I. Introduction
Since the discovery of actin in non-muscle cells, it has been postulated that spatiotemporal regulation of the mechanical behaviors of the filamentous actin (F-actin) cytoskeleton networks may regulate cellular shape change and force generation in cell migration and division [1–3]. Perhaps the simplest idea is that a transition between fluid-like (solution) and solid-like (gel) behavior of cytoskeletal F-actin networks could regulate the direction of cellular protrusions. In homogeneous networks of F-actin formed with a single cross-linking protein in vitro, such “sol-gel” transitions have been observed by changing cross-linker and F-actin concentration and modulating F-actin length [4–6]. F-actin networks formed in vitro demonstrate a broad diversity of mechanical behaviors that, in principle, could be harnessed to regulate the mechanical properties of cells over a variety of time and length scales.
However, the F-actin cytoskeleton in adherent animal cells can hardly be considered as a homogeneous network undergoing ‘sol-gel’ transitions (Fig. 1). A myriad of actin binding proteins work in concert to regulate the kinetics of F-actin assembly and the organization of F-actin networks [7]. Typically, a unique molecular signature and dynamic structure is found in F-actin networks in different regions of the cell and are temporally regulated during cell migration and division [8, 9]. While it is widely appreciated that an intact F-actin cytoskeleton is essential for regulation of cell shape change and the generation of mechanical forces, the mechanical behaviors of the living F-actin cytoskeleton are largely unknown. Moreover, an understanding of how the mechanical behavior of the F-actin cytoskeleton is modulated during physiological behaviors such as cell adhesion, migration and division remains far from complete
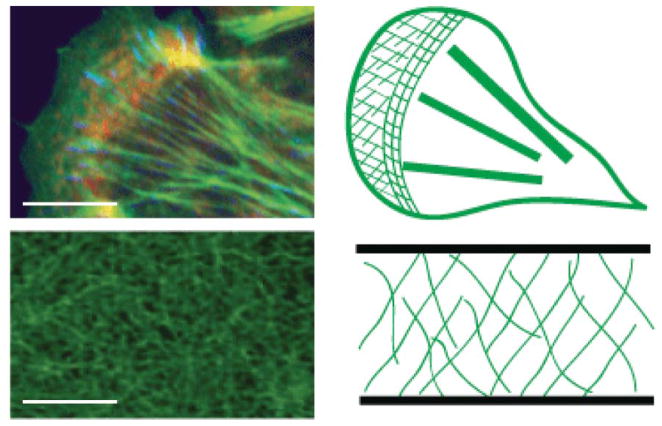
Figure 1.
Fluorescent Images (left) and schematics (right) of F-actin networks observed in living cells (top) and in vitro (bottom). F-actin (green), a focal adhesion marker (blue, paxillin) and myosin II (red) are indicated in the image of the cell; in vitro network is stained for F-actin using fluorescent phalloidin. Cell schematic indicates the lamellipodium (green cross-hatches), lamella (thin gridded lines), stress fibers (thick lines) and cortex (surrounding boundary). By contrast, F-actin networks in vitro are homogeneous networks. Scale bar is 10 microns in both images.
Since this understanding will only come from an integration of measurements performed in vitro with those inside live cells, we review the recent work in both these fields and identify potential intersections for future research.
II. Mechanics of F-actin Networks in vitro
Isotropic, three-dimensional networks of F-actin can be formed in vitro by polymerizing F-actin in the presence of cross-linking proteins (Fig. 1). Although the structure of these networks is quite different from those found in many live cells, these systems have served as models with which to identify basic mechanisms of mechanical response of F-actin networks. Here, we briefly discuss two ubiquitous features of the mechanics of F-actin networks: linear viscoelasticity and nonlinear elastic response. We also discuss recent advances in understanding the physics of ‘active’ F-actin networks where chemical energy is harnessed to establish asymmetric structure and generate local forces [10].
a. Linear Viscoelastic Response
Simple elastic materials are described by a linear stress-strain relationship that relates the deformation (strain) of a material to the force per unit area (stress) exerted; the ratio between the stress and strain is called the elastic modulus. By contrast, in a Newtonian fluid, an applied stress results in a constant deformation rate; the ratio between the stress and strain rate is a measure of viscosity. Generally, networks formed from biopolymers often show mechanical properties that are in between that of a pure fluid or elastic solid and, thus, are said to be ‘viscoelastic’ [11]. The viscoelastic response often depends on the time scale of the measurement as a result of different length scales associated with internal stress relaxation [12].
Permanent cross-linking of F-actin eliminates much of the viscous response by restricting the ability of cross-linkers to unbind from actin filaments at long time scales [13–15]. F-actin networks formed with relatively static cross-links, such as scruin [14], rigor-state heavy meromyosin (HMM) (Kd ≈ 10−8M) [15, 16] and biotin-avidin (Kd ≈ 10−15M) [13] are predominately elastic solids and exhibit a very large degree of tunability, ranging from 0.03 Pa to >300 Pa as a function of cross-linker concentration [14].
Most physiological cross-linkers are dynamic on physiological time-scales and have a finite binding affinity to F-actin [4, 12, 17]. For instance, α-actinin (Kd ≈ 10−6M) has a dissociation rate around 1 s−1 [18]. When compared to static cross-linkers, the lower affinity cross-linkers will decrease, but not completely eliminate, the elastic behavior of the network. An increase in the dissociation rate of cross-linkers shifts the frequency dependent viscous response to higher frequencies [4]. Such modifications to the binding and unbinding rates to actin tune the timescale of the viscoelastic response for F-actin networks [12, 17, 19]. Because cross-linker unbinding allows for rearrangements that play a significant role in viscous response in F-actin networks, it is hypothesized that cross-linker unbinding and rearrangement could enable stress relaxation through cytoskeletal networks at long times to accommodate cell shape change while maintaining a rigid network in response to short time scale perturbations. However, future work is required to delineate these behaviors in live cells.
b. Nonlinear Elastic Response
For most F-actin networks, the mechanical response becomes nonlinear at large stress or strain, such that the stiffness depends on the magnitude of the applied stress or strain [13, 14, 20, 21]. Quite generally, sparse (low F-actin density) networks with a small degree of cross-linking are observed to soften at large strains [14, 22–24]. In contrast, dense (high F-actin density) networks with a high concentration of cross-links are observed to stiffen at intermediate strains followed by a dramatic softening at larger strains [14, 22–24].
Qualitatively, the nature of this stress-stiffening response is similar for networks formed with different actin crosslinking proteins, including α-actinin, filamin, scruin, HMM [14, 25, 26] as well as non-physiological cross-links formed through biotin-avidin bonds [27]. The critical strain at which the onset of stiffening occurs is typically on the order of 5–30% and is dependent on the type of cross-linker, linear elastic modulus and measurement time scale. Typically, the shear elastic stiffness increases by about a factor of two; however, for F-actin networks cross-linked with filamin A, the stiffness can increase by nearly a factor of 100 [28]. Here, the elastic properties of the cross-linking proteins themselves is thought to dominate the network’s elastic properties [28–30].
Recent experiments suggest that living cells may capitalize on this tunability to modify its viscoelastic properties in response to a stimulus. Physiological networks of F-actin shortened with gelsolin and cross-linked with filamin A demonstrate tremendous stress stiffening at strains of approximately 30% [28]. By incorporating motor proteins within the network, this stiffening is observed in the absence of externally applied stress (Koenderink, unpublished). This suggests that, when attached to a resistive substrate, motor-containing F-actin networks may operate in a state of ‘pre-tension’ and that the stiffness of this network could be modulated by the level of myosin activity [31]. The regulation of myosin activity, which occurs through phosphorylation of its regulatory light chain, can occur at much faster than the time scales required for transcriptional regulation of actin-binding proteins. In this way, a cell may be able to tune its stiffness quickly in response to fast strain.
c. ‘Active’ F-actin Networks
F-actin networks within the living cell operate far from chemical equilibrium, and chemical energy, in the form of ATP or GTP, is harnessed to control the assembly, architecture and mechanical properties of F-actin networks in a myriad of ways including the regulation of F-actin assembly, affinity of cross-linking proteins, and activity of myosin-II. Thus, a major challenge for the future is to extend current knowledge of the mechanics of F-actin networks in chemical equilibrium to those driven far from equilibrium to form ‘active’ F-actin networks [10].
Perhaps the best studied model system is the actin-based motility of the pathogen Listeria monocytogenes [32] where the minimal necessary proteins required for motility has been identified and motility has been recapitulated in vitro [33, 34]. Briefly, the localization of ActA drives the preferential assembly of F-actin into a branched dendritic network. The polymerization of semi-flexible F-actin filaments is harnessed to generate forces [35] sufficient for displacement of the sphere. Within the actin filament, ATP is hydrolyzed to ADP and becomes susceptible to filament severing by the protein cofilin. Thus, in this system chemical energy in the form of ATP is utilized to provide a sufficient available pool of actin monomers required for force generation. By directing the assembly of a similar network formed from cytoplasmic extracts at the surface of the tip of an atomic force microscopy, properties of this dendritic network under compression have been measured and showed a transition between stress stiffening and softening behavior under increased strain (Fig. 2)[36].
Figure 2.
Schematic diagrams of four distinct F-actin cytoskeletal networks discussed here showing the geometry of force application (black arrow) for the mechanical measurement of elastic modulus (G′) or viscous modulus (G″). For the lamellar network, force arrow indicates cell-generated force measured in the underlying extracellular matrix.
Reconstitution of active contractile F-actin networks, as a model for the cellular cortex, has been of interest for some time. Initial experiments sought to explore the importance of network gelation, via cross-linking proteins, on the contraction driven by myosin II motors [6, 37]. These experiments identified that a minimal amount of cross-linking was required for macroscopic network contraction whereas further increasing the density of cross-linking proteins inhibited contraction [6, 37]. More recent experiments have further characterized the range of concentrations of cross-linkers and motor proteins over which contractility is observed. These experiments estimate the force generated by this contraction to be on the order of 100 pN per F-actin bundle [38]. Recent models have made predictions for the upper limit of tension generated by actomyosin gels [39].
III. Mechanics of F-actin Networks in Cell Migration
In living cells, the F-actin cytoskeleton encompasses a variety of different structures that are essential for many different aspects of cell physiology. For each process, the F-actin cytoskeleton has a distinct molecular composition and structure, which suggest that the mechanical properties of these networks may be tuned for a specific aspect of cellular physiology. Here we focus on F-actin structures involved in different steps of cell migration, including those essential for cell protrusion (lamellipodia and filopodia), adhesion (lamella and stress fibers) and shape change (cortex). However, the role of F-actin mechanics in other cellular processes, such as endocytosis, trafficking and cell division are other rich areas of study.
a. Mechanics of Lamellipodial Actin Networks
The initial step in cell migration is the protrusion of the leading cell membrane created by a branched, dendritic array, similar to that formed in listeria-based propulsion, called the lamellipodium. Here, it is thought polymerization of F-actin generates mechanical forces to change leading edge cells shape. Adhesions to the extracellular matrix can occur within the lamellipodium, forming small, myosin II independent focal adhesions through which the lamellipodial F-actin networks exert low traction stresses, of the order of 20 Pa, on the extracellular matrix [40]. Depending on the type of technique utilized, the forces associated with cell protrusion have been measured to be quite weak (a few pN per micron) [41] or significantly stronger (several hundred pN per micron) [42]. Filopodia, a protrusive organelle also driven by F-actin polymerization, consist of tightly bundled F-actin and exert significantly less stress than lamellipodial networks [43]. These variations in the absolute force generated by protrusion may arise from differences in geometry or timescale of the measurement, which may probe the effect of cellular adhesion to a varying degree. Additional experiments have shown that myosin-II driven networks proximal to the lamellipodium apply additional tension through this network that promotes the assembly of cellular adhesions [44]. Since migrating cells may need to exert significant stress to invade surrounding extracellular matrix, future work to understand the origins of stall stress for various protrusive organelles could have a significant impact on our ability to control cell migration.
b. Mechanics of the Lamellar Actin Network
Proximal to the leading edge lamellipodium, adhesions to the extracellular matrix form and delineate a transition to a myosin-II containing F-actin network termed the lamella. Here, the F-actin network is transported from the leading edge towards the cell center in a myosin-II-dependent process termed ‘retrograde flow’. Many characteristics of retrograde flow can be captured by modeling the F-actin network as an active polar gel [45].
Understanding how retrograde flow in the lamella is harnessed for efficient cell protrusion has been of interest for many decades. Measurements have shown that increased rate of cell protrusion occurs when retrograde flow decreases [46, 47], suggesting that adhesions to the extracellular matrix act as a ‘molecular clutch’ to modulate interactions between the F-actin cytoskeleton and the immobilized extracellular matrix (Fig. 2) [48]. When the clutch is engaged, focal adhesion proteins bind strongly to actin and transmit forces to the extracellular matrix via their attachment to integrins. When disengaged, focal adhesions bind weakly to actin and contractility allows fast retrograde flow of actin and slippage past the focal adhesion. However, traction force experiments revealed a biphasic relationship between F-actin speed and traction force [40]. Near the cell edge where F-actin speed is fast, F-actin speed and traction force are inversely related, consistent with a simple molecular cutch. However, at larger focal adhesions farther from the cell edge where F-actin motion is overall slower, F-actin speed and traction stress follow a direct relationship. More recent experiments and modeling have shown that such a slip clutch could additionally act as a mechanical sensor [49, 50]. Future work is required to determine how the F-actin retrograde flow is utilized, in concert with mechanosensitive focal adhesions to enable adherent cells to sense the mechanical properties of their surrounding extracellular matrix.
c. Mechanics of Stress Fibers
In some cell types, the lamellar network is further organized into contractile bundles that form parallel or perpendicular to the cell edge [51, 52]. Contractile F-actin bundles form from contraction of the lamellar network [53, 54] and de novo assembly [9, 55, 56]. Typically, one end of a stress fiber terminates at a point of adhesion to the extracellular matrix or another cell while the other end typically becomes woven into the F-actin cortex near the nucleus. It is likely that the contractile F-actin networks linked to cellular adhesions determine the magnitude of forces transmitted to outside the cell as well as the mechanical response of the cell to stresses and strains applied to these adhesions. A wide variety of contractile F-actin networks with different architectures and organization of F-actin polarity are found near cellular adhesions and, it is likely that differences in the mechanical behavior determine the precise nature of the force transmission to the extracellular matrix.
Stress fibers are a specific type of contractile F-actin bundle characterized by repeating units of myosin II motor proteins and α-actinin, reminiscent of the sarcomere structure in muscle cells. The spacing between myosin II along the stress fiber can change over time, indicating a dynamic structure with non-uniform elasticity and forces [57]. For instance, if the stress fiber becomes severed or detached its length contracts in a myosin-II dependent fashion [58, 59]. If myosin II activity is enhanced, the spacing between the myosin II decreases near the peripheral regions, but expands near the central regions, suggesting a non-uniformity in the mechanical properties of these bundles. Atomic Force microscopy measurements have shown that the stiffness of the stress fiber is approximately 12 kPa and that the modulus is constant for strains up to 12% [60]. When myosin II cross-linking and enzymatic activity is perturbed by pharmacological treatment with blebbistatin, the elastic modulus decreases to 8 kPa. Thus, myosin II plays an important role in determining the linear stiffness of stress fibers.
Moreover, the stiffness of blebbistatin-treated stress fibers becomes highly strain sensitive, with the modulus increasing by 60% for strains between 1% and 10% (Fig. 2) [60]. Nonlinearity in the elastic properties of stress fibers have been confirmed by tensile tests, which have shown that the tensile elastic modulus increases from 1.45 MPa at low strains up to 104 MPa at strains near 200% [59]. The nonlinearity of the stress-strain relationship for stress fibers is strikingly similar to those that have been observe in vitro and suggest the importance of nonlinear elasticity in the determination of force transmission.
Interestingly, the rupture force of stress fibers has been measured to be close to 370 nN, nearly a 100-fold larger than the typical amount of force exerted at a cellular adhesion site. Instead, the magnitude of traction stresses is consistent with the amount of force required to extend the length of the stress fiber by 20%; consistent with the idea that stress fibers within the cell exist in a pre-strained state and that nonlinear elastic behavior of stress fibers determines the interplay between intracellular strain and traction stress exerted to the extracellular matrix.
Stress fibers have been modeled as viscoelastic cables, possessing an elastic component and a viscous dissipative element from filaments gliding past each other, in addition to active contractile units from myosin motor protein activity [61, 62]. Future work to identify how the mechanical behaviors of actomyosin structures regulates the assembly and mechanical properties of stress fibers will provide new insight into the regulation of cellular adhesion and migration.
d. Mechanics of the Cell Cortex
The F-actin cortex is a thin, membrane-bound F-actin network that regulates cell shape. The F-actin cortex also utilizes myosin-II contractility to remodel and generate tension along the cellular periphery. Such contractility can describe the convex shape attained by a variety of adherent cells, including fibroblasts and B16 melanoma cells, and a modified Laplace law model which considers the contractile properties of the actin cortex can predict the radius of curvature of the convex arcs [63]. Models assuming only a 2D cable network of a specified lattice geometry and a distribution of focal adhesions can predict finite forces at adhesions [64]. Myosin-II generated tension within the actin cortex is also observed to inhibit local cell protrusion. Inhibition of this tension by perturbations to myosin II or mechanics of the extracellular matrix can enhance local protrusive activity, such as that observed in angiogenesis or neuronal growth [65].
When the F-actin cortex detaches from the cell membrane, a rounded structure known as a ‘bleb’ occurs. Cellular blebbing is observed to occur during apoptosis, but also during cell division and certain types of cell migration. In non-apoptotic blebs, the diameter of the bleb expands after initial formation for approximately 30 seconds, reaching sizes of between 1–2 microns [66, 67]. After expansion, assembly of a new F-actin cortex at the membrane, coupled with myosin-II contractility drives the retraction of the bleb. During this retraction phase, the bleb’s stiffness increases fivefold due to the stiffness of the newly reattached actin cortex [67]. These experiments also suggest that the blebs are an indicator of a high intracellular pressure, on the order of 100 Pa [67]. Further experiments are needed to assess implications of this high pressure on cytoskeletal dynamics and cellular physiology.
A myriad of techniques have been developed to measure the mechanical response of cellular cortex by indenting, shearing or extending the exterior cell membrane through integrin-mediated (or non-specific) adhesion to a micron- or nano-scale probe (Fig. 2) [68]. A primary component of this response is the F-actin cortex, although other contributions from the soluble and insoluble cytoskeleton cannot be neglected. These measurements have shown that the cortex is a viscoelastic solid, displaying a broad spectrum of mechanical relaxations reminiscent of glassy materials [69], and consistent with the broad spectrum of relaxations observed for 3D in vitro F-actin networks [70]. The elastic modulus at 0.1–1 Hz can range from a few hundred Pa for neutrophils and neurons to several thousand Pa for fibroblasts. Moreover, for many adherent cells, the cortical stiffness varies monotonically with the stiffness of the extracellular matrix: stiffer extracellular matrices promote the generation of a stiffer cortex [71]. Similarly, the cortical stiffness increases linearly with the magnitude of cell-generated traction stress or stress applied to the cortex, similar to the nonlinearity observed in in vitro F-actin networks [68]. While viscoelastic models of the F-actin cortex can explain many of the observed measurements of the cellular cortex, recent measurements have identified additional slow propagations which are better described by poroelastic models of F-actin deformation and cytoplasm flow [72].
IV. Outlook
Advances in molecular biology and biochemistry have enabled rapid progress both in the control over F-actin cytoskeletal networks formed in live cells and the ability to reconstitute F-actin networks in vitro. Thus, future work to integrate physical measurements and models of in vitro F-actin networks with observations and measurements of cellular networks will provide a coherent foundation to understand how the dynamics and mechanics of individual macromolecules give rise to the complex, adaptive F-actin cytoskeleton in adherent cells.
The ultimate goal in the field of F-actin cytoskeletal mechanics is to develop predictive physical models to characterize the mechanics of cellular physiology, including adhesion, migration and division. These models will provide insight to the key physical parameters that govern the mechanics of each of these processes and an understanding of how macromolecular interactions give rise to shape change and force generation at the cellular level. While some of these parameters could be ones we have developed to characterize equilibrium materials such as viscosity and elasticity, other behaviors may require the development of new parameters to describe non-equilibrium materials. This insight will open up new opportunities to control and modulate aspects of cellular physiology that rely specific mechanical response of the F-actin cytoskeleton.
Acknowledgments
MLG would like to acknowledge support from a Career Award at the Scientific Interfaces from the Burroughs Wellcome Fund as well as a NIH Director’s Pioneer Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollard TD. Cytoskeletal functions of cytoplasmic contractile proteins. J Supramol Struct. 1976;5(3):317–34. doi: 10.1002/jss.400050306. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Spudich JA. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- 3.Stossel TP. Contractile proteins in cell structure and function. Annu Rev Med. 1978;29:427–57. doi: 10.1146/annurev.me.29.020178.002235. [DOI] [PubMed] [Google Scholar]
- 4.Wachsstock DH, Schwarz WH, Pollard TD. Cross-linker dynamics determine the mechanical properties of actin gels. Biophys J. 1994;66(3 Pt 1):801–9. doi: 10.1016/s0006-3495(94)80856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janmey PA, et al. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990;345(6270):89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- 6.Janson LW, Kolega J, Taylor DL. Modulation of contraction by gelation/solation in a reconstituted motile model. J Cell Biol. 1991;114(5):1005–15. doi: 10.1083/jcb.114.5.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vale RD, Kreis T. Guidebook to the Cytoskeletal and Motor Proteins. 2. Oxford: Oxford University Press; 1999. [Google Scholar]
- 8.Le Clainche C, Carlier MF. Regulation of Actin Assembly Associated With Protrusion and Adhesion in Cell Migration. Physiol Rev. 2008;88(2):489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 9.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231(3):446–54. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher DA, Geissler PL. Active biological materials. Annu Rev Phys Chem. 2009;60:469–86. doi: 10.1146/annurev.physchem.040808.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardel ML, et al. Chapter 19: Mechanical response of cytoskeletal networks. Methods Cell Biol. 2008;89:487–519. doi: 10.1016/S0091-679X(08)00619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieleg O, et al. Transient binding and dissipation in cross-linked actin networks. Phys Rev Lett. 2008;101(10):108101. doi: 10.1103/PhysRevLett.101.108101. [DOI] [PubMed] [Google Scholar]
- 13.MacKintosh FC, Kas J, Janmey PA. Elasticity of semiflexible biopolymer networks. Phys Rev Lett. 1995;75(24):4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 14.Gardel ML, et al. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304(5675):1301–5. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 15.Tharmann R, Claessens MM, Bausch AR. Viscoelasticity of isotropically cross-linked actin networks. Phys Rev Lett. 2007;98(8):088103. doi: 10.1103/PhysRevLett.98.088103. [DOI] [PubMed] [Google Scholar]
- 16.Marston S, Weber A. Dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 2002;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- 17.Lieleg O, Bausch AR. Cross-linker unbinding and self-similarity in bundled cytoskeletal networks. Phys Rev Lett. 2007;99(15):158105. doi: 10.1103/PhysRevLett.99.158105. [DOI] [PubMed] [Google Scholar]
- 18.Wachsstock DH, Schwartz WH, Pollard TD. Affinity of alpha-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophysical journal. 1993;65(1):205–214. doi: 10.1016/S0006-3495(93)81059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward SM, et al. Dynamic viscoelasticity of actin cross-linked with wild-type and disease-causing mutant alpha-actinin-4. Biophys J. 2008;95(10):4915–23. doi: 10.1529/biophysj.108.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storm C, et al. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–4. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Tseng Y, Wirtz D. Strain Hardening of Actin Filament Networks. REGULATION BY THE DYNAMIC CROSS-LINKING PROTEIN alpha -ACTININ. J Biol Chem. 2000;275(46):35886–35892. doi: 10.1074/jbc.M002377200. [DOI] [PubMed] [Google Scholar]
- 22.Head DA, Levine AJ, MacKintosh FC. Deformation of cross-linked semiflexible polymer networks. Phys Rev Lett. 2003;91(10):108102. doi: 10.1103/PhysRevLett.91.108102. [DOI] [PubMed] [Google Scholar]
- 23.Head DA, Levine AJ, MacKintosh FC. Distinct regimes of elastic response and deformation modes of cross-linked cytoskeletal and semiflexible polymer networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68(6 Pt 1):061907. doi: 10.1103/PhysRevE.68.061907. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm J, Frey E. Elasticity of stiff polymer networks. Phys Rev Lett. 2003;91(10):108103. doi: 10.1103/PhysRevLett.91.108103. [DOI] [PubMed] [Google Scholar]
- 25.Tharmann R, Claessens MMAE, Bausch AR. Viscoelasticity of Isotropically Cross-Linked Actin Networks. Physical Review Letters. 2007;98(8):088103. doi: 10.1103/PhysRevLett.98.088103. [DOI] [PubMed] [Google Scholar]
- 26.Esue O, Tseng Y, Wirtz D. Alpha-actinin and filamin cooperatively enhance the stiffness of actin filament networks. PLoS ONE. 2009;4(2):e4411. doi: 10.1371/journal.pone.0004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKintosh FC, Käs J, Janmey PA. Elasticity of Semiflexible Biopolymer Networks. Physical Review Letters. 1995;75(24):4425. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 28.Gardel ML, et al. Stress-dependent elasticity of composite actin networks as a model for cell behavior. Phys Rev Lett. 2006;96(8):088102. doi: 10.1103/PhysRevLett.96.088102. [DOI] [PubMed] [Google Scholar]
- 29.Broedersz CP, Storm C, MacKintosh FC. Nonlinear elasticity of composite networks of stiff biopolymers with flexible linkers. Physical Review Letters. 2008;101(11) doi: 10.1103/PhysRevLett.101.118103. [DOI] [PubMed] [Google Scholar]
- 30.Kasza KE, et al. Nonlinear elasticity of stiff biopolymers connected by flexible linkers. Physical Review E (Statistical, Nonlinear, and Soft Matter Physics) 2009;79(4):041928–5. doi: 10.1103/PhysRevE.79.041928. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282(3):C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 32.Cameron LA, et al. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1(2):110–9. doi: 10.1038/35040061. [DOI] [PubMed] [Google Scholar]
- 33.Carlier MF, et al. Actin-based motility as a self-organized system: mechanism and reconstitution in vitro. C R Biol. 2003;326(2):161–70. doi: 10.1016/s1631-0691(03)00067-2. [DOI] [PubMed] [Google Scholar]
- 34.Akin O, Mullins RD. Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell. 2008;133(5):841–51. doi: 10.1016/j.cell.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Footer MJ, et al. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc Natl Acad Sci U S A. 2007;104(7):2181–6. doi: 10.1073/pnas.0607052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445(7125):295–8. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janson LW, Sellers JR, Taylor DL. Actin-binding proteins regulate the work performed by myosin II motors on single actin filaments. Cell Motil Cytoskeleton. 1992;22(4):274–80. doi: 10.1002/cm.970220407. [DOI] [PubMed] [Google Scholar]
- 38.Bendix PM, et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys J. 2008;94(8):3126–36. doi: 10.1529/biophysj.107.117960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlsson AE. Contractile stress generation by actomyosin gels. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;74(5 Pt 1):051912. doi: 10.1103/PhysRevE.74.051912. [DOI] [PubMed] [Google Scholar]
- 40.Gardel ML, et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183(6):999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnet S, et al. Weak force stalls protrusion at the leading edge of the lamellipodium. Biophys J. 2006;90(5):1810–20. doi: 10.1529/biophysj.105.064600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prass M, et al. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174(6):767–72. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cojoc D, et al. Properties of the force exerted by filopodia and lamellipodia and the involvement of cytoskeletal components. PLoS ONE. 2007;2(10):e1072. doi: 10.1371/journal.pone.0001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannone G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128(3):561–75. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruse K, et al. Contractility and retrograde flow in lamellipodium motion. Phys Biol. 2006;3(2):130–7. doi: 10.1088/1478-3975/3/2/005. [DOI] [PubMed] [Google Scholar]
- 46.Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14(4):763–71. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 47.Jurado C, Haserick JR, Lee J. Slipping or gripping? Fluorescent speckle microscopy in fish keratocytes reveals two different mechanisms for generating a retrograde flow of actin. Mol Biol Cell. 2005;16(2):507–18. doi: 10.1091/mbc.E04-10-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1(9):761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 49.Wang YL. Flux at focal adhesions: slippage clutch, mechanical gauge, or signal depot. Sci STKE. 2007;2007(377):pe10. doi: 10.1126/stke.3772007pe10. [DOI] [PubMed] [Google Scholar]
- 50.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322(5908):1687–91. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- 51.Cramer LP. Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem Soc Symp. 1999;65:173–205. [PubMed] [Google Scholar]
- 52.Cramer LP, Mitchison TJ, Theriot JA. Actin-dependent motile forces and cell motility. Curr Opin Cell Biol. 1994;6(1):82–6. doi: 10.1016/0955-0674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 53.Verkhovsky AB, Borisy GG. Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J Cell Biol. 1993;123(3):637–52. doi: 10.1083/jcb.123.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J Cell Biol. 1995;131(4):989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173(3):383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senju Y, Miyata H. The role of actomyosin contractility in the formation and dynamics of actin bundles during fibroblast spreading. J Biochem. 2009;145(2):137–50. doi: 10.1093/jb/mvn151. [DOI] [PubMed] [Google Scholar]
- 57.Peterson LJ, et al. Simultaneous stretching and contraction of stress fibers in vivo. Mol Biol Cell. 2004;15(7):3497–508. doi: 10.1091/mbc.E03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90(10):3762–73. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deguchi S, Ohashi T, Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J Biomech. 2006;39(14):2603–10. doi: 10.1016/j.jbiomech.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Lu L, et al. Mechanical properties of actin stress fibers in living cells. Biophys J. 2008;95(12):6060–71. doi: 10.1529/biophysj.108.133462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achim B, Ulrich SS. Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction. New Journal of Physics. 2007;(11):425. [Google Scholar]
- 62.Stachowiak M, O’Shaughnessy B. Kinetics of stress fibers. New Journal of Physics. 2008;(2):025002. [Google Scholar]
- 63.Bischofs IB, et al. Filamentous network mechanics and active contractility determine cell and tissue shape. Biophys J. 2008;95(7):3488–96. doi: 10.1529/biophysj.108.134296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul R, et al. Propagation of mechanical stress through the actin cytoskeleton toward focal adhesions: model and experiment. Biophys J. 2008;94(4):1470–82. doi: 10.1529/biophysj.107.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer RS, et al. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol. 2009;19(3):260–5. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charras GT, et al. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175(3):477–90. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charras GT, et al. Life and times of a cellular bleb. Biophys J. 2008;94(5):1836–53. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasza KE, et al. The cell as a material. Curr Opin Cell Biol. 2007;19(1):101–7. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Bursac P, et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4(7):557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 70.Gardel ML, et al. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc Natl Acad Sci U S A. 2006;103(6):1762–7. doi: 10.1073/pnas.0504777103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solon J, et al. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. 2007;93(12):4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenbluth MJ, et al. Slow stress propagation in adherent cells. Biophys J. 2008;95(12):6052–9. doi: 10.1529/biophysj.108.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]