Abstract
Meniscectomies have been shown to lead to osteoarthritis and the success of meniscal replacements remains questionable. It has been suggested that the success of a meniscal replacement is dependent on several factors, one of which is the secure fixation and firm attachment of the replacement to the tibial plateau at the horn locations. To aid in the development of meniscal replacements, the objectives of the current study were to determine the time-dependent and failure properties of human meniscal attachments. In contrast to the time-dependent tests, during uniaxial failure testing a charge-coupled video camera was used to document the local strain and linear modulus distribution across the surface of the attachments. The lateral attachments were statistically smaller in cross-sectional area and longer than the medial attachments. The anterior attachments were statistically longer and had a smaller cross-sectional area than the posterior attachments. From the stress relaxation tests, the load and stress relaxation rates of the medial anterior attachment were statistically greater than the medial posterior attachment. There were no significant differences in the creep, structural properties or the ultimate stress between the different attachments. Ultimate strain varied between attachments as well as along the length of the attachment. Ultimate strain in the meniscus region (10.4±6.9%) and mid-substance region (12.7±16.4%) was smaller than the bony insertion region (32.2±21.5%). The lateral and anterior attachments were also found to have statistically greater strain than the medial and posterior attachments, respectively. The linear modulus was statistically weaker in the bony insertion region (69.7±33.7 MPa) compared to the meniscus region (153±123 MPa) and mid-substance region (195±121 MPa). Overall the anterior attachments (169±130 MPa) were also found to be statistically stronger than the posterior attachments (90.8±64.9 MPa). These results can be used to help design tissue engineered replacement menisci and their insertions and show the differences in material properties between attachment as well as within an attachment.
Keywords: meniscus, knee, horn attachments, enthesis, modulus, material properties
INTRODUCTION
Menisci serve several important functions in the knee joint including load bearing and transmission across the joint (Walker and Erkman, 1975; Shrive et al., 1978; Radin et al., 1984). The geometry, composition, and firm attachment of the menisci to the tibial plateau allow it to bear and distribute load across the knee joint (Fithian et al., 1990; Renstrom and Johnson, 1990; Setton et al., 1999). Menisci are frequently injured and often treated by partial or total meniscectomy if the injury occurs in the avascular zone. Meniscectomies have been shown to lead to decreased contact areas, increased stress, and degeneration of articular cartilage (Baratz et al., 1986; Chen et al., 1996; Szomor et al., 2000; Zielinska and Haut Donahue, 2006). Therefore, meniscal replacements are under investigation. Effective meniscal replacements aim to restore the native contact mechanics of the knee. Factors affecting the function of meniscal replacements include: the method of fixation to the tibial plateau (Chen et al., 1996; Alhalki et al., 1999; Setton et al., 1999; Cole et al., 2003), the size, geometry and material properties of the replacement (Pollard, 1995; Setton et al., 1999).
Meniscal attachments are thought to be ligamentous and previous studies have characterized the time dependent (Maes and Haut Donahue, 2006) and failure properties of bovine meniscal attachments (Villegas et al., 2007). Although these studies have provided more insight on meniscal attachments, one pitfall is that in the bovine knee the lateral posterior enthesis attaches to the femur, unlike a tibial insertion in the human. The current study will expand the knowledge of the mechanical response of human meniscal horn attachments in order to aid in the development of more successful meniscal replacements. The objectives of the present study were to 1) examine the stress relaxation and creep properties of human meniscal attachments, 2) determine the failure properties of human meniscal attachments, and 3) examine the local strain and linear modulus distribution across the surface of the attachments.
MATERIALS AND METHODS
Specimen preparation
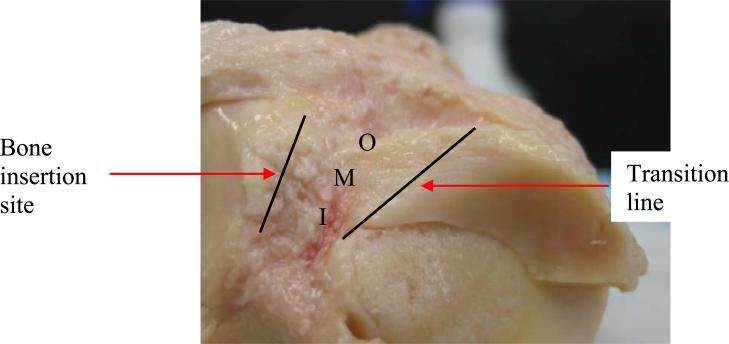
Six human knees (51-67, avg. age 59 yrs; 5 males, 1 female) were obtained from a national tissue bank and frozen (NDRI, Philadelphia, PA). The sample size used in this study was not large enough to study differences due to age or gender. Prior to testing, each knee was thawed at room temperature and dissected. A non-destructive method of accurately measuring the cross-sectional area of irregular shapes was used to measure the area (Race and Amis, 1996; Goodship and Birch, 2005). The length measurements were taken parallel to the collagen fibers at three locations; outer, middle, and inner (Figure 1) and on both the proximal and distal sides of each attachment.
Figure 1.
Superior view of the lateral anterior (LA) attachment, showing the transition from meniscus to attachment and the bone insertion site. Regions of length measurement are also labeled (outer – O, middle – M, inner- I).
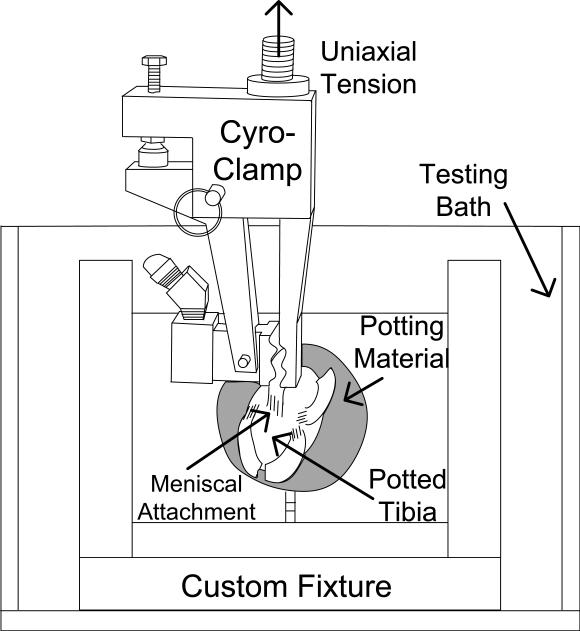
The tibia was potted in a steel tube and mounted in a custom built 5 DOF fixture previously used (Maes and Haut Donahue, 2006). Meniscal attachments were loaded parallel to the collagen fiber orientation and tibial plateau while bathed in 37°C PBS using a servo-hydraulic uniaxial materials testing machine (Model 8872, Instron Corporation, Canton, MA) (Figure 2). To ensure uniaxial tension and reduce slippage, a universal joint was used with a cryo-clamp. Care was taken to ensure that the zone of frozen tissue did not extend beyond the freeze clamp and the freeze line did not penetrate into the tissue being tested.
Figure 2.
Schematic of test setup with fixture/bath assembly, clamp assembly, and loaded specimen.
The meniscus was gripped at the transition line between the meniscal tissue and attachment while the tibia was held fixed. Each attachment was preconditioned for 10 cycles at 10mm/min, between 0% and 3% of the gauge length using a sine wave (Maes and Haut Donahue, 2006). Each attachment of a given specimen was first tested in stress relaxation, followed by a recovery period (Maes and Haut Donahue, 2006) prior to a creep test. Lastly, following the creep test and a recovery period, each attachment was pulled to failure.
The stress relaxation test ramped to a deformation of 3% of the gauge length in 0.5 seconds and then held the deformation for 45 minutes (Hingorani et al., 2004). All four attachments were tested in random order from each specimen. The specimen was then placed at 4 C and allowed to fully recover (Maes and Haut Donahue, 2006). The creep test ramped to the peak load determined from the stress relaxation test (Hingorani et al., 2004) in 0.5 seconds and was held for 45 minutes. Again, attachments were tested in random order and allowed to recover. The creep and stress relaxation rates were determined by plotting against the natural log of time and finding the slope using linear regression. One sample slipped from the grip during time-dependent testing, hence, that knee data was not included in the data analysis and results.
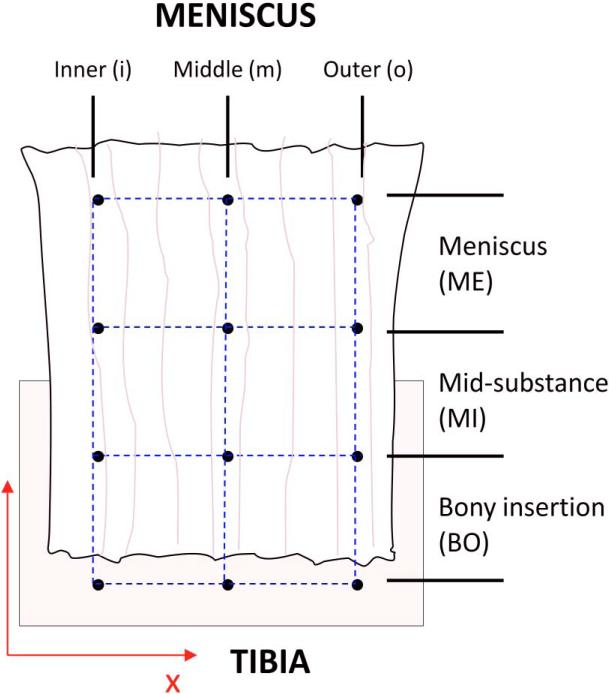
In order to increase the sample size for failure testing data, we tested an additional 2 knees with an average age of 55, 1 female and 1 male. Testing of the additional knees was completed to ensure at least 5 samples of each attachment were tested. Not all sample sizes were equal to 8 however since some samples slipped, errors with the data acquisition system occurred and some attachments failed at the grip interface. The pull to failure test ramped at a rate of 2%sec−1 (Lam et al., 1995; Quapp and Weiss, 1998). A 3×3 grid was created on the tissue surface, dividing the attachment into three horizontal regions and three longitudinal sections (Figure 3). The Meniscus (ME) region was defined as the upper region near the meniscus, the Midsubstance (MI) was defined as the middle region of the attachment, and the Bony Insertion (BO) was defined as the lower region near the insertion into the bone. Longitudinally, the attachment was divided into Outer, Middle, and Inner sections (Figure 3). A charge-couple video camera (Model MicroPix M-1024 CCD camera, Ann Arbor, MI) recorded motion of the markers. Pictures captured from the camera were analyzed using a custom-made processing program (MATLAB, version 7.4 (R2007a)) in order to calculate Green's strain (Villegas et al., 2007). Both structural and material properties were determined from the failure tests. To quantify the structural properties of the attachments the ultimate load (N), the ultimate elongation (mm), and the linear stiffness (N/mm) were determined. The linear stiffness was defined as the slope of the linear region of the load versus displacement plot and was determined using linear regression. To quantify the material properties of the attachments the ultimate stress (MPa), the ultimate strain (%), and the linear modulus (MPa) were determined. The linear modulus was defined as the slope of the linear region of the stress versus strain plot using linear regression.
Figure 3.
Regions of meniscal attachment for strain analysis
Averages and standard deviations were calculated for dimensional properties, stress relaxation and creep properties, structural properties, and ultimate stress for each of the four attachments and a oneway repeated measures analysis of variance (ANOVA) was performed to make comparisons between attachments. The descriptive statistics were divided into longitudinal sections for each region for each attachment as well as longitudinal sections for each region for each side (lateral, medial) and each location (anterior, posterior). A four factor (side, location, horizontal region, longitudinal section) ANOVA was performed to compare the ultimate strains and linear moduli between the side and location of the attachments and between the horizontal regions and longitudinal sections. When significant results were identified by ANOVA, post hoc comparisons were made using Tukey's method (p<0.05). Comparisons were also made between the combination of side and location to test differences in attachments. A significance level of 0.05 was used for all statistical analyses.
RESULTS
The proximal surface (11.9±3.97 mm) was statistically longer than the distal surface (10.3±3.93 mm). The outer side of the attachment (13.1±4.61 mm) was statistically longer than the inner (9.08±2.93 mm) and middle (11.5±3.29 mm). The middle length was also found to be statistically longer than the inner length. Although significant differences were found between the different surfaces and at different locations along the width of the meniscal attachments, the average of all measurements was taken to determine the gauge length for testing.
The MP attachment was statistically shorter than all other attachments and the LP attachment was statistically shorter than the MA and the LA attachments (Table 1). The lateral attachments were 5% longer than the medial attachments (p=0.02), and the anterior attachments were 36% longer than the posterior attachments. The lateral attachments were 6% smaller in cross-sectional area than the medial attachments, and the cross-sectional area of the anterior attachments were 20% smaller than the posterior attachments (Table 1).
Table 1.
Dimensional properties of human meniscal attachments (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior). Average ± standard deviation
| Overall Dimensional Properties | ||
|---|---|---|
| |
Length (mm) |
Cross-Sectional Area (mm2) |
| LA (n=6) | 13.02 ± 3.230*# | 23.22 ± 5.820^# |
| LP (n=6) | 9.795 ± 2.365^# | 21.73 ± 13.17# |
| MA (n=6) | 13.87 ± 3.837# | 18.73 ± 8.470# |
| MP (n=6) |
7.170 ± 2.555 |
30.70 ± 7.700 |
| MEAN |
11.23 ± 4.02 |
23.20 ± 10.10 |
| | ||
| LATERAL (n=12) | 11.53 ± 3.272~ | 22.47 ± 10.15~ |
| MEDIAL (n=12) | 10.91 ± 4.710 | 23.92 ± 10.06 |
| ANTERIOR (n=12) | 13.43 ± 3.538@ | 20.84 ± 7.650@ |
| POSTERIOR (n=12) |
8.573 ± 2.769 |
25.89 ± 11.81 |
Significantly different from LP (p<0.05)
Significantly different from MA (p<0.05)
Significantly different from MP (p<0.05)
Significantly different from medial (p<0.05)
Significantly different from posterior (p<0.05)
The only significant differences in stress relaxation properties were found between the MA and MP attachments (Table 2). No significant statistical differences were found between any of the attachments for the creep properties (p>0.05). The MA attachment showed the largest values for many creep properties, while the MP attachment showed the smallest values for these properties (Table 3).
Table 2.
Stress relaxation properties of human meniscal attachments (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior). Average ± standard deviation. Linear curve fits of the normalized data plotted against the log of time was >80%.
| Overall Stress Relaxation Properties | ||||||
|---|---|---|---|---|---|---|
| |
Load at End (N) |
Load Relaxation Rate (N/ln(s)) |
Normalized Load at End |
Normalized Relaxation Rate (1/ln(s)) |
Stress at End (MPa) |
Stress Relaxation Rate (MPa/ln(s)) |
| LA | 17.17 ± 2.59 | −1.065 ± 0.492 | 0.71 ± 0.09 | −0.042 ± 0.016 | 0.82 ± 0.26 | −0.049 ± 0.020 |
| LP | 15.98 ± 2.14 | −0.881 ± 0.351 | 0.79 ± 0.10 | −0.043 ± 0.018 | 1.18 ± 0.35 | −0.069 ± 0.036 |
| MA | 18.98 ± 3.77 | −1.365 ± 0.103^ | 0.68 ± 0.08^ | −0.050 ± 0.011 | 1.34 ± 0.77 | −0.090 ± 0.043^ |
| MP |
14.61 ± 1.19 |
−0.449 ± 0.142 |
0.85 ± 0.05 |
−0.026 ± 0.011 |
0.43 ± 0.07 |
−0.013 ± 0.004 |
| | ||||||
| MEAN | 16.60 ± 2.81 | −0.919 ± 0.452 | 0.76 ± 0.10 | −0.040 ± 0.016 | 0.91 ± 0.52 | −0.052 ± 0.039 |
Statistically different from MP (p<0.05)
n=5
Table 3.
Creep properties of human meniscal attachments (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior). Average ± standard deviation. Linear curve fits of the normalized data plotted against the log of time was >80%.
| Overall Creep Properties | ||||||
|---|---|---|---|---|---|---|
| |
Displacement at End (mm) |
Displacement Creep Rate (mm/ln(s)) |
Normalized Displacement at End |
Normalized Creep Rate (1/ln(s)) |
Strain at End (%) |
Strain Creep Rate (%/ln(s)) |
| LA | 0.24 ± 0.18 | 0.052 ± 0.028 | 1.037 ± 0.042 | 0.007 ± 0.008 | 1.91 ± 1.47 | 0.414 ± 0.240 |
| LP | 0.20 ± 0.10 | 0.046 ± 0.022 | 1.031 ± 0.026 | 0.007 ± 0.007 | 2.60 ± 1.30 | 0.606 ± 0.290 |
| MA | 0.29 ± 0.17 | 0.060 ± 0.040 | 1.071 ± 0.106 | 0.017 ± 0.016 | 2.58 ± 1.99 | 0.671 ± 0.366 |
| MP |
0.17 ± 0.08 |
0.036 ± 0.016 |
1.012 ± 0.008 |
0.002 ± 0.001 |
3.43 ± 1.58 |
0.745 ± 0.404 |
| | ||||||
| MEAN | 0.22 ± 0.13 | 0.048 ± 0.026 | 1.036 ± 0.054 | 0.008 ± 0.010 | 2.677 ± 1.542 | 0.616 ± 0.327 |
No significant differences present (p>0.05) n=5
While no significant differences between the ultimate load, ultimate elongation, linear stiffness or ultimate stress were found between the four attachments (p>0.05) (Table 4), the ultimate loads and ultimate elongations tended to be larger for the LA and MP attachments compared to the LP and MA attachments. The linear stiffness had a 40% difference among the attachments with the LA attachment approximately double the LP attachment.
Table 4.
Structural and material properties of human meniscal attachments obtained from pull to failure tests (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior). Average ± standard deviation
| Overall Structural Properties |
Material Property Data |
|||
|---|---|---|---|---|
| |
Ultimate Load (N) |
Ultimate Elongation (mm) |
Linear Stiffness (N/mm) |
Ultimate Stress (MPa) |
| All Regions |
All Regions |
All Regions |
All Regions |
|
| LA | 625.2 ± 294.5 | 3.544 ± 1.160 | 215.8 ± 78.76 | 28.68 ± 11.66 |
| LP | 330.3 ± 168.3 | 2.766 ± 1.215 | 129.5 ± 36.90 | 19.93 ± 7.918 |
| MA | 455.5 ± 181.9 | 3.205 ± 1.246 | 169.4 ± 24.19 | 27.26 ± 14.22 |
| MP |
591.6 ± 200.3 |
2.954 ± 0.425 |
207.2 ± 52.79 |
22.00 ± 11.99 |
| |
|
|||
| MEAN | 500.6 ± 232.5 | 3.117 ± 1.024 | 180.5 ± 59.45 | 24.47 ± 11.33 |
No significant differences (p>0.05) n=5
The mean ultimate strain of the lateral attachments (20.1±20.6%) was statistically greater than the medial attachments (17.7±16.5%), while the anterior attachments (20.2±20.5%) were statistically greater than the posterior attachments (17.6±16.9%). Ultimate strains were also statistically different between individual attachments with the MP (12.8±8.50%) statistically smaller than both the LA (20.6±22.2%, p=0.03) and MA (19.7±18.6%, p=0.04) attachments. The ultimate strain of the BO region (32.2±21.5%) was statistically over 50% greater than both the ME (10.4±6.9%, p=0.00) and MI regions (12.7±16.4%, p=0.00) (Table 5).
Table 5.
Ultimate strain data of human meniscal attachments obtained from pull to failure tests (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior). Average ± standard deviations. No statistical differences were found between the outer, middle, and inner sections of the meniscal attachments (p>0.05)
| Overall Failure Material Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
ULTIMATE STRAIN (%) |
||||||||
| Meniscus (ME)^ |
Midsubstance (MI)^ |
Bony Insertion (BO) |
|||||||
| OUTER |
MIDDLE |
INNER |
OUTER |
MIDDLE |
INNER |
OUTER |
MIDDLE |
INNER |
|
| LA# | 9.84 ± 3.31 | 16.50 ± 3.71 | 4.23 ± 3.61 | 12.40 ± 3.40 | 5.55 ± 0.25 | 8.43 ± 3.62 | 36.20 ± 26.6 | 47.64 ± 34.48 | 42.07 ± 23.40 |
| LP | 16.58 ± 10.75 | 9.80 ± 2.27 | 10.84 ± 8.70 | 8.77 ± 5.59 | 33.22 ± 42.30 | 9.28 ± 4.67 | 28.00 ± 17.25 | 27.58 ± 19.43 | 27.45 ± 12.16 |
| MA# | 9.56 ± 5.34 | 7.90 ± 5.90 | 10.72 ± 9.19 | 9.49 ± 4.65 | 17.04 ± 9.82 | 9.35 ± 7.86 | 26.96 ± 15.68 | 42.18 ± 25.47 | 39.19 ± 23.24 |
| MP |
7.57 ± 4.62 |
6.96 ± 3.50 |
12.02 ± 12.76 |
NA |
NA |
NA |
13.47 ± 5.95 |
17.38 ± 9.36 |
19.42 ± 12.01 |
| | |||||||||
| MEAN |
11.35 ± 7.24 |
10.71 ± 5.52 |
9.00 ± 7.98 |
10.32 ± 4.48 |
19.95 ± 28.74 |
8.99 ± 5.02 |
27.57 ± 18.88 |
35.51 ± 25.78 |
33.43 ± 19.61 |
| | |||||||||
| LATERAL1 | 13.21 ± 8.30 | 13.52 ± 4.61 | 7.53 ± 7.18 | 10.78 ± 4.60 | 20.92 ± 33.28 | 8.86 ± 3.96 | 32.10 ± 21.61 | 37.61 ± 28.42 | 34.76 ± 19.19 |
| MEDIAL | 8.71 ± 4.74 | 7.55 ± 4.86 | 11.09 ± 9.16 | 9.49 ± 4.65 | 17.04 ± 9.82 | 9.35 ± 7.86 | 21.90 ± 14.12 | 32.88 ± 23.68 | 31.78 ± 21.32 |
| ANTERIOR2 | 9.72 ± 4.02 | 12.20 ± 6.49 | 7.47 ± 7.42 | 10.94 ± 4.13 | 10.47 ± 8.36 | 8.84 ± 5.47 | 31.58 ± 21.19 | 44.91 ± 28.72 | 40.63 ± 22.04 |
| POSTERIOR | 13.20 ± 9.69 | 8.58 ± 3.00 | 11.17 ± 8.83 | 8.77 ± 5.60 | 33.22 ± 42.30 | 9.28 ± 4.67 | 22.55 ± 15.38 | 23.75 ± 16.39 | 24.44 ± 11.96 |
Significantly different from BO
Significantly different from MP
Significantly different from MEDIAL
Significantly different from POSTERIOR
n=5
The mean linear modulus of the anterior attachments (169±130 MPa) were statistically stronger than the posterior attachments (90.8±64.9 MPa, p=0.00) while no significant differences were found between the lateral and medial attachments. Linear modulus values among the attachments were statistically important, with the LA attachment (161±124 MPa) and MA attachment (179±139 MPa) statistically stronger than the LP attachment (96.3±70.7 MPa, p<0.00) (Table 6). As presented above the ultimate strains were higher in the BO region, which corresponded to a decreased linear modulus at the bone insertion site. The ME region (153±123 MPa) and MI region (195±121 MPa) were statistically over 50% stronger than the BO region (69.7±33.7 MPa, p=0.00). Again no statistic differences were found between the outer, middle, and inner sections of the meniscal attachments (p>0.05) (Table 6).
Table 6.
Linear modulus data of human meniscal attachments obtained from pull to failure tests (LA – lateral anterior, LP – lateral posterior, MA – medial anterior, MP – medial posterior)
| Overall Failure Material Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
LINEAR MODULUS (MPA) |
||||||||
| Meniscus (ME)^ |
Midsubstance (MI)^ |
Bony Insertion (BO) |
|||||||
| OUTER |
MIDDLE |
INNER |
OUTER |
MIDDLE |
INNER |
OUTER |
MIDDLE |
INNER |
|
| LA# | 101.1 ± 37.45 | 126.4 ± 63.40 | 353.8 ± 136.4 | 147.8 ± 94.04 | 283.1 ± 134.8 | 300.6 ± 20.13 | 84.91 ± 30.41 | 69.56 ± 23.70 | 74.39 ± 33.32 |
| LP | 110.4 ± 92.57 | 89.84 ± 30.69 | 89.64 ± 68.11 | 204.9 ± 86.64 | 96.18 ± 99.50 | 115.3 ± 57.36 | 57.04 ± 25.37 | 64.73 ± 26.11 | 57.75 ± 26.75 |
| MA# | 285.1 ± 200.0 | 285.5 ± 75.94 | 202.6 ± 56.71 | 222.0 ± 177.6 | 139.8 ± 55.27 | 262.4 ± 82.43 | 104.7 ± 63.65 | 63.25 ± 28.84 | 66.63 ± 13.19 |
| MP |
99.20 ± 65.62 |
77.35 ± 63.05 |
117.9 ± 83.96 |
NA |
NA |
NA |
75.30 ± 45.30 |
54.82 ± 22.17 |
69.28 ± 35.10 |
| | |||||||||
| MEAN |
149.4 ± 135.2 |
141.5 ± 99.6 |
186.0 ± 130.0 |
198.3 ± 124.0 |
176.0 ± 128.7 |
210.6 ± 102.1 |
79.38 ± 43.46 |
63.17 ± 23.99 |
66.57 ± 26.88 |
| | |||||||||
| LATERAL | 106.7 ± 72.47 | 106.1 ± 48.48 | 207.0 ± 169.3 | 183.5 ± 87.70 | 189.6 ± 148.4 | 184.8 ± 105.8 | 69.71 ± 30.06 | 66.92 ± 23.91 | 65.32 ± 29.62 |
| MEDIAL | 192.6 ± 171.1 | 181.4 ± 128.7 | 165.0 ± 79.03 | 222.0 ± 177.6 | 139.8 ± 55.27 | 262.3 ± 82.42 | 90.01 ± 54.34 | 59.04 ± 24.65 | 67.95 ± 25.03 |
| ANTERIOR1 | 203.3 ± 172.9 | 205.9 ± 106.8 | 269.8 ± 122.2 | 194.4 ± 148.4 | 221.7 ± 126.2 | 278.7 ± 62.84 | 94.81 ± 48.18 | 66.40 ± 25.10 | 70.51 ± 24.23 |
| POSTERIOR | 105.3 ± 77.73 | 84.29 ± 44.78 | 102.2 ± 72.00 | 204.9 ± 86.64 | 96.18 ± 99.50 | 115.3 ± 57.36 | 65.34 ± 35.12 | 60.23 ± 23.75 | 62.99 ± 29.78 |
Significantly different from BO
Significantly different from LP
Significantly different from POSTERIOR
LA (n=5), LP (n=6), MA (n=5), MP (n=6)
DISCUSSION
The research presented here documents for the first time, the geometry, time-dependent, and failure properties of human meniscal attachments. Relaxation of the attachments could affect the attachment response to repetitive loading, which may change the position of the meniscal replacement within the joint. The variations in material properties seen between different attachments and within an individual attachment may be related to different loading conditions in the knee or to biochemical differences. From these complex results it appears that meniscal replacements may need to possess properties that varying with location in the joint, as well as within the attachment to replicate the native attachments.
Specimens in this study underwent a free thaw cycle. While a previous study has shown no significant change in properties of soft tissues after freeze/thaw cycles (Clavert et al., 2001), failure strength and Young's modulus have been found to be statistically smaller for freeze/thaw specimens compared to fresh specimens (Sabiston et al., 1990; Clavert et al., 2001). Thus future studies should consider testing fresh material when possible. Additionally, only the surface strain was measured during the tests, and thus it is uncertain what the strain distribution is throughout the thickness of the attachment. Additionally, the strain measurements were taken from 2D images and no correction factor was used to account for any curvature of the meniscal root surface. With avulsion failures it is believed that the strains and loads seen at failure may not accurately represent the strain and strength of the actual ligament as it does the ligament bone interface (Woo et al., 1983). Considering this, care should be taken in interpreting these results as 50% of the samples failed by bone avulsion at the tibial plateau. Uniform loading of all fibers of the cross-section was difficult to confirm. However, since there were no significant difference in surface strains from the inner to middle to outer sections, it is likely that the cross-section was loaded evenly. Lastly, it is presently unknown if these property variations will be necessary for a meniscal replacement to restore normal knee kinematics and contact behavior. Future studies using finite element simulations can answer this question (Haut Donahue et al., 2003).
While the menisci are also attached via meniscofemoral ligaments (MFL) and the transverse ligament, the study of these attachments was beyond the scope of this study. Properties of the MFL have previously been documented (Gupte et al., 2002). The transverse ligament connects the anterior horns of the medial and lateral menisci. Although its material properties are still unknown, it would require a different experimental set-up to test 2 soft tissue interfaces
It has previously been shown that the anterior region of the human meniscus displaces more than the posterior region during motion (Thompson et al., 1991; Vedi et al., 1999; Rankin et al., 2006). The geometric results show that the anterior attachments are longer and smaller in cross-sectional area in comparison to the shorter and larger posterior attachments. Perhaps this loading environment has resulted in meniscal attachments that have adapted to fit in the joint space. The lateral meniscus has also been shown to translate more than the medial meniscus (Thompson et al., 1991; Vedi et al., 1999; Rankin et al., 2006) and in the same fashion the lateral attachments were found to be longer and smaller in cross-sectional area than the medial attachments. Although significant differences were found in the geometry of the meniscal attachments, no significant structural differences in ultimate elongation or stiffness were found.
The results of the stress relaxation tests demonstrated that the meniscal attachments are time dependent, with significant differences between the MA and MP attachments. In the stress relaxation study of bovine attachments (Maes and Haut Donahue, 2006), it is interesting to note that the normalized load and the stress at the end of the test are approximately half of that in the current human study and the normalized creep rate was 20-200 times larger for the bovine.
Due to the ligamentous nature of the attachments, their properties can be compared to other ligaments. From a number of studies linear stiffness values have ranged from 30-120 N/mm, ultimate loads from 290-1100 N, and ultimate stress from 13-70 MPa for various knee ligaments (Johnson et al., 1994; Harner et al., 1995; Staubli et al., 1999; Gupte et al., 2002; LaPrade et al., 2005). In the current study the average values were found to be 172 N/mm, 479 N, and 25.2 MPa for the linear stiffness, ultimate load, and ultimate stress, respectively, in general agreement with other ligamentous structures of the knee. These data should be interpreted with care as the variability is quite high and whether the variability is a result of a large natural variation or inherent to the methods used is not known. However, a similar set-up has been used to test other soft tissues with success (Haut and Powlison, 1990; Haut and Haut, 1997; Donahue et al., 2001). While no significant differences were found between the ultimate load of the medial and lateral posterior attachments, the medial posterior attachments had a substantially larger ultimate load compared to the lateral posterior attachments. Clinically it is often shown that the medial attachment is torn more often (Smith et al., 2002), and hence, a possible explanation would be that in vivo the meniscofemoral ligaments (MFL) contribute to the strength of the lateral posterior attachment. Ultimate strain, load and elongation were not reported if the attachment failed at the grip interface. Using sensitivity data from Haut Donahue et al., 2003 and the standard deviation from this study, the power of failure data was only 70% (Haut Donahue et al., 2003). Hence, the lack of statistically significant differences in the failure data should be interpreted with consideration of the power of the test.
The ultimate strain distribution across the surface of the attachment was found to be inhomogeneous. Similar to other studies of knee ligaments (Woo et al., 1983; Lam et al., 1995; Gardiner et al., 2001), the greatest strains in the current study were found nearest to the insertion site compared to that of the midsubstance region. Collagen fibril crimp frequency was found to be largest in the BO zone compared to the ME and MI zones of bovine meniscal attachments (Villegas et al., 2008). Higher crimp frequency allows for more stretching of the fibers, corresponding to greater strains in this region. Large strains at the insertion site correlates with reduced linear modulus in this region compared to the midsubstance, another phenomenon that was captured in the current study. Several studies have shown avulsion as a common mode of failure in knee structures (Woo et al., 1983; Lam et al., 1995; Gao et al., 1996) which corresponds to larger strains being found near the insertion sites of the ligaments to bone. The present study reaffirms this finding with 50% of the attachments failing by tibial avulsion.
The clinical implications of these results indicate that the design of meniscal replacements will be a challenging process in which independent detail will need to be given to each of the four attachments. If the same properties were applied to all four attachments for example the posterior attachments low mobility may be compromised changing joint mechanics. This knowledge can also help in the design of meniscal replacements used with knowledge of the different loading and motions of the meniscus in these different locations. Most importantly it was found that the strain and therefore linear modulus distribution along the length of the attachment surface are different. The attachments of meniscal replacements will likely need to replicate this variation in material properties along the length to replicate the gradient of strength from the meniscus body down into the underlying bone at the insertion site and prevent failure. Posterior root tears are becoming increasing evident (Brody et al., 2006; Jones et al., 2006; Choi et al., 2008; Lee et al., 2008; Ahn et al., 2009; De Smet et al., 2009), and hence, this data will not only have relevance in the design of meniscal replacement, but for the repair of root tears.
ACKNOWLEDGEMENTS
This study was supported in part by the National Institutes of Health (AR051906-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors affirm that they have no financial affiliation or involvement with any commercial organization that has direct financial interest in any matter included in this manuscript.
REFERENCES
- Ahn JH, Lee YS, et al. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16(1):77–80. doi: 10.1016/j.knee.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Alhalki MM, Howell SM, et al. How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. American Journal of Sports Medicine. 1999;27(3):320–328. doi: 10.1177/03635465990270030901. [DOI] [PubMed] [Google Scholar]
- Baratz ME, Fu FH, et al. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. American Journal of Sports Medicine. 1986;14(4):270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- Brody JM, Lin HM, et al. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805–10. doi: 10.1148/radiol.2393050559. [DOI] [PubMed] [Google Scholar]
- Chen MI, Branch TP, et al. Is it important to secure the horns during lateral meniscal transplantation? A cadaveric study. Arthroscopy. 1996;12(2):174–181. doi: 10.1016/s0749-8063(96)90007-9. [DOI] [PubMed] [Google Scholar]
- Choi NH, Son KM, et al. Arthroscopic all-inside repair for a tear of posterior root of the medial meniscus: a technical note. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):891–3. doi: 10.1007/s00167-008-0581-3. [DOI] [PubMed] [Google Scholar]
- Clavert P, Kempf JF, et al. Effects of freezing/thawing on the biomechanical properties of human tendons. Surg Radiol Anat. 2001;23(4):259–62. doi: 10.1007/s00276-001-0259-8. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Carter TR, et al. Allograft meniscal transplantation: background, techniques, and results. Instr Course Lect. 2003;52:383–96. [PubMed] [Google Scholar]
- De Smet AA, Blankenbaker DG, et al. MR diagnosis of posterior root tears of the lateral meniscus using arthroscopy as the reference standard. AJR Am J Roentgenol. 2009;192(2):480–6. doi: 10.2214/AJR.08.1300. [DOI] [PubMed] [Google Scholar]
- Donahue TL, Gregersen C, et al. Comparison of viscoelastic, structural, and material properties of double-looped anterior cruciate ligament grafts made from bovine digital extensor and human hamstring tendons. J Biomech Eng. 2001;123(2):162–9. doi: 10.1115/1.1351889. [DOI] [PubMed] [Google Scholar]
- Fithian DC, Kelly MA, et al. Material properties and structure-function relationships in the menisci. Clinical Orthopedics and Related Research. 1990;252:19–31. [PubMed] [Google Scholar]
- Gao J, Rasanen T, et al. The morphology of ligament insertions after failure at low strain velocity: an evaluation of ligament entheses in the rabbit knee. J Anat. 1996;189(Pt 1):127–33. [PMC free article] [PubMed] [Google Scholar]
- Gardiner JC, Weiss JA, et al. Strain in the human medial collateral ligament during valgus loading of the knee. Clinical Orthopaedics & Related Research. 2001;391:266–274. doi: 10.1097/00003086-200110000-00031. [DOI] [PubMed] [Google Scholar]
- Goodship AE, Birch HL. Cross sectional area measurement of tendon and ligament in vitro: a simple, rapid, non-destructive technique. J Biomech. 2005;38(3):605–8. doi: 10.1016/j.jbiomech.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Gupte CM, Smith A, et al. Meniscofemoral ligaments-structural and material properties. J Biomech. 2002;35(12):1623–9. doi: 10.1016/s0021-9290(02)00238-5. [DOI] [PubMed] [Google Scholar]
- Harner CD, Xerogeanes JW, et al. The human posterior cruciate ligament complex: an interdisciplinary study. Ligament morphology and biomechanical evaluation. Am J Sports Med. 1995;23(6):736–45. doi: 10.1177/036354659502300617. [DOI] [PubMed] [Google Scholar]
- Haut Donahue TL, Hull ML, et al. How the stiffness of meniscal attachments and meniscal material properties affect tibio-femoral contact pressure computed using a validated finite element model of the human knee joint. Journal of Biomechanics. 2003;36(1):19–34. doi: 10.1016/s0021-9290(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Haut RC, Powlison AC. The effects of test environment and cyclic stretching on the failure properties of human patellar tendons. J Orthop Res. 1990;8(4):532–40. doi: 10.1002/jor.1100080409. [DOI] [PubMed] [Google Scholar]
- Haut TL, Haut RC. The state of tissue hydration determines the strain-rate sensitive stiffness of human patellar tendon. Journal of Biomechanics. 1997;30(1):79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- Hingorani RV, Provenzano PP, et al. Nonlinear viscoelasticity in rabbit medial collateral ligament. Ann Biomed Eng. 2004;32(2):306–12. doi: 10.1023/b:abme.0000012751.31686.70. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Tramaglini DM, et al. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- Jones AO, Houang MT, et al. Medial meniscus posterior root attachment injury and degeneration: MRI findings. Australas Radiol. 2006;50(4):306–13. doi: 10.1111/j.1440-1673.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Lam TC, Shrive NG, et al. Variations in rupture site and surface strains at failure in the maturing rabbit medial collateral ligament. Journal of Biomechanical Engineering. 1995;117(4):455–61. doi: 10.1115/1.2794207. [DOI] [PubMed] [Google Scholar]
- LaPrade RF, Bollom TS, et al. Mechanical properties of the posterolateral structures of the knee. Am J Sports Med. 2005;33(9):1386–91. doi: 10.1177/0363546504274143. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jee WH, et al. Radial tear of the medial meniscal root: reliability and accuracy of MRI for diagnosis. AJR Am J Roentgenol. 2008;191(1):81–5. doi: 10.2214/AJR.07.2945. [DOI] [PubMed] [Google Scholar]
- Maes JA, Haut Donahue TL. Time dependent properties of bovine meniscal attachments: stress relaxation and creep. J Biomech. 2006;39(16):3055–61. doi: 10.1016/j.jbiomech.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684–687. doi: 10.1016/0749-8063(95)90110-8. [DOI] [PubMed] [Google Scholar]
- Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998;120(6):757–63. doi: 10.1115/1.2834890. [DOI] [PubMed] [Google Scholar]
- Race A, Amis AA. Cross-sectional area measurement of soft tissue. A new casting method. J Biomech. 1996;29(9):1207–12. doi: 10.1016/0021-9290(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Radin EL, de Lamotte F, et al. Role of the menisci in the distribution of stress in the knee. Clinical Orthopaedics and Related Research. 1984;(185):290–4. [PubMed] [Google Scholar]
- Rankin M, Noyes FR, et al. Human meniscus allografts’ in vivo size and motion characteristics: magnetic resonance imaging assessment under weightbearing conditions. Am J Sports Med. 2006;34(1):98–107. doi: 10.1177/0363546505278706. [DOI] [PubMed] [Google Scholar]
- Renstrom P, Johnson RJ. Anatomy and biomechanics of the menisci. Clinincs in Sports Medicine. 1990;9(3):523–538. [PubMed] [Google Scholar]
- Sabiston P, Frank C, et al. Transplantation of the rabbit medial collateral ligament. II. Biomechanical evaluation of frozen/thawed allografts. J Orthop Res. 1990;8(1):46–56. doi: 10.1002/jor.1100080106. [DOI] [PubMed] [Google Scholar]
- Setton LA, Guilak F, et al. Biomechanical factors in tissue engineered meniscal repair. Clin Orthop. 1999;(367 Suppl):S254–72. doi: 10.1097/00003086-199910001-00025. [DOI] [PubMed] [Google Scholar]
- Shrive NG, JJ OC, et al. Load-bearing in the knee joint. Clinical Orthopedics. 1978;131:279–87. [PubMed] [Google Scholar]
- Smith GN, Mickler EA, et al. Severity of medial meniscus damage in the canine knee after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2002;10(4):321–6. doi: 10.1053/joca.2002.0520. [DOI] [PubMed] [Google Scholar]
- Staubli HU, Schatzmann L, et al. Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med. 1999;27(1):27–34. doi: 10.1177/03635465990270011301. [DOI] [PubMed] [Google Scholar]
- Szomor ZL, Martin TE, et al. The protective effects of meniscal transplantation on cartilage. An experimental study in sheep. J Bone Joint Surg Am. 2000;82(1):80–8. doi: 10.2106/00004623-200001000-00010. [DOI] [PubMed] [Google Scholar]
- Thompson WO, Thaete FL, et al. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19(3):210–5. doi: 10.1177/036354659101900302. discussion 215-6. [DOI] [PubMed] [Google Scholar]
- Vedi V, Williams A, et al. Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg Br. 1999;81(1):37–41. doi: 10.1302/0301-620x.81b1.8928. [DOI] [PubMed] [Google Scholar]
- Villegas DF, Hansen TA, et al. A quantitative study of the microstructure and biochemistry of the medial meniscal horn attachments. Ann Biomed Eng. 2008;36(1):123–31. doi: 10.1007/s10439-007-9403-x. [DOI] [PubMed] [Google Scholar]
- Villegas DF, Maes JA, et al. Failure properties and strain distribution analysis of meniscal attachments. J Biomech. 2007;40(12):2655–62. doi: 10.1016/j.jbiomech.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clinical Orthopedics. 1975;109:184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- Woo SL, Gomez MA, et al. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1(1):22–9. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- Zielinska B, Haut Donahue TL. 3D Finite Element Model of Medial Meniscus Meniscectomy;Changes in Contact Behavior. Journal of Biomechanical Engineering. 2006;128(1):115–123. doi: 10.1115/1.2132370. [DOI] [PubMed] [Google Scholar]