Abstract
Background & Aims
CCL20 is a chemokine that regulates the homeostatic and inflammatory trafficking of leukocytes to the small intestine, and regulates the development of the gastrointestinal lymphoid architecture. T cells expressing Th2 cytokines are critical for experimental food allergy, and we hypothesized that CCL20 is involved in the localization of these cells to the gut.
Methods
We evaluated the role of CCR6 in allergic diarrhea induced by sensitization and oral challenge with ovalbumin (OVA) using CCR6+/+ and −/− mice.
Results
CCR6−/− mice were protected from OVA-induced diarrhea, but surprisingly were not impaired in mastocytosis or allergen-specific IgE. CCR6−/− mice were also protected from T cell-mediated diarrhea induced by anti-CD3 antibody. Allergic diarrhea was associated with an increased expression of Th2 cytokines within the intestinal mucosa that was significantly reduced in CCR6 −/− mice. Inhibition of lymphocyte homing by treatment with FTY720 did not impair allergic diarrhea, indicating that reactivation of T cells could occur locally within the small intestine. Finally, T cell transfer studies demonstrated that CCR6 was required both on the transferred T cells and in the recipient mouse in order to manifest allergic disease in the gastrointestinal tract.
Conclusions
These studies highlight a mast cell- and IgE-independent role for CCR6-bearing T cells in the pathogenesis of gastrointestinal allergic disease.
INTRODUCTION
Food allergic reactions are initiated by allergen cross-linking of IgE bound to intestinal mast cells, mast cell degranulation, and release of mast cell products that act directly on the intestinal epithelium, or indirectly through enteric nerves, to induce changes in intestinal ion secretion and barrier function 1, 2. Mice systemically sensitized to ovalbumin (OVA) and repeatedly orally challenged with OVA develop a mast cell and IgE-dependent acute diarrhea associated with a Th2 inflammation in the small intestine 3. We have previously shown that mesenteric lymph node (MLN) CD4+ T cells from mice with allergic diarrhea can transfer allergic disease to naïve mice 4, highlighting the role of T lymphocytes in an IgE- and mast cell-driven model system. Forbes et al recently showed that transgenic expression of the single T cell cytokine IL-9 within the intestine could lead to a local mastocytosis and diarrhea replicating experimental models of allergen-driven experimental food allergy 5. Inhibition of IL-4 and IL-13 given early during repeated oral allergen challenge can also inhibit allergic symptoms 6. Allergen-specific T cells producing Th2 cytokines have been shown to be present in the intestinal mucosa of human subjects with food allergic diseases 7, 8, including non-IgE-mediated food allergic disease. The factors responsible for recruitment of pathogenic T cells to the intestine in food allergic disorders are not known, and we hypothesized that mucosally-expressed chemokines would be critical for the homing of T cells to the gut in experimental food allergy.
CCL20 (MIP-3α) is a chemokine that is expressed by gastrointestinal epithelium 9, is regulated by NF-κB 10, and is overexpressed in inflammatory bowel disease 10, 11. We have recently shown that ligation of the low affinity IgE receptor on intestinal epithelial cells leads to release of functional CCL20 12. Expression of CCL20 is highest in the follicle-associated epithelium of the Peyer’s patch 13, 14, but it is also expressed by mouse and human enterocytes 9. The cognate receptor for CCL20 is CCR6, and is expressed on memory T cells, B lymphocytes, and dendritic cells (DCs). CCR6−/− mice have impaired mucosal but not systemic humoral responses to immunization and rotavirus infection 15. In addition, CCR6−/− mice have alterations in the architecture of organized lymphoid tissue in the gastrointestinal tract, including Peyer’s patches, isolated lymphoid follicles, and cryptopatches 16–18. We hypothesized that this ubiquitous mucosal chemokine would play a role in the homing of T lymphocytes to the gastrointestinal tract in experimental food allergy, and tested this using CCR6+/+ and −/− mice.
METHODS
Allergic Diarrhea
CCR6−/− mice were generated previously by S. Lira 15, back-crossed for 10 generations to the Balb/c background and maintained in SPF conditions. Balb/c mice were purchased from NCI (Frederick, MD). All experiments were performed with the approval of the Institutional Animal Care and Use Committee. Female age-matched CCR6+/+ and CCR6−/− mice (5–8 weeks of age) were sensitized to ovalbumin (OVA) as previously described4. Symptoms were monitored for 30 minutes after feeding, and diarrhea was marked as present or absent.
Cholera Toxin and CD3-Induced Diarrhea
Cholera toxin-induced diarrhea was generated by administering 50 μg of cholera toxin (CT, List Biological Laboratories, Campbell, CA) by intragastric gavage to CCR6+/+ or CCR6−/− mice. After 3 h, mice were euthanized and 3 intestinal loops/mouse prepared for weight/length and wet/dry weight ratios. Intestinal segments were weighed, dried in an oven (80 °C, 48 h), and re-weighed. Wet/dry weight ratios are reflective of fluid secretion and edema.
T cell-mediated diarrhea was induced as previously described 19, 20. Briefly, mice were administered 0.2 mg of anti-CD3 (eBioscience, functional grade) by ip injection or PBS as control. After 2 h, mice were euthanized and intestinal loops were prepared as above.
Cell culture
Mesenteric lymph nodes (MLN) were isolated and cells were cultured with media alone or OVA (100 μg/ml) for 72 hours. Supernatants were collected for cytokine determination by ELISA (eBioscience, San Diego, CA).
Adoptive Transfer
Donor mice were sensitized and fed with OVA to induce allergic diarrhea. MLN cells were isolated, re-stimulated with OVA as above prior to washing and iv transfer to naïve mice (4 × 106 cells/mouse). For CD4+ T cell transfer, CD4+ T cells were negatively selected (StemCell, Vancouver, BC) prior to transfer of 2 × 106 cells/mouse. Recipient mice were orally challenged with OVA every second day starting 24 h after transfer, and diarrhea symptoms monitored as above.
Alternatively, 3 × 106 CD4+ T cells from DO11.10 mice were CFSE-labeled and transferred to naïve CCR6+/+ or CCR6−/− mice. After 24 h, recipient mice were fed with 50 mg of OVA. After 72 h, cells in the MLN were isolated for analysis of proliferation.
FTY720 administration
FTY720 was a gift from V. Brinkmann (Novartis Pharma AG) to JSB. To block lymphocyte egress from the lymph nodes, mice were treated orally with 0.3 mg/kg FTY720 daily beginning one day prior to initiation of oral OVA challenges and daily thereafter. Efficacy was verified by measurement of circulating CD4+ and CD8+ T cells.
RT-PCR
RNA was isolated from jejunum, and real-time RT-PCR was performed as previously described 4.
Histology and Immunostaining
Jejunal segments were fixed in formalin and paraffin. Jejunal mast cells were detected by chloroacetate esterase staining according to published protocols of mast cell detection 21.
IgE Measurement
OVA-specific IgE in serum and intestinal lavage fluid was measured by capture ELISA as previously described 4.
RESULTS
The CCL20-CCR6 chemokine axis is necessary for gastrointestinal allergic symptoms
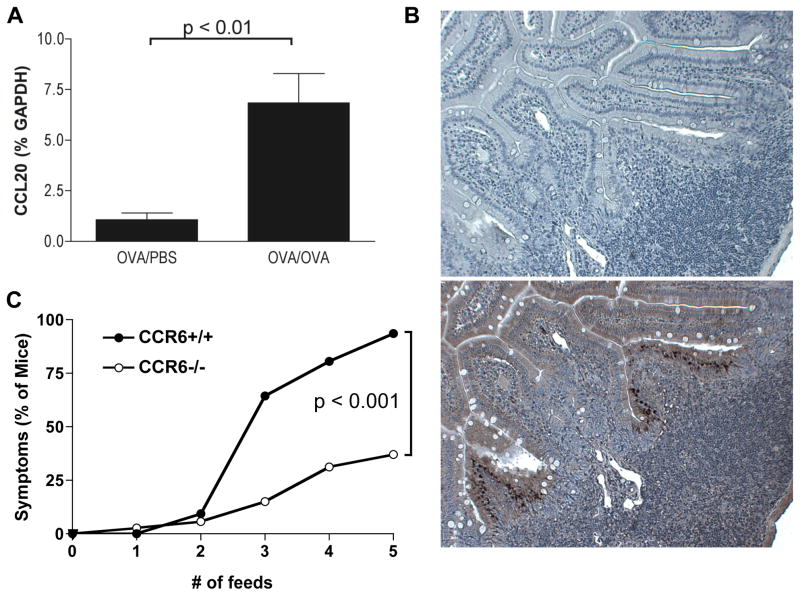
In a screen of local chemokine expression during the course of allergic diarrhea, CCL20 mRNA expression was significantly upregulated compared to control mice that were sensitized but unfed with OVA (Figure 1a). Immunostaining for CCL20 showed a diffuse positive expression pattern in enterocytes, and an intense immunoreactivity in M cells as has previously been reported 14 (Figure 1b). CCL20 is known to be an important chemokine for mucosal lymphocyte homeostasis, and to test the role of this chemokine in allergic diarrhea, we used mice that were genetically deficient for the receptor CCR6. Wild-type mice sensitized to OVA developed acute self-limiting symptoms beginning after the third oral challenge with OVA (> 50% of mice had symptoms by the third feed, and approximately 90% by the fifth feed). In contrast, CCR6−/− mice were significantly protected from the onset of allergic diarrhea (Figure 1c).
Figure 1. CCL20 is upregulated during allergic diarrhea and CCR6 is required for diarrhea symptoms.
CCR6+/+ and CCR6−/− mice were sensitized and orally challenged with OVA. A. CCL20 mRNA expression in the jejunum of CCR6+/+ and CCR6−/− mice. Data are expressed as fold-changed compared to unchallenged controls. B. Immunostaining for CCL20 in the small intestine (bottom panel shows anti-CCL20 staining, top panel is isotype control). Note the dense immunoreactivity in the follicle-associated epithelium. C. Onset of symptoms (% of mice with visible diarrhea) after each feed was recorded. n = 33 (+/+) and 36 (−/−).
CCR6-Independent IgE and Mast Cell Responses
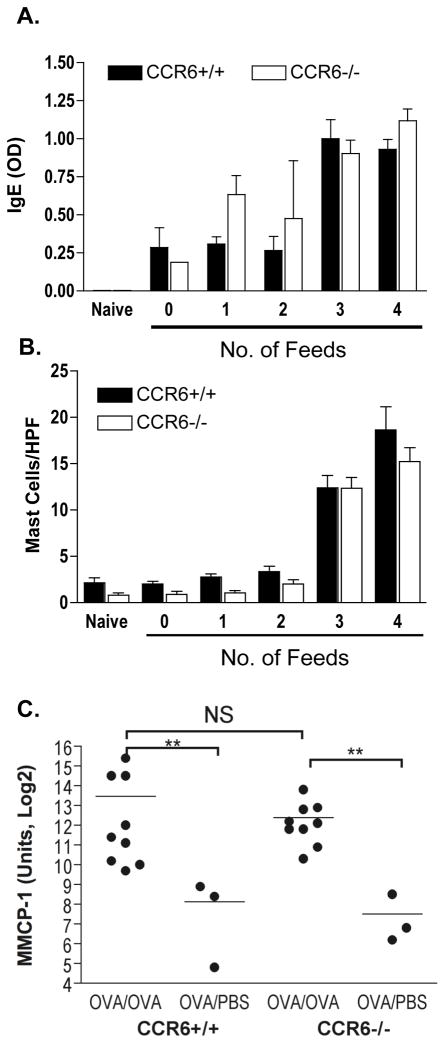
It has previously been demonstrated 3 that diarrhea symptoms in this model are dependent on mast cells and IgE. Surprisingly, we observed that neither gastrointestinal mast cells nor serum IgE were significantly impaired in CCR6−/− mice (Figure 2). Serum OVA-specific IgE was measured by ELISA in mice sacrificed after 0 to 4 oral challenges (every second day) with OVA. OVA-specific IgE was significantly boosted after the third oral challenge, and was not significantly different between CCR6+/+ and CCR6−/− mice. This was also true for OVA-specific IgE detected in intestinal lavage (data not shown). A significant increase in tissue mast cells was also observed, beginning after the third OVA challenge. CCR6−/− mice were not impaired in tissue mast cells (Figure 2b). This was confirmed by mRNA expression of the mast cell protease MMCP-1, that was significantly upregulated in both CCR6+/+ and −/− mice (Figure 2c). Mast cell numbers were further confirmed by flow cytometry (Figure S1). Therefore, we conclude that CCR6 deficiency does not prevent the onset of allergic diarrhea by interfering with either tissue mast cells or allergen-specific IgE.
Figure 2. Allergen-induced Jejunal Mast Cells and serum IgE are CCR6-independent.
CCR6+/+ and CCR6−/− mice were sensitized and with OVA. 4 mice were sacrificed after each feed of OVA (from 0 to 4). Top panel: Serum OVA-specific IgE. Middle panel: Jejunum mast cell counts per high power field (HPF). Bottom panel: MMCP-1 mRNA expression in jejunum from sacrificed after 4 (OVA/OVA) or 0 (OVA/PBS) feeds of OVA. ** p < 0.01, NS = not significant.
CCR6 is required for T cell-mediated, but not toxin-driven, diarrhea
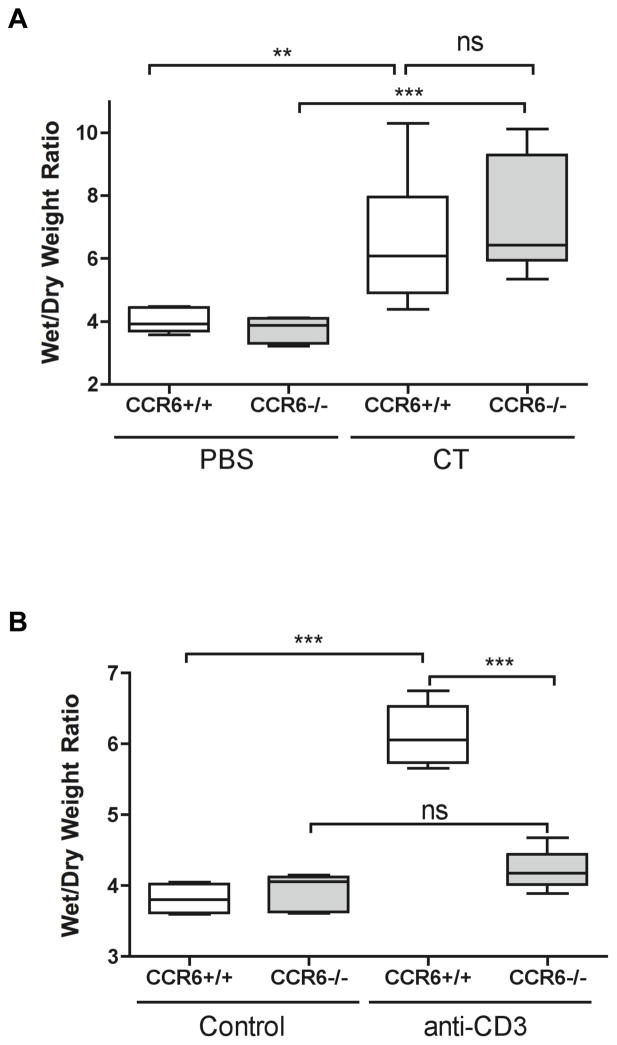
To determine if CCR6−/− mice have an impairment in their epithelial secretion mechanisms, we tested two other models of acute diarrhea. Mice were orally administered 50 μg cholera toxin (CT), and after 3 h ligated loops were prepared to quantify fluid secretion by wet/dry weights. CCR6+/+ mice responded to CT with a significant fluid loss throughout the small intestine, and this was independent of CCR6 (Figure 3). We next used a T cell-driven model of diarrhea induced by administration of anti-CD3 antibodies. Naïve CCR6+/+ and CCR6−/− mice were injected with 0.2 mg of anti-CD3, and 2 h later intestinal loops were prepared to assess fluid secretion in the small intestine (Figure 3). Administration of 0.2 mg of anti-CD3 antibody to CCR6+/+ mice led to fluid secretion in the proximal and mid-small intestine by 2 h post-injection in CCR6+/+ mice. In contrast, CCR6−/− mice were protected from CD3-induced diarrhea, similar to our findings with allergic diarrhea. We next isolated cells from the lamina propria of CCR6+/+ and CCR6−/− mice. There was no difference in either the total number of lamina propria cells or the percentage of CD4+ T cells (Fig S2). When isolated cells from the lamina propria were stimulated in vitro with anti-CD2/CD28 antibodies, cytokine secretion was also not significantly different in cells isolated from CCR6−/− mice (Fig S2). Therefore CCR6 deficiency does not appear to be associated with a change in the constitutive homing of effector T cells into the small intestinal mucosa.
Figure 3. CCR6 is required for T cell-mediated, but not toxin-induced diarrhea.
A: CCR6+/+ and CCR6−/− mice were fed 50 μg of cholera toxin or PBS as control. After 3 h, mice were euthanized and ligated loops were prepared from small intestine. Wet/dry weights were calculated as in methods. n = 3/group. B: CCR6+/+ and CCR6−/− mice were injected with 0.2 mg of anti-CD3 antibody or left un-injected as control. After 2 h, ligated loops were prepared as above. n = 7/group. ** p < 0.01; *** p < 0.001; ns = not significant.
CCR6 is required for local Th2 cytokine expression in the jejunum
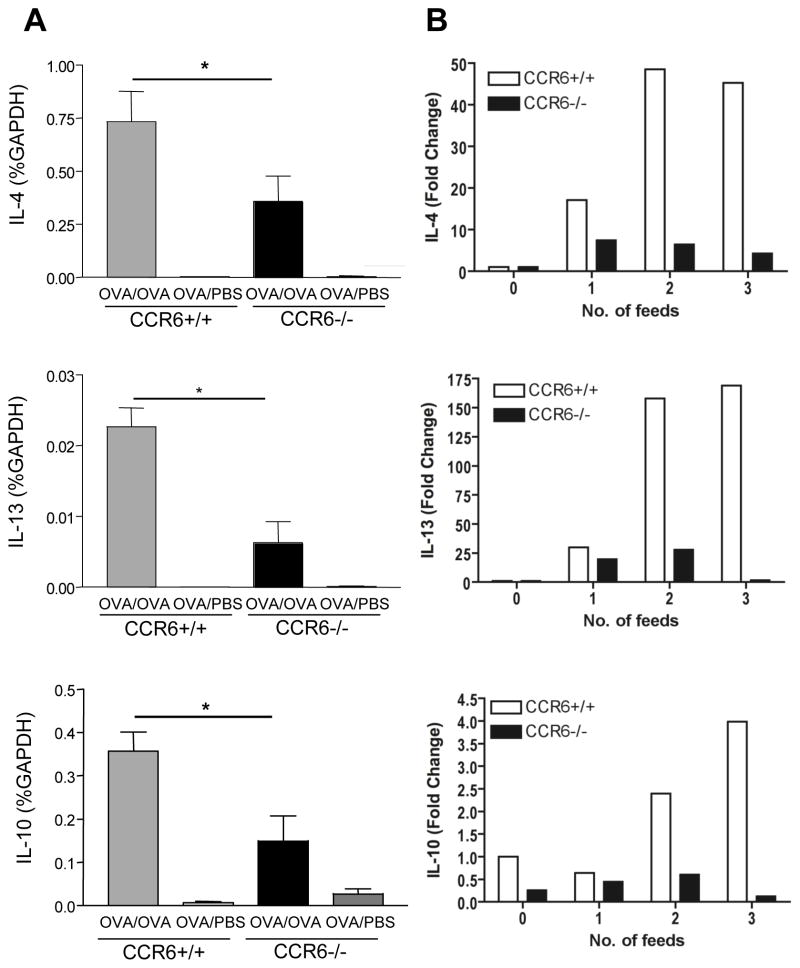
As T cell-driven diarrhea was CCR6-dependent, we next assessed cytokine expression in the jejunum of mice with allergic diarrhea. CCR6+/+ and CCR6−/− mice were sensitized to OVA and given four oral challenges of OVA, or were unfed as controls. Real-time PCR was performed for the cytokines IL-4, IL-13, and IL-10 as we and others have previously shown that these cytokines are locally upregulated during allergic diarrhea 3, 4. CCR6+/+ mice had a significant upregulation of IL-4, IL-13, and IL-10 that was significantly reduced in CCR6−/− mice (Figure 4). IFN-γ, IL-17, TNFα, and IL-18 were neither significantly upregulated during allergic diarrhea nor significantly different between CCR6+/+ and CCR6−/− mice (Fig S3). This was observed throughout the OVA challenge period as shown by time course of cytokine expression. Thus, the characteristic Th2-skewed inflammation in the jejunum of mice with allergic diarrhea was impaired by CCR6 deficiency.
Figure 4. Allergen-induced Th2 cytokine expression in the small intestine is impaired in CCR6 −/− mice.
Left panels: CCR6+/+ and CCR6−/− mice were sensitized to OVA and challenged with OVA (OVA/OVA) or PBS (OVA/PBS) as control. Mice were euthanized within 1–2 h after symptom onset. (n= 10 samples/group) Right panels: Mice were sacrificed after each OVA feeding (4 mice/group). RNA was isolated from jejunum, and RT-PCR for IL-4 (top panel), IL-13 (middle panel) and IL-10 (bottom panel) was performed on individual samples (left panels) or pooled samples from the time course (right panels).
CCR6 does not influence Th2 reactivation in the MLN
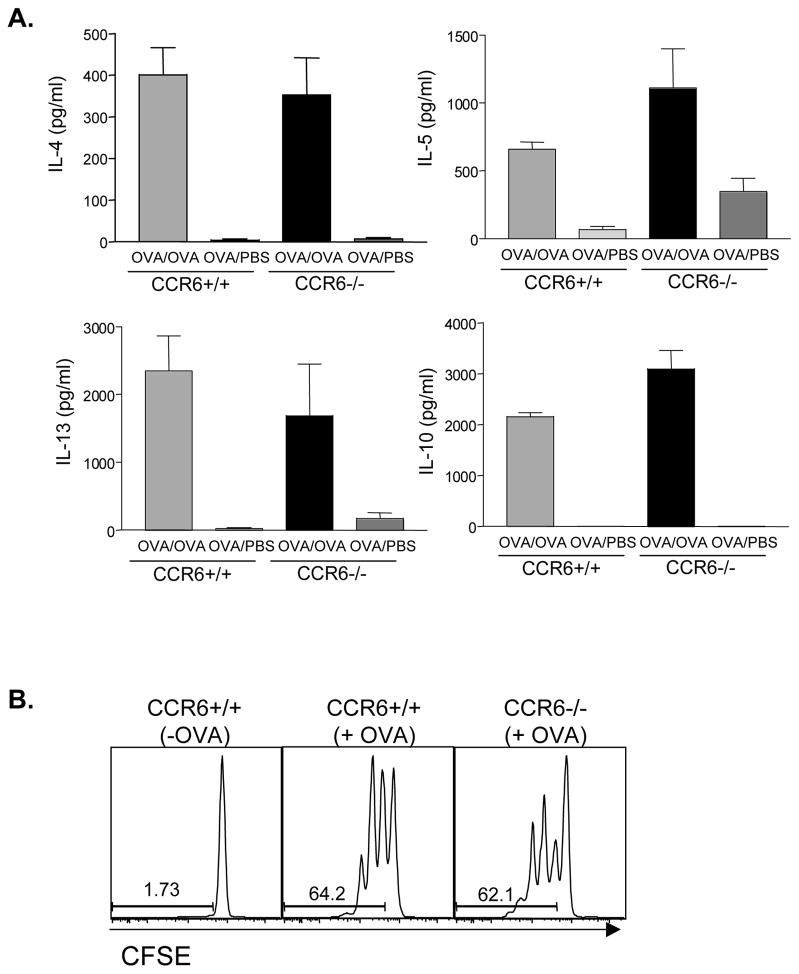
We have previously shown that activation of Th2 lymphocytes in the allergic diarrhea model occurs in the MLN in response to oral OVA challenge 4. To test the role of CCR6 in T cell reactivation in mucosal tissues, we re-stimulated MLN cells from CCR6+/+ and −/− mice with OVA and determined cytokine output (Figure 5). CCR6+/+ mice with allergic diarrhea had a significant increase in OVA-specific IL-4, IL-5, IL-13, and IL-10 secretion that was unimpaired in CCR6−/− mice. Naïve T cell priming was also assessed by transferring CFSE-labeled DO11.10 CD4+ T cells to CCR6+/+ and −/− mice. Feeding of mice resulted in a robust proliferation of DO11.10 cells in the MLN of both CCR6+/+ and −/− mice (Figure 5). Upregulation of the gut homing molecule α4β7 on T cells activated within the MLN was also unimpaired in CCR6−/− mice (data not shown). Thus antigen delivery to the lymph node and presentation to T cells is not impaired in CCR6−/− mice. In addition, we performed in vitro antigen presentation assays with purified lamina propria DCs, and observed that DCs from CCR6+/+ and CCR6−/− mice were equal in their ability to induce cytokine secretion from responder CD4+ T cells (Figure S4).
Figure 5. Th2 responses in the mesenteric lymph node are normal in CCR6 −/− mice.
CCR6+/+ and CCR6−/− mice were sensitized to OVA and challenged with OVA (OVA/OVA) or PBS (OVA/PBS) as control. MLN cells were re-stimulated with OVA and IL-4, IL-5, IL-13, and IL-10 were measured in culture supernatants (Panel A). CFSE-labeled OVA-specific DO11.10 cells were transferred to naïve CCR6+/+ and CCR6−/− mice. Mice were fed 50 mg OVA (+OVA) or remained unfed as control (−OVA). After 96 h, T cell proliferation in the MLN was assessed by CFSE dilution (Panel B).
T cell re-activation during oral challenge does not require homing from the lymph node
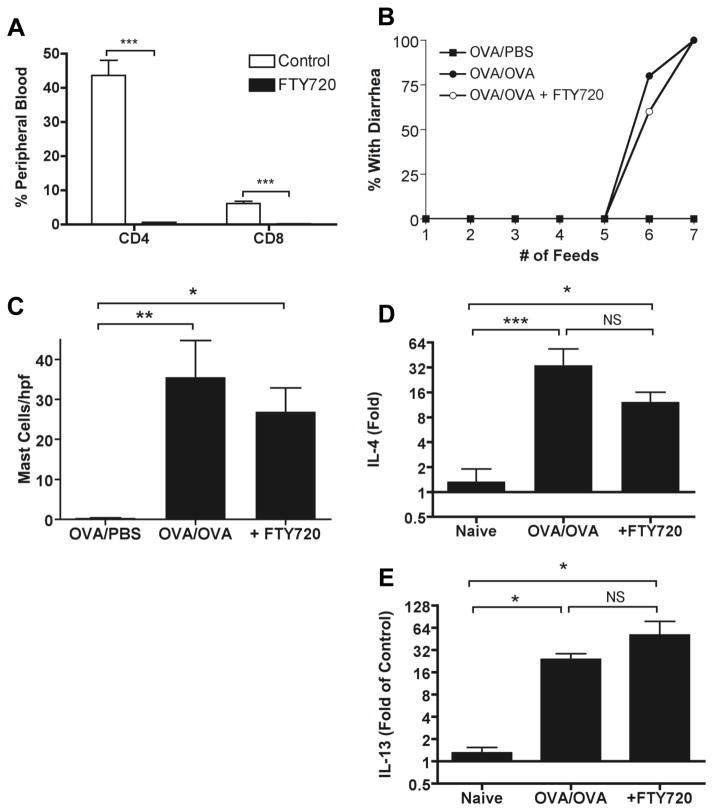
To investigate further the role of T cell homing in induction of disease, we used the sphingosine 1-phosphate 1 receptor agonist FTY720 that has been shown to inhibit the egress of lymphocytes from lymph nodes, including MLN and Peyer’s patches 22–24. FTY720 was administered orally to OVA-sensitized mice prior to oral OVA challenge. Efficacy of FTY720 was verified by measuring circulating CD4+ and CD8+ T cells (Figure 6). FTY720 resulted in an almost complete elimination of circulating CD4+ and CD8+ T cells, although there was no major effect of FTY720 on the resident CD4+ T cell population in the lamina propria (Figure S5). Blockade of lymphocyte egress from the lymph nodes with FTY720 had no effect on onset of allergic diarrhea, tissue mast cells, or local Th2 cytokine production. Therefore once sensitization has already occurred and memory has been established, reactivation of T cells can readily occur in the lamina propria without trafficking to and from lymph nodes.
Figure 6. T cell reactivation occurs locally in the absence of homing from lymph nodes.
Mice were treated with FTY720 daily beginning one day prior to beginning the OVA feeds. Efficacy of the FTY720 treatment was checked by flow cytometry of peripheral blood (A), which showed a near abolishment of circulating CD4 and CD8 T cells. FTY720 treatment had no effect on onset of OVA-induced diarrhea (B), jejunal mastocytosis (C), or jejunal IL-4 (D) or IL-13 (E) expression. Data are representative of two independent experiments, with a total of 10 mice per group.
T cell transfers demonstrate requirement for CCR6 on both effector T cells and in the recipient for disease onset
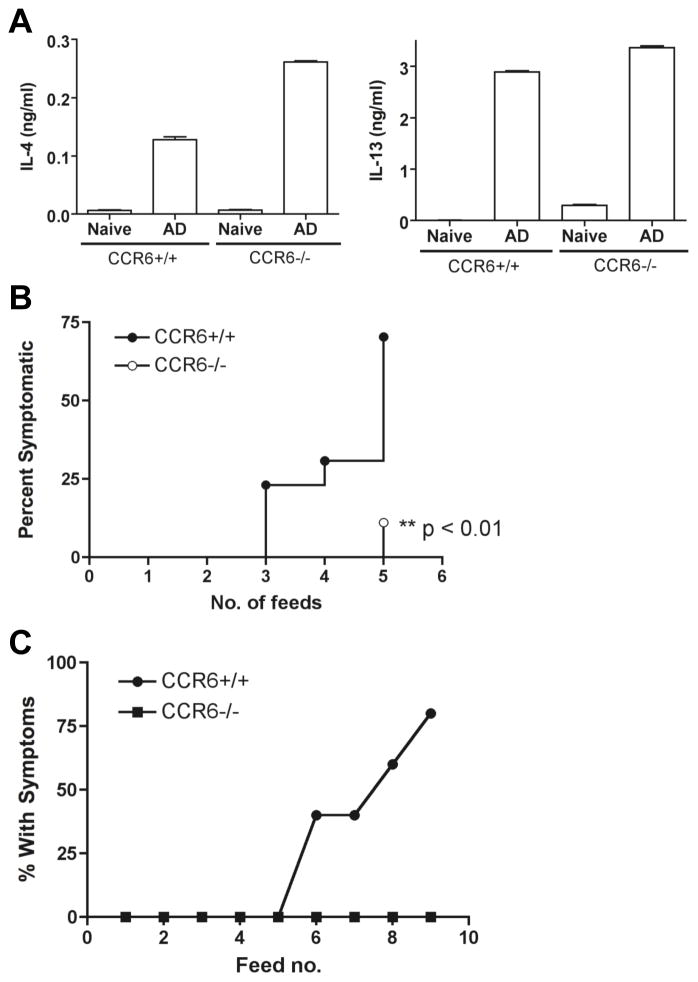
Administration of MLN cells (total or CD4+ T cells) from mice with allergic diarrhea can transfer disease and bypass the need for systemic sensitization in naïve mice 4. MLN cells from CCR6−/− mice have normal cytokine responses as compared to CCR6+/+ mice, so we tested the ability of these cells to transfer disease (Figure 7). MLN cells from CCR6+/+ and −/− mice (after sensitization and oral OVA challenge) were transferred to naïve Balb/c mice. Recipient mice were then repeatedly challenged with OVA. Mice receiving MLN cells from CCR6+/+ mice developed diarrhea. In contrast, mice receiving MLN cells from CCR6−/− mice had significantly reduced onset of allergic diarrhea. Pre-transfer IL-4 and IL-13 levels were confirmed to be similar in cells from CCR6 +/+ and −/− mice (Figure 7), suggesting that while the effector function of these cells is not reduced, either homing of these cells to the small intestine or reactivation of these cells in vivo is defective. We next performed cell transfers to determine if wild-type CCR6+/+ CD4+ T cells could restore the disease phenotype to CCR6−/− mice (Figure 7). Wild-type mice receiving purified CD4+ T cells from CCR6+/+ MLN developed diarrhea in response to oral OVA challenge. In contrast, CCR6−/− mice were completely resistant to the transfer of allergic diarrhea using CCR6+/+ T cells from the MLN.
Figure 7. CCR6 on T cells is necessary, but not sufficient, for allergic diarrhea.
MLN cells from OVA-sensitized and fed (AD) CCR6+/+ or CCR6−/− mice were re-stimulated with OVA in vitro before transferring to naïve Balb/c mice. Mice were then fed with OVA every second day and diarrhea symptoms recorded. (A) The cytokine output of the transferred cells prior to transfer. (B) Symptoms of wild-type mice receiving primed CCR6+/+ or CCR6−/− T cells as above. (C) Donor cells were CCR6+/+, and recipients were CCR6+/+ or CCR6−/− as indicated.
DISCUSSION
T lymphocytes play an important role in IgE-mediated food allergic disorders, and have also been presumed to be central to the pathogenesis of non-IgE-mediated gastrointestinal allergic disorders such as eosinophilic gastrointestinal disorders. We hypothesized that recruitment of T cells to the intestinal mucosa through chemokine-driven pathways would be instrumental to the development of gastrointestinal allergic disease. We examined the role of the mucosal chemokine CCL20 and its receptor CCR6 in the development of experimental food allergy using CCR6−/− mice. CCR6 was necessary for allergen-induced diarrhea symptoms, and our results point to a critical role for CCR6 in both T cell homing and activation in the gastrointestinal tract.
CCR6−/− mice were significantly protected from the onset of allergic diarrhea, despite mounting a normal IgE and mast cell response. This suggests an alternative mechanism of disease pathogenesis in this model of allergic diarrhea. Brandt et al have clearly shown that mast cells and IgE are required for allergen-induced diarrhea 3. Our results show that T cells are also required, and not solely upstream of the generation of IgE and mast cells. IL-4 and IL-13 have been shown to directly increase epithelial permeability and reduce glucose-coupled sodium absorption in mouse intestine 25. This would be of significant clinical interest as there are several food-allergic disorders that have been loosely categorized as “cell-mediated” without having a clear mechanism explaining the pathway from allergen exposure to gastrointestinal dysfunction. Recently Brandt et al demonstrated that targeting IL-4 during oral challenge of mice could prevent allergic diarrhea, but once diarrhea had been established, IL-4 was no longer required for acute symptom onset 6. Together these results suggest that mast cells and Th2 cytokine production are both independently necessary for establishing allergic symptoms and inflammation in the gut.
CCR6 deficiency could potentially be acting at the level of the epithelial cell as it has been reported that CCL20 can influence epithelial ion secretion in human intestinal epithelial cell lines 26. This may be particularly relevant for the alternative CCR6 ligand HBD-2, which is secreted luminally and has been shown to promote epithelial restitution 27. Murine beta-defensin 14 has also been shown to have activity on CCR6 28, but the expression of defensins in gastrointestinal allergic disease is not yet known. However, we feel that epithelial CCR6 is unlikely to be a major factor as two independent T cell mediated models of diarrhea were CCR6-dependent while toxin-induced diarrhea was not influenced by CCR6 deficiency. CD3 treatment mediates diarrhea by a TNF-α-dependent mechanism leading to alteration of epithelial transporters and manipulation of epithelial barrier function 20, 29. Our data point to memory T cell recruitment and/or activation of those memory T cells being defective in CCR6−/− mice. When total cells were isolated from the lamina propria of naïve CCR6+/+ or CCR6−/− mice, there was no defect in stimulation-induced cytokine secretion. This data suggests that activation of cells in vivo is deficient in CCR6−/− mice, and we speculate that this is due to changes in mucosal architecture (loss of lymphoid structures) associated with CCR6 deficiency. We examined T cell cytokine expression more closely in our allergic diarrhea model.
Our data show that allergen-induced Th2 cytokine expression in the small intestine is dependent on CCR6, despite normal Th2 cytokine expression in the draining lymph node. This suggests that either activation within the lamina propria or homing from the lymph node to the lamina propria is defective in CCR6−/− mice. CCR6 is present on memory T cells, and on a subset of CD4+ T cells in the lamina propria 17. CCR6 is upregulated on proliferating OT-II transgenic T cells after activation in the mesenteric lymph node 30, supporting a role for CCR6 in homing to the lamina propria after activation in the lymph node. There are clearly other mechanisms responsible for T cell recruitment to the lamina propria, such as CCR9 and the homing molecule α4β7, evidenced by the fact that CCR6−/− mice do not have a major impairment in mucosal T cells, and in fact had previously been reported to have increased intraepithelial and lamina propria CD4+ populations 15. We directly addressed the role of T cell homing during allergen challenge by using FTY720, a sphingosine 1-phosphate receptor 1 agonist that blocks lymphocyte egress from the lymph nodes. FTY720 had no effect when it was administered after sensitization and during oral OVA challenge. This data supports the conclusion that T cell re-activation in response to oral OVA challenge is occurring locally within the intestinal mucosa. FTY720 has been shown to be effective on blocking egress from both MLN and Peyer’s patch 22–24, and efficacy of FTY720 treatment was verified in our experiments by showing a near abolishment of circulating CD4 and CD8 T cells. FTY720 ameliorates experimental colitis 31, 32, demonstrating efficacy in the gastrointestinal tract. FTY720 partially impairs allergic symptoms when given prior to allergen challenge in a model of allergic diarrhea localized to the large intestine 33, and more complete inhibition was observed when FTY720 was administered prior to sensitization. Allergen-driven inflammation in the large intestine may be more likely to be dependent on re-activation within the lymph nodes as it is unlikely that significant amounts of intact antigen would remain to be absorbed into the large intestinal lamina propria.
Transfer of primed Th2-secreting T cells to naïve mice eliminates the need for systemic sensitization with OVA and alum. Transfer of wild-type T cells to wild-type recipients resulted in allergic diarrhea when the mice were repeatedly fed with OVA. Transfer of CCR6−/− cells that secreted similar levels of Th2 cytokines could not transfer disease. Thus CCR6 on CD4+ T cells is necessary for transfer of allergic diarrhea. However, CCR6 on CD4+ T cells is not sufficient to transfer disease, as we observed that adoptive transfer of wild-type CD4+ T cells (or whole MLN populations) to CCR6−/− mice did not lead to OVA-induced diarrhea. These data are very similar to those previously shown by Lundy et al 34, whereby splenic T cells from wild-type mice could transfer allergic airway hyperresponsiveness to wild-type mice, but not CCR6−/− mice. They demonstrated that there was a deficiency in DC numbers in the CCR6−/− lung, and that the DCs from CCR6−/− mice were less able to trigger IL-5 secretion from pulmonary T cells. In the gastrointestinal tract, we observed the previously reported result 35 that CCR6 is not expressed on lamina propria DCs. In addition, we did not observe any functional defect in the ability of DCs from CCR6−/− mice to prime T cells in vivo as shown in Figure 5B, or in vitro as shown in Supplemental Figure 6. Therefore our data do not support a role for CCR6 on DCs in OVA-specific T cell activation. This is in contrast to the T cell response to microbial pathogens, such as Salmonella, which is dependent on DC expression of CCR6 35. Salmonella, as particulate antigens, are more likely to be taken up via the Peyer’s patch in which CCR6 is abundantly expressed on DCs. CCR6 deficiency has been shown to lead to changes in mucosal lymphoid tissue architecture. Number and size of Peyer’s patches is reduced 18, isolated lymphoid follicles (ILF) are reduced 16, and M cell numbers are also decreased in CCR6−/− mice 36. Takayama et al directly addressed the role of Peyer’s patches in allergic diarrhea, and found in fact that they played a suppressive role through facilitating the expansion of CD4+ CD25+ FoxP3+ regulatory T cells 37. Therefore the diminished Peyer’s patches in CCR6−/− mice would be expected to enhance, rather than reduce allergic diarrhea and inflammation. However, the role of isolated lymphoid follicles in allergic diarrhea or as an inductive or reactivation site for T lymphocytes has not been addressed. Mouse small intestine normally contains 100–200 of these structures that bring together B cells, T cells, and DCs 38. CCR6 deficiency results in a decrease in the total number of these ILFs 16. We hypothesize that these organized structures may be required for optimal reactivation of T lymphocytes in the gastrointestinal tract, and explain why wild-type CD4+ cells transferred to CCR6−/− mice do not fully achieve their pathogenic potential in the gastrointestinal tract, and why CCR6−/− mice are also resistant to CD3-induced diarrhea, despite the normal function of these T cells in vitro. We speculate that an altered GALT anatomy could potentially inhibit the ability of memory T cells to be reactivated by either antigen or anti-CD3 antibodies.
In summary, we show that CCR6 plays a role in the development of gastrointestinal allergic disease, in part through expression on CD4+ T lymphocytes. Our data utilizing adoptive transfers indicates that in a normal recipient, the absence of CCR6 on a single cell type (CD4+ T cells) prevents disease development. In addition, provision of wild-type pathogenic T cells cannot induce disease in CCR6−/− mice. These findings suggest a role for CCR6 by one of two mechanisms: either in homing of T cells to appropriate mucosal locations for reactivation, or secondly for optimal activation of T cells in the small intestine, which we speculate is due to the absence of organized solitary isolated lymphoid follicles in CCR6−/− mice. In addition, our data points to intriguing mast cell and IgE-independent effects of T lymphocytes in gastrointestinal allergic disease that may be of particular relevance in the pathogenesis of “cell-mediated” allergic disorders of the gastrointestinal tract.
Supplementary Material
Figure S1. Flow cytometric detection of lamina propria mast cells. Lamina propria cells were isolated from CCR6+/+ and CCR6−/− mice with allergic diarrhea (OVA/OVA) or control (PBS/OVA) mice and stained with anti-FcεRI and c-kit antibodies. Mice with allergic diarrhea had an expansion of the FcεRI+c-kit+ population. This was also observed in CCR6−/− mice that did not develop allergic diarrhea symptoms.
Figure S2. Lamina Propria T cells in CCR6+/+ and CCR6−/− mice. Cells were isolated from the lamina propria of CCR6+/+ and CCR6−/− mice (4/group). Cells were stained with the pan-leukocyte marker CD45 and CD4. The proportion of CD4+ T cells was unchanged in CCR6−/− mice (top left). In addition, the total yield of cells per small intestine (right) was also unchanged. Cells were stimulated with anti-CD2 and anti-CD28 for 72 h in complete media, and cytokine release measured by ELISA (bottom panel).
Figure S3. Impact of CCR6 on lamina propria expression of Th1 and proinflammatory cytokines. CCR6+/+ and −/− mice were sensitized and fed with OVA four times (OVA/OVA), or were sensitized and left unfed as controls (OVA/PBS). Jejunum was removed, RNA isolated, and RT-PCR performed for IFN-γ, IL-17, TNFα, and IL-18. Expression was normalized to the housekeeping gene GAPDH.
Figure S4. Induction of T cell cytokine production by CCR6+/+ or CCR6−/− dendritic cells from the lamina propria. Lamina propria cells were isolated from CCR6+/+ and CCR6−/− mice, followed by positive selection for CD11c+ DCs. CD4+ T cells were isolated from DO11.10 mice, and co-cultured with DCs plus OVA peptide. Cytokine secretion from CD3/CD28 re-stimulated T cells was measured by ELISA. Data are the mean + SEM from three individual isolations.
Figure S5. Impact of FTY720 treatment on resident lamina propria CD4+ T cells. CCR6+/+ and CCR6−/− mice were fed with FTY720 on two consecutive days prior to isolation of lamina propria cells from the small intestine. Cells were stained with the pan-leukocyte marker CD45 and CD4, and cells were acquired on a flow cytometer. Percent of CD4+ T cells among the total CD45+ population was calculated.
Acknowledgments
This work was supported by NIH funds DK071576, AI044236, EPA grant R834064, and support from the Food Allergy Initiative (to MCB). ABB was supported by a fellowship from the Crohn’s and Colitis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures. The authors have no conflicts of interest to disclose.
Experimental procedures and analysis were performed by ABB, AKK, HG, and MCB, JSB and SAL provided critical reagents and mice; JSB, SAL, and LAM made significant intellectual contributions to the experimental design, and the manuscript was written by MCB.
References
- 1.Berin MC, Kiliaan AJ, Yang PC, Groot JA, Kitamura Y, Perdue MH. The influence of mast cells on pathways of transepithelial antigen transport in rat intestine. J Immunol. 1998;161:2561–6. [PubMed] [Google Scholar]
- 2.Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87:687–93. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight AK, Blazquez AB, Zhang S, Mayer LF, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 5.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, Rothenberg ME. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. J Allergy Clin Immunol. 2009;123:53–8. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin XP, Magnusson J, Ahlstedt S, Dahlman-Hoglund A, Hanson LL, Magnusson O, Bengtsson U, Telemo E. Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J Allergy Clin Immunol. 2002;109:879–87. doi: 10.1067/mai.2002.123238. [DOI] [PubMed] [Google Scholar]
- 8.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707–13. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Imai T, Baba M, Ishikawa I, Uehira M, Nomiyama H, Yoshie O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol. 1999;29:633–42. doi: 10.1002/(SICI)1521-4141(199902)29:02<633::AID-IMMU633>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–9. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 11.Puleston J, Cooper M, Murch S, Bid K, Makh S, Ashwood P, Bingham AH, Green H, Moss P, Dhillon A, Morris R, Strobel S, Gelinas R, Pounder RE, Platt A. A distinct subset of chemokines dominates the mucosal chemokine response in inflammatory bowel disease. Aliment Pharmacol Ther. 2005;21:109–20. doi: 10.1111/j.1365-2036.2004.02262.x. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Chehade M, Liu W, Xiong H, Mayer L, Berin MC. Allergen-IgE complexes trigger CD23-dependent CCL20 release from human intestinal epithelial cells. Gastroenterology. 2007;133:1905–15. doi: 10.1053/j.gastro.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderle P, Rumbo M, Sierro F, Mansourian R, Michetti P, Roberts MA, Kraehenbuhl JP. Novel markers of the human follicle-associated epithelium identified by genomic profiling and microdissection. Gastroenterology. 2005;129:321–7. doi: 10.1053/j.gastro.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 16.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–40. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugering A, Kucharzik T, Soler D, Picarella D, Hudson JT, 3rd, Williams IR. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J Immunol. 2003;171:2208–15. doi: 10.4049/jimmunol.171.5.2208. [DOI] [PubMed] [Google Scholar]
- 18.Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutierrez J, Torres M, Martinez AC, Marquez G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol. 1999;276:R715–23. doi: 10.1152/ajpregu.1999.276.3.R715. [DOI] [PubMed] [Google Scholar]
- 20.Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–47. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, Stevens RL. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160:5537–45. [PubMed] [Google Scholar]
- 22.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol. 1998;160:5037–44. [PubMed] [Google Scholar]
- 23.Yanagawa Y, Masubuchi Y, Chiba K. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats, III. Increase in frequency of CD62L-positive T cells in Peyer’s patches by FTY720-induced lymphocyte homing. Immunology. 1998;95:591–4. doi: 10.1046/j.1365-2567.1998.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henning G, Ohl L, Junt T, Reiterer P, Brinkmann V, Nakano H, Hohenberger W, Lipp M, Forster R. CC chemokine receptor 7-dependent and -independent pathways for lymphocyte homing: modulation by FTY720. J Exp Med. 2001;194:1875–81. doi: 10.1084/jem.194.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, Ogawa H, Dwinell MB, McCole DF, Eckmann L, Kagnoff MF. Chemokine receptor CCR6 transduces signals that activate p130Cas and alter cAMP-stimulated ion transport in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2005;288:C321–8. doi: 10.1152/ajpcell.00171.2004. [DOI] [PubMed] [Google Scholar]
- 27.Vongsa RA, Zimmerman NP, Dwinell MB. CCR6 regulation of the actin cytoskeleton orchestrates human beta defensin-2- and CCL20-mediated restitution of colonic epithelial cells. J Biol Chem. 2009;284:10034–45. doi: 10.1074/jbc.M805289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Identification and Biological Characterization of Mouse beta-defensin 14, the orthologue of human beta-defensin 3. J Biol Chem. 2008;283:5414–9. doi: 10.1074/jbc.M709103200. [DOI] [PubMed] [Google Scholar]
- 29.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–15. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–54. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- 31.Fujii R, Kanai T, Nemoto Y, Makita S, Oshima S, Okamoto R, Tsuchiya K, Totsuka T, Watanabe M. FTY720 suppresses CD4+ CD44 high CD62L- effector memory T cell-mediated colitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G267–74. doi: 10.1152/ajpgi.00496.2005. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima T, Ito T, Kishi D, Kai Y, Tamagawa H, Nezu R, Kiyono H, Matsuda H. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflamm Bowel Dis. 2004;10:182–92. doi: 10.1097/00054725-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Kurashima Y, Kunisawa J, Higuchi M, Gohda M, Ishikawa I, Takayama N, Shimizu M, Kiyono H. Sphingosine 1-phosphate-mediated trafficking of pathogenic Th2 and mast cells for the control of food allergy. J Immunol. 2007;179:1577–85. doi: 10.4049/jimmunol.179.3.1577. [DOI] [PubMed] [Google Scholar]
- 34.Lundy SK, Lira SA, Smit JJ, Cook DN, Berlin AA, Lukacs NW. Attenuation of allergen-induced responses in CCR6−/− mice is dependent upon altered pulmonary T lymphocyte activation. J Immunol. 2005;174:2054–60. doi: 10.4049/jimmunol.174.4.2054. [DOI] [PubMed] [Google Scholar]
- 35.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–32. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lugering A, Floer M, Westphal S, Maaser C, Spahn TW, Schmidt MA, Domschke W, Williams IR, Kucharzik T. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer’s patches. Am J Pathol. 2005;166:1647–54. doi: 10.1016/S0002-9440(10)62475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takayama N, Igarashi O, Kweon MN, Kiyono H. Regulatory role of Peyer’s patches for the inhibition of OVA-induced allergic diarrhea. Clin Immunol. 2007;123:199–208. doi: 10.1016/j.clim.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometric detection of lamina propria mast cells. Lamina propria cells were isolated from CCR6+/+ and CCR6−/− mice with allergic diarrhea (OVA/OVA) or control (PBS/OVA) mice and stained with anti-FcεRI and c-kit antibodies. Mice with allergic diarrhea had an expansion of the FcεRI+c-kit+ population. This was also observed in CCR6−/− mice that did not develop allergic diarrhea symptoms.
Figure S2. Lamina Propria T cells in CCR6+/+ and CCR6−/− mice. Cells were isolated from the lamina propria of CCR6+/+ and CCR6−/− mice (4/group). Cells were stained with the pan-leukocyte marker CD45 and CD4. The proportion of CD4+ T cells was unchanged in CCR6−/− mice (top left). In addition, the total yield of cells per small intestine (right) was also unchanged. Cells were stimulated with anti-CD2 and anti-CD28 for 72 h in complete media, and cytokine release measured by ELISA (bottom panel).
Figure S3. Impact of CCR6 on lamina propria expression of Th1 and proinflammatory cytokines. CCR6+/+ and −/− mice were sensitized and fed with OVA four times (OVA/OVA), or were sensitized and left unfed as controls (OVA/PBS). Jejunum was removed, RNA isolated, and RT-PCR performed for IFN-γ, IL-17, TNFα, and IL-18. Expression was normalized to the housekeeping gene GAPDH.
Figure S4. Induction of T cell cytokine production by CCR6+/+ or CCR6−/− dendritic cells from the lamina propria. Lamina propria cells were isolated from CCR6+/+ and CCR6−/− mice, followed by positive selection for CD11c+ DCs. CD4+ T cells were isolated from DO11.10 mice, and co-cultured with DCs plus OVA peptide. Cytokine secretion from CD3/CD28 re-stimulated T cells was measured by ELISA. Data are the mean + SEM from three individual isolations.
Figure S5. Impact of FTY720 treatment on resident lamina propria CD4+ T cells. CCR6+/+ and CCR6−/− mice were fed with FTY720 on two consecutive days prior to isolation of lamina propria cells from the small intestine. Cells were stained with the pan-leukocyte marker CD45 and CD4, and cells were acquired on a flow cytometer. Percent of CD4+ T cells among the total CD45+ population was calculated.