Abstract
Individuals exposed to psychological stressors may experience a long-term resetting of behavioral and neuroendocrine aspects of their “stress response” so that they either hyper or hypo-respond to subsequent stressors. These effects of psychological or traumatic stressors may be mimicked in rats using the resident-intruder model of social defeat.
The social defeat model has been characterized to model aspects of the physiology and behavior associated with anxiety and depression. The objective of this study was to determine if behaviors elicited following repeated social defeat can also reflect aspects of ethologically relevant stresses associated with existing post traumatic stress disorder (PTSD) models. Socially defeated rats displayed weight loss and an enhanced and prolonged response to acoustic startle which was displayed for up to 10 days following repeated social defeat. These data indicate that the severe stress of social defeat can produce physiologic and behavioral outcomes which may reflect aspects of traumatic psychosocial stress.
Keywords: Social defeat, Emotional Stress, Acoustic Startle
1. Introduction
Severe psychosocial stressors can precipitate the development of mood disorders such as post traumatic stress disorder (PTSD). PTSD is a mood disorder that can occur following repeated or extreme physical or psychosocial stress (Miller & McEwen, 2006; Yehuda, McFarlane, & Shalev, 1998); and most likely interacts with an existing genetic liability (Wakizono et al., 2007; Yehuda & Antelman, 1993). A major feature of this condition is intrusive memories of the traumatic event that are often triggered by sensory input (van der Kolk, 2006). The individual genetic or epigenetic and environmental characteristics of susceptibility to this disorder are not yet clear.
Animal models of traumatic stress are being characterized for behavioral responses which may resemble aspects of PTSD (Lister, 1990; Miller et al., 2006; Yehuda, Flory, Southwick, & Charney, 2006). The complexity of mood disorders like PTSD, however, makes the development of an appropriate animal model for PTSD challenging. In addition, behavioral tests in animal models of anxiety-like behavior cannot directly measure psychological symptoms such as flashbacks and nightmares (Miller et al., 2006). Instead, behavioral testing in animal models of traumatic stress can measure physiological and behavioral endophenotypes associated with PTSD (Cohen et al., 2004; Khan & Liberzon, 2004). Currently, rodent models of PTSD encompass the use of acute or repeated stressors such as immobilization, forced swim (Khan et al., 2004), underwater trauma, predator exposure (Cohen et al., 2004), or inescapable foot shock (Garrick, Morrow, Shalev, & Eth, 2001).
Social defeat is an ethologically relevant stressor which utilizes the natural establishment of social rank in male rodents. During social defeat an aggressive resident male rat fights off an intruding male which has entered his territory. As a consequence of social defeat, the intruder male displays subordinate posturing to prevent further attack from the resident male rat. Following this interaction, subordination is reinforced as the intruder male receives visual, olfactory and auditory stimuli from the resident male while being separated by a partition (Martinez, Calvo-Torrent, & Herbert, 2002).
Following social defeat, intruder male rats have been well documented for exhibiting anxiety and depressive-like behaviors (Blanchard et al., 1995; Blanchard, McKittrick, & Blanchard, 2001; Koolhaas, De Boer, De Rutter, Meerlo, & Sgoifo, 1997a; Miczek, 1991). Physiologic effects associated with increased anxiety-like behavior following social defeat have been shown to persist for up to 14 days following social defeat (Koolhaas, Meerlo, De Boer, Strubbe, & Bohus, 1997b). Among the symptoms observed in the subordinate male (intruder) are weight loss, increased heart rate, sleep disturbances, increased body temperature (Koolhaas et al., 1997b) and hypothalamo-pituitary adrenal axis disturbances (Bhatnagar & Vining, 2003). Intruder rats may also display anxiety-like behavior when exposed to novel stressors (Frank et al., 2006; Koolhaas et al., 1999; Ruis et al., 1999; Von Frijtag et al., 2000). Therefore, like the ethologically relevant stresses of predator odor and under water trauma, social defeat, may also be an appropriate stressor to investigate for the elicitation of physiological responses and behaviors which can be encompassed by PTSD-associated responses to trauma.
Patients with PTSD can exhibit an enhanced startle response (Grillon & Baas, 2003). In rats, an observed difference in the acoustic startle response is a behavioral measure of anxiety-like behavior (Walker & Davis, 2002). Following exposure to severe stressors, rats do not readily habituate to acoustic startle (Garrick et al., 2001). Although socially defeated rats are known to exhibit an enhanced response to acoustic startle following morphine withdrawal (Miczek, 1991), they have not been observed to exhibit a prolonged enhancement in the startle response without measuring their response to drug withdrawal. In this study, our goal was to determine if rats exposed to repeated social defeat, and absent of drug withdrawal, exhibit an enhanced and prolonged startle response. These data may contain components which can be used to enhance existing PTSD models.
2. Materials and Methods
2.1 Animals
Experimental subjects were male Long Evans rats obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Rats in all experiments were maintained on a 12:12 hr light: dark cycle (lights on at 0700hr) with food and water freely available. A normal schedule of care and cage cleaning was performed by the animal facility staff.
Male resident Long Evans rats weighing 400-500g were paired for one week with 3 month old virgin females weighing (300g) in (54cm × 25cm × 22cm) polycarbonate cages to establish territory of the resident. Male intruders weighing 325-350g were housed individually for one week before each experiment in (32cm × 22cm × 20cm) polycarbonate cages under similar conditions. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Emory University.
2.2 The Social Defeat Model
The social defeat model used in this study was adapted from experiments by others (Nikulina, Covington, Ganschow, Hammer, & Miczek, 2004). One week after pairing, male resident rats with female rats, the male residents were trained to attack novel intruder male opponents twice a day for 4 days. Once the female was removed, a novel intruder male was placed in the cage of the resident male. Training consisted of measuring the attack latency of the resident male to novel intruder male opponents. Attack latency of male residents was scored and only those resident males with an attack latency of <60s were used in the study (Buwalda et al., 2001). Females were re-introduced into the cage following the training period.
During the social defeat procedure, the female was removed from the resident's cage and a naïve intruder male was placed into the resident's cage behind a wire mesh partition for 5min. Following this 5min period, the partition was removed and the resident-intruder interaction occurred for a maximum of 5min or until the intruder displayed 5s of a continuous submissive supine posture. Following the resident-intruder interaction, the resident and intruder were again separated by a partition for 60min (Covington & Miczek, 2001). During this period the intruder experienced psychosocial stress via exposure to visual, olfactory and auditory cues from the resident. The intruder was returned to its home cage after 60min. This procedure was repeated every third day (i.e., days 1, 4, 7 and 10) for a total of 4 social defeat episodes (Covington et al., 2001; Miczek & Mutschler, 1996).
A cage transfer control group (CTC) was used. The CTC group consisted of naïve male rats which were placed into a new empty cage with the same dimensions as its home cage. The CTC group and the social defeat (SoD) group were placed in a cage for the same duration of time, but the SoD group animals were placed in the cage of a male resident. The CTC group was used as a control for handling, cage transfer and exposure to a novel cage. All animals were also weighed before the SoD occurred (day1), and again on days 10 and 22. For this experiment, (n=10) for the CTC and SoD treatment groups.
2.3 The Response to Acoustic Startle Following SoD
Next, we assessed the acoustic startle response in another set of socially defeated animals to determine if this response was still enhanced 10 days following the last SoD. Methods for measuring acoustic startle were adapted from experiments performed by others (Chabot & Taylor, 1992; Walker et al., 2002). On day 7 following SoD, all animals received a 5min acclimation period to the startle chamber in the presence of 65dB background noise. The next day, each animal was placed in a startle chamber with lights off. Startle responses were measured using the SR Lab startle reflex system (San Diego Instruments, San Diego, CA). In individual chambers, animals were exposed to 5min of 65dB background noise followed by a series of 30 startle-eliciting noise bursts (10 each at 95, 110, and 125dB; 50ms pulse duration). The three noise bursts were presented at an interstimulus interval of 30s in a pseudorandom order; startle noise bursts were presented with the constraint that each noise burst, was presented once within a block of the three different noise bursts. The following day, the startle response was measured in the same manner, but with the chamber lights on. The standard lighting of the chamber was 420 lux and was at a constant level when the lights were turned on. One week later, the startle response measurement was repeated at the same light intensity. For the SoD and CTC treatment groups, (n=11) animals.
2.4 Statistics
Body weight in both the CTC and SoD groups was analyzed. A two-way repeated measures ANOVA (body weight × day) was conducted with Bonferroni post hoc comparisons when appropriate using graphpad Prism 3.0 statistical software.
In an analysis of the overall acoustic startle amplitude displayed between treatment groups, the mean of the startle amplitudes at 95, 110 and 125dB was compared across all experimental days of acoustic startle. A paired t-test comparing the CTC and SoD groups was then performed using Prism 3.0 statistical software.
Next, the overall effect of experimental day between the CTC and SoD groups was analyzed. To determine the overall effect of the experimental day, the mean of the startle amplitudes at 95, 110 and 125dB was compared between experimental days, using paired t-tests with graphpad Prism 3.0 statistical software.
An analysis of the effect of treatment, noise level and treatment day was conducted using a three-way repeated measures ANOVA (treatment × day × noise level) using SPSS 15.0 statistical software. A test for sphericity was conducted. Where sphericity was not met, a Greenhouse–Geisser correction test was performed. To determine the effects of the noise levels during the days of startle testing, paired t-tests were conducted using graphpad Prism 3.0 statistical software.
Furthermore, two-way repeated measures ANOVA (treatment × noise burst trial) with Bonferroni post tests were conducted to determine the response of the CTC and SoD groups to acoustic startle across all 10 noise burst trials at the 95dB, 110dB and 125dB noise levels using graphpad Prism 3.0 statistical software. A Grubbs outlier test was performed before all statistical tests were conducted, website: (http://www.graphpad.com/quickcalcs/Grubbs1.cfm). An alpha level of 0.05 was selected for all statistical tests.
3. Results
3.1 Reduction in Body Weight Gain
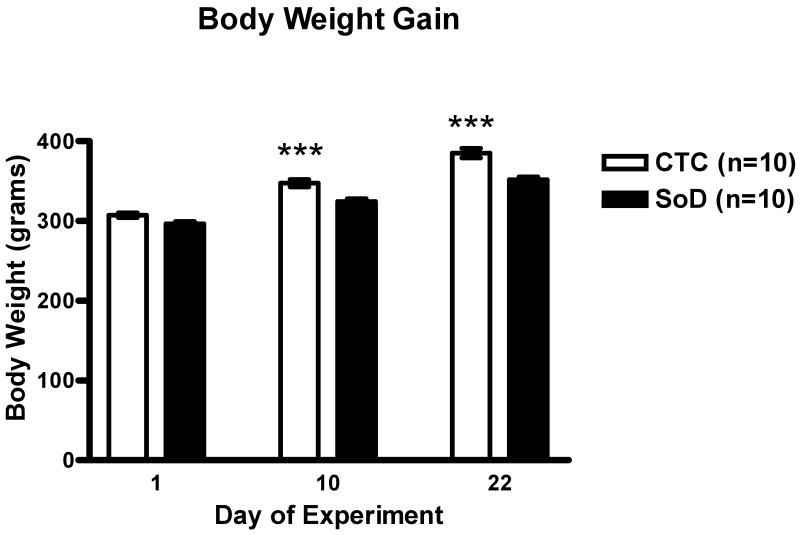
Following social defeat, the SoD group exhibited a reduction in weight gain as compared to the CTC group. There was an observed effect of treatment (F[1,27]= 49.90, p<0.0001) and day on body weight (F [2,27]=117.1, p<0.0001) and a treatment × day on body weight interaction was observed (F[2,27]=4.339, p<0.05). Post hoc analysis revealed a significant increase in body weight by the CTC group compared to the SoD group on day 10 (***p<0.0001) and day 22 (***p<0.0001) (Figure 1).
Figure 1. Reduction in Body Weight Gain Following Social Defeat.
The SoD group exhibited a reduction in body weight gain compared to the CTC group. Post hoc analysis revealed significant differences on day 10 (***p<0.001) and day 22 (***p<0.001). All data are represented as mean ± SEM.
3.2 A Larger Acoustic Startle Following Social Defeat
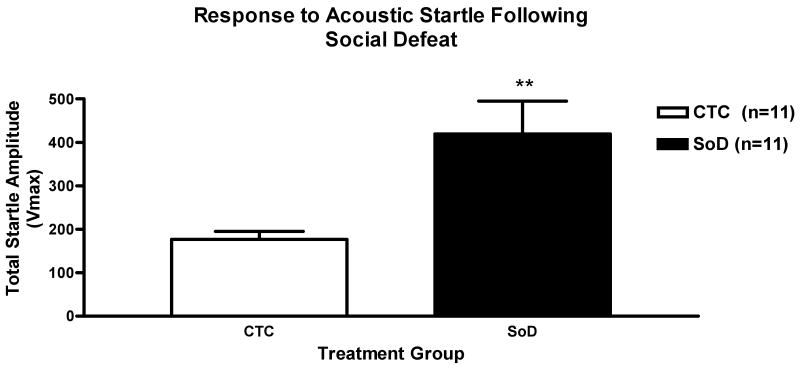
The effect of treatment was also measured. There was a significant increase in the mean startle amplitude for the SoD group as compared to the CTC group (**p<0.01) (Figure 2).
Figure 2. An increased Response to Acoustic Startle Following SoD.
Following acoustic startle, the total of the startle amplitudes measured from the SoD group was significantly larger than the CTC group (**p<0.01). A measurement of the average noise level amplitudes for each treatment group was obtained at 95dB, 110dB and 125dB, during experimental days 12, 13 and 21. All data are represented as the mean startle amplitude of the noise burst levels (95, 110, 125dB) from all days of the acoustic startle ± SEM.
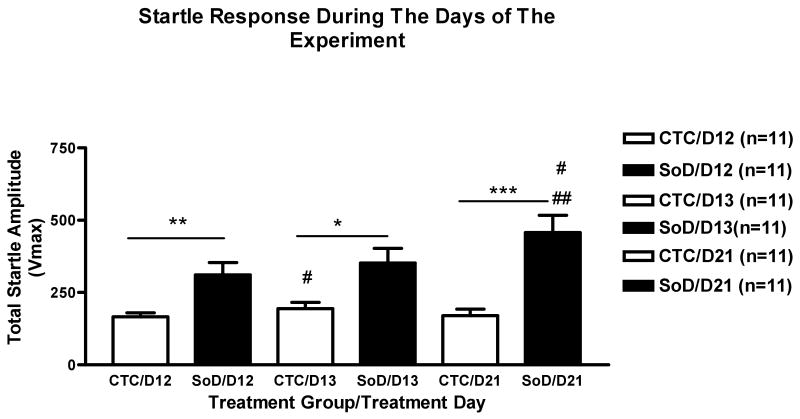
Next the effect of experimental day of acoustic startle testing was analyzed. The SoD group exhibited a significant increase in the mean startle amplitude as compared to the CTC group, during experimental day 12 (**p<0.01) day13 (*p<0.05) and day 21 (***p<0.001). The CTC group showed an enhanced acoustic startle response between days 12 and 13 (#p<0.05). The SoD group showed an enhanced startle response between days 12 and 21 (##p<0.01) and days 13 and 21 (#p<0.05) (Figure 3).
Figure 3. Startle Response During Days 12, 13 and 21.
The SoD group exhibited a larger response to acoustic startle during all experimental days. For CTC vs SoD, (*p<0.05), (**p<0.01) and (***p<0.001). The CTC group exhibited an increase in startle between days 12 and 13 (#p<0.05). The SoD group exhibited a larger response to acoustic startle between days 12, and 21 (##p<0.01) and days 13 and 21 (#p<0.05). All data are represented as the mean Vmax of all noise burst levels (95dB, 110dB, 125dB) ± SEM.
From an analysis of the startle response between the CTC and SoD groups, Greenhouse-Giesser corrections revealed significant effects of noise (F[1.709,40]=4.465, p=0.018) and day (F[1.240,40]=98.48, p<0.01). In addition, a day × treatment interaction (F[1.240,40] = 11.801, p<0.01), and a noise level × day interaction (F[2.724,80] = 2.937, p<0.05) were observed. Sphericity was assumed for a noise × treatment interaction (F[2,40] = 0.526) and a noise × treatment × day (F[4,80] = 0.165) interaction.
To determine the effect of noise level between the CTC and SoD treatment groups across all of the experimental days, the mean startle responses between the CTC and SoD groups for each experimental day, were analyzed. At the 95 dB noise level there was an increase in the startle response of the SoD group, compared to the CTC group during day 21 (*p<0.05).
At the110dB noise level there was an increase in the startle response of the SoD group as compared to the CTC group at days 12, and 13 (**p<0.01) and day 21 (*p<0.05). The SoD group also exhibited an enhanced startle response at the 125dB noise level during days 12, 13 and 21 (**p<0.01) as compared to the CTC group.
The acoustic startle response within the CTC and SoD treatment groups between days was also analyzed. Between days 12 and 13, The CTC group exhibited a significant decrease at the 95dB noise level (###p<0.001). No significant difference was observed at the 110 and 125dB noise levels. Between days 12 and 21, there was a significant decrease in the startle response at the 95dB noise level (#p<0.05). No significant difference in startle amplitude was observed at the 110 and 125dB noise levels. Finally, between days 13 and 21, there was a significant decrease in the startle response at the 95dB (##p<0.01), 110dB (#p<0.05) and 125dB (#p<0.05) noise levels.
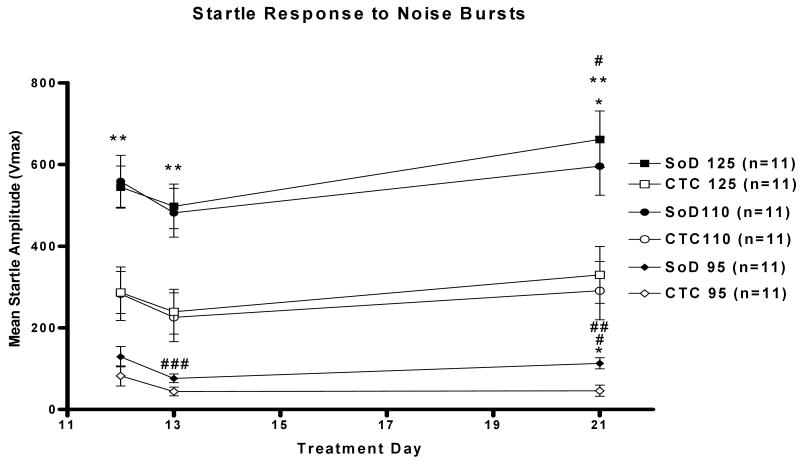
An analysis of the SoD group between days of startle testing only revealed a significant increase in the startle response between days 13 and 21 (#p<0.05) at the 95dB noise level. No significant difference was observe at the 110 or 125dB noise level between days 12 and 21 or days 13 and 21 (Figure 4).
Figure 4. Increased Response of The SoD Group to Startle Noise Bursts.
The SoD group exhibited a larger response to acoustic startle as compared to the CTC group at the 95db, 110dB and 125 dB noise levels during days 12, 13 and 21. At 95dB noise level, the SoD group exhibited a larger acoustic startle response as compared to the CTC group on day 21 (*p<0.05). Similarly at the 110dB noise level, the SoD group exhibited a larger startle response than the CTC group (**p<0.01) on days 12 and 13 and (*p<0.05) day 21. Also at the 125dB noise level, the SoD group had a larger startle response as compared to the CTC group (**p<0.01) on days 12, 13 and 21. Within groups analysis showed that the CTC and SoD groups also exhibited significant differences between days (#p<0.05), (##p<0.01), and (###p<0.001). All data are represented as mean startle amplitude (Vmax) ± SEM.
Comparison of startle across all 10 trials at 95dB, 110dB and 125dB was conducted to determine if there was a difference in the overall magnitude of the acoustic startle response between the CTC and SoD groups. At 95dB noise level there was a main effect of treatment (F[1,20]=179.1,***p<0.001). No main effect of trial and no interaction were observed. At the 110dB noise level there were main effects of treatment (F[1,20]=227.6,***p<0.001) and trial (F[9,20]=2.857, *p<0.05). No interaction was observed. At the 125dB noise level there was a main effect of treatment (F[1,20]=236.7,***p<0.001). There was no effect of trial and no interaction was observed (data not shown).
4. Discussion
The most salient finding from this study is that, social defeat stress can produce an enhanced and prolonged acoustic startle response in the absence of the specific trauma. These data may be reflective of the lack of acoustic startle habituation observed in some PTSD patients following psychosocial stress. As such, social defeat stress may be useful to include in animal models of PTSD.
Our first experiment was designed to determine if our social defeat stress paradigm could produce a reduction in weight gain. Following social defeat, we observed a reduction in body weight gain in the SoD group as compared to the CTC group, by observed main effects of treatment and the day body weight was measured. This reduced rate of weight gain in the SoD group is consistent with previous observations of the reduction in weight gain observed following social defeat stress (Bhatnagar, Vining, Iyer, & Kinni, 2006; Blanchard et al., 1995).
To further measure the effect of social stress by the SoD group, we tested the SoD and CTC groups in the acoustic startle test. The acoustic startle test is a test in which enhancement of the response to startle above a baseline level can be used as a measure of anxiety-like behavior in rats (Garrick et al., 2001; Walker et al., 2002). Sensitization of the startle response in rats has been observed following fear conditioning, predator stress, inescapable shock, and the exposure to varying acute stressors (Adamec, Bartoszyk, & Burton, 2004; Garrick et al., 2001; Sawamura et al., 2004). Although socially defeated rats exhibit an enhanced acoustic startle response following withdrawal (Miczek, 1991), it was not known if an enhanced startle response could be maintained over a prolonged period in socially defeated rats as compared to non-stressed cage-transfer control animals. Our data reveal that socially defeated animals exhibited an increase in the startle response as compared to the CTC group and that the increase in startle is observed during all days of startle testing.
Furthermore, data analysis of the effects of treatment, noise level and day of testing indicate that socially defeated rats respond differently than the CTC group by day and noise level. Specifically, an increase in acoustic startle was observed in the SoD group as compared to the CTC group at 95dB during day 21. At the 110dB and 125dB noise burst levels, the SoD group also showed an increase in startle as compared to the CTC group, during all days of testing.
Where as the CTC group habituated to acoustic startle, the SoD group did not habituate. Within group analysis between days of startle testing revealed that the CTC group exhibited a reduction in the startle response at the 95dB noise levels between the days 12 and 13, days 12 and 21 and days 13 and 21. Also a reduction was observed at the 110 and 125dB noise levels between days 13 and 21. Contrary to the CTC group, the SoD group exhibited an increase in acoustic startle at the 95dB noise level between days 13 and 21.
Although bright light has been reported to facilitate the acoustic startle response (Walker et al., 2002), an analysis of the effect of lighting condition did not reveal an affect of lighting condition in the CTC or SoD groups. Absence of an effect of lighting condition in the SoD group could represent a ceiling effect in the startle response. Alternatively, the increase in startle observed between days 13 and 21 at 95dB (startle with lights on) could be a result of re-exposure to a traumatic context of the startle box.
As previously mentioned, the CTC group exhibited significantly decreased startle responses between startle testing in the dark vs. light. This may indicate that the lighting conditions were not bright enough to elicit an increase in startle by the CTC group. Alternatively, handling stress has been reported to be necessary for facilitation of light-enhanced startle (Walker et al., 2002). In our study neither, the CTC or SoD groups were handled so that we could examine the effects of social defeat stress. Future studies using our social defeat paradigm may need to investigate the role of handling stress on facilitation of acoustic startle.
To determine if a ceiling effect occurred in the SoD group, the acoustic startle response was compared across all 10 noise level trials in the CTC and SoD groups. We observed that the SoD group exhibited an enhanced magnitude of response across all trials at 95dB, 110dB and 125dB, during days 12, 13 and 21 as compared to the CTC group. These data indicate that the startle response of the SoD group is enhanced above that of the CTC group, and that socially defeated rats can exhibit an increased response to acoustic startle that is independent of drug withdrawal.
These data are also of particular interest because they may reflect the same increase in startle observed in predator odor and underwater exposure models of PTSD in rats (Cohen et al., 2004). Like social defeat, the predator odor and underwater trauma models are ethologically relevant to the rat. Following exposure to cat odor, (predator odor) anxiety-like behavior can be observed in the elevated plus maze and in an increased acoustic startle response has been observed up to 1 month following this trauma. The underwater trauma model, models the brief traumatic experience of suffocation or smothering. Following this trauma, exposed animals have also been observed to also exhibit fear associated behaviors in the elevated plus maze and an increase in the acoustic startle response 2 weeks following the last trauma. Our data reflect an increase in acoustic startle 2 weeks following the last social defeat that does not habituate over time. These data may indicate that the social defeat model can contribute toward an enhanced stress response which mirrors the behavior observed following predator odor and underwater trauma.
Of particular interest, patients with chronic PTSD exhibit can exhibit an enhanced response to acoustic startle between 1 and 4 weeks after the initial trauma (Yehuda et al., 1998). Therefore, it may be reasonable to suggest that the prolonged enhancement to startle in the SoD group could reflect be a hyperactive behavior, modeling a similar phenotypic startle response observed in persons with PTSD (Morgan, Grillon, Lubin, & Southwick, 1997).
Future studies should expand upon these data to determine if our social defeat paradigm can accurately model PTSD-like behavior. The investigation of anxiolytic and antidepressant drug treatments on the acoustic startle response following social defeat can be examined. These experiments may determine if our social defeat paradigm can model the pharmacologic and behavioral responses associated with PTSD. In addition, experiments can be implemented to determine if the acoustic startle response can be modified by exposure to a trauma cue related to social defeat stress.
Another area of investigation would be to determine if the intensity of the social defeat trauma can be correlated with the enhanced and prolonged acoustic startle response observed in our study. This can be investigated by, varying the number of social defeats and measuring the outcome of the acoustic startle responses. Additionally, to determine if PTSD-like behaviors increase over time, multiple types of anxiety tests need to be implemented over different time intervals following social defeat.
Additionally, future studies should perform assessments of other symptomatologies of PTSD such as disturbed sleep architecture (Pawlyk, Jha, Brennan, Morrison, & Ross, 2005) and sympathetic nervous system responsiveness (Debiec & LeDoux, 2006), using telemetric monitoring. Also the influence of genetic or epigenetic liabilities should be examined.
4.1 Conclusion
The observations of reduced weight gain and enhanced exploratory/ arousal behaviors in the SoD group, are consistent with behavior observed in emotionally stressed rats. In addition, we observed a robust and enhanced acoustic startle response which was prolonged in socially defeated rats. Taken together, the reduction in weight gain and the increased and prolonged acoustic startle exhibited by the SoD group may reflect resulting outcomes of traumatic psychosocial stress.
Acknowledgments
We wish to thank Dr. Mike Kuhar (Emory University) for the purchase of Long Evans rats, and Pfizer Pharmaceuticals for an unrestricted grant to Dr. Zackary Stowe (Emory University) for the purchase of the Coulburn Truscan system, and Zachary Stowe for the purchase of the acoustic startle chambers.
John Vincent Knight Pulliam was supported by the American Psychological Association Minority Fellowship Program (MH18882), and the Facilitating Academic Careers in Engineering and Science Program at Emory University (NSF agreement #0450303, sub award # I-66-606-63). We thank Drs. Ericka M. Boone, Frederick Gregory and John Ehlen for their critical reading of this manuscript.
Role of the Funding Source: We wish to thank Dr. Mike Kuhar (Emory University) for the purchase of Long Evans rats, and Pfizer Pharmaceuticals for an unrestricted grant to Dr. Zackary Stowe (Emory University) for the purchase of the acoustic startle chambers. Drs. Kuhar and Stowe had no role in the data collection, writing or submission of this manuscript.
John Vincent Knight Pulliam was supported by the American Psychological Association Minority Fellowship Program (MH18882), and the Facilitating Academic Careers in Engineering and Science Program at Emory University (NSF agreement #0450303, sub award # I-66-606-63). These funding sources were used to support the graduate training of John Pulliam.
Footnotes
Declaration of Interest: None.
Conflict of Interest Statement: Although no conflict of interest exists, Pfizer Pharmaceudicals provided an unrestricted grant to Dr. Zachary Stowe (Emory University). These monies were used to purchase the Coulbourn Truscan system and the acoustic startle chambers from San Diego Instruments.
Contributors: John Pulliam, Ahmad M. Dawaghreh, Ernest Alema-Mensah and Paul M. Plotsky are submitting the manuscript entitled “A Posttraumatic Stress Disorder Phenotype Can Be Modeled Following Social Defeat in Male Long Evans Rats.”
John Pulliam, the primary author of the manuscript was responsible for data collection for the manuscript. In addition, John Pulliam wrote the initial drafts of the manuscript.
The statisticians Ahmad M. Dawaghreh, Ernest Alema-Mensah, have made a significant contribution to the analysis and interpretation of the data. They have also made a contribution by editing of the results section for the manuscript.
The authors, John Pulliam and Paul M. Plotsky shared in the writing and editing of all sections of the manuscript. All of the authors consent to have their names included in the manuscript and approve the final version of the manuscript.
Sincerely,
John Pulliam & Paul Plotsky
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John V.K. Pulliam, Email: JPulliam@msm.edu.
Ahmad M. Dawaghreh, Email: aalmahmoud@msm.edu.
Ernest Alema-Mensah, Email: eamensah@msm.edu.
Paul M. Plotsky, Email: PPlotsky@emory.edu.
References
- Adamec R, Bartoszyk GD, Burton P. Effects of systemic injections of vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004;504:65–77. doi: 10.1016/j.ejphar.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Horm Behav. 2003;43:158–65. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–71. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Buwalda B, Felszeghy K, Horvath KM, Nyakas C, de Boer SF, Bohus B, Koolhaas JM. Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol Behav. 2001;72:349–54. doi: 10.1016/s0031-9384(00)00414-5. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Circadian modulation of the rat acoustic startle response. Behav Neurosci. 1992;106:846–52. doi: 10.1037//0735-7044.106.5.846. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–70. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–98. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–4. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Frank E, Salchner P, Aldag JM, Salome N, Singewald N, Landgraf R, Wigger A. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav Neurosci. 2006;120:60–71. doi: 10.1037/0735-7044.120.1.60. [DOI] [PubMed] [Google Scholar]
- Garrick T, Morrow N, Shalev AY, Eth S. Stress-induced enhancement of auditory startle: an animal model of posttraumatic stress disorder. Psychiatry. 2001;64:346–54. doi: 10.1521/psyc.64.4.346.18600. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Khan S, Liberzon I. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology (Berl) 2004;172:225–9. doi: 10.1007/s00213-003-1634-4. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997a;640:69–72. [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997b;21:775–82. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–40. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats. Psychopharmacology (Berl) 1991;104:181–6. doi: 10.1007/BF02244176. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–64. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Lubin H, Southwick SM. Startle reflex abnormalities in women with sexual assault-related posttraumatic stress disorder. Am J Psychiatry. 1997;154:1076–80. doi: 10.1176/ajp.154.8.1076. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Shimizu K, Nibuya M, Wakizono T, Suzuki G, Tsunoda T, Takahashi Y, Nomura S. Effect of paroxetine on a model of posttraumatic stress disorder in rats. Neurosci Lett. 2004;357:37–40. doi: 10.1016/j.neulet.2003.12.039. [DOI] [PubMed] [Google Scholar]
- van der Kolk BA. Clinical implications of neuroscience research in PTSD. Ann N Y Acad Sci. 2006;1071:277–93. doi: 10.1196/annals.1364.022. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–46. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Wakizono T, Sawamura T, Shimizu K, Nibuya M, Suzuki G, Toda H, Hirano J, Kikuchi A, Takahashi Y, Nomura S. Stress vulnerabilities in an animal model of post-traumatic stress disorder. Physiol Behav. 2007;90:687–95. doi: 10.1016/j.physbeh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–10. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry. 1993;33:479–86. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–96. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44:1305–13. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]