Abstract
Lambs vaccinated with Haemonchus contortus excretory/secretory (ES) glycoproteins in combination with the adjuvant Alhydrogel are protected against H. contortus challenge infection. Using glycan microarray analysis we showed that serum from such vaccinated lambs contains IgG antibodies that recognize the glycan antigen Galα1-3GalNAc-R and GalNAcβ1-4(Fucα1-3)GlcNAc-R. Our studies revealed that H. contortus glycoproteins contain Galα1-3Gal-R as well as significant levels of Galα1-3GalNAc-R, which has not been previously reported. Extracts from H. contortus adult worms contain a galactosyltransferase acting on glycan substrates with a terminal GalNAc, indicating that the worms possess the enzymatic potential to synthesize terminal Gal-GalNAc moieties. These data illustrate that glycan microarrays constitute a promising technology for fast and specific analysis of serum anti-glycan antibodies in vaccination studies. In addition, this approach facilitates the discovery of novel, antigenic parasite glycan antigens that may have potential for developing glycoconjugate vaccines or utilization in diagnostics.
Keywords: Haemonchus contortus, Toxocara canis, Antigenicity, Glycosylation, α-galactose, Carbohydrate, Glycan microarray
1. Introduction
Infections by gastro-intestinal nematodes are wide-spread and cause substantial damage, both in terms of well-being of livestock and economic losses by farmers. Haemonchus contortus is a common gastro-intestinal nematode, which resides in the abomasum of sheep and feeds on the host’s blood. Treatment by antihelminthic drugs is an effective way to control infection, although increasing drug resistance requires another and urgent approach to combat these infections (Jackson and Coop, 2000). Many studies focussed on the identification of immunogenic protein antigens of H. contortus and the analysis of their potential to induce protective immunity by vaccination (Vervelde et al., 2002; Knox et al., 2003; Redmond and Knox, 2006). Several native antigens, including “hidden” gut-derived antigens, can induce protection against H. contortus (Knox et al., 2003). However, attempts to induce protection employing recombinant forms of these antigens are not encouraging, suggesting that specific post-translational modifications, such as glycosylation, may contribute to the protective properties of these proteins (Vervelde et al., 2002).
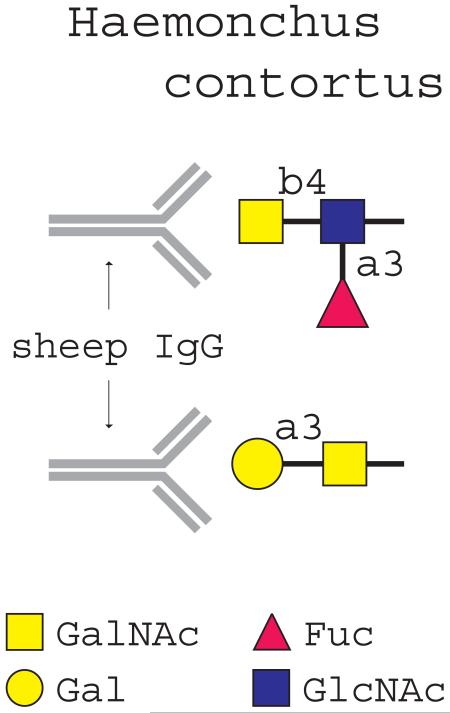
Glycosylation can greatly contribute to the immunogenicity of proteins, especially when the glycans are foreign to the host. Glycans are abundant on the surface and secretory products of helminths, and are well exposed to the environment. Both glycans of the parasitic trematode Schistosoma mansoni (Okano et al., 1999, 2001) and nematode-glycans (Tawill et al., 2004) have the capacity to trigger T-helper 2 (Th2) type responses and the production of glycan-specific antibodies in their hosts (Okano et al., 1999, 2001). Individuals infected with Schistosoma species and chimpanzees immunized with radiation-attenuated cercariae showed high levels of anti-glycan serum IgG to the glycan antigens GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc (LDN-DF) and Fucα1-3GalNAcβ1-4GlcNAc (F-LDN), glycan motifs that are not found in mammals (van Remoortere et al., 2001, 2003a, 2003b; van Die and Cummings, 2006). Recent data showed that vaccination with natural excretory/secretory (ES) antigens from H. contortus in Alhydrogel, a strong Th2 type response-inducing adjuvant, induced protection in lambs against challenge infection with H. contortus, whereas a similar vaccination protocol using dimethyl dioctadecyl ammonium bromide (DDA) as adjuvant was ineffective (Vervelde et al., 2003). In these vaccination trials, induction of protection was significantly correlated with the presence of high levels of serum IgG against the glycan epitope GalNAcβ1-4(Fucα1-3)GlcNAc (LDNF), suggesting that this glycan structure may contribute to the induction of protective immunity (Vervelde et al., 2003).
Novel developments in glycan microarray technology now allow the simultaneous detection of antibodies directed against a large number of glycan antigens using very small serum samples (Blixt et al., 2004). To explore whether vaccination with H. contortus ES antigens induces multiple anti-glycan antibodies, the same sera as used in our previous studies were screened on a glycan-array containing more than 250 different glycan antigens. The data indicate that vaccination of lambs with ES antigens indeed resulted in eliciting multiple anti-glycan antibodies, which varied depending on the adjuvant used. In addition to anti-LDNF IgG, a high level of IgG recognizing the glycan antigen Galα1-3GalNAc was observed only in sera of the protected lambs, which were vaccinated with ES antigens in Alhydrogel. Our data revealed that glycoproteins from different developmental stages of H. contortus contain a terminal Galα1-3GalNAc-R moiety, a glycan antigen that to our knowledge has not been reported before on helminth glycoproteins.
2. Materials and methods
2.1. Materials
Sera from lambs were obtained from studies described previously (Vervelde et al., 2003). Essentially, Black Bless sheep were immunized s.c. three times at 3 week intervals (at day 0, day 21 and day 42) with H. contortus-derived ES products in Alhydrogel or DDA. Two weeks after the last immunization (day 56), all sheep were challenged with H. contortus L3s. H. contortus ES antigens were obtained as previously described (Vervelde et al., 2003). The lectin GSI-B4-biotin was purchased from Sigma (St. Louis, MO, USA). Goat anti-mouse-peroxidase (PO), streptavidin-PO and streptavidin-alkalic phosphatase were purchased from Jackson Immunoresearch (West Grove, USA). The anti-mouse-alkalic phosphatase was purchased from Zymed laboratories, Inc. (San Francisco, USA) and mouse anti-sheep IgG was from Serotec (Kidlington, UK). The anti-Galα1-3Gal antibody M86 (Galili et al., 1998) was a kind gift from Dr. U. Galili (University of Massachusetts Medical School, USA). Monocytes were isolated from buffycoat (Sanquin, Amsterdam, the Netherlands) with CD14 MACS beads (Miltenyi biotec, Auburn, USA) according to the manufacturer’s protocol. Galα1-3Gal-polyacrylamide (PAA), Galα1-3GalNAc-PAA and glucitol-PAA were purchased from Lectinity (~20% substitution, Lectinity, Finland) and LDNF-BSA was synthesized as previously described (van Remoortere et al., 2000). p-Nitrophenyl-N-acetyl-β-D-GalNAc (GalNAcβ-pNP), GalNAcα-pNP, Galβ-pNP, Galα-pNP, Galβ1-4GlcNAcβ-pNP (LN-pNP) were purchased from Sigma (St. Louis, MO, USA). GalNAcβ1-4GlcNAc-O-(CH2)8COOCH3 was a kind gift from Ole Hindsgaul (University of Alberta, Canada). Fucα1-2Galβ1-3GlcNAc-O(CH2)7CH3, Fucα1-2Galβ1-4GlcNAc-O-(CH2)8COOCH3 and Galβ1-3GlcNAc-O-(CH2)8COOCH3 were a kind gift from Monica Palcic (University of Alberta, Canada).
2.2. Glycan array
Glycan array screening was performed by Core H of the Consortium for Functional Glycomics (CFG) (University of Oklahoma, Oklahoma, USA). The glycan array is a microarray containing a library of natural and synthetic glycans with amino linkers printed onto N-hydroxysuccinimide (NHS)-derivatized glass slides to form a covalent amide linkage. All glycan structures used and their CFG numbers (#), as well as standard procedures for glycan array testing are available at the CFG website (http://www.functionalglycomics.org/fg/). The array used was printed array Version 2.1 containing glycan structures with CFG # 1-264.
Glycan-array slides were incubated with pooled serum (day 49, 1:100 dilution), and subsequently with Alexa-labeled mouse anti-sheep IgG secondary antibodies in PBS containing 0.5% Tween-20. The samples (100 μl) were applied directly onto the surface of a single slide, covered with a microscope cover slip and then incubated in a humidified chamber for 60 min. Slides were subsequently washed by successive rinses in (i) PBS-0.05% Tween, (ii) PBS, (iii) deionized water, and immediately subjected to imaging. Fluorescence intensities were detected by using a ScanArray 5000 (PerkinElmer) confocal scanner. Image analyses were carried out using IMAGENE image analysis software (BioDiscovery, El Segundo, CA, USA). No background subtractions were performed. The array was done twice. Data were plotted by using Microsoft EXCEL software.
2.3. Preparation of helminth proteins
Helminth homogenates were prepared from H. contortus (adults and L3s), Dictyocaulus viviparus (adults and L3s), Trichinella spiralis (L3s and ES antigens), Toxocara canis (adults), Caenorhabditis elegans (adults), Fasciola hepatica (adults), S. mansoni (adults and cercariae) as described by De Bose-Boyd et al. (1998). For Western blotting, frozen worms were thawed and resuspended in 100 mM Tris-HCl, pH 8, containing protease inhibitors. For ELISA assays, the proteins of the helminth homogenates were precipitated by adding 4 vol. of (-20°C) acetone. Subsequently, the mixture was incubated for 1 h at -20°C, the protein pellet collected by centrifugation for 10 min at 13,000 g and re-suspended in ELISA coating buffer. For galactosyltransferase assays, H. contortus adult worms were homogenized in 50 mM Na cacodylate buffer, pH 7, on ice using five pulses of 10 s with a Polytron PT 1200 (Kinematic AG Littau, Switzerland). After sonification, Triton-X-100 was added to a final concentration of 1% and the mixture was incubated on ice for 30 min. The supernatant was collected after centrifugation for 10 min 11,000 g at 4°C, and the protein concentration was determined using the BCA protein Assay (Pierce).
2.4. Affinity purification of anti-Galα1-3GalNAc antibodies from serum of immunized lambs
To purify antibodies specific for Galα1-3GalNAc, 0.75 ml pooled serum (day 49) was used, derived from the lambs immunized with ES antigens in Alhydrogel after vaccination. The serum was incubated with Galα1-3GalNAc-PAA-Biotin (1 mg/ml) for 30 min at room temperature in PBS containing 0.1% SDS (BDH Laboratory Supplies, Poole, England). The formed immune complexes were subsequently captured with streptavidin-agarose beads by incubation at room temperature for 1 h on a roller bank. The beads were collected by centrifugation and washed with PBS containing 0.1% SDS. The bound antibody was eluted from the beads with 0.1 M glycine-HCl, pH 2.8, immediately neutralized with 1 mM Tris, pH 7.5, (Pierce, Rockford,USA), and assayed for Galα1-3GalNAc specificity by ELISA.
2.5. ELISA and Western blotting
Helminth extracts, glucitol-PAA, Galα1-3Gal-PAA, Galα1-3GalNAc-PAA, LDNF-BSA (10 μg/ml) and lysates of monocytes (10 μg/ml) were coated overnight on NUNC maxisorb plates (Roskilde, Denmark). After blocking (60 min 37°C) with 1% ELISA-grade BSA (Fraction V, fatty acid free; Calbiochem, San Diego, USA) in TSM (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2) and washing with TSM containing 0.1% Tween-20, glycan-specific antibodies or biotinylated GSI-B4 were added for 60 min at 37°C. For the ELISA with sheep-derived serum, bound antibodies were detected by incubation with mouse anti-sheep IgG (Serotec, UK), followed by detection with goat anti-mouse PO, both at 37°C for 60 min. In the case of incubation with biotinylated GSI-B4, unbound GSI-B4 was washed away with TSM and binding was detected with streptavidin-PO conjugate. The reaction was developed by TMB substrate and O.D. measured by spectrophotometry.
For Western blotting, the proteins (15 μg) within the helminth extracts were separated by SDS-PAGE (Mini-PROTEAN 3 System, BioRad, Hercules, USA) under reducing conditions on a 12.5% polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was blocked overnight in 1% BSA/PBS solution and probed for 1 h at room temperature with GSI-B4-biotin. After washing and incubation with streptavidin-alkaline phosphatase conjugate, bound lectin was detected using x-phosphate/5-bromo-4-chloro-3-inodylphosphate (Promega) and 4-nitrobluetetrazolium chloride (Promega, Leiden, the Netherlands).
2.6. Galactosyltransferase assays
Galactosyltransferase activity of H. contortus homogenates and bovine α1,3galactosyltransferase (Sigma, Saint Louis, Missouri, USA) were determined essentially as previously described (Joziasse et al., 1990). Enzyme assays, using H. contortus extract or bovine α1,3galactosyltransferase as the enzyme source, were done in a 25 μl reaction mixture containing 0.5 mM UDP-[14C]-Gal (6 Ci/mol) (Amersham Biosciences, Buckinghamshire, UK), 20 mM MnCl2, 4 mM ATP, 0.5% Triton X-100, 100 mM Na-cacodylate buffer, pH 7.2, and 1 mM acceptor substrate. Control assays lacking an acceptor were performed to correct for endogenous acceptors. After incubating the samples for 17 h at 37°C, the mixture was passed through SepPak C-18 cartridges (Palcic et al., 1994) (Waters Corporation, Massachusetts, USA). The UDP-[14C]-galactose incorporation was measured by liquid scintillation (Packard TRI CARB, A Camberra Company, Ontario, Canada). The average enzymatic activity of two independent experiments was defined in nmol/ml/h.
3. Results
3.1. Presence of anti-glycan antibodies in sera of lambs immunized with ES antigens of H. contortus
We have previously reported that lambs are protected against the parasitic nematode H. contortus after vaccination with ES glycoproteins using Alhydrogel as an adjuvant. When DDA was used as an adjuvant, no protection was seen (Vervelde et al., 2003). In lambs vaccinated with ES antigens in Alhydrogel, but not in any other group, a significant increase was found in antibody levels against the LDNF antigen, and the anti-LDNF IgG response was significantly correlated with protection.
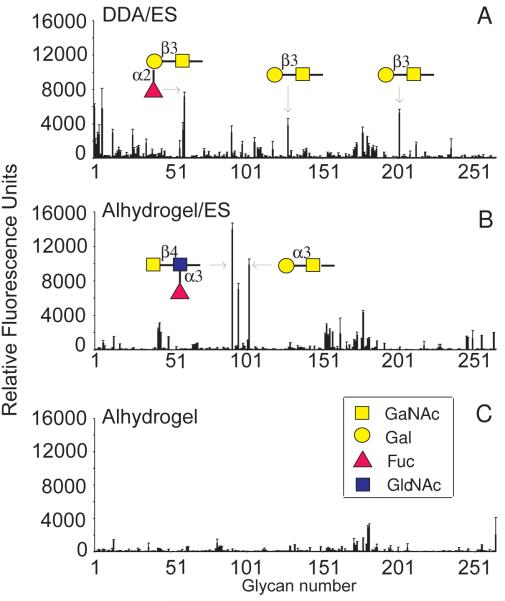
To determine whether the protected lambs have, in addition to serum antibodies to LDNF, antibodies against other glycan antigens, the same sera as used in our previous studies were screened for antibodies recognizing specific oligosaccharides within a large library of glycan antigens, using the glycan-array facility of the CFG (http://www.functionalglycomics.org) (Blixt et al., 2004). The data in Fig. 1 show that sera from lambs vaccinated with ES antigens in Alhydrogel and DDA contained antibodies that recognized multiple glycan antigens on the array, in contrast to pooled serum from a control group that only received Alhydrogel. Remarkably, the sera from the vaccinated lambs vaccinated with ES antigens and different adjuvants did not recognize the same glycan antigens. Similar to our previous observations, sera from lambs immunized with ES antigen in Alhydrogel recognized the LDNF antigen (#91, referring to the number assigned to this epitope in the array). In addition, the Galα1-3GalNAc glycan antigen (#102) was clearly recognized, as well as an oligosaccharide containing the blood group B-antigen (#95) which contains a terminal α1-3Gal. However, the related structure Galα1-3Galβ-R was not recognized by serum antibodies. In contrast, lambs vaccinated with ES antigens in DDA, a vaccination protocol that did not induce protection, contained serum antibodies recognizing both Galβ1-3GalNAc (#128, #201) and a fucosylated derivative, Fucα1-2Galβ1-3GalNAc (#59, #60).
Fig. 1.
Glycan array analysis of anti-glycan antibodies in the sera of lambs immunized with Haemonchus contortus excretory/secretory (ES) antigens. Pooled sera (1:100 diluted in PBS) from lambs immunized with H. contortus ES antigens in combination with adjuvant dimethyl dioctadecyl ammonium bromide (DDA) or Alhydrogel, contain IgG antibodies to different glycan antigens as determined by glycan array analysis. Pooled sera from lambs that received ES antigens in DDA contain mostly IgG antibodies recognizing Galβ1-3GalNAc, or α2-fucosylated Galβ1-3GalNAc (A), whereas sera from lambs that received ES antigens in Alhydrogel contain mostly serum IgG recognizing Galα1-3GalNAc and GalNAcβ1-4(Fucα1-3)GlcNAc-R (LDNF) (B).. Sera from lambs that received only adjuvant without ES antigens did not contain significant anti-glycan antibody levels (C).
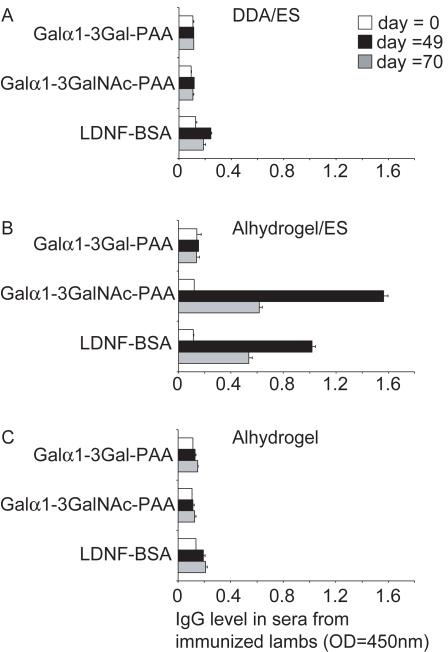
To validate the data found in the glycan array, an ELISA was performed, in which neoglycoconjugates carrying different selected glycan antigens were coated. The amount of IgG-specific antibodies against Galα1-3GalNAc, LDNF and Galα1-3Gal in the sera of lambs immunized with ES antigens in combination with Alhydrogel or DDA, and the sera of control lambs immunized with Alhydrogel only, was measured. Similar to the results of the glycan array, high levels of IgG antibodies against both LDNF and Galα1-3GalNAc were detected only in the lambs immunized with ES antigens in Alhydrogel (Fig. 2), whereas no antibodies could be detected recognizing the Galα1-3Gal-epitope. The antibody levels observed were highest at day 49 of the immunization protocol, similar to what has been previously observed for the anti-LDNF antibody levels (Fig. 2).
Fig. 2.
Anti-glycan IgG in the sera of lambs vaccinated with Haemonchus contortus excretory/secretory (ES) products analyzed by ELISA. The amount of IgG against Galα1-3GalNAc-polyacrylamide (PAA), GalNAcβ1-4(Fucα1-3)GlcNAc-BSA (LDNF) and Galα1-3Gal-PAA, was determined by ELISA in pooled sera (1:100 diluted in PBS) of lambs immunized with ES antigens in dimethyl dioctadecyl ammonium bromide (DDA) (A) or ES antigens in Alhydrogel (B) or with Alhydrogel only (C), on different days in the immunization schedule (Vervelde et al., 2003). The data from two independent experiments, performed in duplicate, are shown and error bars represent the S.D.
3.2. Terminal α-Gal on glycoproteins from H. contortus and other helminths
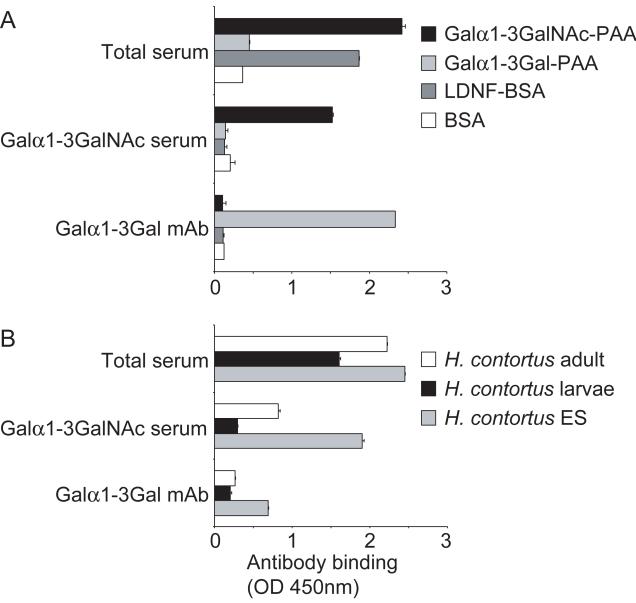
The presence of antibodies recognizing the glycan antigen Galα1-3GalNAc in sera of lambs immunized with H. contortus ES antigens predicts that such glycans are synthesized within H. contortus. To establish the presence of terminal Galα1-3GalNAc-R moieties in glycoconjugates of H. contortus, antibodies recognizing Galα1-3GalNAc from the sera of lambs immunized with ES antigens in Alhydrogel were affinity purified by immunoprecipitation of the serum with Galα1-3GalNAc-PAA-biotin coupled to streptavidin beads. The antibodies eluted from the beads showed binding to Galα1-3GalNAc-PAA in ELISA as expected, whereas no binding to LDNF-BSA or Galα1-3Gal-PAA could be detected (Fig. 3A), thereby establishing that the antibody was highly purified. The immunopurified anti-Galα1-3GalNAc antibodies recognized glycoproteins in both H. contortus adults and ES antigens, whereas a lower binding was observed to L3s (Fig. 3B). In parallel, the presence of Galα1-3Gal glycan epitopes, recently described to occur in the nematode Parelaphostrongylus tenuis (Duffy et al., 2006), was investigated within H. contortus using the anti-Galα1-3Gal monoclonal antibody (mAb) M86 that does not recognize Galα1-3GalNAc epitopes (Galili et al., 1998) (Fig. 3A). The results show that the anti-Galα1-3Gal mAb M86 recognizes H. contortus ES glycoproteins, indicating the presence of terminal Galα1-3Gal glycan epitopes (Fig. 3B).
Fig. 3.
Glycoproteins of Haemonchus contortus contain Galα1-3GalNAc as well as Galα1-3Gal antigens. A) Anti-Galα1-3GalNAc antibodies (indicated as Galα1-3GalNAc serum) were affinity purified from total serum of protected lambs (indicated as total serum), as described in Materials and methods. The Galα1-3GalNAc antibodies specifically recognize Galα1-3GalNAc-polyacrylamide (PAA), and not Galα1-3Gal-PAA or GalNAcβ1-4(Fucα1-3)GlcNAc-R (LDNF)-BSA, as was demonstrated by ELISA with these neoglycoconjugates (coated at 5 μg/ml). By contrast, the monoclonal antibody (mAb) M24 specifically detects Galα1-3Gal-PAA, which is in agreement with the reported Galα1-3Gal specificity of this antibody (Galili et al., 1998). B) The anti-Galα1-3GalNAc antibodies recognize adult worm proteins (coated at 10 μg/ml) and excretory/secretory (ES) glycoproteins (coated at 2 μg/ml) of H. contortus, whereas lower recognition of L3s (coated at 10 μg/ml) was detected, as shown by ELISA. mAb M24 (anti-Galα1-3Gal) shows binding to H. contortus ES glycoproteins, whereas binding to the other stages was hardly detectable.
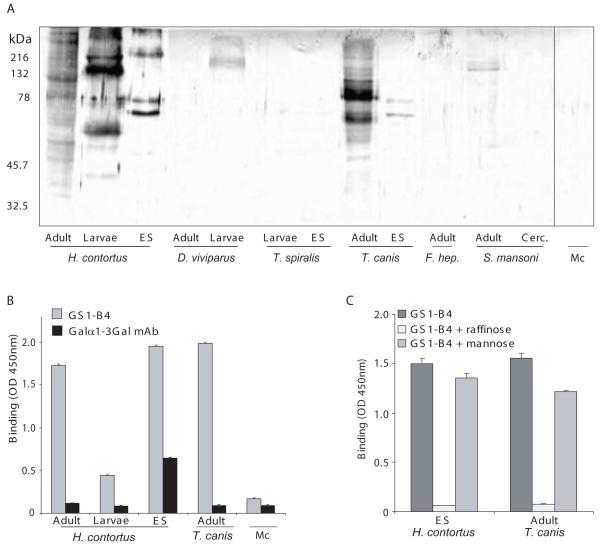
To investigate whether other helminth species contain glycan antigens terminating in α-Gal, homogenates of different nematode and trematode species were tested with the lectin GSI-B4 by immunoblot and ELISA. GSI-B4 is a lectin showing a high specificity for α-Gal, whereas β-Gal is hardly bound (Murphy and Goldstein, 1977) (Fig. 4). GSI-B4 binds to glycoproteins from different life stages of H. contortus (Fig. 5A and B), and binding of GSI-B4 was observed to T. canis glycoproteins (Fig. 5A-C). The binding of GSI-B4 to H. contortus ES antigens and T. canis glycoproteins is specific, since it could be inhibited by raffinose but not by mannose (Fig. 5C). A very low or no detectable binding of GSI-B4 was observed to glycoproteins derived from D. viviparous, T. spiralis, F. hepatica and S. mansoni, or to a control glycoprotein mixture derived from human monocytes (Fig. 5A), indicating that terminal α-Gal is not a very common feature on glycoproteins of nematodes or trematodes. Toxocara canis adult worms did not show reactivity with the anti-Galα1-3Gal mAb (Fig. 5B), which may indicate that the α-Gal within T. canis glycoproteins is not present in an α1-3-linkage to Gal, but the exact structural details should be further investigated. In summary, our data provide evidence that H. contortus expresses both Galα1-3Gal and Galα1-3GalNAc containing glycoproteins. Remarkably, glycoproteins containing terminal α-Gal epitopes are not frequently detected in other nematodes or trematodes, suggesting certain species-specificity.
Fig. 4.
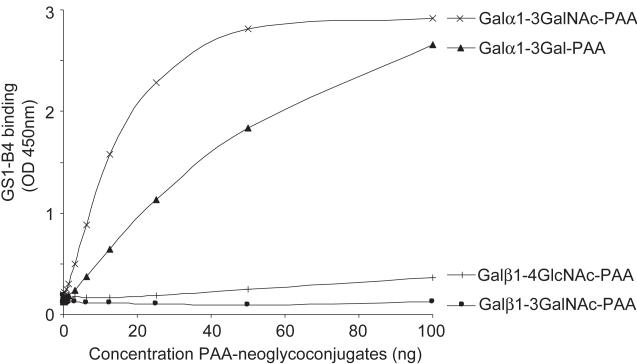
The lectin GSI-B4 recognizes neoglycoconjugates with terminal αGal and shows higher affinity to Galα1-3GalNAc-PAA than to Galα1-3Gal-PAA. Neoglycoconjugates carrying different glycan antigens coupled to Polyacrylamide (PAA) were coated in similar concentrations ranging from 0 - 100 ng (0 - 2 μg/ml in coating buffer) and analysed for reaction with GSI-B4-PO (5 μg/ml) in ELISA.
Fig. 5.
Glycoproteins from Haemonchus contortus and Toxocara canis react with the αGal-specific lectin GSI-B4. A) Proteins (15 μg) of different helminth species and stages (indicated as in the figure, Dictyocaulus viviparus, Trichinella spiralis, Schistosoma mansoni, Fasciola hepatica ( =F. hep)) were separated by SDS-PAGE and transferred to nitrocellulose. Monocyte-derived proteins (Mc) were included in the assay as a negative control. Western blots were incubated with the αGal-specific lectin GSI-B4 (biotin-labeled, 5 μg/ml). Molecular size markers (in kDa) are indicated to the left. B) H. contortus and T. canis proteins strongly react with GSI-B4, as was demonstrated by ELISA using GSI-B4-PO at 5 μg/ml. Analysis of the binding of the Galα1-3Gal-specific monoclonal antibody (mAb) M24 (Galili et al., 1998) showed that H. contortus excretory/secretory (ES) proteins, but not T. canis worm glycoproteins, contain detectable levels of terminal Galα1-3Gal. C) The staining of H. contortus and T. canis proteins with GSI-B4-PO (5 μg/ml) is specific, as was shown by the capacity of raffinose (10 mM), but not mannose (10 mM), to block the binding.
3.3. Extracts of H. contortus contain galactosyltransferase(s) acting on oligosaccharides with a terminal GalNAc
To determine whether H. contortus expresses an enzyme capable of catalyzing the transfer of a Gal from UDP-Gal to substrates with terminal GalNAc, a homogenate of H. contortus adult worms was used as an enzyme source. The acceptor specificity of the putative H. contortus galactosyltransferase(s) compared with that of bovine α3-galactosyltransferase is shown in Table 1. Results indicate that H. contortus expresses galactosyltransferase(s) with activity towards several substrates with a terminal GalNAc, whereas no activity was detected towards any acceptor tested with a terminal Gal. The products formed by this enzyme activity, Gal-GalNAc-R, could not be cleaved by either α- or β-galactosidase (Sigma; data not shown), even when added in 100-fold excess, preventing the determination of the type of anomeric linkage of the Gal in the formed products. The bovine α1,3GalT, which was tested in parallel as a control, has a clear preference for mono- or oligosaccharides with terminal β-linked Gal (Table 1) as previously demonstrated (Joziasse et al., 1990). In conclusion, H. contortus contains galactosyltransferase activities that are clearly distinct from bovine α1,3GalT. These galactosyltransferases may be responsible for the synthesis of both Galα-GalNAc and/or Galβ-GalNAc sequences in H. contortus.
Table 1.
Galactosyltransferase activity in an enzyme extract derived from Haemonchus contortus adult worms
| Acceptor | Relative galactosyltransferase (GalT) activity |
|
|---|---|---|
| H. contortus GalT | Bovine α1,3-GalT | |
| GalNAcα-O-pNP | 100a | <1 |
| GalNAcβ-O-pNP | 13 | <1 |
| GalNAcβ1-4GlcNAc-R1b | 40 | 5 |
| Galβ1-4GlcNAc-pNP | <1 | 51 |
| Galβ-O-pNP | <1 | 41 |
| Galα-O-pNP | <1 | 4 |
| Fucα1-2Galβ1-3GlcNAc-R2 | <1 | 1 |
| Fucα1-2Galβ1-4GlcNAc-R1 | <1 | 2 |
| Galβ1-3GlcNAc-R1 | <1 | 100a |
All acceptor substrates in the assays have been used at a concentration of 1 mM. The acceptor specificity of the H. contortus enzyme extract has been compared with the activity of commercial bovine (α1,3-galactosyltransferase (α1,3-GalT). For both enzymes, the acceptor substrate that showed the highest activity has been set at 100%. In the assays with H. contortus extract, 100% activity represents an enzyme activity of 1 nmol/ml/h, and for the α1,3-GalT 100% activity represents an activity 49 nmol/ml/h.
Values set at 100%
R1 = -O-(CH2)8COOCH3; R2 = -(CH2)7CH3
4. Discussion
Lambs can be protected against challenge infection with the parasitic nematode H. contortus by vaccination with ES glycoproteins using Alhydrogel as an adjuvant (Vervelde et al., 2003). In the studies described, a high IgG antibody level against LDNF was observed in sera of the animals vaccinated with ES antigens in Alhydrogel, which was significantly correlated with protection (Vervelde et al., 2003). Here, we extend these findings by showing that the sera of the protected lambs also contained a high level of IgG antibodies against the glycan epitope Galα1-3GalNAc, as shown by glycan micro-array screening and confirmed by ELISA. Vaccination of lambs with ES antigens in DDA, which was not associated with protection, also showed induction of anti-glycan antibodies. Remarkably, these anti-glycan antibodies were directed to other glycan antigens than seen in the lambs vaccinated with ES antigens in Alhydrogel
The presence of serum antibodies against Galα1-3GalNAc antigens in the immunized animals indicates that H. contortus ES antigens contain these glycan moieties, and that these glycans are antigenic. The data show, to our knowledge for the first time, that Galα1-3GalNAc epitopes are present in H. contortus ES antigens and on glycoproteins of adult worms. Within glycolipids, Galα1-3GalNAc epitopes have been shown as a conserved structural motif within the arthro-series carbohydrate backbone of glycolipids in C. elegans (Gerdt et al., 1999), Onchocerca volvulus (Wuhrer et al., 2000) and Ascaris suum (Friedl et al., 2003), however it is unknown whether these glycolipids are immunogenic.
Screening of several helminth species with the lectin GSI-B4, which recognizes terminal α-Gal irrespective of its linkage, showed that this modification is not very common among nematodes and trematodes. In addition to H. contortus, a significant binding of GSI-B4 was detected with T. canis, whereas no or very low binding was observed with S. mansoni, F. hepatica, T. spiralis or D. viviparus glycoproteins. The presence of α-Gal as a capping structure of protein-linked glycans has been previously observed in some helminth species. In the dog cestode Echinococcus granulosus, N-glycans carry antennae capped with Galα-Gal (Khoo et al., 1997). In the nematode Parastrongylus tenuis which commonly infects white-tailed deer, Galα1-3Galβ1-4GlcNAc is present as a dominant antenna of complex type N-glycans in adult worms. Since deer, similarly to most non-human mammals synthesize Galα1-3Gal, Duffy et al. (2006) suggested that the presence of similar terminal glycan moieties in the worm may represent a form of molecular mimicry that could enable the nematode to evade the immune response of the host (Galili et al., 1988; Damian, 1997). Our data show a similar situation in H. contortus, which infects sheep that most likely synthesize Galα1-3Gal epitopes. No antibodies recognizing Galα1-3Gal could be detected in the sera of lambs immunized with H. contortus ES antigens, whereas our data (Fig. 3B) indicate that H. contortus ES glycoproteins express terminal Galα1-3Gal and Galα1-3GalNAc moieties.
The use of different adjuvants resulted in the induction of selective anti-glycan antibodies. The significance of this observation in relation to the induction of protection of the lambs to challenge infection is not fully clear. However, the induction of significant antibody levels to the glycan epitopes Galβ1-3GalNAc and Fucα1-2Galβ1-3GalNAc in lambs vaccinated with ES antigens in DDA might at least predict the presence of such glycan antigens in H. contortus and their antigenicity in sheep. Interestingly, a C. elegans α1,2-fucosyltransferase was recently characterized with the potential to generate the sequence Fucα1-2Galβ1-3GalNAcα-R, and various highly antigenic methylated forms of the Fucα1-2Galβ1-3GalNAc moiety have been demonstrated in T. canis (Khoo et al., 1991; Schabussova et al., 2007). These data suggest that Fucα1-2Galβ1-3GalNAcα-R may be a common structure in H. contortus, C. elegans and T. canis.
The presence of various terminal Gal residues in H. contortus implies that this nematode expresses enzymes capable of catalyzing the transfer of a Gal residue to oligosaccharides with a terminal GalNAc. Our results demonstrate that H. contortus adults indeed possess such galactosyltransferase activity. The potential of galactosyltransferase(s) from H. contortus to use acceptors containing a terminal GalNAc residue clearly differs from the preference of the bovine α1,3-galactosyltransferase, which only shows an efficient activity towards acceptors containing a terminal Gal (Joziasse et al., 1990). Unfortunately, the nature of the glycosidic bond between the Gal and GalNAc residues could not be determined using α-, or β-galactosidases. This could be the result of a reduced accessibility of the enzymes to the formed product, which is an uncommon substrate for the α- and β-galactosidases. The lack of activity towards H-type antigens may indicate that H. contortus does not express galactosyltransferases able to synthesize blood group B antigen, which contains a Galα1-3Gal moiety.
Our data illustrate that glycan microarrays constitute a promising technology for fast and specific analysis of serum anti-glycan antibodies in vaccination studies. In addition, this approach facilitates the discovery of novel, antigenic parasite glycan antigens that may have potential for developing glycoconjugate vaccines or utilization in diagnostics. It should be emphasized, however, that the glycan-array used in this study contains many (mostly known) glycan structures, but a great variety of glycan structures exist that are not present on the array. Very few H. contortus glycan structures have been structurally characterized (Haslam et al., 1996, 1998). It is expected that these nematodes express a large array of different glycan structures, possibly including highly antigenic structures, which are not present on available glycan-arrays. The availability of specific “pathogen-arrays” would allow enormous progress in this field.
In summary, the glycan-array screening reported here resulted in the discovery of the antigenic glycan structure Galα1-3GalNAc on glycoproteins of H. contortus, which has not been previously reported. The production of antibodies against this structure by sheep, protected by immunization with ES antigens in combination with Alhydrogel, suggests that these antibodies may contribute to immune protection, which is an interesting possibility warranting follow-up studies. An example illustrating the protective capacity of anti-glycan antibodies is the rapid expulsion of T. spiralis from rats by anti-tyvelose mono- and polyclonal antibodies (Ellis et al., 1994). Interestingly, vaccination of sheep using a galactose-containing protein complex from H. contortus, H-Gal-GP, showed a protective effect (Smith et al., 1994, 1999). Similar to ES antigens, H-Gal-GP contains the LDNF antigen, next to undefined Gal moieties. Recently it was shown that the protective effect that was obtained with this H-Gal-GP is unlikely to be caused by the LDNF epitope present on the metalloprotease that is part of the protein complex (Geldhof et al., 2005). Unfortunately, the structure of the Gal-containing “H-Gal-GP” is not yet known. It would be interesting to investigate whether protective epitope(s) on the H-Gal-GP complex could include Galα1-3GalNAc antigen.
Acknowledgement
We thank Core H of the Consortium of Functional Glycomics (University of Oklahoma, Oklahoma, USA) for performing the glycan array screening. We thank Dr. Jacqueline Poot (Intervet Int., Boxmeer, the Netherlands), Dr. Elena Pinelli and Dr. Joke van der Giessen (RIVM Bilthoven, the Netherlands), and Dr. Lodewijk Tielens (Utrecht University, the Netherlands) for providing us with diverse helminth material. We thank Dr. U. Galili (University of Massachusetts Medical School, USA) for the kind gift of the M86 antibody and Dr. Monica Palcic and Ole Hindsgaul (Carlsberg Laboratory, Copenhagen, Denmark) for oligosaccharides. This work was supported by the Netherlands Technology Foundation (STW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian RT. Parasite immune evasion and exploitation: reflections and projections. Parasitology. 1997;115:S169–175. doi: 10.1017/s0031182097002357. [DOI] [PubMed] [Google Scholar]
- DeBose-Boyd RA, Nyame AK, Cummings RD. Molecular cloning and characterization of an alpha1,3 fucosyltransferase, CEFT-1, from Caenorhabditis elegans. Glycobiology. 1998;8:905–917. doi: 10.1093/glycob/8.9.905. [DOI] [PubMed] [Google Scholar]
- Duffy MS, Morris HR, Dell A, Appleton JA, Haslam SM. Protein glycosylation in Parelaphostrongylus tenuis--first description of the Galalpha1-3Gal sequence in a nematode. Glycobiology. 2006;16:854–862. doi: 10.1093/glycob/cwl001. [DOI] [PubMed] [Google Scholar]
- Ellis LA, Reason AJ, Morris HR, Dell A, Iglesias R, Ubeira FM, Appleton JA. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- Friedl CH, Lochnit G, Zahringer U, Bahr U, Geyer R. Structural elucidation of zwitterionic carbohydrates derived from glycosphingolipids of the porcine parasitic nematode Ascaris suum. Biochem J. 2003;369:89–102. doi: 10.1042/BJ20021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- Galili U, LaTemple DC, Radic MZ. A sensitive assay for measuring alpha-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation. 1998;65:1129–1132. doi: 10.1097/00007890-199804270-00020. [DOI] [PubMed] [Google Scholar]
- Geldhof P, Newlands GF, Nyame K, Cummings R, Smith WD, Knox DP. Presence of the LDNF glycan on the host-protective H-gal-GP fraction from Haemonchus contortus. Parasite Immunol. 2005;27:55–60. doi: 10.1111/j.1365-3024.2005.00744.x. [DOI] [PubMed] [Google Scholar]
- Gerdt S, Dennis RD, Borgonie G, Schnabel R, Geyer R. Isolation, characterization and immunolocalization of phosphorylcholine-substituted glycolipids in developmental stages of Caenorhabditis elegans. Eur J Biochem. 1999;266:952–963. doi: 10.1046/j.1432-1327.1999.00937.x. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, Dell A. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J Biol Chem. 1996;271:30561–30570. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Coles GC, Reason AJ, Morris HR, Dell A. The novel core fucosylation of Haemonchus contortus N-glycans is stage specific. Mol Biochem Parasitol. 1998;93:143–147. doi: 10.1016/s0166-6851(98)00020-6. [DOI] [PubMed] [Google Scholar]
- Jackson F, Coop RL. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:S95–107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- Joziasse DH, Shaper NL, Salyer LS, Van den Eijnden DH, van der Spoel AC, Shaper JH. Alpha 1----3-galactosyltransferase: the use of recombinant enzyme for the synthesis of alpha-galactosylated glycoconjugates. Eur J Biochem. 1990;191:75–83. doi: 10.1111/j.1432-1033.1990.tb19095.x. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Maizels RM, Page AP, Taylor GW, Rendell NB, Dell A. Characterization of nematode glycoproteins: the major O-glycans of Toxocara excretory-secretory antigens are O-methylated trisaccharides. Glycobiology. 1991;1:163–171. doi: 10.1093/glycob/1.2.163. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Nieto A, Morris HR, Dell A. Structural characterization of the N-glycans from Echinococcus granulosus hydatid cyst membrane and protoscoleces. Mol Biochem Parasitol. 1997;86:237–248. doi: 10.1016/s0166-6851(97)00036-4. [DOI] [PubMed] [Google Scholar]
- Knox DP, Redmond DL, Newlands GF, Skuce PJ, Pettit D, Smith WD. The nature and prospects for gut membrane proteins as vaccine candidates for Haemonchus contortus and other ruminant trichostrongyloids. Int J Parasitol. 2003;33:1129–1137. doi: 10.1016/s0020-7519(03)00167-x. [DOI] [PubMed] [Google Scholar]
- Murphy LA, Goldstein IJ. Five alpha-D-galactopyranosyl-binding isolectins from Bandeiraea simplicifolia seeds. J Biol Chem. 1977;252:4739–4742. [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA., Jr. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–6717. [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Harn DA., Jr. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- Palcic MM, Pierce M, Hindsgaul O. Synthetic neoglycoconjugates in glycosyltransferase assay and purification. Methods Enzymol. 1994;247:215–227. doi: 10.1016/s0076-6879(94)47016-2. [DOI] [PubMed] [Google Scholar]
- Redmond DL, Knox DP. Further protection studies using recombinant forms of Haemonchus contortus cysteine proteinases. Parasite Immunol. 2006;28:213–219. doi: 10.1111/j.1365-3024.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Schabussova I, Amer H, van Die I, Kosma P, Maizels RM. O-methylated glycans from Toxocara are specific targets for antibody binding in human and animal infections. Int J Parasitol. 2007;37:97–109. doi: 10.1016/j.ijpara.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Smith SK, Pettit D, Newlands GF, Redmond DL, Skuce PJ, Knox DP, Smith WD. Further immunization and biochemical studies with a protective antigen complex from the microvillar membrane of the intestine of Haemonchus contortus. Parasite Immunol. 1999;21:187–199. doi: 10.1046/j.1365-3024.1999.00217.x. [DOI] [PubMed] [Google Scholar]
- Smith WD, Smith SK, Murray JM. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 1994;16:231–241. doi: 10.1111/j.1365-3024.1994.tb00345.x. [DOI] [PubMed] [Google Scholar]
- Tawill S, Le Goff L, Ali F, Blaxter M, Allen JE. Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect Immun. 2004;72:398–407. doi: 10.1128/IAI.72.1.398-407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I, Cummings RD. Glycans modulate immune responses in helminth infections and allergy. Chem Immunol Allergy. 2006;90:91–112. doi: 10.1159/000088883. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM, van den Eijnden DH. Various stages of Schistosoma express Lewis(x), LacdiNAc, GalNAcbeta1-4 (Fucalpha1-3)GlcNAc and GalNAcbeta1-4(Fucalpha1-2Fucalpha1-3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology. 2000;10:601–609. doi: 10.1093/glycob/10.6.601. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, van Dam GJ, Hokke CH, van den Eijnden DH, van Die I, Deelder AM. Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect Immun. 2001;69:2396–2401. doi: 10.1128/IAI.69.4.2396-2401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Remoortere A, Bank CM, Nyame AK, Cummings RD, Deelder AM, van Die I. Schistosoma mansoni-infected mice produce antibodies that cross-react with plant, insect, and mammalian glycoproteins and recognize the truncated biantennaryN-glycan Man3GlcNAc2-R. Glycobiology. 2003a;13:217–225. doi: 10.1093/glycob/cwg025. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, Vermeer HJ, van Roon AM, Langermans JA, Thomas AW, Wilson RA, van die I, van den Eijnden DH, Agoston K, Kerekgyarto J, Vliegenthart JF, Kamerling JP, van dam GJ, Hokke CH, Deelder AM. Dominant antibody responses to Fucalpha1-3GalNAc and Fucalpha1-2Fucalpha1-3GlcNAc containing carbohydrate epitopes in Pan troglodytes vaccinated and infected with Schistosoma mansoni. Exp Parasitol. 2003b;105:219–225. doi: 10.1016/j.exppara.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Vervelde L, Van Leeuwen MA, Kruidenier M, Kooyman FN, Huntley JF, Van Die I, Cornelissen AW. Protection studies with recombinant excretory/secretory proteins of Haemonchus contortus. Parasite Immunol. 2002;24:189–201. doi: 10.1046/j.1365-3024.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- Vervelde L, Bakker N, Kooyman FN, Cornelissen AW, Bank CM, Nyame AK, Cummings RD, van Die I. Vaccination-induced protection of lambs against the parasitic nematode Haemonchus contortus correlates with high IgG antibody responses to the LDNF glycan antigen. Glycobiology. 2003;13:795–804. doi: 10.1093/glycob/cwg107. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Rickhoff S, Dennis RD, Lochnit G, Soboslay PT, Baumeister S, Geyer R. Phosphocholine-containing, zwitterionic glycosphingolipids of adult Onchocerca volvulus as highly conserved antigenic structures of parasitic nematodes. Biochem J. 2000;348(Pt 2):417–423. [PMC free article] [PubMed] [Google Scholar]