Abstract
Systemic administration of thiazolidinediones reduces peripheral inflammation in vivo, presumably by acting at peroxisome proliferator-activated receptor γ (PPARγ) in peripheral tissues. Based on a rapidly growing body of literature indicating the CNS as a functional target of PPARγ actions, we postulated that brain PPARγ modulates peripheral edema and the processing of inflammatory pain signals in the dorsal horn of the spinal cord. To test this in the plantar carrageenan model of inflammatory pain, we measured paw edema, heat hyperalgesia, and dorsal horn expression of the immediate-early gene c-fos after intracerebroventricular (ICV) administration of PPARγ ligands or vehicle. We found that ICV rosiglitazone (0.5–50 µg) or 15d-PGJ2 (50–200 µg), but not vehicle, dose-dependently reduced paw thickness, paw volume and behavioral withdrawal responses to noxious heat. These anti-inflammatory and anti-hyperalgesia effects result from direct actions in the brain and not diffusion to other sites, because intraperitoneal and intrathecal administration of rosiglitazone (50 µg) and 15d-PGJ2 (200 µg) had no effect. PPARγ agonists changed neither overt behavior nor motor coordination, indicating that non-specific behavioral effects do not contribute to PPAR ligand-induced anti-hyperalgesia. ICV administration of structurally dissimilar PPARγ antagonists (either GW9662 or BADGE) reversed the anti-inflammatory and anti-hyperalgesic actions of both rosiglitazone and 15d-PGJ2. To evaluate the effects of PPARγ agonists on a classic marker of noxious stimulus-evoked gene expression, we quantified Fos protein expression in the dorsal horn. The number of carrageenan-induced Fos-like immunoreactive profiles was less in rosiglitazone-treated rats as compared to vehicle controls. We conclude that pharmacological activation of PPARγ in the brain rapidly inhibits local edema and the spinal transmission of noxious inflammatory signals.
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs) are transcription factors belonging to the nuclear receptor superfamily (Kota BP, 2005). The α, β/δ, and γ isoforms of PPAR receptors (Berger et al., 2005; Michalik and Wahli, 2006) are activated by fatty acids, eicosanoids, and synthetic ligands. Activated PPARs form functional heterodimers with retinoid X receptors (RXR). This complex interacts with various co-activators and a specific peroxisome proliferator response element (PPRE) on the promoter region of target genes to alter transcription (Tan et al., 2005).
The PPARγ isotope has received considerable attention for its role as a lipid sensor. PPARγ activation leads to adipocyte differentiation and induces gene expression of enzymes that facilitate lipid uptake and synthesis (Lehrke M, 2005). Synthetic PPARγ agonists of the thiazolidinedione (TZD) class, such as rosiglitazone, act as insulin sensitizers and have become important in the treatment of type 2 diabetes.
In addition to diabetes, PPAR ligands represent a promising therapeutic strategy for other diseases including those associated with inflammation (Abdelrahman et al., 2005; Moraes et al., 2006). For example, systemic administration of PPARα or PPARγ ligands reduce peripheral inflammation in vivo (Cuzzocrea et al., 2004; Oliveira et al., 2007; Taylor et al., 2002), in part by acting at PPARs located in liver or at the site of inflammation (Devchand et al., 1996; Napimoga et al., 2008). While most attention has been paid to PPAR function in peripheral tissues, it is becoming increasingly clear that pharmacological activation of PPARγ may alleviate certain CNS pathology (Abdelrahman et al., 2005). CNS sites of action of PPARγ ligands are supported by recent reports of PPARγ expression in brain (Moreno et al., 2004) and spinal cord (Shibata et al., 2008). Also, we and others have recently reported that supraspinal (intracerebroventricular) administration of PPARα ligands (perfluoroctanoic acid) reduced peripheral edema and/or inflammatory hyperalgesia (D'Agostino et al., 2009; D'Agostino et al., 2007; Taylor et al., 2005), and that intrathecal administration of PPARγ ligands, rosiglitazone and 15d-PGJ2, reduced behavioral signs of neuropathic pain (Churi et al., 2008). Whether supraspinal administration of PPARγ ligands reduces inflammatory pain and edema remains unclear. To address this question, the present studies evaluated the effects of intracerebroventricular administration of PPARγ agonists on edema, pain-like behavior, and noxious stimulus-evoked gene expression in a key site of spinal nociceptive transmission. Specifically, we quantified the dorsal horn expression of the immediate-early gene c-fos, a classic neural marker of pain (Harris, 1998).
METHODS
Animals
Male Sprague-Dawley rats weighing 250–350g were used in this study. All animals were cared for in accordance to the guidelines set forth by the National Institutes of Health regarding the proper treatment and use of laboratory animals. All experiments involving animals were approved by the Tulane University Institutional Animal Care and Use Committees.
Intracerebroventricular cannulae implantation
Guide cannulae (Plastics one, Roanoke, VA) for intracerebroventricular (ICV) injection were placed one wk before experimentation. Surgical anesthesia was achieved with isoflurane (5% for induction; 1.5–2% for maintenance). Rats were placed in a stereotaxic apparatus fitted with blunt ear bars (Stoelting, Kiel, WI). After an incision to expose the cranium, the dorsal surface of the skull was leveled by zeroing the dorso-ventral coordinate at lambda and bregma. A 26-G stainless steel guide cannula (Plastics One, Roanoke, VA) was lowered to the right lateral brain ventricle using the following stereotaxic coordinates: 0.7 posterior to bregma, 1.5 mm lateral from midline and 3.3–4.0 below the skull surface (Paxinos and Watson, 1997). Initial placement of the cannula was verified by slow downward movement of saline when the tubing was opened and raised (Taylor et al., 1994). The cannula was fixed to the skull with 2–3 small screws and dental cement. After hardening of the cement and suturing of the incision, a 30G stylet (Plastics One, Roanoke, VA) was secured within the guide.
Carrageenan-Induced Paw Inflammation
100 µl of 1% (behavioral studies) or 3% (immunohistochemical studies) carrageenan (type IV, lambda, Fluka, Milwaukee, WI) was prepared in saline and subcutaneously injected into the mid-plantar region of the right hindpaw. 3% carrageenan was used to maximize spinal Fos expression. Paw edema was assessed in all animals with two methods. First, while gently restraining the rat, dorsal-to-ventral paw thickness was measured with a Mitutoyo Pocket Gauge micro-caliper (Western Tool, Oakland, CA). Second, while gently restraining the rat, each hind limb was submerged to the ankle hairline within a plethysmometer, yielding a measure of paw volume (Ugo Basile, Italy). The average of 3 measurements at each time point was calculated.
Thermal Paw Withdrawal Test
Rats were acclimated to an 8” × 8” clear plexiglass box on a plexiglass floor (Ugo Basile, Italy) for at least 1 hr. Absorbent paper towels, initially placed under the animal, were removed at least 30 min before testing. To facilitate acclimation and response reliability, fluctuations in room noise and vibrations and temperature were minimized. The thermal stimulus consisted of a radiant heat source (8V, 50W lamp, Ugo Basile, Italy) positioned under the glass floor directly beneath the hind paw. When triggered, a timer was activated, and light passed through a small aperture at the top of a movable case. At specified time-points, the stimulus was applied to the right paw and the latency to paw withdrawal was recorded. The average of 3 measurements was calculated. If the rat did not respond within 30 sec, the heat was discontinued to prevent tissue damage. One day before testing, voltage intensity was adjusted to standardize the average latency to paw withdrawal ± 1 sec from one day to the next.
Drug Injection and Experimental Timeline
Prior to injection of rosiglitazone (Cayman Chemical, Ann Arbor, MI), 15d-PGJ2 (Cayman Chemical, Ann Arbor, MI), GW9662 (Sigma, St. Louis, MO), and/or BADGE (Sigma, St. Louis, MO), we evaluated paw thickness, paw volume, and/or paw withdrawal latency to heat stimulation. Next, with gentle restraint we attached drug-loaded polyethylene tubing to the cannula. Receptor agonists were administered 30 min prior to induction of inflammation, while receptor antagonists were administered 45 min prior to inflammation. For the paw volume studies, vehicle controls did not differ from one experiment to the next, and were therefore combined into a group of 12 for data presentation and analysis. Intrathecal injections were administered under light isoflurane anesthesia (5% induction, then 2–2.5% maintenance in oxygen). Rosiglitazone or vehicle was administered in a volume of 10 µL using a 25 µL Hamilton microsyringe fitted with a 27-gauge needle. The needle was inserted into the subarachnoid space through the intervertebral foramen. A tail or foot flick response was used as verification of correct placement of the needle. ICV injections were delivered in a 5 µl volume via a 25 µl Hamilton syringe fitted with a 27-G needle attached to approximately 10cm of 20-G polyethylene tubing, which was connected to the 26-G guide cannula. Progress of the remote injection was visually confirmed by observation of movement of a small air bubble within the PE-20 tubing. Injectors were left in place an additional min to minimize backflow within the catheter. The animal was returned to its testing box. At t=0, carrageenan was injected and inflammation and paw withdrawal latency was evaluated at t=1, 2, 3, and 4 hr.
Immunohistochemistry for Fos Protein
Three hours after carrageenan injection carrageenan-induced inflammation was measured via changes in paw thickness as described previously. Rats were then deeply anesthetized (Pentobarbital, 60mg/kg i.p. Sigma, St Louis, MO) and perfused intracardially with 200 ml of ice-cold phosphate buffered saline 0.1M (PBS) followed by 300 ml of fixative (10% formalin in 0.1M PBS). The cord was then removed and post-fixed for 3 hr in 10% formalin and then cryoprotected (30% sucrose in 0.1M PBS). Transverse sections were cut at 40 µm on a freezing microtome and collected in 0.1M PBS. The sections were washed three times in 0.1M PBS and immersed in 0.3% H2O2 for 30 min. Sections were pretreated with 1% normal goat serum to block non-specific binding and then incubated in rabbit polyclonal Fos protein antibody (1:20,000, Calbiochem, San Diego, CA) overnight at room temperature on a slow rocker. The tissue was then washed three times in 0.1M PBS, again blocked in 1% normal goat serum, and incubated in biotinylated goat anti-rabbit 2° antibody (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hrs at room temperature. Next, the sections were washed and exposed to avidin-biotin-peroxidase complex for 1hr at room temperature. The chromogen was developed with 0.01% hydrogen peroxide and visualized with 0.05% diaminobenzidine. The tissue was washed with PB, mounted onto Superfrost/Plus slides, air dried, dehydrated in a series of graded alcohols, cleared in xylene and cover-slipped.
Quantification and Analysis of Fos-Positive Profiles
Sections were captured under bright-field illumination with a 10× objective using Meta Imaging Series software (Metamorph 4.500). Blinded to treatment, Fos-immunoreactive (Fos-IR)-positive profiles were counted using NIH Image J software (Abramoff et al., 2004; Rasband, 1997–2007). Images were first transformed into 16-bit grayscale and then relative size (µm2) and circularity of Fos-IR positive profiles were determined from a random subset of images using the wand tool. Next, the region of interest (ROI) was outlined and each image thresholded manually to the beginning of the upward slope of the density histogram using the image-adjust-threshold command. Three separate regions were analyzed in the ipsilateral and contralateral portions of the spinal cord: lamina I–II, lamina III–IV and lamina V. In both the ipsilateral and contralateral sections of the spinal cord, highlighted profiles in the ROI were automatically counted using the analyze-analyze particles command. This automated method provides essential identical counts to those obtained manually (Brightwell and Taylor, 2009).
Data Analysis and Statistics
Behavioral studies
To determine dose-response relationships, data were analyzed by two-way ANOVA with Dose as the between-subjects factor and Time as the repeated measure, and the F-values reported in the text of the Results. If the resulting F-value was significant (p<0.05), then post-hoc t-tests with Bonferroni correction were performed to obtain individual comparisons at each time point, and significant differences of each drug dose as compared to vehicle control are denoted in the Figures. Fos quantification. Data were analyzed by two-way ANOVA. If the effects of an individual variable proved significant, these analyses were followed by post-hoc t-tests with Bonferroni correction. P<0.05 was considered significant.
RESULTS
Supraspinal administration of PPAR γ agonists dose-dependently decrease edema
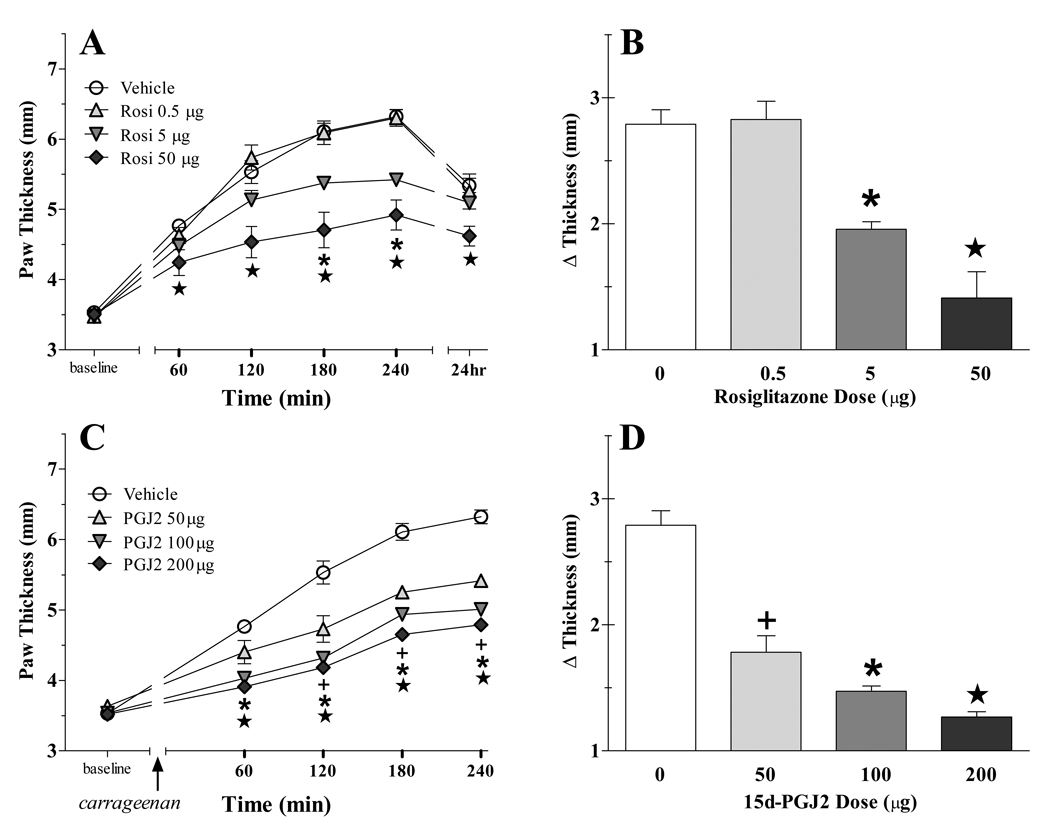
We previously reported that systemic administration of PPARγ agonists robustly reduce the peripheral edema associated with the intraplantar injection of carrageenan (Taylor et al., 2002), but the site of action was not determined. To test the hypothesis that activation of PPARγ in the brain reduces peripheral edema, we used the intracerebroventricular (ICV) route to administer two compounds known to selectively activate the PPARγ isoforms: rosiglitazone (Lehmann et al., 1995) and 15d-PGJ2 (Forman et al., 1995; Kliewer et al., 1995). As illustrated in Figure 1, both rosiglitazone (F[3,100]=15.40, p<0.0001) and 15d-PGJ2 (F[3,84]=37.51, p<0.0001) dose-dependently inhibited carrageenan-induced increase in paw thickness. Table 1 illustrates that rosiglitazone (F[3,100]=4.706, p<0.01) and 15d-PGJ2 (F[4,88]=14.90, p<0.0001) dose-dependently inhibited carrageenan-induced increase in paw volume.
Figure 1. PPAR γ agonists dose-dependently decrease edema.
Vehicle or drugs were injected ICV after the baseline measurement of paw thickness. Thirty min later, carrageenan was subcutaneously injected into the hind paw. Panels A and C display the time-course data, while Panels B and D display the data as the difference between baseline and the mean of the 240 min time point. Either rosiglitazone (A–B) or 15d-PGJ2 (C–D) reduced the development of edema. n=6–11 per group. Values represent mean ± SEM. (+), (*), and (★)represent significant difference (P<0.05 vs vehicle by Bonferroni post-tests following two-way ANOVA) from low, medium and high dose, respectively.
Table 1. Effect of PPAR γ ligands on carrageenan-induced increase in paw volume.
Paw volume (ml) values represent mean ± SEM at 240 min after carrageenan injection. For the intracerebroventricular (ICV) studies, p<0.05 refers to statistical difference from the combined ICV control group (see Methods). For the intraperitoneal studies (IP), p<0.05 refers to statistical difference from the IP vehicle (saline) group.
| Route | Agonist | Antagonist | n | Paw Volume | p<0.05?? |
|---|---|---|---|---|---|
| ICV | Vehicle (Saline + DMSO) | 12 | 2.94 ± 0.05 | ||
| ICV | Rosi .5µg | ------------- | 6 | 2.86 ± 0.12 | No |
| 5µg | ------------- | 6 | 2.45 ± 0.11 | Yes | |
| 50µg | ------------- | 5 | 2.32 ± 0.08 | Yes | |
| ICV | PGJ2 50µg | ------------- | 6 | 2.59 ± 0.05 | Yes |
| 100µg | ------------- | 4 | 2.43 ± 0.05 | Yes | |
| 200µg | ------------- | 4 | 2.26 ± 0.02 | Yes | |
| ICV | ------------ | BADGE 50µg | 6 | 2.95 ± 0.11 | No |
| ICV | ------------ | GW9662 100µg | 4 | 3.00 ± 0.09 | No |
| ICV | Rosi 50µg | BADGE 50µg | 6 | 2.97 ± 0.13 | No |
| Rosi 50µg | GW9662 100µg | 4 | 3.07 ± 0.13 | No | |
| ICV | PGJ2 200µg | BADGE 100µg | 4 | 3.03 ± 0.03 | No |
| PGJ2 200µg | GW9662 100µg | 4 | 3.06 ± 0.04 | No | |
| Vehicle (Saline) | 3 | 3.28 ± 0.15 | |||
| IP | Rosi 30mg/kg | ------------- | 5 | 2.86 ± 0.15 | No |
| Rosi 100mg/kg | ------------- | 4 | 2.62 ± 0.16 | Yes | |
PPARγ receptors mediate the anti-inflammatory actions of rosiglitazone and 15d-PGJ2
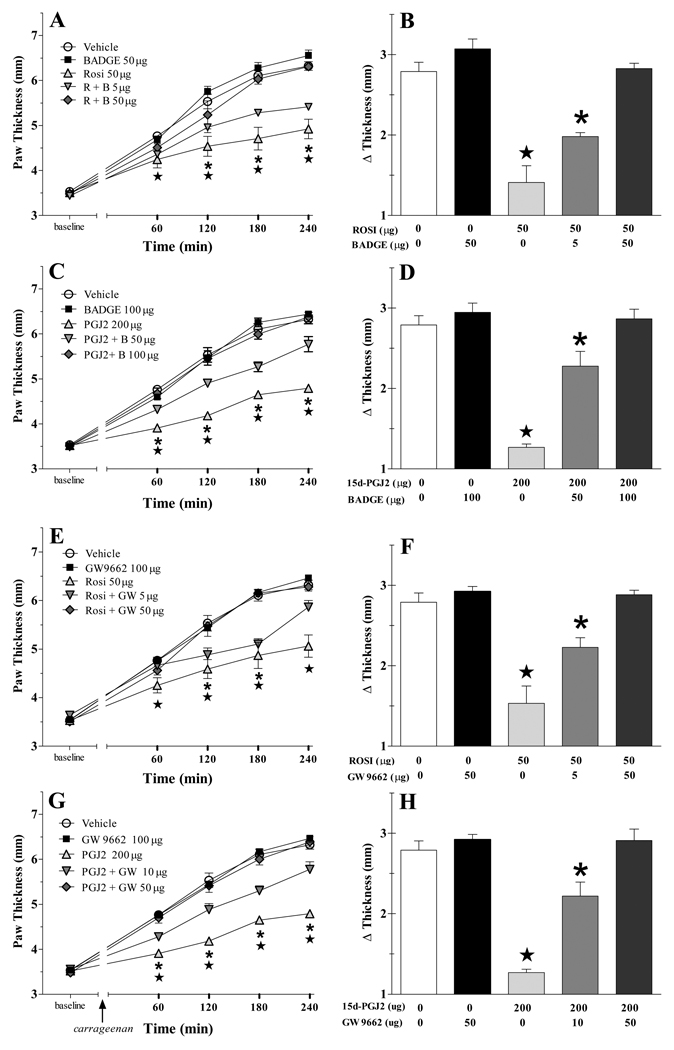
To test the hypothesis that PPARγ receptors mediate the anti-inflammatory actions of rosiglitazone and 15d-PGJ2, we used two compounds thought to selectively antagonize the PPARγ isoforms: GW9662 (Miyahara et al., 2000) and BADGE. While the selectivity of BADGE is controversial (Bishop-Bailey et al., 2000; Fehlberg et al., 2002; Wright et al., 2000), the advantages of testing our hypothesis with two structurally dissimilar compounds outweighs this uncertainty. As illustrated in Fig 2, neither BADGE nor GW9662 changed edema when administered alone. However, BADGE dose-dependently reversed the decrease in paw thickness produced by either rosiglitazone (F[4,120]=16.7, p<0.0001) or 15d-PGJ2 (F[4,92]=31.2, p<0.0001), respectively. Similarly, GW9662 dose-dependently reversed the anti-edema effects of either rosiglitazone (F[4,100]=11.2, p<0.0001) or 15d-PGJ2 (F[4,92]=30.0, p<0.0001). Furthermore, as illustrated by the paw volume data of Table 1, pre-treatment with either antagonist reduced the anti-inflammatory effects of rosiglitazone and 15d-PGJ2 (P<0.005 in every case).
Figure 2. PPARγ receptors mediate the anti-inflammatory actions of rosiglitazone and 15d-PGJ2.
The PPARγ antagonist BADGE attenuated the anti-inflammatory actions of either rosiglitazone (R, panels A–B) or 15d-PGJ2 (PGJ2, panels C–D). Similarly, GW9667 attenuated the actions of rosiglitazone (panels E–F) or 15d-PGJ2 (panels G–H). Antagonists were injected ICV, followed 15 min later by vehicle, rosiglitazone (Rosi) or 15d-PGJ2 (PGJ2), and then 30 min later by carrageenan was injected into the hindpaw. n= 6–11 per group. Values represent mean ± SEM. (★) and (*) represent significant difference (P<0.05 vs vehicle by Bonferroni post-tests following two-way ANOVA) from agonist alone and agonist + low dose antagonist, respectively.
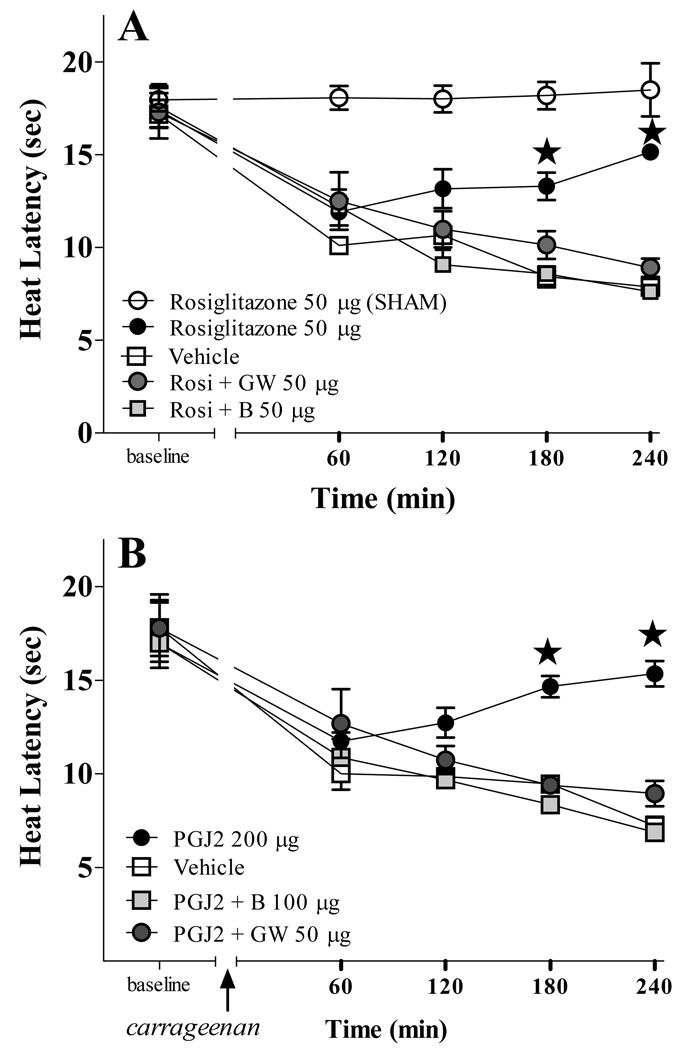
PPARγ mediates the anti-hyperalgesic effects of supraspinal rosiglitazone and 15d-PGJ2
We next evaluated paw withdrawal threshold to noxious heat. As illustrated in Fig 3, ICV rosiglitazone and 15d-PGJ2 significantly reduced thermal hyperalgesia (F[3,68]=8.905, p<0.001) and (F[3,52]=6.093, p<0.01), respectively, following the intraplantar injection of carrageenan. Rosiglitazone had no effect in Sham controls (intraplantar injection of saline instead of carrageenan). To test the hypothesis that PPARγ receptors mediate the anti-hyperalgesic actions of rosiglitazone and 15d-PGJ2, we pre-administrated two PPARγ selective antagonists. Fig 3A shows that either BADGE (F[2,70]=37.43, p<0.0001) or GW9662 (F[2,65]=28.00, p<0.0001) reversed the anti-hyperalgesic actions of 50 µg rosiglitazone. Fig 3B shows that BADGE (F[2,50]=27.32, p<0.0001) and GW9662 (F[2,50]=17.50, p<0.0001) also reversed anti-hyperalgesic actions of 200 µg 15d-PGJ2.
Figure 3. PPARγ receptors mediate the anti-hyperalgesic actions of rosiglitazone and 15d-PGJ2.
The intraplantar injection of carrageenan but not saline (SHAM) decreased paw withdrawal latency to heat. Either rosiglitazone (Rosi, panel A) or 15d-PGJ2 (PGJ2, panel B) decreased this hyperalgesia. Rosi did not change latency in SHAM. Pretreatment with the PPARγ antagonists GW9662 (GW) or BADGE (B) prevented the anti-hyperalgesic effects of the agonists. n= 4–7 per group. Values represent mean ± SEM. ★P<0.05 agonist alone vs. vehicle by Bonferroni post-tests following two-way ANOVA.
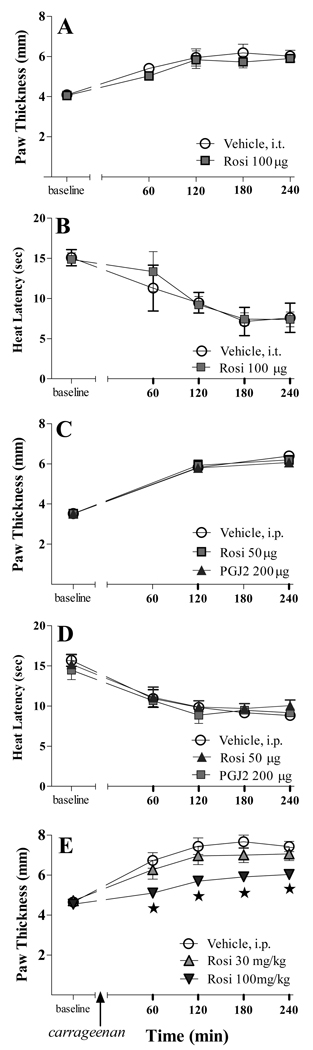
Intrathecal or systemic delivery of microgram doses of PPARγ agonists do not change inflammatory hyperalgesia or edema
PPARγ agonists could diffuse from or leak out of the ICV space and into the spinal cord or the peripheral circulation, respectively, thus acting at PPAR receptors outside of the brain. To test this, we administered the highest ICV dose of rosiglitazone (50 µg/rat) or 15d-PGJ2 (200 µg/rat) via the intraperitoneal and intrathecal routes. As illustrated in Fig 4A–B, intrathecal high-dose rosiglitazone reduced neither carrageenan-induced edema nor hyperalgesia. Furthermore, systemic administration of these doses of rosiglitazone or 15d-PGJ2 did not change (p>0.05) edema as assessed by paw thickness (Fig 4C), heat hypersensitivity (Fig 4D), nor edema as assessed by paw volume (Table 1). As expected from previous studies, only at a thousand-fold higher dose (100 mg/kg = 30 mg/rat) did systemic delivery of rosiglitazone decrease paw thickness (F[2,45]=21.71, p<0.0001, Fig 4E) and paw volume (F[2,45]=22.43, p<0.0001, Table 1). These data indicate that supraspinal sites mediate the anti-edema and anti-hyperalgesic effects of ICV PPARγ agonists.
Figure 4. Microgram doses of neither rosiglitazone nor 15d-PGJ2 change inflammatory hyperalgesia or edema.
Intrathecal (i.t.) administration of microgram doses of rosiglitazone did not reduce carrageenan-induced edema (A) or hyperalgesia (B). Systemic administration of these doses did not change (p>0.05) paw thickness (C) or heat latency (D). Only at hundreds-fold higher doses did systemic delivery of rosiglitazone decrease paw thickness (E). These data indicate that the anti-edema and anti-hyperalgesic effects of microgram doses of ICV PPARγ agonists do not result from diffusion to peripheral or spinal sites.
PPARγ agonists inhibit the noxious stimulus-evoked activity of dorsal horn neurons
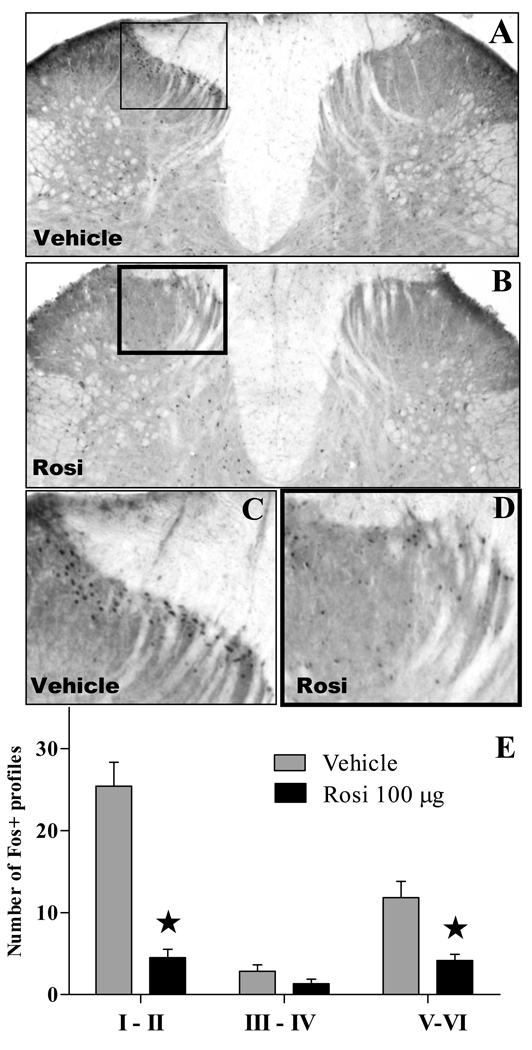
To test the hypothesis that PPARγ agonists increase supraspinal inhibition of spinal nociceptive processing, we evaluated the dorsal horn expression of Fos, a marker of spinal neuron activation. As reported previously by numerous laboratories, the intraplantar injection of carrageenan increased the number of Fos-IR profiles in the ipsilateral but not contralateral dorsal horn, with greatest numbers observed in lamina I–II (Fig 5). Pretreatment with i.c.v. rosiglitazone reduced Fos-IR by roughly 75% throughout the ipsilateral dorsal horn (F[1,30]=60, p<0.0001), including lamina I–II (Fig 5E), but had no effect at the contralateral dorsal horn [p>0.05, not shown].
Figure 5. Rosiglitazone reduces carrageenan-induced Fos expression in the dorsal horn.
Representative photomicrographs of the L4–L5 dorsal horn showing Fos-like immunoreactivity of control animals (A, C) or rats treated 100µg of rosiglitazone i.c.v. (B, D) following the intraplantar injection of carrageenan. Panel D depicts average number of Fos-positive profiles at each laminar region of the L4–L5 dorsal horn on the side ipsilateral to the carrageenan injection. n = 6. Values represent mean ± SEM. *p<0.0001 vs vehicle by Bonferroni post-tests following two-way ANOVA.
ICV PPARγ agonists do not produce behavioral side effects
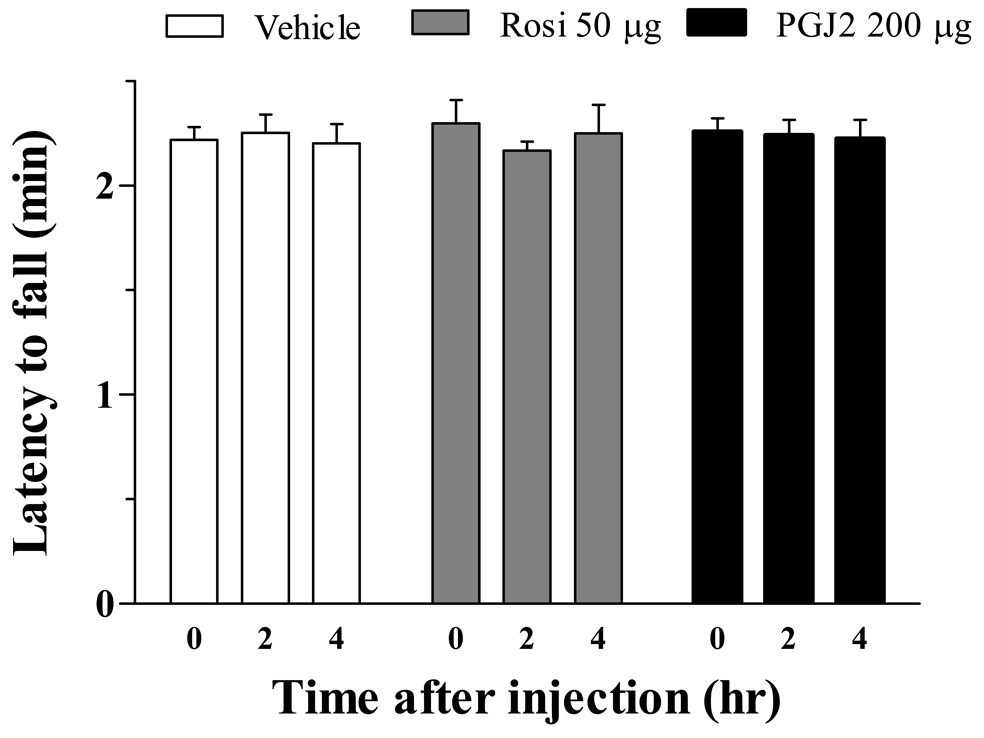
Neither of the receptor agonists or antagonists produced overt signs of sedation, hyperactivity, or illness. To determine whether 15d-PGJ2 or rosiglitazone altered more subtle systems such as motor coordination, we assessed duration spent on an accelerating rotarod. As illustrated in Figure 6, neither 15d-PGJ2 nor rosiglitazone produced ataxia when delivered at the maximal ICV dose used in the previous studies (p>0.05).
Figure 6. PPARγ agonists delivered centrally do not affect motor coordination.
Motor coordination was assessed by evaluating time spent on an accelerating rotarod (4–40 rpm, over 10 min). Drug was administered after baseline measurement at t=0, measurements were repeated at 120 and 240 min later. n= 3 per group. Values represent mean ± SEM.
DISCUSSION
Our studies demonstrate for the first time that ICV rosiglitazone or 15d-PGJ2 act directly in the brain to reduce behavioral withdrawal responses to noxious heat and paw edema. The number of carrageenan-induced Fos-like immunoreactive profiles in dorsal horn (a classic marker of noxious stimulus-evoked gene expression) was less in rosiglitazone-treated rats as compared to vehicle controls. ICV administration of structurally dissimilar PPARγ antagonists (either GW9662 or BADGE) reversed the anti-inflammatory and anti-hyperalgesic actions of both rosiglitazone and 15d-PGJ2. We conclude that pharmacological activation of PPARγ in the brain rapidly inhibits the spinal transmission of noxious inflammatory signals and local edema.
Supraspinal sites mediate the actions of ICV administration of PPARγ agonists
Neither intrathecal nor intraperitoneal injection of 50–200 µg of PPARγ agonists mimicked the effects of identical ICV doses. Only at much higher doses (~1000× higher) did systemic administration of rosiglitazone decrease edema, as described previously (Cuzzocrea et al., 2004; Taylor et al., 2002). We conclude that diffusion of PPARγ agonists out of the cerebral ventricles is insufficient to explain our results, and that PPAR receptors within the brain (rather than spinal cord or periphery) mediate the anti-edema and anti-hyperalgesic effects observed after ICV administration of rosiglitazone (0.5–50 µg) or 15d-PGJ2 (50–200 µg).
PPARγ immunoreactivity and mRNA transcripts are distributed in multiple regions of the brain, including the dorsal horn and supraspinal pain modulatory centers such as the periaqueductal gray, rostral ventral medulla and amygdala (Churi et al., 2008; Cullingford et al., 1998; Moreno et al., 2004). Intracerebroventricular injection of rosiglitazone and 15d-PGJ2 may act at one or more of these regions to modulate inflammatory pain. Indeed, recent studies indicate that PPARγ agonists alter CNS function. For example, the in vivo administration of pioglitazone exerts neuroprotective effects in the brain in animal models of Alzheimer’s disease, Parkinson’s disease, stroke and multiple sclerosis (Bernardo A, 2006; Sundararajan et al., 2006). As with peripheral nerve injury, brain ischemia increases PPARγ expression in neurons, supporting the idea that injury alters neuronal PPARγ signaling (Victor et al., 2006).
Supraspinal PPARγ suppresses inflammation-evoked neuronal activity at the dorsal horn
It is unlikely that non-specific side effects contribute to the PPARγ ligand-induced reduction of behavioral hypersensitivity to sensory stimuli. First, the anti-allodynic effects of 15d-PGJ2 and rosiglitazone returned to pre-injection baseline within 4 hr; the reversible nature of this action argues against drug-induced injury to the nervous system. Second, rotarod testing demonstrated no effect of anti-allodynic doses of rosiglitazone or 15d-PGJ2 on motor coordination. And third, the anti-edema actions of rosiglitazone were reversible, largely resolving within 24 hr.
Rather than non-specific effects, we believe that ICV administration of PPARγ agonists inhibit the development of inflammatory pain by reducing nociceptive transmission at the spinal cord. Numerous studies indicate that the hindpaw injection of carrageenan induces Fos protein expression, a marker of neuronal activity, at the dorsal horn of the L4–L5 spinal cord (Buritova et al., 1996; Coggeshall, 2005; Nackley et al., 2003). Although there is general (but not universal) agreement that increased Fos expression is a marker of increased neuronal activity (Coggeshall, 2005; Harris, 1998), it remains to be determined whether these neurons contribute to pain excitation or inhibition. Our results demonstrate that rosiglitazone dramatically reduced Fos expression, indicating that ICV PPARγ agonists either enhance descending inhibition, reduce descending facilitation, or induce hormonal modulation of spinal pain transmission (Ren and Dubner, 2002; Urban and Gebhart, 1999). Clues from ICV studies with PPARα agonists raise the additional possibility of supraspinal inhibition of pain signaling in dorsal root ganglion (D'Agostino et al., 2009). Further studies are required to evaluate the effects of PPARγ agonists on the activity of CNS neurons at specific brain regions that contribute to descending pain modulation.
Supraspinal inhibition of peripheral inflammation
Our current and previous studies demonstrated that systemic administration of the PPARγ ligands troglitazone and rosiglitazone reduced peripheral inflammation in vivo (Taylor et al., 2002). Further studies will be required to determine whether these or other TZDs cross the blood brain barrier into the central nervous system. If they cross the blood brain barrier at sufficient concentrations, our results would suggest that actions in the brain might facilitate or synergize with the well-documented peripheral anti-inflammatory actions of PPARγ ligands.
The idea that the brain can modulate peripheral inflammation has been reviewed (Lotti et al., 2002). For example, ICV administration of PPARα agonists, salicylates, acetaminophen, or α-melanocyte-stimulating hormone reduce peripheral inflammation, possibly by triggering descending inhibitory signals (Catania et al., 1991; D'Agostino et al., 2007; Lotti et al., 2002; Taylor et al., 2005). Descending signals traveling through the spinal cord, dorsal root ganglion and/or sympathetic chain may inhibit the release of inflammatory mediators such as histamine and substance P (Catania et al., 1991). Alternatively, descending signals to the spinal cord may lead to inhibition of dorsal root reflexes associated with neurogenic inflammation, ultimately leading to decreased substance P-mediated plasma extravasation and decreased CGRP-mediated increases in blood flow (Brain and Cambridge, 1996; Lembeck and Holzer, 1979; Sluka, 2002). Further studies are required to determine whether PPARγ receptors in the brain act via similar mechanisms to reduce cutaneous inflammation.
Transcription-independent mechanism of steroid receptor action
Steroid receptor ligands are well-known to exert numerous actions via long-term, transcription-dependent, genomic mechanisms. However, there is a growing recognition for the importance of transcription-independent (non-genomic) mechanisms to the actions of PPAR ligands (Gardner et al., 2005). For example, PPARγ acts in a non-genomic manner to suppress NF-κB, STAT-1, and AP-1 signaling pathways (Moraes et al., 2006). Also, Loverme et al. demonstrated in mice that systemic administration of PPARα agonists rapidly (within 30 min) reduced behavioral signs of nociception after the intraplantar injection of dilute formalin (LoVerme J, 2006). Similarly, the present studies show that both 15d-PGJ2 and rosiglitazone rapidly reduce edema and inflammatory pain, often within 60 min of administration. Because this relatively short time course may be insufficient for altered transcription and translation of pain-related proteins, we suggest that non-genomic mechanisms in the brain may contribute to the anti-allodynic actions of PPARγ ligands.
Summary
Maeda et al recently reported that systemic administration of pioglitazone reduced behavioral signs of neuropathic pain (Maeda et al., 2008), raising the possibility that this FDA-approved drug can be effective as an analgesic agent. Previous studies indicate that peripheral sites contribute to the anti-hyperalgesic actions of 15d-PGJ2 in models of inflammatory pain (Napimoga et al., 2008; Pena-dos-Santos et al., 2009), while PPARγ sites in the spinal cord contribute to the anti-allodynic actions of 15d-PGJ2 and rosiglitazone in models of neuropathic pain (Churi et al., 2008; Maeda et al., 2008). Here we show that supraspinal PPARγ sites can also contribute to the pain-reducing actions of 15d-PGJ2 and rosiglitazone. Our results add supraspinal sites to the list of targets considered for future studies designed to evaluate the mechanism of analgesic action of PPARγ agonists. Indeed, unlike rosiglitazone or 15d-PGJ2, pioglitazone is known to cross the blood-brain-barrier (BBB), and thus its systemic administration can theoretically act upon PPARγ receptors in the CNS as well as the periphery, perhaps to exert the anti-hyperalgesic effects reported by Maeda et al (Maeda et al., 2008).
Acknowledgements
Supported by R01NS062306, R21DA018732 and K02DA019656 to BKT
Abbreviations
- ICV
intracerebroventricular
- 15d-PGJ2
15-deoxy-Δ12,14-Prostaglandin J2
- BADGE
Bisphenol A diglycidyl ether
- GW9662
2-Chloro-5-nitrobenzanilide
- PPAR
peroxisome proliferator-activated receptor
- CNS
central nervous system
- RXR
retinoid X receptor
- PPRE
peroxisome proliferator response element;
- TZD
thiazolidinedione
- ANOVA
analysis of variance
- CGRP
Calcitonin gene related peptide
- NF-κB
nuclear factor-kappa B
- STAT-1
signal transducer and activator of transcription-1
- AP-1
activator protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–781. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Berger J, Akiyama T, Meinke P. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bernardo AML. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D, Hla T, Warner TD. Bisphenol A diglycidyl ether (BADGE) is a PPARgamma agonist in an ECV304 cell line. Br J Pharmacol. 2000;131:651–654. doi: 10.1038/sj.bjp.0703628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Cambridge H. Calcitonin gene-related peptide: vasoactive effects and potential therapeutic role. Gen Pharmacol. 1996;27:607–611. doi: 10.1016/0306-3623(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Taylor BK. Noradrenergic Neurons in the Locus Coeruleus Contribute to Neuropathic Pain. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buritova J, Honore P, Chapman V, Besson J-M. Enhanced effects of co-administered dexamethasone and diclofenac on inflammatory pain processing and associated c-Fos expression in the rat. Pain. 1996;64:559–568. doi: 10.1016/0304-3959(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Catania A, Arnold J, Macaluso A, Hiltz ME, Lipton JM. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc Natl Acad Sci U S A. 1991;88:8544–8547. doi: 10.1073/pnas.88.19.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain. 2008;9:639–649. doi: 10.1016/j.jpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Cullingford TE, Bhakoo K, Peuchen S, Dolphin CT, Patel R, Clark JB. Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem. 1998;70:1366–1375. doi: 10.1046/j.1471-4159.1998.70041366.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Mattace Raso G, Cuzzocrea S, Loverme J, Piomelli D, Meli R, Calignano A. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur J Pharmacol. 2009;613:54–59. doi: 10.1016/j.ejphar.2009.04.022. [DOI] [PubMed] [Google Scholar]
- D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther. 2007;322:1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPAR-alpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Fehlberg S, Trautwein S, Goke A, Goke R. Bisphenol A diglycidyl ether induces apoptosis in tumour cells independently of peroxisome proliferator-activated receptor-gamma, in caspase-dependent and -independent manners. Biochem J. 2002;362:573–578. doi: 10.1042/0264-6021:3620573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman LJ, Hock CE, Harwell M, Estilow-Isabell S. Comparison of the effects of immobilization and pressure overload induced cardiac hypertrophy on immunoreactive beta-endorphin. Life Sci. 1995;57:2041–2047. doi: 10.1016/0024-3205(95)02192-l. [DOI] [PubMed] [Google Scholar]
- Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Mol Pharmacol. 2005;68:933–941. doi: 10.1124/mol.105.012260. [DOI] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kota BPHT, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51:85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Lehrke MLM. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lembeck F, Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lotti T, Bianchi B, Ghersetich I, Brazzini B, Hercogova J. Can the brain inhibit inflammation generated in the skin? The lesson of gamma-melanocyte-stimulating hormone. Int J Dermatol. 2002;41:311–318. [PubMed] [Google Scholar]
- LoVerme JRR, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci. 2008;108:341–347. doi: 10.1254/jphs.08207fp. [DOI] [PubMed] [Google Scholar]
- Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr., Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, Verri WA, Jr., Cunha FQ, Ferreira SH. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther. 2008;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- Oliveira AC, Bertollo CM, Rocha LT, Nascimento EB, Jr., Costa KA, Coelho MM. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR alpha, and pioglitazone, an agonist of PPAR gamma. Eur J Pharmacol. 2007;561:194–201. doi: 10.1016/j.ejphar.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Orlando, FL: Academic Press, Inc; 1997. [Google Scholar]
- Pena-dos-Santos DR, Severino FP, Pereira SA, Rodrigues DB, Cunha FQ, Vieira SM, Napimoga MH, Clemente-Napimoga JT. Activation of peripheral kappa/delta opioid receptors mediates 15-deoxy-(Delta12,14)-prostaglandin J2 induced-antinociception in rat temporomandibular joint. Neuroscience. 2009;163:1211–1219. doi: 10.1016/j.neuroscience.2009.07.052. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Bethesda, Maryland, USA: U. S. National Institutes of Health; ImageJ. 1997–2007 http://rsb.info.nih.gov/ij.
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci. 2002;22:5687–5693. doi: 10.1523/JNEUROSCI.22-13-05687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int. 2006;49:136–144. doi: 10.1016/j.neuint.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Tan NS, Michalik L, Desvergne B, Wahli W. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol. 2005;93:99–105. doi: 10.1016/j.jsbmb.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Dadia N, Yang CB, Krishnan S, Badr M. Peroxisome proliferator-activated receptor agonists inhibit inflammatory edema and hyperalgesia. Inflammation. 2002;26:121–127. doi: 10.1023/a:1015500531113. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Holloway D, Printz MP. A unique central cholinergic deficit in the spontaneously hypertensive rat: physostigmine reveals a bradycardia associated with sensory stimulation. J Pharmacol Exp Ther. 1994;268:1081–1090. [PubMed] [Google Scholar]
- Taylor BK, Kriedt C, Nagalingam S, Dadia N, Badr M. Central administration of perfluorooctanoic acid inhibits cutaneous inflammation. Inflamm Res. 2005;54:235–242. doi: 10.1007/s00011-005-1350-0. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, Serhan CN, Spiegelman BM. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]