Abstract
Background and Aims
The recommended timing of surveillance colonoscopy for individuals with adenomatous polyps is based on adenoma histology, size, and number. The burden and cost of surveillance colonoscopy are significant. The aim of this study was to examine the utilization of surveillance colonoscopy on a community wide basis.
Methods
We retrospectively queried participants in the Prostate, Lung, Colorectal, and Ovarian Cancer screening trial in nine U.S. communities about use of surveillance colonoscopy. Subjects whose initial colonoscopy demonstrated advanced adenoma (AA), non-advanced adenoma (NAA), or non-adenomatous findings (NA) were included. Colonoscopy exams were confirmed by reviewing colonoscopy reports.
Results
Of 3,876 subjects selected for inquiry, 3,627 (93.6%) responded. The cumulative probability of a surveillance colonoscopy within 5 years was 58.4% (N=1342) in the AA group, 57.5% in those with ≥3 NAA (N=117), 46.7% in those with 1-2 NAA (N=905), and 26.5% (N=1263) in subjects without an adenoma. Within 7 years, 33.2% of subjects with advanced adenoma received ≥ 2 surveillance exams versus 26.9% for those with ≥3 NAA, 18.2% for those with 1 or 2 NAA, and 10.4% for those with non-adenomatous findings. Incomplete colonoscopy, family history of colorectal cancer, or interval adenomatous findings could explain only a minority of surveillance colonoscopy in low risk subjects.
Conclusions
In community practice there is substantial over utilization of surveillance colonoscopy among low risk subjects and under utilization among subjects with advanced adenoma. Interventions to better align surveillance colonoscopy use with risk for advanced lesions is needed.
Introduction
Colorectal cancer screening is widely endorsed and encouraged (1-3), because identification and removal of adenomatous polyps can reduce the subsequent incidence of and mortality from colorectal cancer (4,5). Because of the risk of development of recurrent or metachronous adenomas and interval cancer, monitoring with surveillance colonoscopy is recommended, especially for subjects with a history of adenomatous polyps. The burden of surveillance colonoscopy is substantial, estimated to be responsible for about ¼ of the colonoscopies performed annually, and is among the most commonly cited indications for colonoscopy (6).
Increasing the rate of colorectal cancer screening with resultant adenoma detection will further stimulate the need for surveillance colonoscopy, and promises to further strain the capacity to deliver colonoscopy services (7,8).
Surveillance guidelines have emphasized risk stratification, with surveillance at 3 years for subjects with an advanced adenoma or ≥ 3 adenomas, and less frequent surveillance among subjects with non-advanced adenoma (recommended every 5 – 10 years) or no adenomas (recommended every 10 years) (9). Surveys of surgeons, gastroenterologists, and primary care physicians suggest that physicians endorse surveillance colonoscopy at more frequent intervals than guidelines recommend (10,11). No studies have measured the actual use of surveillance colonoscopy on a community wide basis nor have they examined how surveillance is being employed in relation to prior histologic findings.
We evaluated surveillance colonoscopy use in a sample of subjects enrolled in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial, an ongoing randomized, controlled, community-based study of cancer screening which includes flexible sigmoidoscopy. The trial provided sigmoidoscopy screening at centralized screening centers. Individuals were referred to their primary care physician for the decision regarding follow up diagnostic testing such as colonoscopy for polypoid abnormalities found on screening flexible sigmoidoscopy (12). After the baseline diagnostic colonoscopy, subsequent surveillance colonoscopy was managed and performed by local medical providers and was not performed or arranged by trial investigators.
To evaluate the utilization of surveillance colonoscopy, we telephoned and interviewed a selection of PLCO subjects in nine regional study centers about surveillance colonoscopy utilization. Individuals whose baseline colonoscopy demonstrated advanced adenoma, non-advanced adenoma, or non-adenomatous findings were included. Colonoscopy use was confirmed by obtaining corroborating colonoscopy reports. The yield of surveillance colonoscopy in this cohort has been reported (13). Here, we examine the utilization of colonoscopy in relation to prior histologic findings.
Methods
Sponsored by the National Cancer Institute, the details of the design, conduct, and recruitment into the PLCO trial have been reported (14). In the initial trial design, 60 cm flexible sigmoidoscopy was performed in the intervention group at baseline and again 3 years later. Subjects randomized after 1998 received their second examination 5 years later. Randomization began in November 1993 and was completed in July 2001, with nearly 155,000 people aged 55 to 74 enrolled. Participants were recruited into PLCO through mass mailings. Among the eligibility criteria were: 1) age 55-74; 2) not currently undergoing treatment for cancer except basal cell or squamous cell skin cancer; 3) no known prior cancer of the colon, rectum, prostate, lung, or ovaries; 4) no surgical removal of the colon, one lung, ovary (prior to October 1996) or prostate; 5) and no colonoscopy, sigmoidoscopy, or barium enema in the past three years (for individuals randomized after April 1995). The ten PLCO centers are located in: Washington, D.C.; Detroit, MI; Salt Lake City, UT; Denver, CO; Honolulu, HI; Minneapolis, MN; Marshfield WI; Pittsburgh, PA; St. Louis, MO and Birmingham, AL. Screening centers obtained written informed consent from each participant and the institutional review board approved the PLCO protocol at each center.
Participant Outcome and Follow Up
Subjects enrolled in this study of colonoscopy utilization come from the PLCO trial. Included subjects had a polypoid abnormality on the initial screening flexible sigmoidoscopy and follow-up diagnostic colonoscopy within 18 months, and were classified based on the histologic results of their diagnostic colonoscopy into 3 groups: 1) advanced adenoma, 2) non-advanced adenoma, and 3) non-adenoma. To insure at least 5 years of follow-up since the initial colonoscopy, PLCO subjects randomized prior to Jan 1, 2000 were eligible. Subjects in Alabama were excluded due to the small number of subjects randomized before January 2000. Eligible subjects were randomly sampled for inclusion with sampling frequencies determined by baseline group and subjects’ year 3 or year 5 flexible sigmoidoscopy (FSG) screening status. Subjects who did not return for the year 3 or 5 screen, as well as subjects with advanced adenomas at baseline colonoscopy, were over-sampled because these individuals were of special interest in evaluating surveillance colonoscopy use and outcome. Sampling weights were as follows: 50% and 100% for the advanced adenoma group with and without year 3 or 5 FSG respectively, 30% and 50% for the non-advanced adenoma group with and without year 3 or 5 FSG, and 20% and 75% for the non-adenoma group with and without year 3 or 5 FSG, respectively.
Subjects were alerted by letter about an impending phone interview on colonoscopy use and were contacted between March 2005 and June 2006. To enhance accurate reporting, the differences between colonoscopy and sigmoidoscopy were reviewed at the beginning of the phone interview. Participants were reminded of the name of the physician who performed and the date of occurrence of colonoscopy known to PLCO staff (the diagnostic colonoscopy occurring after the abnormal FSG screen) and then queried about subsequent procedures. Participants were asked to estimate the date and identify the provider of subsequent procedures. When a colonoscopy was reported, the screening center collected the corroborating colonoscopy report and abstracted it in a standardized fashion. Advanced adenomas were defined as size ≥ 1 cm, presence of tubulovillous or villous histology, or presence of severe or high grade dysplasia. Preparation quality was obtained by the abstractors from the colonoscopy reports and recorded as inadequate, adequate or not available.
To estimate co-morbidity among participants we calculated a modified co-morbidity index (15) using data from a medical questionnaire administered at randomization and from a supplemental questionnaire obtained between April 2006 and May 2007. The index was modified in that we could not ascertain the status of the following health variables: dementia, AIDS, renal failure, history of peptic ulcer disease, congestive heart failure, and peripheral vascular disease.
As a means of verifying the reported absence of colonoscopy, we contacted the primary care physician and when appropriate, the specialist physician, of a separate group of 99 PLCO trial subjects who were interviewed and who indicated they did not undergo colonoscopy, to determine whether records existed documenting a colonoscopy procedure. These included 53 subjects who had a initial screening flexible sigmoidoscopy negative for abnormalities, 37 subjects who were non compliant and did not appear for their initial screening sigmoidoscopy, and 9 subjects who had an abnormal FSG with subsequent colonoscopy, but who reported no subsequent surveillance colonoscopy exam.
Statistical Analysis
A Kaplan-Meier analysis was performed to estimate the probability of getting a colonoscopy within each of the groups. Time was measured from the date of the diagnostic colonoscopy. To obtain a conservative estimate of surveillance use and to account for subjects brought back early for clinical concerns, repeat colonoscopy exams performed within 6 months of the baseline exam were considered part of the baseline exam and histologic findings from those exams were included with the baseline results. Subjects were censored if they underwent a year 3 or year 5 FSG screen which was abnormal for polypoid findings and colonoscopy which followed an abnormal year 3 or 5 screen was not included. The Kaplan-Meier analysis was performed using weights that were inversely proportional to the sampling frequencies.
Logistic regression was used to estimate risk factors for failing to receive colonoscopy among subjects with advanced adenoma and for subjects without an adenoma receiving multiple colonoscopy exams.
Role of the Funding Source
The funding source did not influence the conduct or reporting of the results.
Results
Of 3876 subjects selected for inquiry, 3,627 (93.6%) responded. Non response was primarily due to death (N=125, 3.2%) and inability to contact or refusal to participate (N=124, 3.2%). The characteristics of the included population are presented in Table 1 and are reflective of the overall PLCO population (12). Sixty percent were men, 93.1% were white and the majority had some college or a college degree (68.6%). Based on the most advanced lesion at diagnostic colonoscopy after screening FSG, 1342 subjects had advanced adenoma (AA), 1022 had non-advanced adenoma (NAA) (N=117 with ≥3 NAA and N=905 with 1-2 NAA), and 1263 had non adenomatous findings (NA). Subjects were followed for a median of 8.9 years (range 5.4 – 12.0).
Table 1.
Characteristics of Study Population (N=3,627)
| Characteristics | N (%) |
|---|---|
| Non-Hispanic White | 3378 (93.1) |
| Male | 2175 (60.0) |
| Age (mean) | 63.4 |
| Education1 | |
| Less than High School | 268 (7.4) |
| High School Degree | 863 (23.8) |
| Some College, Technical School | 1213 (33.4) |
| College Degree | 1274 (35.1) |
| Family History of Cancer 2 | 458 (12.6) |
| Baseline Colonoscopy Result | |
| Advanced Adenoma | 1342 (37.0) |
| Non-advanced Adenoma | 1022 (28.2) |
| ≥3 | 117 (11.4) |
| 1-2 | 905 (88.6) |
| Non-adenoma 3 | 1263 (34.8) |
| Median Years of Follow-up (range) | 8.9 (5.4-12.0) |
| Baseline Colonoscopy 4 1994-1995 | 853 (23.5) |
| 1996-1997 | 1945 (53.6) |
| 1998-1999 | 829 (22.9) |
| Subsequent PLCO Trial Screening Flexible Sigmoidoscopy | |
| None | 2938 (81.0) |
| Year 3 (T3) | 328 (9.0) |
| Year 5 (T5) | 361 (10.0) |
9 (0.2%) = not available
In a first degree relative.
Includes subjects with hyperplastic polyp histology (n=717), other non-adenomatous histology (n=49), subjects with polyps of unknown histology (n=264), and subjects with no polyps on baseline colonoscopy (n=233).
Three subjects were tested in 1993 and 9 in 2000.
The cumulative probability of a surveillance colonoscopy (Table 2) within 5 years was 58.4% in the advanced adenoma group, 57.5% in those with ≥3 non-advanced adenomas, 46.7% in those with 1-2 non-advanced adenoma, and 26.5% in subjects with no adenoma (p<0.0001 for AA or ≥3 NAA vs. 1-2 NAA or NA, and for 1-2 NAA vs. NA). A Kaplan-Meier probability curve of utilization is presented in Figure 1.
Table 2.
Cumulative Kaplan-Meier Estimates of Probability of Surveillance Colonoscopy
| Baseline Colonoscopy Result | ||||
|---|---|---|---|---|
| Advanced Adenoma (AA) (N=1342) | Non-Advanced Adenoma (NAA) (N=1022) | Non-Adenoma (NA) (N=1263) | ||
| ≥3 NAA (N=117) | 1-2 NAA (N=905) | |||
| Year | % | |||
| 1 | 5.3 | 2.3 | 1.0 | 1.0 |
| 2 | 20.3 | 13.5 | 7.0 | 4.2 |
| 3 | 30.7 | 19.5 | 15.1 | 8.8 |
| 4 | 50.2 | 45.8 | 33.6 | 17.3 |
| 5* | 58.4 | 57.5 | 46.7 | 26.5 |
| 6 | 67.9 | 67.0 | 59.2 | 35.7 |
| 7 | 72.2 | 74.7 | 66.6 | 45.1 |
| 8 | 76.5 | 77.2 | 69.8 | 52.5 |
| 9 | 79.3 | 81.9 | 74.2 | 57.9 |
- AA vs. 1-2 NAA (p <0.0001)
- AA vs. NA (p <0.0001)
- ≥3 NAA vs. NA (p < .0001)
- 1-2 NAA vs. NA (p < .0001)
- ≥3 NAA vs. 1-2 NAA (p=0.03)
- ≥3 NAA vs. AA (p=0.8, NS)
Figure 1.
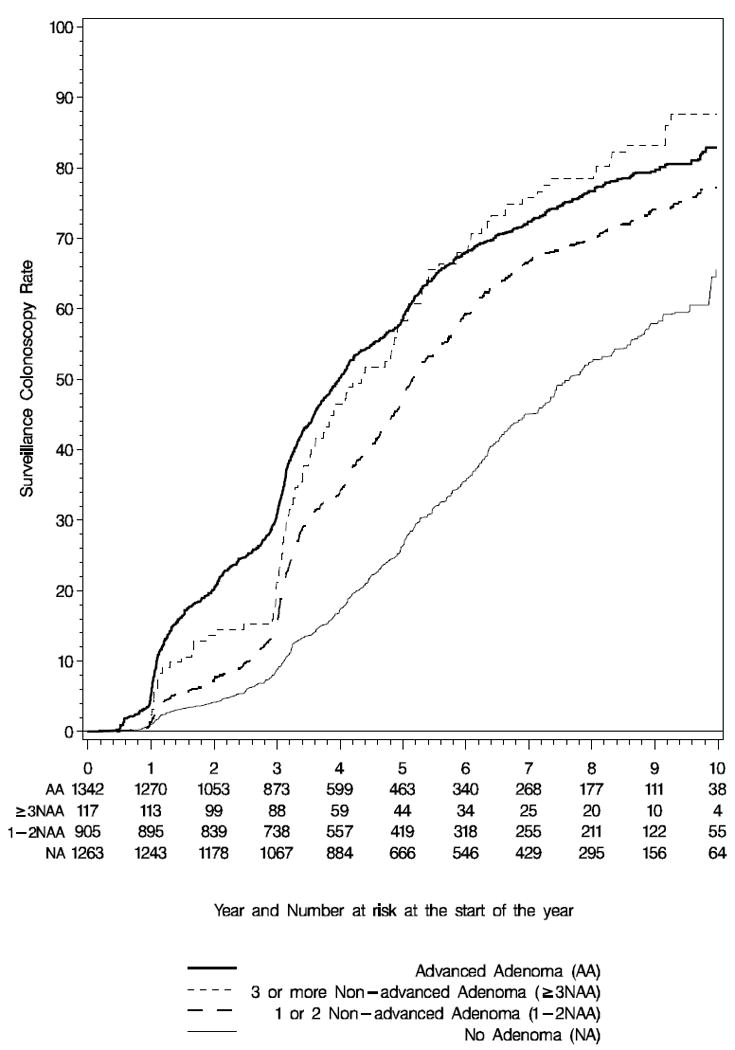
Kaplan-Meier probability curve for surveillance colonoscopy use by year from baseline diagnostic colonoscopy for subjects with Advanced Adenoma (Solid Line), ≥3 Non-advanced Adenoma (dotted line), 1-2 Non-advanced Adenoma (dashed line), and no adenoma (thin line).
Some subjects received more than one surveillance colonoscopy. In Table 3 the number and weighted percent of colonoscopy exams within 7 years, among subjects with ≥ 7 years of follow-up (N=3052) is charted. Within 7 years, 33.2% of subjects with advanced adenoma received ≥ 2 surveillance exams versus 26.9% for those with ≥3 NAA, 18.2% for those with 1 or 2 NAA, and 10.4% for those with non-adenomatous findings. All pair wise comparisons were significant at a p≤0.0001 except for AA vs. ≥3 NAA (p=0.2) and ≥3 NAA vs. 1 or 2 NAA (p=.02).
Table 3.
Number of Surveillance Colonoscopy Exams Within the First 7 years of Follow-up1
| Baseline Colonoscopy Result | ||||
|---|---|---|---|---|
| Advanced Adenoma (AA) (N=1079) | Non-Advanced Adenoma (NAA) (N=947) | Non-Adenoma (NA) (N=1026) | ||
| ≥ 3 NAA (N=110) | 1-2 NAA (N=837) | |||
| Colonoscopy Exams | N (weighted %)2 | |||
| 0 | 268 (27.3) | 25 (23.5) | 255 (31.9) | 429 (52.2) |
| 1 | 437 (39.5) | 54 (49.6) | 423 (49.9) | 443 (37.3) |
| 2 | 275 (24.2) | 19 (17.2) | 136 (15.7) | 123 (8.3) |
| 3 | 70 (6.5) | 11 (8.9) | 17 (1.9) | 19 (1.3) |
| 4 | 20 (1.7) | 1 (0.8) | 5 (0.5) | 6 (0.6) |
| ≥5 | 9 (0.7) | 0 | 1 (0.1) | 6 (0.4) |
| Mean (among all subjects) 3,4 | 1.18 | 1.14 | 0.90 | 0.62 |
| Mean (among subjects with ≥ 1 colonoscopy) 4 | 1.63 | 1.49 | 1.32 | 1.29 |
This only includes subjects with ≥7 years of follow up (N=3052 and excludes subjects with a positive year 3 or year 5 screening flexible sigmoidoscopy.
These percentages are weighted percentages, based on the sampling percentage within each group.
All pair wise comparisons are significant at p < 0.001 except for AA compared with ≥3 NAA (p=0.5) and ≥3 NAA compared with 1 or 2 NAA (p=.004).
The mean number of colonoscopies among all subjects is 0.85 and among all subjects with ≥1 colonoscopy is 1.40.
Among subjects with advanced adenoma, 27.3% did not undergo surveillance colonoscopy within 7 years (Table 3). To evaluate the effect of co-morbid illness on underutilization of surveillance among subjects with advanced adenoma or ≥ 3 non-advanced adenomas we calculated a modified co-morbidity index score. Of 896 with surveillance, 791 (88.3%) had an index of 0 or 1, and 105 (11.7%) had a score ≥2, whereas of 293 without surveillance, 247 (84.3%) had an index of 0 or 1, and 46 (15.7%) had a score ≥2, (p=.08). If we restricted the sample of advanced adenoma subjects to those under age 70 with a co-morbidity index of 0 or 1, the results were similar, with 25.4% not having surveillance.
In a logistic regression model to assess the lack of surveillance after 7 years in subjects with advanced adenoma (N=1079) that included age, gender, family history of colorectal cancer (CRC), time of enrollment in the trial, education, inadequate preparation/incomplete colonoscopy, and comorbidity, the factors associated with lack of surveillance were age, earlier enrollment, and family history. Subjects 70-74 at time of entry into the trial were more likely to not undergo a surveillance exam as compared to those 55-69 (OR=1.5, 95% CI 1.0-2.2, p=0.03). After 7 years, 35.6% in the 70-74 year old age group as compared to 26.1% in the 55-69 year group did not receive surveillance. Subjects without a family history of CRC in a first degree relative were also more likely to not have surveillance (26.3% vs. 15.5% of those with a family history) (OR=2.2, 95% CI 1.4-3.6 (p<.001)), as were subjects enrolled earlier in the trial (subjects slated for year 3 as opposed to year 5 repeat screening) (OR=1.4, 95% CI 1.0-1.8 (p=.04)).
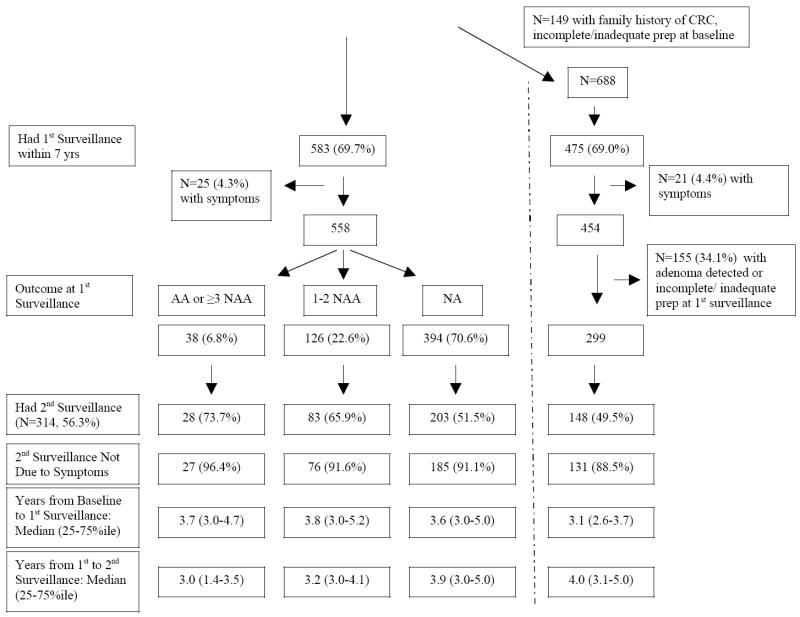
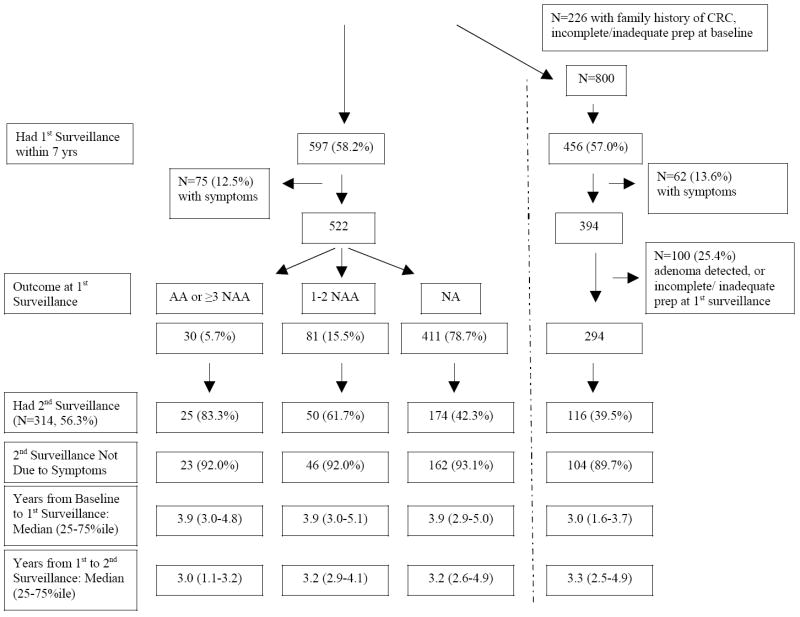
The utilization of 1st and 2nd surveillance exams among subjects with ≥7 years of follow-up with 1 or 2 non advanced adenomas or no adenomas at baseline colonoscopy is presented in Figure 2A-B. The median time in years (25th and 75th percentile) from the baseline to the first surveillance and from the first to the second surveillance is presented on the bottom of each panel. In the far right of the figures, results of surveillance in “pure” subgroups, in subjects with no family history of CRC, complete colonoscopy with adequate preparation at baseline and at 1st surveillance, without symptoms, and with no adenoma diagnosed at the 1st surveillance exam are presented.
Figure 2. Surveillance Colonoscopy Use by Baseline Histologic Status.
CRC = Colorectal Cancer, AA= advanced adenoma, NAA=non-advanced adenoma, NA= no adenoma. The pathways on the far right demonstrate utilization in the subgroup without a family history of CRC, with complete, adequate colonoscopy, and without symptoms.
Figure 2A describes subjects with 1-2 non-advanced adenomas at baseline. Only 4.3% of the 69.7% who underwent a 1st surveillance exam did so for symptoms. Among the 394 (70.6%) whose 1st surveillance exam showed no adenoma, 203/394 (51.5%) had a second surveillance exam, only 18 of which (8.9%) were performed for symptoms. The median time for the 2nd surveillance exam in these asymptomatic subjects, after 1 or 2 non-advanced adenomas at baseline and no adenoma at the 1st surveillance was 3.9 years (Figure 2A). In the subgroup without family history, symptoms, or incomplete/inadequate preparation, 131/299 (43.8%) underwent a 2nd surveillance examination at a median of 4.0 years from the 1st surveillance exam. Utilization of 2nd surveillance in this group was similar to that of all subjects without an adenoma at the 1st surveillance (185/394, 47.0%).
Of the 1026 subjects with no adenoma at the baseline exam, 597 (58.2%) underwent a first surveillance within 7 years, only 75 (12.5%) of whom did so for symptoms (Figure 2B). The 1st surveillance exam was performed at a median of 3.9 years from the baseline exam. Among 411 subjects whose 1st surveillance exam again showed no adenoma, 162 had a second surveillance exam in the absence of symptoms. This second exam was performed at a median of 3.2 years from the 1st. Of these 162 individuals, 31 (19.1%) had a family history of CRC and 18 (11.1%) had either an inadequate preparation or an incomplete colonoscopy at the 1st surveillance, leaving over 69% without an identifiable reason for a repeat surveillance exam. In the “pure” subgroup without a family history and with complete colonoscopy, 104/294 (35.4%) underwent a 2nd surveillance at a median of 3.3 years from the 1st exam.
In a logistic model including age, gender, family history of colorectal cancer (CRC), time of enrollment in the trial, education, inadequate preparation/incomplete colonoscopy examining risk factors for receiving a 1st surveillance colonoscopy within 7 years among subjects with no adenoma at baseline and in the absence of symptoms (N=951), only family history was significantly associated with surveillance (OR=2.0, 95%CI 1.3-2.9, p<.001).
We examined the extent to which incomplete colonoscopy due to lack of cecal visualization or inadequate preparation at the baseline examination may have contributed to surveillance utilization among low risk subjects. A total of 574 subjects with either no adenoma at baseline colonoscopy (N=273) or 1 or 2 non-advanced adenomas at baseline colonoscopy (N=301) had a 1st surveillance colonoscopy within 4 years, in the absence of symptoms. Among colonoscopy reports of those with no adenoma, only 8/273 (2.9%) and among those with 1 or 2 NAA, 11/301 (3.7%), indicated lack of cecal visualization at the baseline colonoscopy, and 18/273 (6.6%) and 16/301 (5.3%) respectively indicated an inadequate preparation at the baseline colonoscopy.
We examined the extent to which multiple physician examiners may have contributed to utilization of colonoscopy among low risk subjects. Among 162 asymptomatic subjects (Figure 2B) with no adenoma at baseline nor at the 1st surveillance, with a second surveillance exam, 109/162 (67.3%) had all 3 exams performed by either the same physician, at the same location, or within the same physician practice and only 8 patients (4.9%) were examined under 3 different circumstances.
Among subjects (N=99) who reported not having a colonoscopy but in whom records were pursued to see if the subject’s negative report could be contradicted, no report of a colonoscopy procedure could be recovered in 91 (91.9%).
Indication for colonoscopy performance showed that of the 4853 colonoscopies in 3627 subjects, only 353 (7.3%) were attributed to symptoms, 3936 (81.1%) were performed for follow up of a previous polyp, 58 (1.2%) for family history, 269 (5.5%) as a consequence of an interval PLCO screening exam, and 237 (4.8%) for other reasons. Analysis of the results across the 9 screening centers demonstrated a range of surveillance at 5 years for subjects with advanced adenoma from 32.6 to 75.6%, and for the non adenoma group from 13.6 to 34.1%.
The yield of surveillance exams in this cohort including adenomatous findings and the detection of 5 interval cancers has been reported (13).
Discussion
Our results among hundreds of colonoscopy practitioners in 9 regional U.S. areas demonstrate substantial over utilization of surveillance colonoscopy among low risk subjects without adenomatous polyps or symptoms and significant under utilization among high risk subjects with a history of advanced adenoma. We also found evidence of relatively early utilization of colonoscopy among subjects with 1 or 2 non-advanced adenoma, with 33.6% undergoing surveillance colonoscopy within 4 years, when guidelines have advised testing in 5 years (2), and more recently in 5 – 10 years (9).
Among all subjects without an adenoma at the baseline colonoscopy, 26.5% underwent surveillance colonoscopy within 5 years and 45.1% within 7 years. Among those followed for ≥ 7 years, over 10% underwent two or more surveillance colonoscopies within 7 years. In a detailed accounting of surveillance colonoscopy use in asymptomatic subjects without an adenoma at baseline or at 1st surveillance (Figure 2B), in over 69% neither a family history nor an incomplete colonoscopy could explain the performance of a 2nd surveillance exam, and in the subgroup without a family history, symptoms or incomplete or inadequate colonoscopy, over 35% had a 2nd surveillance at a median of 3.3 years from the 1st. Including the baseline exam, over 90% of these subjects had 3 colonoscopies within a 9 year period. This level of utilization contrasts to current guidelines which advise that colonoscopy can be deferred for 10 years after an exam in which no adenoma is detected (9), although only indirect data are available to support this recommendation (16). These data confirm significant utilization of surveillance colonoscopy among low risk subjects in relation to guidelines, consistent with what surveys have suggested (10, 11).
These data are important for studies that model the cost effectiveness of colonoscopy-based colorectal cancer screening. Surveillance colonoscopy utilization is an important component of cost, comprising about 25% of all colonoscopy procedures (6), and studies have used differing, but often idealized intervals when simulating utilization in a screening cohort (17). To the extent that utilization is more on the order of what is described here, idealized intervals such as a colonoscopy every 10 years when a colonoscopy exam without an adenoma is not detected underestimate the real costs of a colonoscopy screening program by underestimating the use of surveillance procedures when non-adenomatous polyps are found.
In an analysis of factors associated with repeat colonoscopy among subjects without an adenoma at baseline, only a family history of CRC was significantly associated with utilization. In the most recent recommendations on surveillance, family history was offered as a factor to be used in deciding the timing of surveillance between 5 to 10 years for subjects with one or two small adenomas (9), but not in reference to the interval after a negative exam, except among those relatively few, high risk subjects with a first degree relative diagnosed before age 60 or with two first degree relatives affected. Prospective studies on the impact of family history on surveillance outcome are limited (9) and additional research is needed.
As a possible explanation of early surveillance, incomplete colonoscopy due to lack of cecal visualization or inadequate preparation was infrequently identified. Similarly, lack of communication among physicians seemed to be potentially responsible for only a minority of overutilization. Among a cohort of 162 asymptomatic subjects with no adenoma at baseline or at 1st surveillance who underwent a 2nd surveillance, in over 67% all 3 exams were performed by the same physician, by the same practice, or in the same location.
Among subjects with advanced adenoma, under utilization was observed. Subjects with advanced adenomas are advised to undergo a surveillance exam within 3 years because of their increased risk for subsequent CRC (18), and the 3 year follow up recommendation has been in place for many years (19). Only 31% had a surveillance exam within that time frame, and only 58.4% underwent surveillance within 5 years. Analysis of factors associated with lack of surveillance in the advanced adenoma group shows that older subjects were less likely to obtain testing, perhaps reflecting an appropriate attention to co-morbid conditions. We could not identify however, a significant difference in comorbidity when comparing those with and without surveillance exams. Individuals with a family history of CRC were 2.2 fold more likely to have had a surveillance exam, perhaps reflecting participant and patient concern regarding a possible synergism between family history and the presence of an advanced adenoma. Nonetheless, it remains unclear why a significant amount of the recommended surveillance in subjects with advanced adenoma did not occur.
Several strengths of this investigation should be emphasized. The likelihood of our having obtained relatively complete follow-up information is high, as the timing, results and details of the baseline colonoscopy were already available. As a result, the primary care physician as well as the specialty physician who performed the initial colonoscopy were known, and could help stimulate the patient’s memory to more accurately obtain records. Furthermore, relatively few instances of medical records documenting colonoscopy were found in subjects who reported not undergoing the procedure. Conversely, record retrieval confirmed self reported colonoscopy and our data rely on documented colonoscopy use. Our estimates of surveillance colonoscopy use are conservative since we included subjects who underwent interval negative screening sigmoidoscopy as part of the PLCO trial, and these subjects were, as one would expect, less likely to undergo surveillance colonoscopy.
Between 1995 and 2005, when the colonoscopy procedures reported upon in this study occurred, surveillance recommendations varied across professional organizations (19). For example, the recommended interval for surveillance was unspecified for subjects without an adenoma. Because of the lack of consensus, implementation of surveillance may have been more variable, and viewing surveillance through the lens of current guidelines may be skewed. Attention to appropriate surveillance utilization has increased in recent years (9) and our results largely predate this attention. Nonetheless, the large sample size and broad geographic representation do provide substantive insight and raise concern.
It should be recognized that strict adherence to guidelines for surveillance will not necessarily result in an optimal outcome for individual patients. Recent data show that although surveillance guidelines do accurately identify subjects at higher risk for recurrence of advanced adenoma, they are far from perfect (20). Examination of the yield of surveillance colonoscopy in this cohort demonstrates that risk stratification does identify individuals at higher and lower risk for recurrence (13). Other cohorts show a similar pattern of over and underutilization of surveillance, with risk of advanced adenoma recurrence corresponding to baseline adenoma status (21). Overall, the data suggest that resource consumption can be better managed by aligning use with risk of recurrence.
Limitations should also be acknowledged. To determine whether co-morbid conditions were responsible for under utilization among subjects with advanced adenoma, we had to rely in part on assessments from the time of randomization, which preceded the timing of surveillance by a number of years. Furthermore, we could not collect all the health variables that comprise the co-morbidity index, and thus may be underestimating co-morbidity in our subjects. However, missing health outcomes are unlikely to be present in our healthy cohort (22). Some of the over utilization in the group without adenomas may have come from entry into the screening process via flexible sigmoidoscopy. Uncertainty and doubt may have ensued from an initial sigmoidoscopy which detected an abnormality, followed by a colonoscopy which found no polyp, stimulating physicians to order surveillance exams earlier than they might ordinarily have. However, within the no adenoma group, utilization was similar between subjects who had a finding, albeit a non adenomatous finding such as hyperplastic polyps (N=1030), and those who had no polyps (N=233). Furthermore, colonoscopy procedures were predominately for surveillance not for symptoms, and clinical circumstances such as incomplete colonoscopy or family history did not account for much of the utilization. We could not account for early surveillance due to incomplete polypectomy or concerns about the adequacy of polypectomy. However, we aggregated initial colonoscopy procedures occurring within 6 months to account for early surveillance to monitor polypectomy sites, and most of the observed surveillance, as documented in table 2, occurred in later years. While some of the variability in implementation of surveillance may be attributable to different physician approaches to the potential yield and risk associated with delaying interval examinations, a broad over utilization of surveillance in low risk subjects in all geographic regions was demonstrated. Similar observations have been seen among subjects participating in adenoma prevention trials, where a 10-30% incidence of an extra colonoscopy or sigmoidoscopy above the already frequent testing mandated by participation in the clinical trial occurred (19). Finally, the subjects enrolled were volunteers in a clinical trial who may be more health conscious and thus desirous and demanding of more frequent surveillance.
In conclusion, in community practice of surveillance colonoscopy there is substantial over utilization of surveillance colonoscopy among subjects without adenomas or with low risk adenomas, and under utilization among subjects with advanced adenoma. Interventions to better align surveillance colonoscopy use with risk for advanced lesions is needed (13,23). Further research to enhance the evidence base supporting current surveillance recommendations and further efforts to encourage adherence with guidelines should be pursued.
Acknowledgments
Supported by a contract from the National Cancer Institute N01-CN2551
The authors gratefully acknowledge the coordinators and interviewers, who are so essential to the function of the PLCO trial.
Georgetown University: Colleen McGuire, Johnsonette Ginyard, Tiffanie Hammond, Amy Tran; Henry Ford Health System: Karen Broski, Kathy Pratt, Debi Emmer, Sherrie Stanifer, Joann Kok; Marshfield Clinic: Karen Lappe, Virginia Fischer, Deb Multerer, Amy Vieth; Pacific Health Research Institute, Hawaii: Victoria Jenkins, Kathleen Bow, Becky Kendro; Washington University: Heidi Lowery, Kate Naughton, Barb Ritter; University of Colorado: Sheryl Ogden, Peggy Fyles, Sally Tenorio; University of Minnesota: Deb Engelhard, Jill Cordes, Aaron Zirbes, Candace Borgen, Sophie Breer, Candace Muller, Darcie Baxter, Marianne Rice, Cheri Haselhuhn, DeeAnn Swavely, Darlene Heath, Natalya Portnov; University of Pittsburgh: Betsy Gahagan, Carol Lucas; University of Utah: Lisa Gren, Julie Varner, Eddy Wicklander, Jake Wolf
Footnotes
PLCO cancer screening trial registered as: NCT00002540
There are no conflicts of interest reported by the authors relevant to this manuscript. The investigators had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.U.S.Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Annals of Internal Medicine. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 2.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, Kirk L, Litin S, Simmang C Gastrointestinal Consortium. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 3.Colorectal cancer screening. Recommendation statement from the Canadian task force on preventive health care. Canadian Family Physician. 2001;47:1811–1815. [PMC free article] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. New England Journal of Medicine. 1993;329(27):1977–81. doi: 10.1056/NEJM199312303292701. see comment. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [letter; comment]. [see comments] [DOI] [PubMed] [Google Scholar]
- 6.Lieberman DA, De Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterology. 2000;118:619–624. doi: 10.1016/s0016-5085(00)70269-1. [DOI] [PubMed] [Google Scholar]
- 7.Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151–1162. doi: 10.1053/j.gastro.2005.07.059. [see comment][comment] [DOI] [PubMed] [Google Scholar]
- 8.Vijan S, Inadomi J, Hayward RA, Hofer TP, Fendrick AM. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Alimentary Pharmacology & Therapeutics. 2004:507–515. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- 9.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, Byers T, Hyman N, Kirk L, Thorson A, Simmang C, Johnson D, Rex DK US Multi-Society Task Force on Colorectal Cancer, American Cancer Society. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1875. doi: 10.1053/j.gastro.2006.03.012. [Review] [83 refs] [DOI] [PubMed] [Google Scholar]
- 10.Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Annals of Internal Medicine. 2006;145:654–659. doi: 10.7326/0003-4819-145-9-200611070-00007. summary for patients in Ann Intern Med. 2006 Nov 7;145(9):I26; PMID: 17088574. [DOI] [PubMed] [Google Scholar]
- 11.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Annals of Internal Medicine. 2004;141:264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Weissfeld JL, Schoen RE, Pinsky PF, Bresalier RS, Church T, Yurgalevitch S, Austin JH, Prorok PC, Gohagan JK PLCO Project Team. Flexible sigmoidoscopy in the PLCO cancer screening trial: results from the baseline screening examination of a randomized trial. Journal of the National Cancer Institute. 2005;97(13):989–97. doi: 10.1093/jnci/dji175. [DOI] [PubMed] [Google Scholar]
- 13.Pinsky PF, Schoen RE, Weissfeld JL, Church T, Yokochi LA, Doria-Rose VP, Prorok P. The yield of surveillance colonoscopy by adenoma history and time to examination. Clinical Gastroenterology & Hepatology. 2009;7(1):86–92. doi: 10.1016/j.cgh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, Mandel JS, Oberman A, O’Brien B, Oken MM, Rafla S, Reding D, Rutt W, Weissfeld JL, Yokochi L, Gohagan JK Prostate Lung Colorectal and Ovarian Cancer Screening Trial ProjectTeam. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials. 2000;21:Suppl–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295:2366–2373. doi: 10.1001/jama.295.20.2366. see comment. [DOI] [PubMed] [Google Scholar]
- 17.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [summary for patients in .] [Review] [25 refs] [DOI] [PubMed] [Google Scholar]
- 18.Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. New England Journal of Medicine. 1992;326:658–662. doi: 10.1056/NEJM199203053261002. see comments. [DOI] [PubMed] [Google Scholar]
- 19.Schoen RE. Surveillance after positive and negative colonoscopy examinations: issues, yields, and use. American Journal of Gastroenterology. 2003;98:1237–1246. doi: 10.1111/j.1572-0241.2003.07492.x. [DOI] [PubMed] [Google Scholar]
- 20.Laiyemo AO, Murphy G, Albert PS, Sansbury LB, Wang Z, Cross AJ, Marcus PM, Caan B, Marshall JR, Lance P, Paskett ED, Weissfeld J, Slattery ML, Burt R, Iber F, Shike M, Kikendall JW, Lanza E, Schatzkin A. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Annals of Internal Medicine. 2008;148:419–426. doi: 10.7326/0003-4819-148-6-200803180-00004. see comment. [DOI] [PubMed] [Google Scholar]
- 21.Laiyemo AO, Pinsky PF, Marcus PM, Lanza E, Cross AJ, Schatzkin A, Schoen RE. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clinical Gastroenterology & Hepatology. 2009;7:562–567. doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinsky PF, Miller A, Kramer BS, Church T, Reding D, Prorok P, Gelmann E, Schoen RE, Buys S, Hayes RB, Berg CD. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. American Journal of Epidemiology. 2007;165:874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, Bond JH, Nelson DB, Triadafilopoulos G, Ramirez FC, Collins JF, Johnston TK, McQuaid KR, Garewal H, Sampliner RE, Esquivel R, Robertson D. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077–85. doi: 10.1053/j.gastro.2007.07.006. see comment. [DOI] [PubMed] [Google Scholar]