Drs. Michael Sanguinetti (University of Utah) and Mark Keating (Novartis Institute for Biomedical Research, Cambridge, MA). CHO cells were used for transient transfections. Variant KCNQ1 was engineered by site-directed mutagenesis using standard techniques [28], and plasmid sequences were verified by resequencing prior to use. To study IKs, 2 μg WT or mutant KCNQ1 plasmid was co-transfected with KCNE1 in a bicistronic vector (KCNE1-IRES-eGFP, 2 μg) into CHO cells with 12 μl of FuGENE6 (Roche) in 0.5 ml serum-free medium for 30 min, after which standard medium was restored for 48 hours in culture. The cells were removed from the dish by brief trypsinization and stored in standard medium for experiments within the next 12 hours.
Whole-cell voltage clamp
Cells showing green fluorescence were chosen for study. Voltage-clamp studies were performed using methods previously reported [29]. To obtain current-voltage (I-V) relations for IKs, cells were held at -80 mV. Activating currents were elicited with 5-sec depolarizing pulses from -60 to +100 mV in 20 mV steps, and tail currents were recorded upon return to -40 mV. Pulses were delivered every 30 sec. Current densities (in pA/pF) were obtained after normalization to cell surface area. I-V relationships were analyzed by fitting the Boltzmann equation to the data: I=Imax/{1+exp[(Vt-V0.5)/k]}, where Imax is the maximal current, Vt is the test potential, V0.5 is the membrane potential at which 50% of the channels are activated, and k is a slope factor. Time constants for activation and deactivation were obtained by fitting mono-exponential functions to the current data using CLAMPFIT software.
The effects of WT NPPA peptide (and mutant NPPA S64R peptide fragment on IKs were assessed by adding peptide (100 nM) to the superfusate for 30 min pretreatment prior to recording IKs. The dose responses of IKs to WT and to mutant S64R were assessed by repeating the experiments with peptide concentrations of each ranging from 0.1 to 100 nM. To further address the role of receptor-mediated initiation of the cGMP-dependent ANP pathway in modifying IKs by WT and mutant S64R peptide, experiments were performed with the addition of two other membrane-permeable agents to the ANP pretreatment: the competitive ANP receptor antagonist anantin (500 nM) and the cGMP analog 8'-Br-cGMP (200 μM).
Solutions and drugs
To record IKs, the internal pipette filling solution contained (in mM): KCl 200, K4BAPTA 5, K2ATP 5, MgCl2 1 and HEPES 10. The solution was adjusted to pH 7.2 with KOH, yielding a final [K+]i of ~235 mM, as we have described previously [29, 30]. The external solution was normal Tyrode's, containing (in mM) NaCl 130, KCl 4, CaCl2 1.8, MgCl2 1, HEPES 10, and glucose 10, and was adjusted to pH 7.35 with NaOH. Experiments were conducted at 22-23°C. WT NPPA peptide fragment (AA sequence: CQVLSEPNEEAG) and 8'-Br-cGMP were purchased from Sigma-Aldrich Co. (St Louis, MO). The mutant NPPA S64R peptide fragment (AA sequence: CQVLREPNEEAG) was commercially synthesized by Peptide 2.0 Co. (Chantilly, VA). Anantin was obtained from Bachem Americas Inc. (Torrance, CA).
Computational Simulations of IKs and of the Human Atrial AP
Markov models of IKs were generated using the 17-state formulation described by Silva and Rudy [31] (Figure S-1 of the online data supplement). The current-voltage curves described above for WT KCNQ1 cotransfected with KCNE1 were used to generate the model of WT-IKs. As noted below, both mutant KCNQ1-IAP54-56 and mutant NPPA S64R peptide fragment generated similar in vitro effects on IKs function. Accordingly, the current-voltage curves for KCNQ1-IAP54-56 IKs were used to generate the “variant” IKs that here refers to both KCNQ1-IAP54-56 IKs and S64R fragment IKs. The details of model generation for WT-IKs and variant IKs are provided in Figures S-2, S-3 and Table S-1 of the online data supplement.
The Courtemanche-Ramirez-Nattel (CRN) simulation of human atrial APs was implemented [32]. The CRN IKs formulation was replaced with the Markov model of the human WT or variant IKs described above. Steady-state AP simulations were generated at pacing cycle length (CL) ranging from 300 to 4000 ms to compare the behavior of the simulation with implementation of WT and variant IKs (Figure S-4). Details of the CRN AP simulation are provided in the online data supplement (Table S-1). Electronic versions of the WT and variant IKs models as well as the CRN AP simulation are available upon request.
A simulation study was performed to evaluate the effects of premature atrial stimulation on morphology and duration of APs. Two premature atrial stimuli with a coupling interval of 400 ms were applied to the end of a 12-pulse train of 1000 ms pacing CL. APs as well as transmembrane current interactions were compared for WT and variant IKs.
Statistical analysis
Data are expressed as mean ± SEM. For comparisons among means of more than two groups, ANOVA was used, with post hoc pair-wise comparisons by Duncan's test if significant differences among means were detected. If only two groups were being compared, Student's t-test was used. A P-value <0.05 was considered statistically significant.
Results
Mutation screening
During the 3-year enrollment period, 245 patients with AF were approached and 231 (94%) subjects agreed to participate in the study. This study cohort included 98 patients (42%) with lone AF and 133 with AF associated with heart disease. The majority of subjects were Caucasian (94%), and 5% were African-American. AF was diagnosed at a mean age of 50 ± 14 years.
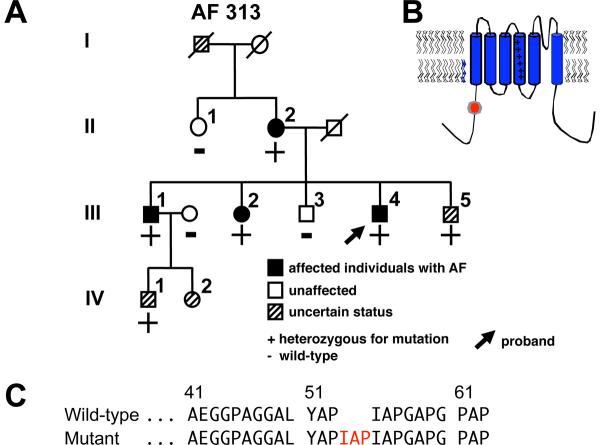
In the study cohort of 231 individuals with AF, screening for KCNQ1 mutations in genomic DNA identified a unique sequence variant in a case of familial AF (Vanderbilt AF 313). In the proband, a 9-bp duplication was identified resulting in insertion of the amino acids isoleucine (I), alanine (A) and proline (P) at locations 54 to 56 (IAP54-56) in the N-terminus of the KCNQ1 protein (Figure 2). The variant was also confirmed in three affected family members. The sequence change was not found in Caucasian, Chinese Han, or Asian population controls but was identified in 2.1% (2/94, the denominator refers to the number of chromosomes that were successfully screened and is therefore twice the number of African-American controls) of African-American individuals. As the controls were obtained from the anonymous Coriell repository, no clinical information about these individuals is available.
Figure 2.
KCNQ1 mutation identified in a kindred with familial atrial fibrillation (AF). (A) Family pedigree of Vanderbilt AF 313. Solid symbols denote AF and striped symbols individuals of uncertain phenotype. Male subjects are shown as squares and female subjects as circles. The proband (arrow) and the presence (+) or absence (-) of the KCNQ1-IAP54-56 indel are indicated. (B) Location of the variant in the KCNQ1 protein. (C) Portion of the KCNQ1 N-terminus amino acid wild-type and mutant sequence.
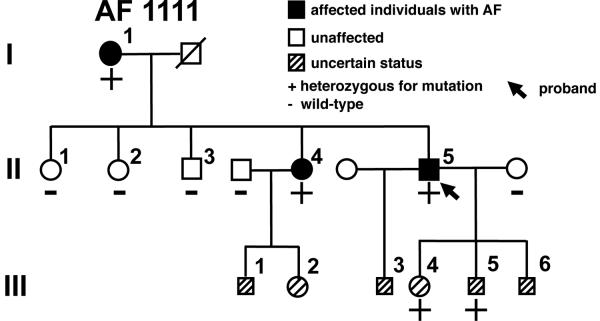
Genomic DNA sequencing of NPPA identified a novel missense mutation A190C (adenine to cytosine substitution at nucleotide 190) in exon 2 in a proband with familial AF (Vanderbilt AF 1111) (Figures 1 and 3). The mutation was also confirmed in two affected family members but was absent in unaffected family members. Furthermore, the mutation was not identified in Caucasian, Chinese Han, Asian, or African-American population controls. The mutation is also conserved across many specifies (dog, rat and mouse). Because of its location in the proANP peptide, subsequent post-translational modifications result in the arginine substitution being reflected not within ANP, but within the vessel dilator peptide (Figure 1). No other KCNQ1 or NPPA non-synonymous variants were identified in these 231 subjects.
Figure 3.
NPPA mutation identified in a kindred with familial AF. Shown is the family pedigree of Vanderbilt AF 1111. Symbols as in Figure 1A.
Family phenotype evaluation
The KCNQ1-IAP54-56 proband in Vanderbilt AF 313 (III-4) presented with palpitations at age 38 but was not diagnosed with AF until age 42 (Table 1). Although the proband initially responded to sotalol therapy, he gradually began to experience more frequent symptomatic recurrences of paroxysmal AF over a 7 to 8 year period. Flecainide was substituted for sotalol and has been effective at suppressing symptomatic AF. A detailed family history showed that most of the affected family members developed lone AF at a relatively young age and all presented with symptomatic paroxysmal AF (Table 1). To date, they have all been managed successfully with antiarrhythmic drugs. Evaluation of the ECG recordings showed that the corrected QT interval was normal during sinus rhythm for all family members studied (QT: 412±25 ms; QTc: 431±6 ms). Although two family members (III-5 and IV-1, Figure 2) have episodic palpitations that last up to 20 mins and occur every couple of months, we have not been able to document AF; therefore, we have classified their status as uncertain.
Table 1.
Clinical phenotype of living genotype-positive individuals in Vanderbilt AF 313 and AF 1111.
| AF kindred and pedigree number | Age at onset (yr) | Age at diagnosis (yr) | Rhythm | Symptoms | Echo | LA size (mm) | QT/QTc (ms) |
|---|---|---|---|---|---|---|---|
| AF 313 | |||||||
| II-2 | 48 | 52 | PAF | Yes | LVH | 46 | 368/430 |
| III-1 | 46 | 54 | PAF | Yes | Normal | 44 | 390/440 |
| III-2 | 48 | 56 | PAF | Yes | Normal | 42 | 428/430 |
| III-4 | 38 | 42 | PAF | Yes | Normal | 36 | 440/436 |
| III-5 | 44 | - | NSR | Yes | Normal | 40 | 430/420 |
| IV-1 | 36 | - | NSR | Yes | Normal | 38 | 420/428 |
| AF 1111 | |||||||
| I-1 | 42 | 44 | PAF | Yes | LVH | 44 | 390/405 |
| II-4 | 46 | 50 | PAF | Yes | Normal | 45 | 410/420 |
| II-5 | 36 | 42 | PAF | Yes | Normal | 42 | 380/410 |
| III-4 | 28 | - | NSR | Yes | Normal | 41 | 390/410 |
| III-5 | 30 | - | NSR | Yes | Normal | 42 | 400/415 |
PAF = paroxysmal atrial fibrillation; LA = left atrial; LVH = left ventricular hypertrophy ; NSR = normal sinus rhythm.
The NPPA-S64R proband in Vanderbilt AF 1111 (II:5) developed symptoms of palpitations, lightheadedness, and dizziness at age 36 and was diagnosed with paroxysmal lone AF at age 42 years when an event recorder documented an episode of AF associated with rapid ventricular response. As his AF episodes are short-lived and infrequent, occurring less than once per month, he has been successfully managed with only metoprolol. A family history (Figure 3) revealed that the proband's older sister and mother also were diagnosed with paroxysmal AF at a relatively young age (44 and 50 years, respectively). The proband's mother (I:1) initially presented with rapid palpitations and was found to be in rapid AF but subsequently also developed hypertension and sick sinus syndrome necessitating a permanent pacemaker when she was 58 years old. Currently, the mother's AF is controlled with a combination of metoprolol and digoxin. The proband's sister (II-4) also has highly symptomatic paroxysmal lone AF that is responsive to flecainide. Two of the proband's children also describe infrequent palpitations. Although consistent with AF, to date we have not been able to document AF; therefore we have designated them as of uncertain phenotype. A number of individuals in generation III have declined genetic or clinical screening, so their AF status is unknown. ANP levels were compared between family members affected with AF who were mutation carriers and those unaffected and WT for the S64R variant and were found not to be significantly different (24±8.2 pg/ml, N=3 [affected mutation carriers] vs. 26±11 pg/ml, N=6 [unaffected WT], P>0.05).
The common in vitro phenotype - a large and rapidly-activating IKs
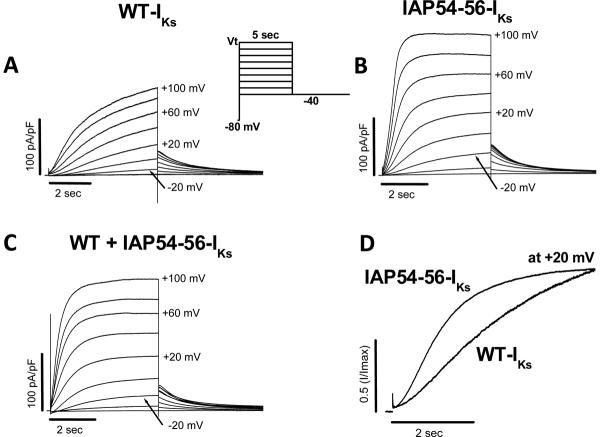
Expression of WT KCNQ1 + KCNE1 in CHO cells resulted in slowly-activating currents characteristic of cardiac IKs. Coexpression of KCNQ1-IAP54-56 with KCNE1 generated currents that were much larger and activated much earlier than WT-IKs (Figure 4); e.g., at +20 mV, peak current was 75±8 pA/pF (KCNQ1-IAP54-56) vs. 25±5 pA/pF (WT) after 5-sec pulses (n=7-8 each, P<0.001). Coexpression of WT and KCNQ1-IAP54-56 with KCNE1 resulted in a phenotype intermediate between the two. Figure 4D highlights the accelerated activation seen with the variant.
Figure 4.
Comparison of currents recorded from (A) wild-type (WT)-IKs (KCNQ1 + KCNE1), (B) IAP54-56-IKs (KCNQ1-IAP54-56 + KCNE1) and (C) co-expression of the WT-IKs + IAP54-56-IKs channels in CHO cells during 5-sec depolarization steps from a holding potential of -80 mV to test potentials in the range -40 to +100 mV followed by repolarization to -40 mV (voltage protocol shown in inset). (D) Comparison of activation of WT and IAP54-56-IKs. Traces obtained at +20 mV are redrawn from (A) and (B) at +20 mV.
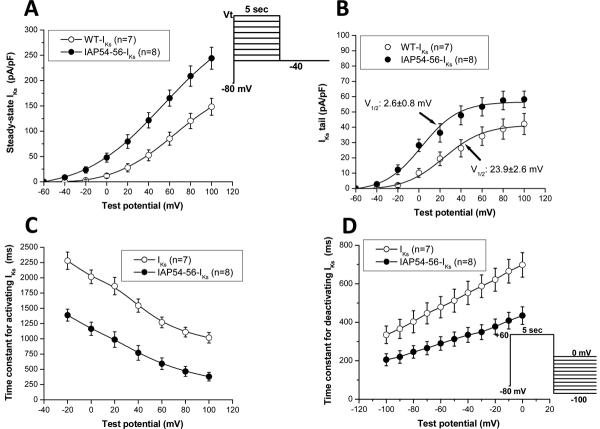
Summary data for the KCNQ1-IAP54-56 mutant are presented in Figure 5 and Table 2. In addition to the increased amplitude (Figure 5A), there was a -21 mV shift in the voltage dependence of activation (Figure 5B), indicating that at plateau voltages the probability of channel opening is further enhanced in the mutant. The mutant accelerated both activation (Figure 5C) and deactivation (Figure 5D) over all voltages tested.
Figure 5.
Current/voltage (I/V) relationships for WT-IKs and mutant (IAP54-56-IKs) channels. (A) Activating IKs as a function of voltage, normalized to cell surface area and measured at the end of a 5-sec depolarizing pulse. (B) Normalized tail current amplitude at -40 mV as a function of depolarizing potential. Fits to Boltzmann distributions are shown. (C) Time constant of IKs activation as a function of voltage. Time constants of IKs deactivation as a function of voltage are shown in (D).
Table 2.
The effects of KCNQ1 mutant IAP54-56, WT NPPA peptide fragment and mutant NPPA S64R peptide fragment on IKs properties.
| n | Activating IKs (pA/pF, +20 mV) | IKs tail (pA/pF) | V1/2 (mV) | τ-activation (ms, +20 mV) | τ-deactivation (ms, -40 mV) | |
|---|---|---|---|---|---|---|
| Control-1 | 7 | 25.2±5.1 | 18.9±45 | 23.9±2.6 | 1864.2±143.3 | 552.7±60.3 |
| IAP54-56-IKs | 8 | 75.1±8.2 † | 36.4±6.1 † | 2.6±0.8 † | 987.4±127.5 † | 334.5±38.6 † |
| Control-2 | 7 | 23.2±7.7 | 17.2±2.6 | 21.7±2.4 | 1859.8±125.7 | 564.5±53.2 |
| WT NPPA fragment | 7 | 53.1±9.2 ‡ | 30.3±4.2 ‡ | 10.6±1.3 ‡ | 1448.8±106.4 * | 414.9±37.5 * |
| Mutant NPPA S64R fragment | 7 | 77.9±9.7 ‡,∥ | 46.2±5.9 ‡,∥ | 0.8±1.1 ‡,∥ | 850.3±83.1 ‡,∥ | 307.7±30.5 ‡,§ |
Values are shown as mean±SEM; The activating IKs was elicited with 5-sec pulsing to +20 mV from a holding potential of -80 mV and the IKs tail was recorded upon pulsing back to -40 mV;
P<0.05 vs. control-2
P<0.001 vs. control-1
P<0.001 vs. control-2
P<0.05 vs. WT NPPA peptide fragment
P<0.001 vs. WT NPPA peptide fragment; there was no statistical difference between two control groups.
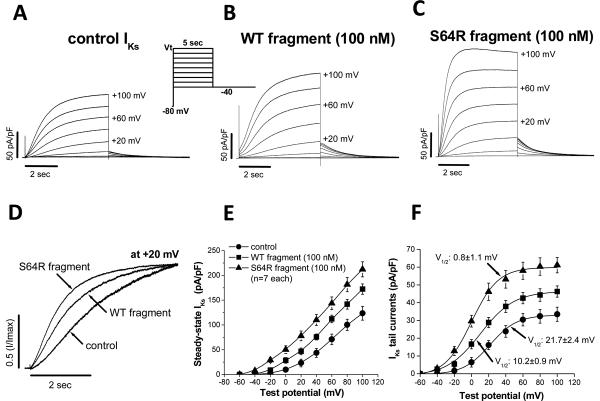
Application of WT NPPA peptide fragment (100 nM) to CHO-cells expressing WT KCNQ1 + KCNE1 yielded a moderate increase in cardiac IKs (Figure 6A and 6B; Table 2), in good agreement with previous studies [33]. However, a much more dramatic augmentation, remarkably similar to that observed with IAP54-56-IKs, was observed with NPPA S64R peptide fragment (100 nM, Figure 6C). At +20 mV, peak current was 78±10 pA/pF (S64R) vs. 23±8 pA/pF (WT) after 5-sec pulses (n=7 each, P<0.001). Activating traces at +20 mV normalized to current amplitude at the end of the pulse from Figures 6A, 6B, and 6C are shown in Figure 6D to emphasize the changes in activation. The voltage dependence of activation and of deactivation are shown in Figures 6E and 6F respectively. As with KCNQ1-IAP54-56, the S64R fragment produced a ~20mV negative shift in the voltage-dependence of tail current (Figure 6F). The results for WT-IKs and the effects of KCNQ1-IAP54-56 and NPPA S64R peptide fragment on IKs are summarized in Table 2.
Figure 6.
Effects of WT NPPA peptide fragment and mutant NPPA peptide fragment-S64R on IKs. (A) control IKs. (B) Increased IKs on exposure to WT fragment. (C) Accelerated activation and further increase of IKs with S64R fragment. (D) Superimposition of activating IKs at +20 mV from panels (A), (B), and (C). (E) Steady state IKs normalized to cell surface area (measured at the end of a 5-sec depolarizing pulse). (F) Normalized tail current amplitude at -40 mV as a function of depolarizing potential. Fits to Boltzmann distributions are shown.
ANP interacts with IKs via the ANP receptor initiation of a cGMP-dependent pathway present in mammalian cells (e.g., CHO cells) [33, 34]. We hypothesized that the alterations in IKs magnitude and kinetics due to the NPPA peptide fragment are mediated through the ANP receptor. Guanylate cyclase, the putative ANP receptor [35], is known to be present on the membrane surface of CHO cells [34], and thus provided an appropriate model for testing this hypothesis. We pretreated IKs-expressing CHO cells with the competitive ANP receptor antagonist, anantin (500 nM), for 30 min prior to exposure to the NPPA S64R peptide fragment (100 nM) [36]. We found that pretreatment with anantin completely eliminated IKs augmentation and acceleration seen with the S64R fragment alone (Figure S-5 of the online data supplement) indicating that the effect of the S64R fragment on IKs is mediated by the endogenous ANP receptor. Further, to investigate the involvement of intracellular cGMP we examined the effect of a cGMP analog, 8'-Br-cGMP, on IKs. Following acute exposure to 200 μM 8'-Br-cGMP, IKs was increased in a similar manner to that observed with the S64R fragment (Figure S-6 of the online data supplement), supporting the notion that IKs modulation by the NPPA peptide fragment occurs via intracellular cGMP.
Having established the role of the ANP receptor in modulating the interaction between the NPPA peptide fragment and IKs, we sought to determine the effects of different concentrations of the WT fragment and mutant S64R fragment on IKs channels. We conducted dose-response experiments with WT fragment and S64R fragment concentrations ranging from 0.1 to 100 nM. While WT fragment increased IKs in a dose-dependent manner with an IC50 value of 8.6± 1.0 nM, the enhancement of the current by S64R mutant was much more potent with an IC50 value of 1.1± 0.2 nM (Figure S-7 of the online data supplement). Also, the mutant markedly accelerated the IKs activation.
Computational simulation of mutant IKs
A 17-state Markov model for IKs was implemented and fit to the WT and variant IKs current profiles described above. The most apparent differences between the two fits were in the transitions between open states: O1⇒O2 and O1⇐O2 transition rates [ψ, ω] were 0.52 and 0.59 ms-1 for variant IKs and 2e-4 and 2e-7 ms-1 for WT-IKs, respectively, at -10 mV transmembrane potential (Table S-1 of the online data supplement).
Computational simulation of human atrial AP
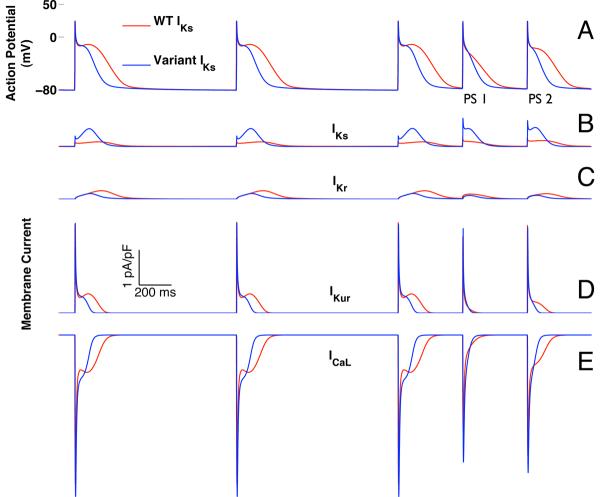
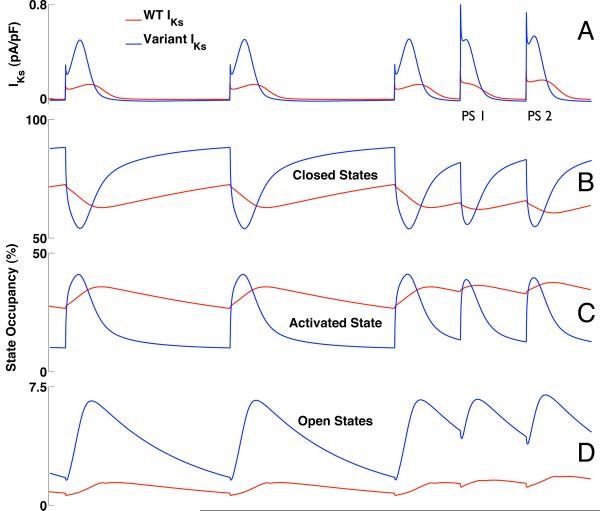
Atrial AP simulations with implementations of WT-IKs or variant IKs were then compared (Figure 7A). At CL 1000 ms, open state IKs channel occupancy was larger for variant IKs which led to 4-fold increase in peak current versus WT-IKs (0.5 pA/pF for variant IKs vs. 0.12 pA/pF for WT-IKs) (Figure 8A) despite a lower overall conductance for the variant IKs (variant GKs = 0.86 × WT GKs; Tables S-1.3 and S-1.4). While the mean closed state occupancies for variant IKs and WT-IKs were similar, there was greater open state occupancy for variant IKs (6% for variant IKs vs. 1.4% for WT-IKs), due to a much more dynamic transition of channels from the closed state, through the activated state, into the open state (Figure 8B-D). By contrast, WT-IKs channels showed relatively little state variation over the course of the AP. The 4-fold increase in peak variant IKs compared to WT-IKs resulted, as expected, in a decrease in steady state AP duration (188 ms for variant IKs vs. 297 ms for WT-IKs at CL 1000 ms) for all pacing CLs (Supplement Figure S-4).
Figure 7.
Simulated double premature atrial depolarizations (400 ms coupling intervals) after a twelve-pulse pacing train (1000 ms cycle length). Data from simulations that include WT-IKs are shown in red, and those incorporating variant IKs, based on IAP54-56, are in blue (A). The first atrial premature stimulus (PS1) simulated with variant IKs was shorter than that for WT-IKs (126 vs. 193 ms). AP durations for the second premature stimulus (PS2) were 138 ms and 243 ms for variant IKs and WT-IKs, respectively. In both simulations, PS1 exhibited loss of the dome associated with membrane repolarization. However, with WT-IKs the dome was restored with PS2 while it remained absent with variant IKs. Changes in individual membrane currents are shown in (B) IKs; (C) IKr; (D) IKur; and (E) ICa-L.
Figure 8.
IKs and channel state occupancy during the simulation of Figure 7 (1000 ms pacing train followed by double premature atrial depolarizations with 400ms coupling interval). WT-IKs is shown in red; variant IKs is in blue (A). Variant IKs exhibits much more dynamic variation in state occupancy during activation as channels transition from the closed states (B) through the activated state (C) into the open states (D). `Closed States' refers to the sum of closed states R1 and R2; `Open States' (D) refers to the sum of open states O1 and O2 (see Figure S-1A of the online data supplement).
During pacing at CL 1000 ms, the dome during phase II of the AP was less pronounced with variant IKs (Figure 7A). With simulations of premature atrial stimuli, the dynamic nature of these morphologic differences was accentuated. With double premature atrial stimuli at 400ms coupling interval, the dome was blunted for both WT-IKs and variant IKs with the first premature stimulus (PS1). However, the dome was restored with the second premature stimulus (PS2) for the WT-IKs but not the variant IKs simulation.
The dome during phase II of the AP reflects a period where the depolarizing effect of the L-type calcium current (ICaL) outweighs the repolarizing potassium currents, primarily IKs, the rapid delayed rectifier potassium current (IKr), and the ultra-rapid delayed rectifier potassium current (IKur) (Figures 7B-E). For both WT-IKs and variant IKs, loss of the dome with PS1 is due to loss of this phase II calcium current. Failure of the PS2 dome to recover in the simulation with variant IKs reflects lack of calcium current recovery as a consequence of the altered AP trajectory imposed by variant IKs.
The repolarizing current IKur showed similar dynamics (though in the opposite direction) as ICaL with smaller variation and hence smaller effect than those seen with ICaL. In this simulation, IKr did not contribute significantly to repolarization dynamics.
Discussion
In this study we identified mutations in KCNQ1 (IAP54-56) and NPPA (S64R) in moderate-sized Caucasian kindreds with early onset familial AF and normal QT intervals. Although both these genes have previously been linked with familial AF, this is the first study to demonstrate that augmented potassium current is a shared phenotype across these diverse genetic defects associated with familial AF. IKs gain-of-function mutations have previously been reported for potassium channel defects, but the notion that mutations in disparate genes, KCNQ1 and NPPA, lead to the same arrhythmia phenotype raises the possibility of subtype specific therapy for familial AF. The KCNQ1-IAP54-56 variant was also identified in a 2.1% of healthy African-American individuals, suggesting it may be a common risk allele in some populations.
One prevailing conceptual model proposed for AF pathogenesis describes reduced atrial refractory period as a substrate for re-entrant arrhythmias [37]. This model is supported by reports of gain-of-function mutations in genes encoding subunits of cardiac channels responsible for generating IKs (KCNQ1/KCNE2) and IK1 (KCNJ2) which are predicted to decrease AP duration [10, 12, 13]. Such understanding provides a therapeutic rationale for prolonging the atrial refractory period for the treatment of AF with antiarrhythmic drugs. In the present study, functional and simulation studies demonstrated that KCNQ1 and NPPA variants are gain-of-function mutations that shorten the AP duration both at steady state and in the setting of PACs - the most common (> 90%) initiating event in the onset of paroxysmal AF [38] - and as such are a likely cause of AF in these families. Although a shortened AP duration would be expected to shorten the QT interval, this was not observed in affected family members carrying the KCNQ1 variant. The mechanisms whereby some KCNQ1 mutations produce AF and others generate ventricular phenotypes remains to be determined. One possible explanation for the phenotypic variability in KCNQ1-linked disease, including that seen in AF or long QT syndrome, may reflect chamber- or disease-specific interactions between the channel and its partners. The latter may be well-described proteins, such as β-subunits or entirely novel proteins.
Studies of kindreds with AF suggest a genetic basis for the condition, and mutations in several cardiac potassium channel genes have been linked to familial AF [12-14]. Although specific mutations in the KCNQ1 gene have been observed in families with AF [10, 39], the role of such mutations in AF remains unclear; often such isolated or “private” mutations are in residues of unknown function, effects on channel conductance are variable, and in most cases it may be difficult to discriminate rare polymorphisms of no functional significance from true mutations. However, the low prevalence of KCNQ1 mutations in large AF cohorts, including ours, suggests that mutations in this gene are not a major cause of AF [40, 41]. In both AF313 and AF1111, multiple family members presented with early onset lone AF, and our segregation analysis supports the novel KCNQ1 and NPPA variants as being associated with AF. Although these variants were absent in the non-African-American control groups, and the in vitro data confirms that the variants are functional, we cannot completely exclude the possibility that the identified variants are not functional, risk-conferring polymorphisms in the Caucasian population. The KCNQ1-IAP54-56 variant however was identified in 2.1% of healthy African-American individuals, suggesting it may be a common risk allele in this population.
ANP, the primary biologically active peptide encoded by NPPA, is a circulating natriuretic hormone that plays an important role in regulation of intravascular blood volume and vascular tone through natriuresis, diuresis, and vasodilatation. It acts through stimulation of the intracellular second messenger cyclic GMP. In congestive heart failure and hypertension, ANP is secreted and released into the circulation in response to atrial wall stretch. Importantly through cGMP signaling, ANP also modulates sodium, calcium, and potassium channel currents in cardiac myocytes [42-44].
Studies have shown that NPPA transcription and processing yields a separate biologically active protein fragment called vessel dilator that is distinct from ANP (Figure 1) [23, 25]. Resequencing of NPPA identified a rare S64R variant that encodes for the vessel dilator in AF1111. The ANP assay does not directly measure the vessel dilator levels. However, one may reasonably infer that ANP concentration is an indirect measure of the fragment since proANP is the immediate progenitor of both. We found no significant differences in ANP levels between affected individuals with AF carrying the S64R variant and unaffected family members who were WT type for the variant, although elevated levels of ANP have been described in several different AF cohorts [45, 46]. The mutant form of this NPPA protein fragment dramatically augments and accelerates cardiac IKs, an effect that shortens the atrial AP duration. Our study demonstrated that the mutant NPPA S64R peptide fragment-induced alterations in IKs magnitude and kinetics were initiated at the ANP receptor since the gain-of-function was prevented by application of the competitive ANP receptor antagonist anantin. The increased potency of the NPPA S64R peptide fragment compared to WT is evidence of increased affinity for the receptor and likely reflects the mechanism by which IKs augmentation is effected by the mutant fragment. With activity via the ANP receptor, the biologic activity of this NPPA protein fragment is likely similar to that of ANP, which has been shown in intact animal atria to cause a dose-dependent, autonomically-mediated shortening of atrial monophasic AP duration and the effective refractory period [47]. An augmented cardiac potassium current could thus provide a substrate for development of AF by shortening the atrial AP. It is likely, however, that not only mutations or rare variants in NPPA and cardiac ion channel genes but also variants in signaling pathways that link the two could increase the susceptibility to developing AF.
Studies have clearly shown that ANP not only modulates cardiac potassium currents but also sodium and calcium currents in cardiac myocytes [33, 42, 48, 49]. As the NPPA-encoded vessel dilator has physiologic effects similar to mature ANP, it is quite likely that the mutant S64R peptide also modifies these channels as well. Although this study demonstrated that the mutant NPPA S64R peptide fragment modulates IKs and provides one possible mechanism for increased AF susceptibility, it does not rule out the possibility that other cardiac ion channels may also be contributing to the demonstrated effect on atrial AP duration. Ongoing studies are trying to determine the precise role of additional ion channels in modulating the effect on atrial AP duration.
Computational simulation of the effect of the IKs gain-of-function demonstrates the anticipated shortening of APs. In addition, simulations of premature stimulation reveal loss of the dome during phase II of the AP. This “triangularization” of the AP has been implicated as generating dispersion of repolarization time, and thus a fibrillation prone substrate, in Brugada Syndrome [50]. The atria are known to display marked heterogeneity of repolarization, in part as a function of region [51, 52], so a similar mechanism seems likely here. While the mutant NPPA S64R peptide fragment yielded more dramatic IKs augmentation, the addition of WT fragment to WT-IKs also resulted in modest IKs gain-of-function (Figure 6). These data suggest that NPPA activation, especially when exaggerated (e.g. due to atrial stretch), may augment IKs and thus predispose to AF; findings of increased ANP levels in patients with AF are also consistent with this hypothesis [53].
This study demonstrates the unique ability of computational simulation to explore the complex interplay between multiple ions, ion channels, ion exchangers and membranes underlying the observed phenotype. AP simulation is also the most efficient means to address the AP consequences of individual mutations and has the great advantage that changes in the gating of other normal currents can be inferred. In this study, loss of the phase II dome for both WT-IKs and variant IKs during premature atrial depolarization is shown to be primarily driven by a marked decrease in ICaL. Recovery of the dome reflects rapid recovery of the calcium current - which is markedly attenuated in the simulation implementing variant IKs.
Clinical Implications
Our study establishes that augmented potassium current with AP shortening and consequent altered calcium current is a common mechanism in diverse familial AF syndromes. As such, a mechanism-based approach targeting prolongation of the human AP can be proposed not only for AF probands but also family members diagnosed with the arrhythmia. Class III antiarrhythmic drugs such as sotalol would be one rational approach. The association of AP shortening with the establishment of a substrate for AF, as highlighted by our study of IKs augmentation, suggests a novel therapeutic role for agents that prolong the AP. For individuals or kindreds with AF mutations known to shorten AP, such therapeutic agents would theoretically be beneficial in preventing or terminating AF. As highlighted in our study, such agents may interact with the repolarizing channels directly (e.g. Class III Vaughan-Williams agents), but may also point to new agents that act indirectly on the channels.
Limitations
Several limitations of the present study warrant consideration. First, the AF kindreds are small and therefore segregation analysis was limited. Second, the broad applicability of this study is limited because mutations in KCNQ1 [40, 41] and NPPA are rare in general and not a common cause of AF. However, identifying novel mutations in AF signaling pathways will provide insight into underlying pathophysiology of the arrhythmia and suggest other members of this biologic pathway as candidates for AF susceptibility. A third limitation is that comprehensive resequencing of the population-based controls was not performed. Although it is possible that additional novel KCNQ1 and NPPA may have been identified, distinguishing pathogenic mutations from rare variants that have no relationship to the disease remains a challenge. Finally, computational simulation of human physiology is limited by the inherent difficulty in validating in human subjects the models upon which they are based. The models of WT-IKs and variant IKs, while based on a good fit to the experimental data, are not necessarily unique; an alternate set of model parameters may yield similar fit to experimental data yet yield a different trajectory when applied to an AP simulation. The ultimate model validation would require in vivo measurement of individual APs and their comprising membrane currents in humans carrying the mutation being modeled - a technological feat that is not currently possible.
Conclusion
In summary, we have for the first time to our knowledge uncovered a shared phenotype across two genetic defects that augment cardiac potassium current and are predicted to shorten cardiac atrial AP duration and predispose to the development of AF. The identification of a final common mechanism has implications for the potential therapeutic therapies for this common and morbid condition.
Supplementary Material
Acknowledgement
We are deeply indebted to Dr. Al George for insight and guidance in the preparation of this manuscript.
Funding Sources: This work was supported in part by NIH grants HL75266 and AHA award 0940116N to Dr. Darbar and HL65962 to Dr. Roden.
ABBREVIATIONS
- AF
Atrial fibrillation
- AP
Action potential
- NPPA
Natriuretic peptide precursor A
- ANP
Atrial natriuretic peptide
- WT
wild-type
- IKs
slow delayed rectifier potassium current
- ICaL
L-type calcium current
- IKr
rapid delayed rectifier potassium current
- IKur
ultra-rapid delayed rectifier potassium current
- I
Isoleucine
- A
Alanine
- P
Proline
- C
Cysteine
- PS1
First premature stimulus
- PS2
Second premature stimulus
- LQTS
long QT syndrome
- CRN
Courtemanche-Ramirez-Nattel
- CL
Cycle length
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- [1].Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- [2].Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- [3].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- [4].Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- [5].Gersh BJ, Tsang TS, Seward JB. The changing epidemiology and natural history of nonvalvular atrial fibrillation: clinical implications. Trans Am Clin Climatol Assoc. 2004;115:149–60. [PMC free article] [PubMed] [Google Scholar]
- [6].Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr., Ilstrup DM, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317(11):669–74. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- [7].Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41(12):2185–92. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- [8].Ellinor PT, Yoerger DM, Ruskin JN, Macrae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118(2):179–84. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- [9].Fox CS, Parise H, D'Agostino RB, Sr., Lloyd-Jones DM, Vasan RS, Wang TJ, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851–5. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- [10].Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- [11].Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–80. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- [12].Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75(5):899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, et al. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332(4):1012–9. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- [14].Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15(14):2185–91. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- [15].Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–35. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Watanabe H, Darbar D, Ingram CR, Jiramongkolchai K, Chopra SS, Kucera G, et al. Abstract 356: Loss of Function Mutations of Sodium Channel Beta-1 and Beta-2 Subunits Associated with Atrial Fibrillation and ST-segment Elevation. Circulation. 2007;116(16):II–54a. _MeetingAbstracts. [Google Scholar]
- [17].Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, et al. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359(2):158–65. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nemer M, Antakly T, Argentin S, Lavigne JP, Drouin J. Cloning and expression of the atrial natriuretic factor gene. Clin Physiol Biochem. 1988;6(34):163–70. [PubMed] [Google Scholar]
- [19].Vesely DL. Atrial natriuretic peptides in pathophysiological diseases. Cardiovasc Res. 2001;51(4):647–58. doi: 10.1016/s0008-6363(01)00256-5. [DOI] [PubMed] [Google Scholar]
- [20].Lewicki JA, Greenberg B, Yamanaka M, Vlasuk G, Brewer M, Gardner D, et al. Cloning, sequence analysis, and processing of the rat and human atrial natriuretic peptide precursors. Fed Proc. 1986;45(7):2086–90. [PubMed] [Google Scholar]
- [21].Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97(15):8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Martin DR, Pevahouse JB, Trigg DJ, Vesely DL, Buerkert JE. Three peptides from the ANF prohormone NH(2)-terminus are natriuretic and/or kaliuretic. Am J Physiol. 1990;258(5):F1401–8. doi: 10.1152/ajprenal.1990.258.5.F1401. [DOI] [PubMed] [Google Scholar]
- [23].Vesely DL, Douglass MA, Dietz JR, Gower WR, Jr., McCormick MT, Rodriguez-Paz G, et al. Three peptides from the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce diuresis, natriuresis, and/or kaliuresis in humans. Circulation. 1994;90(3):1129–40. doi: 10.1161/01.cir.90.3.1129. [DOI] [PubMed] [Google Scholar]
- [24].Nasser A, Dietz JR, Siddique M, Patel H, Khan N, Antwi EK, et al. Effects of kaliuretic peptide on sodium and water excretion in persons with congestive heart failure. Am J Cardiol. 2001;88(1):23–9. doi: 10.1016/s0002-9149(01)01579-x. [DOI] [PubMed] [Google Scholar]
- [25].Vesely DL, Dietz JR, Parks JR, Baig M, McCormick MT, Cintron G, et al. Vessel dilator enhances sodium and water excretion and has beneficial hemodynamic effects in persons with congestive heart failure. Circulation. 1998;98(4):323–9. doi: 10.1161/01.cir.98.4.323. [DOI] [PubMed] [Google Scholar]
- [26].Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5(9):1304–9. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr., et al. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66(2):353–63. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- [28].Rhodes TE, Abraham RL, Welch RC, Vanoye CG, Crotti L, Arnestad M, et al. Cardiac potassium channel dysfunction in sudden infant death syndrome. J Mol Cell Cardiol. 2008;44(3):571–81. doi: 10.1016/j.yjmcc.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang T, Kanki H, Roden DM. Phosphorylation of the IKs channel complex inhibits drug block: novel mechanism underlying variable antiarrhythmic drug actions. Circulation. 2003;108(2):132–4. doi: 10.1161/01.CIR.0000082708.86266.B8. [DOI] [PubMed] [Google Scholar]
- [30].Kanki H, Kupershmidt S, Yang T, Wells S, Roden DM. A structural requirement for processing the cardiac K+ channel KCNQ1. J Biol Chem. 2004;279(32):33976–83. doi: 10.1074/jbc.M404539200. [DOI] [PubMed] [Google Scholar]
- [31].Silva J, Rudy Y. Subunit Interaction Determines IKs Participation in Cardiac Repolarization and Repolarization Reserve. Circulation. 2005;112(10):1384–91. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol (Heart Circ Physiol 44) 1998;275(1):H301–21. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- [33].Shimizu K, Shintani Y, Ding WG, Matsuura H, Bamba T. Potentiation of slow component of delayed rectifier K(+) current by cGMP via two distinct mechanisms: inhibition of phosphodiesterase 3 and activation of protein kinase G. Br J Pharmacol. 2002;137(1):127–37. doi: 10.1038/sj.bjp.0704843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nunez L, Vaquero M, Gomez R, Caballero R, Mateos-Caceres P, Macaya C, et al. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 2006;72(1):80–9. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- [35].Maack T. Receptors of Atrial Natriuretic Factor. Annual Review of Physiology. 1992;54(1):11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- [36].Nachshon S, Zamir O, Matsuda Y, Zamir N. Effects of ANP receptor antagonists on ANP secretion from adult rat cultured atrial myocytes. Am J Physiol. 1995;268(3):E428–32. doi: 10.1152/ajpendo.1995.268.3.E428. [DOI] [PubMed] [Google Scholar]
- [37].Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- [38].Vincenti A, Brambilla R, Fumagalli MG, Merola R, Pedretti S. Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace. 2006;8(3):204–10. doi: 10.1093/europace/euj043. [DOI] [PubMed] [Google Scholar]
- [39].Otway R, Vandenberg JI, Guo G, Varghese A, Castro ML, Liu J, et al. Stretch-sensitive KCNQ1 mutation A link between genetic and environmental factors in the pathogenesis of atrial fibrillation? J Am Coll Cardiol. 2007;49(5):578–86. doi: 10.1016/j.jacc.2006.09.044. [DOI] [PubMed] [Google Scholar]
- [40].Ellinor PT, Moore RK, Patton KK, Ruskin JN, Pollak MR, Macrae CA. Mutations in the long QT gene, KCNQ1, are an uncommon cause of atrial fibrillation. Heart. 2004;90(12):1487–8. doi: 10.1136/hrt.2003.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ellinor PT, Petrov-Kondratov VI, Zakharova E, Nam EG, Macrae CA. Potassium Channel Gene Mutations Rarely Cause Atrial Fibrillation. BMC Med Genet. 2006;7:70. doi: 10.1186/1471-2350-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sorbera LA, Morad M. Atrionatriuretic peptide transforms cardiac sodium channels into calcium-conducting channels. Science. 1990;247(4945):969–73. doi: 10.1126/science.2154853. [DOI] [PubMed] [Google Scholar]
- [43].Le Grand B, Deroubaix E, Couetil JP, Coraboeuf E. Effects of atrionatriuretic factor on Ca2+ current and Cai-independent transient outward K+ current in human atrial cells. Pflugers Arch. 1992;421(5):486–91. doi: 10.1007/BF00370260. [DOI] [PubMed] [Google Scholar]
- [44].Lonardo G, Cerbai E, Casini S, Giunti G, Bonacchi M, Battaglia F, et al. Atrial natriuretic peptide modulates the hyperpolarization-activated current (If) in human atrial myocytes. Cardiovasc Res. 2004;63(3):528–36. doi: 10.1016/j.cardiores.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [45].Tuinenburg AE, Van Veldhuisen DJ, Boomsma F, Van Den Berg MP, De Kam PJ, Crijns HJGM. Comparison of Plasma Neurohormones in Congestive Heart Failure Patients With Atrial Fibrillation Versus Patients With Sinus Rhythm. Am J Cardiol. 1998;81(10):1207–10. doi: 10.1016/s0002-9149(98)00092-7. [DOI] [PubMed] [Google Scholar]
- [46].Rossi A, Enriquez-Sarano M, Burnett JC, Jr., Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35(5):1256–62. doi: 10.1016/s0735-1097(00)00515-5. [DOI] [PubMed] [Google Scholar]
- [47].Stambler BS, Guo GB. Atrial natriuretic peptide has dose-dependent, autonomically mediated effects on atrial refractoriness and repolarization in anesthetized dogs. J Cardiovasc Electrophysiol. 2005;16(12):1341–7. doi: 10.1111/j.1540-8167.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- [48].Clemo HF, Baumgarten CM, Ellenbogen KA, Stambler BS. Atrial natriuretic peptide and cardiac electrophysiology: autonomic and direct effects. J Cardiovasc Electrophysiol. 1996;7(2):149–62. doi: 10.1111/j.1540-8167.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- [49].Kecskemeti V, Pacher P, Pankucsi C, Nanasi P. Comparative study of cardiac electrophysiological effects of atrial natriuretic peptide. Mol Cell Biochem. 1996:160–161. 53–9. doi: 10.1007/BF00240031. [DOI] [PubMed] [Google Scholar]
- [50].Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1(2):210–7. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Feng J, Yue L, Wang Z, Nattel S. Ionic Mechanisms of Regional Action Potential Heterogeneity in the Canine Right Atrium. Circ Res. 1998;83(5):541–51. doi: 10.1161/01.res.83.5.541. [DOI] [PubMed] [Google Scholar]
- [52].Wang J, Liu L, Feng J, Nattel S. Regional and functional factors determining induction and maintenance of atrial fibrillation in dogs. Am J Physiol (Heart Circ Physiol 40) 1996;271(1):H148–58. doi: 10.1152/ajpheart.1996.271.1.H148. [DOI] [PubMed] [Google Scholar]
- [53].Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45(1):82–6. doi: 10.1016/j.jacc.2004.09.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.