Summary
Phencyclidine is an N-methyl D-aspartate receptor (NMDAR) blocker that has been reported to induce neuronal apoptosis during development and schizophrenia-like behaviors in rats later in life. Brain derived neurotrophic factor (BDNF) has been shown to prevent neuronal death caused by NMDAR blockade, but the precise mechanism is unknown. This study examined the role of the phosphatidylinositol-3 kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) pathways in BDNF protection of PCP-induced apoptosis in corticostriatal organotypic cultures. It was observed that BDNF inhibited PCP-induced apoptosis in a concentration dependent fashion. BDNF effectively prevented PCP-induced inhibition of the ERK and PI-3K/Akt pathways and suppressed GSK-3β activation. Blockade of either PI-3K/Akt or ERK activation abolished BDNF protection. Western blot analysis revealed that the PI-3K inhibitor LY294002 prevented the stimulating effect of BDNF on the PI-3K/Akt pathway, but had no effect on the ERK pathway. Similarly, the ERK inhibitor PD98059 prevented the stimulating effect of BDNF on the ERK pathway, but not the PI-3K/Akt pathway. Co-application of LY294002 and PD98059 had no additional effect on BDNF-evoked activation of Akt or ERK. However, concurrent exposure to PD98059 and LY294002 caused much greater inhibition of BDNF-evoked phosphorylation of GSK-3β at serine 9 than did LY294002 alone. Finally, either BDNF or GSK-3β inhibition prevented PCP-induced suppression of cyclic-AMP response element binding protein (CREB) phosphorylation. These data demonstrate that the protective effect of BDNF against PCP-induced apoptosis is mediated by parallel activation of the PI-3K/Akt and ERK pathways, most likely involves inhibition of GSK-3β and activation of CREB.
Keywords: PCP (phencyclidine), NMDAR (N-methyl-D-aspartate receptor), BDNF (brain-derived neurotrophic factor), PI-3K (phosphoinositide-3 kinase), ERK (extracellular signal-regulated kinase), GSK-3β (Glycogen Synthase Kinase 3 β), Akt
Introduction
The non-competitive glutamatergic NMDA receptor antagonist, phencyclidine (PCP), has long been recognized to induce acute schizophrenia-like symptoms in humans (Javitt and Zukin, 1991; Luby et al., 1962). Schizophrenia is a severe neuropsychiatric disorder which affects approximately one percent of the population worldwide. Unfortunately, its etiology and pathophysiology are poorly defined. Reduced glutamatergic and increased dopaminergic transmission have been hypothesized to represent a major deficit in schizophrenia (Jentsch and Roth, 1999; Marc et al., 1999). Since the symptoms of schizophrenia do not usually appear until early adulthood, Weinberger et al (1987) postulated that the etiology of schizophrenia may involve developmental factors. It suggested that aberrant apoptosis during early brain development disrupts normal neuronal circuitry formation and may underlie the expression of some mental diseases in later life, including schizophrenia (Benes, 2000; Weinberger, 1987). Interestingly, it has been consistently shown that blocking the NMDAR during brain development triggers neuronal death in brain regions in a pattern that is similar to the neuropathology observed in post-mortem brains of schizophrenics (Ikonomidou et al., 1999; Wang and Johnson, 2005). Furthermore, animals that received perinatal administration of PCP or MK801 developed schizophrenia-like behaviors in later life, some of which could be prevented by anti-psychotics (Anastasio and Johnson, 2008; Beninger et al., 2002; Wang et al., 2001). These data suggest that a perinatal PCP insult may be suitable for modeling the pathology of schizophrenia and that knowledge of the mechanisms of PCP-induced apoptosis in developing brain may provide insight into the cellular basis of schizophrenia-like behaviors and suggest novel pharmacotherapeutic approaches.
The mechanisms underlying neuronal death following NMDAR blockade are incompletely understood. Recently, this laboratory reported that PCP inhibits the phosphatidylinositol-3 kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) pathways that normally serve a trophic function during early development (Lei et al., 2008; Xia et al., 2008). Further, stimulating these pathways prevented PCP-induced apoptosis. The potential for preventing PCP-induced apoptosis by diminishing the pro-survival effects of NMDAR activation caused us to further investigate the role of brain-derived neurotrophic factor (BDNF), a neurotrophin that promotes neuronal survival via the ERK and PI-3K/Akt pathways (Hetman et al., 1999). Indeed, several laboratories have demonstrated that NMDAR-regulated survival is mediated via BDNF (Jiang et al., 2005; Xu et al., 2007) and decreased BDNF signaling might participate in NMDAR antagonist-induced neurotoxicity (Fumagalli et al., 2003; Semba et al., 2006). BDNF has also been shown to prevent MK801-induced death in cultured cortical neurons, though the precise mechanisms were not investigated (Hansen et al., 2004). However, using HSP-70 expression as an indicator of toxicity, it was reported that activation of the BDNF receptor, TrkB, had no influence on MK-801-induced neurotoxicity in the posterior cingulate cortex of mouse brain (Väisänen et al., 2003). This inconsistency prompted us to investigate the effect of BDNF on PCP-induced neuronal death in organotypic corticostriatal cultures, using caspase-3 activation and DNA fragmentation as two indices for neuronal apoptosis. We also determined the cellular mediators of BDNF activity, mainly focusing on the roles of the ERK and PI-3K/Akt pathways.
Methods
Animals
Timed pregnant female Sprague–Dawley rats were obtained on day 14 or 18 of pregnancy from Charles River. They were housed individually with a regular 12-hour light: 12-hour dark cycle (lights on at 07:00 h, off at 19:00 h) with food and water available ad libitum. On postnatal day (PN) 2.5, the pups were killed by decapitation and their brains were removed and processed for slice culture as described below. All experiments were conducted in accordance with the NIH and the University of Texas Medical Branch at Galveston Institutional Animal Care and Use Committee.
Reagents
PCP was acquired from the National Institute on Drug Abuse (Rockville, MD, USA) and dissolved in distilled water. Slice culture media including Hank’s balanced salt solution, heat inactivated horse serum, OPTI-MEM medium, neurobasal medium and B-27 supplement were purchased from Invitrogen Corporation (Carlsbad, CA, USA). 10% D-(+)-Glucose solution, 200 mM L-glutamine, and penicillin/streptomycin solution were purchased from Sigma-Aldrich (St. Louis, MO, USA). 7-amino-4-trifluorocumarin (AFC), the caspase-3 substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluorocumarin (Ac-DEVD-AFC) and the caspase-3 inhibitor z-DEVD-FMK were purchased from MP Biomedicals (Livermore, CA, USA). Primary antibodies against phopho-GSK-3β (Ser9), phospho-AKT (Ser473), phospho-p44/42 ERK (Thr202/Tyr204) were obtained from Cell Signaling Technology, Inc. (Danvers, MA 01923). Mouse monoclonal anti-actin antibody, HRP-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Chemicon (Temecula, CA). Deoxynucleotidyl transferase (TdT) and biotin-16-dUTP were purchased from Roche Diagnostics (Indianapolis, IN, USA). The ABC Elite Kit and Vector SG peroxidase substrate were purchased from Vector Laboratories (Burlingame, CA, USA). LY294002 (PI-3K inhibitor), AR-A014418 (GSK-3β inhibitor), PD98059 (ERK inhibitor) were purchased from CalBiochem/EMD Biosciences. BDNF was purchased from Sigma (St. Louis, MO, USA). The Akt inhibitor, triciribine (TCN, (Yang et al., 2004), was a generous gift from the laboratory of Dr. Xiaodong Cheng at the University of Texas Medical Branch, Galveston, TX.
Organotypic slice culture
Corticostriatal slice cultures were prepared as previously described (Sherwood and Timiras, 1970; Xia et al., 2008). In brief, 2.5 day-old rat pups were sacrificed by decapitation and the brains were cut into 400-μm-thick coronal sections under sterile conditions. Three adjacent frontal corticostriatal slices with morphology comparable to levels between A5.3 and A6.8 mm in P10 rats (Wang and Johnson, 2007) were cultured in inserts that have a porous and translucent membrane (Culture Plate Insert, MILLIPORE Co, Bedford, MA) at the interface between a CO2-enriched atmosphere and medium. The initial culture medium was a mixture of 25% inactivated horse serum, 25% Hank’s balanced salt solution, and 50% OPTI-MEM culture medium, supplemented with 25 mM D-glucose and 1% penicillin/streptomycin. After three days, it was switched to serum-free, Neurobasal medium supplemented with 25 mM D-glucose, 1 mM glutamine, 2% B-27, and 1% penicillin/streptomycin; it was changed twice a week thereafter. Experiments were performed on DIV 10.
Caspase-3 Activity Assay
Caspase-3 activity in slices was measured as previously described (Wang and Johnson, 2007). Briefly, slices were homogenized in ice-cold lysis buffer containing 25 mM HEPES (pH 7.4), 5 mM MgCl2, 1.5 mM EDTA, 1.0 mM EGTA, 1 mM dithiothreitol, 0.1% Triton X-100 and 1% protease inhibitor cocktail. After centrifuging the homogenates at 13,000 g for 5 min at 4°C, supernatants were collected for measurement of protein levels and caspase-3 enzymatic activity. All samples were prepared in two parallel sets. One set of the sample supernatants were incubated with the selective caspase-3 inhibitor, z-DEVD-FMK (0.5 μM) in an equal volume of assay buffer (100 mM HEPES, pH 7.4, 2 mM dithiothreitol, 0.1% CHAPS, and 1% sucrose), while the other set was incubated with equal volume of assay buffer without z-DEVD-FMK. Fifteen minutes after initiating the incubation, the enzymatic reaction was started by incubating 50 μl samples with 50 μl of the caspase-3 substrate Ac-DEVD-AFC (25 μM) that was dissolved in assay buffer in 96-well plate at 37°C for 60 min in darkness. The resulting fluorescent product, 7-amino-4-trifluoromethyl-cumarin (AFC), was monitored using a microplate fluorometer (Fluoroskan Ascent, Labsystems, Helsinki, Finland) at excitation and emission wavelengths of 405 and 510 nm, respectively. Enzyme activity in each sample was calculated according to a standard curve constructed with concentrations of AFC ranging from 19.5 to 20,000 nM. Caspase-3 activity was calculated as the difference in apparent activities in duplicate-samples incubated with and without the caspase-3 inhibitor, and then normalized to protein concentration. The final caspase-3 activity shown in the figures was presented as percentage of the corresponding control group.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
After drug treatment, slices were fixed with ice-cold 2% paraformaldehyde in 0.1 M PB (pH 7.2) at room temperature for 1 hour and then washed with 0.01M PBS (pH 7.2). After dehydration and re-hydration in a sequential series of ethanol concentrations (70%, 90%, 100%), the slices were incubated with pepsin (0.04% in 10 mM HCl) for 15 min to digest protein and with 0.3% hydrogen peroxide in methanol for 10 min to quench endogenous peroxidase. Slices were then placed in TdT (terminal deoxynucleotidyl transferase) reaction buffer (30 mM Tris-HCl, pH 7.2, 140 mM Na cacodylate and 1 mM CoCl2) for 15 min after a thorough wash with PBS. To stain fragmented DNA, slices were reacted with biotin-16-dUTP (10 nmoles/ml) and TdT (200 U/ml) in the TdT buffer in a humidified chamber for 2 hours at 37°C and then incubated with ABC reagents (Avidin-Biotin-peroxidase Complex) for 60 min. Sliced were then stained using the Vector SG peroxidase substrate kit. Stained TUNEL-positive cells were quantified in a photomicrograph of layers II–IV of the parietal cortex in one microscopic field of each slice was taken using a computer-based image analysis program, SimplePCI (Compix Inc. Imaging Systems, Cranberry Township, PA).
Western blot analysis
Slices were collected and homogenized in 0.3 ml of sucrose buffer (320 mM sucrose, 25 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA) containing the protease inhibitor cocktail, phenylmethlylsulfonyl fluoride (PMSF, 10 μl/ml), phosphatase inhibitor cocktail I and II. After sitting on ice for 15 min, homogenates were centrifuged at 1,000 g for 5 min. The resulting pellets (P1) were dissolved in Tris buffer (50 mM Tris-HCl, 150 mM NaCl, 0.25% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA) that was supplemented with 0.1% SDS, protease inhibitors cocktail, phenylmethlylsulfonyl fluoride (PMSF, 10 μl/ml) and phosphatase inhibitor cocktails I and II and used as the nuclear fraction. The supernatants (S1) were further centrifuged at 10,000 g for 30 min. The resultant supernatants (S2) were collected as the cytoplasmic fraction and the pellet was discarded. After measuring protein concentration, equal amounts of protein (30 μg) were loaded and separated on 10% SDS-polyacrylamide gels with a Tris–glycine running buffer system and then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween-20 (TBST), the membranes were incubated overnight at 4°C with specific antibodies [anti-phospho-Ser-9-GSK-3β (1:2,000), anti-phospho-Ser473-Akt (1:2,000), anti-phospho-Thr202/Tyr204 ERK1/2 (1: 2,000), anti-phospho-Ser133-CREB, ERK1/2, Akt, GSK-3β] and then incubated with HRP-conjugated anti-mouse or anti-rabbit secondary antibody for 1 hour at room temperature. After extensive washes in TBST, blots were visualized by enhanced chemiluminescence (ECL regular or plus) according to the manufacturer’s instruction. Phosphoproteins and non-phosphoproteins were probed on different membranes. To ensure equivalent protein loading, membranes were stripped and reprobed with anti-actin antibody, which was subsequently used to normalize the western analyses.
The bands were scanned and densitometrically analyzed using an automatic image analysis system (Alpha Innotech Corporation, San Leandro, CA). All target proteins were quantified by normalizing to β-actin re-probed on the same membrane and then calculated as percentage of the corresponding control group.
Statistical analysis
All experimental data are presented as mean ± S.E. One-way ANOVA, followed by Student-Newman-Keuls test for multiple comparisons, was used to determine differences among more than two groups. Differences were considered significant when p<0.05.
Results
BDNF prevents PCP-induced apoptosis
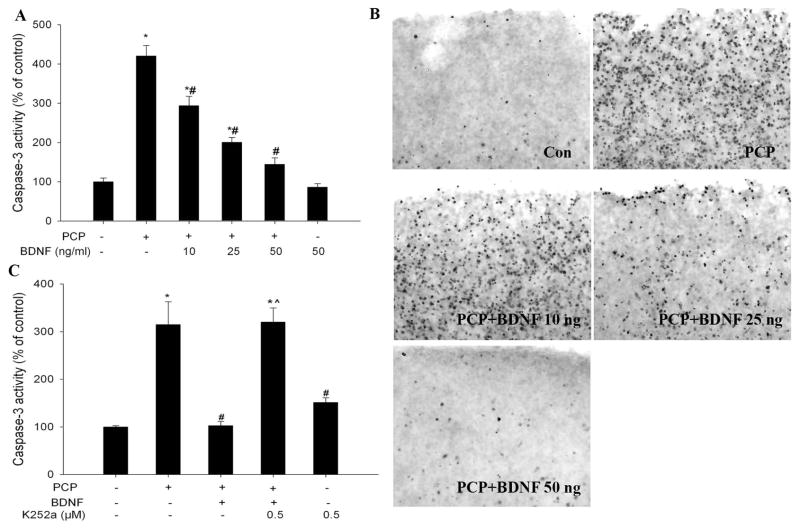
Cultured corticostriatal slices were challenged with 3 μM PCP and cell death was assessed 12 h after the insult by measuring caspase-3 and DNA fragmentation using TUNEL staining. A series of increasing concentrations of BDNF was added to the medium 1 hour before PCP treatment. As has been demonstrated previously (Wang and Johnson, 2007), PCP caused robust caspase-3 activation and wide-spread DNA fragmentation in the superficial layers of the cortex (Layer II to IV) (Fig 1A and 1B). BDNF pretreatment dose-dependently prevented PCP-induced caspase-3 activation and DNA fragmentation; full protection was observed at a concentration of 50 ng/ml (Fig 1. A and B).
Figure 1.
TrkB receptor activation is required for BDNF protection against PCP-induced cell death. A: BDNF prevented PCP-induced aspase-3 activation in a concentration-dependent manner; B: Representative TUNEL staining showing that BDNF dose-dependently prevented PCP-induced apoptosis in the superficial layers of the cortex. C: The TrkB inhibitor, K252a, abolished the protective effect of BDNF. K252a (500 nM) was added to the medium 1 h before BDNF and PCP (3 μM) was added 1 h after BDNF. Slices were collected 12 h after the addition of PCP. N=5–6 for all experiments. *: p< 0.05, vs Con (no treatment); # p<0.05 vs PCP; ^: p<0.05, vs PCP+BDNF (one-way ANOVA).
To clarify the mechanism of BDNF’s protective effect, the Trk B receptor inhibitor, K252a (500 nM), was added to medium 1 hour prior to BDNF (50 ng/ml). In the presence of K252a, BDNF could no longer inhibit PCP-induced caspase-3 activation (Fig 1C). K252a alone did not cause significant caspase-3 activation. These data indicate that BDNF protection against PCP neurotoxicity is mediated through the Trk B receptor.
The role of PI-3K/Akt and ERK pathways
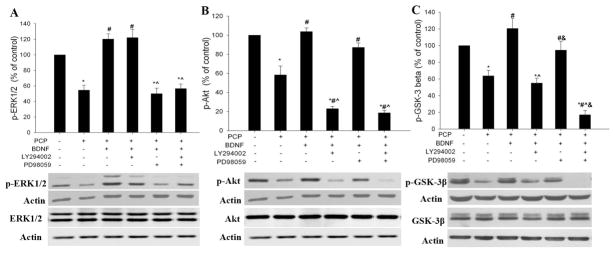
Upon binding to the TrkB receptor, BDNF activates several signaling pathways that are important for neuronal survival, including the ERK and PI-3K/Akt pathways (Hetman et al., 1999). PCP has been found to decrease levels of the active or phosphorylated forms of ERK and Akt in developing brains (Xia et al., 2008). Therefore, we investigated whether protection of BDNF against PCP neurotoxicity involves activation of these two pathways. We examined the effect of BDNF on the activity of the two signaling cascades by Western analysis using antibodies that specifically recognize the activated/phosphorylated forms of ERK and Akt. Preincubation with BDNF (50 ng/ml) was found to effectively prevent ERK and Akt inhibition by PCP (Fig 3. A, B).
Fig 3.
Relationships between the PI-3K/Akt and ERK pathways that are activated by BDNF. The ERK inhibitor, PD98059 (30 μM), or the PI-3K inhibitor, LY294002 (30 μM), or both, were added 1 h before BDNF (50 ng/ml) treatment. PCP (3 μM) was added 1 h after BDNF. Slices were collected 8 h after PCP for western blot analysis of protein expression and phosphorylation of ERK1/2, Akt, and GSK-3β. The phospho-proteins and their non-phospho-counterparts were probed on different membranes and the expression level of all target proteins was normalized to the corresponding actin density probed on the same membrane. N=6. *: p< 0.05, vs control (no treatment); # p<0.05 vs PCP; ^: p<0.05, vs PCP+BDNF; &: p<0.05, vs PCP+ BDNF+ LY294002 (one-way ANOVA).
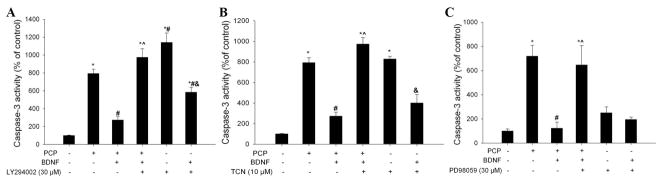
In order to determine whether activation of these pathways are important for BDNF protection, the activation of PI-3K/Akt or ERK pathways by BDNF was blocked pharmacologically with a PI-3K inhibitor (LY294002, 30 μM), an Akt inhibitor (TCN, 10 μM), or an ERK inhibitor (PD98059, 30 μM). We found that in the presence of these signaling inhibitors, BDNF protection against PCP-evoked caspase-3 activation was completely abrogated (Fig 2A, B, C). This strongly suggests that BDNF protects against PCP-induced apoptosis by counteracting the inhibitory effect of PCP on the PI-3K/Akt and ERK pro-survival pathways and further, that both pathways are indispensable. Control experiments revealed that LY294002 and TCN alone induced significant caspase-3 activation in the corticostriatal slices (p<0.05, vs control). Interestingly, BDNF was able to reverse the toxicity of each inhibitor (p<0.05, inhibitor alone vs inhibitor +BDNF).
Figure 2.
BDNF protection against PCP-induced neurotoxicity is mediated by activation of PI-3K/Akt and ERK pathways. Inhibition of activation of PI-3K by LY294002 (30 μM) (A), Akt by TCN (10 μM) (B), or ERK by PD98059 (30 μM) (C) each attenuated BDNF protection against PCP neurotoxicity. Each inhibitor was added 1 h before BDNF (50 ng/ml). PCP (3 μM) was added 1 h after BDNF. Slices were collected 12 h after PCP treatment. N=6. *: p< 0.05, vs control (no treatment); # p<0.05 vs PCP; ^: p<0.05, vs PCP+BDNF; &: p<0.05, vs kinase inhibitors alone (one-way ANOVA).
Relationship between the PI-3K/Akt and ERK pathways
To define the relationship between the ERK and PI-3K/Akt pathways involved in BDNF protection in the cortical slice system, we determined the effect of inhibiting one pathway with a specific kinase inhibitor on the action of BDNF on the other pathway. The results revealed that the ERK inhibitor PD98059 blocked BDNF’s effect on phosphorylation of ERK, but not that of Akt (Fig. 3A); likewise, the PI-3K inhibitor LY294002 specifically blocked BDNF activation of Akt, but had no effect on ERK (Fig 3. B). Concurrent exposure to PD98059 and LY294002 did not further decrease BDNF-evoked phosphorylation of Akt or ERK. Protein expression of ERK or Akt was not changed by the above treatments. These data suggest that the two pathways are stimulated by BDNF in parallel. However, they may act cooperatively on a downstream target.
The role of GSK-3β in BDNF protection
GSK-3β is a pro-apoptotic factor that is normally phosphorylated at serine 9 and inactivated (Cross et al., 1995). The PI-3K/Akt pathway has been reported to promote neuronal survival through inhibition of GSK-3β activity by increasing phosphorylation of the serine 9 residue (Pap and Cooper, 1998). We previously found that inhibition of GSK-3β prevented PCP-induced neuronal death (Lei et al., 2008; Xia et al., 2008), suggesting the involvement of GSK-3β activation. In this study, we investigated the role of GSK-3β in BDNF protection. Slices were pre-incubated with BDNF for 1 hour before they were challenged with PCP and collected 8 hours later for western blot analysis of GSK-3β-ser-9 phosphorylation. As previously reported (Xia et al., 2008), PCP caused significant GSK-3β activation/dephosphorylation and BDNF pretreatment effectively prevented this activation (Fig 3. C).
To investigate the mechanism by which BDNF inhibits GSK-3β, we assessed the effect of BDNF on GSK-3β phosphorylation in the presence of the PI-3K inhibitor, LY294002, or the ERK inhibitor, PD98059, or both. The results of this experiment revealed that blocking the PI-3K/Akt pathway with LY294002 significantly attenuated BDNF-evoked phosphorylation of GSK-3β on serine 9. On the other hand, blocking ERK with PD98059 showed no effect. However, concurrent exposure to PD98059 and LY294002 caused a significantly greater inhibition of BDNF-evoked phosphorylation of GSK-3β at serine 9 than did LY294002 (Fig. 3C). These data suggest that ERK signaling may act as a regulator for the action of the PI-3K/Akt pathway on GSK-3β-ser phosphorylation.
The role CREB in BDNF protection
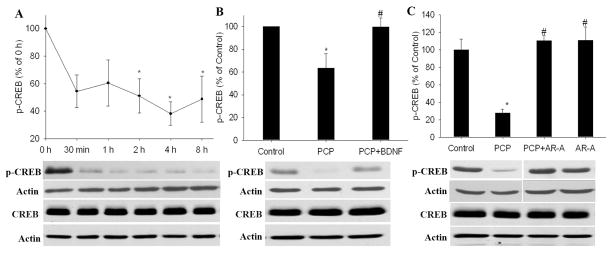
BDNF has been found to exert its neuroprotective effects through phosphorylation of CREB at serine 133 (Finkbeiner, 2000; Finkbeiner et al., 1997). Therefore, we proposed that BDNF inhibition of GSK-3β results in CREB activation. We first examined the effect of PCP on CREB activity. Slices were incubated with PCP for various times (0, 0.5, 2, 4 and 8 h) and then collected for western blot analysis of phospho-CREB-ser133. We found that PCP decreased CREB phosphorylation in a time-dependent manner. Two hours after PCP treatment, the phospho-CREB level was decreased by about 50% and remained at this level until the end of the experiment (8 h) (Fig 4.A). BDNF pretreatment prevented the decrease of phospho-CREB induced by PCP (Fig 4.B). Suppressing GSK-3β activity with the GSK-3β inhibitor AR-A014418 also restored the phospho-CREB level to normal (Fig 4. C). None of these treatments altered the protein expression of CREB.
Fig 4.
The GSK-3 inhibitor AR-A014418 mimicked the stimulatory effect of BDNF on PCP-induced CREB dephosphorylation/inactivation. BDNF (50 ng/ml) or AR-A014418 (AR-A, 10 μM) was added 1 h before PCP (3 μM). Slices were collected 8 h after PCP treatment for western blot analysis of phospho-CREB and CREB. Phospho-CREB and CREB were probed on different membranes and normalized to the corresponding actin probed on the same membrane. N= 3–6*: p< 0.05, vs control (0 h, no treatment); # p<0.05 vs PCP (one-way ANOVA).
Discussion
BDNF and NMDAR-mediated glutamate transmission have been long recognized as two important resources for supporting neuronal survival during brain development (Balazs, 2006; Binder and Scharfman, 2004). This study investigated for the first time the mechanisms of BDNF protection in neuronal apoptosis caused by the NMDAR blocker, PCP. It was observed that exogenous application of BDNF prevented PCP-induced apoptosis in cultured brain slices from developing brains and further, that this protective effect of BDNF is dependent on stimulation of the ERK and PI-3K/Akt signaling cascades.
Among the Trk family, BDNF binds specifically to TrkB receptor with high affinity, but also binds to the p75 neurotrophin receptor (p75NTR) with low affinity. Activation of TrkB has been shown to be essential for the survival-promoting actions of BDNF (Reichardt, 2006). In this study, inhibition of Trk B activation with K252a abolished the neuroprotective effect of BDNF, suggesting that the survival-promoting effect of BDNF is indeed, mediated through TrkB receptors. Upon binding of BDNF, the TrkB receptor activates multiple signaling cascades, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway as well as the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway (Hetman et al., 1999). Depending on the cell type and the nature of apoptotic insult, these signaling pathways are differentially involved in BDNF protection. For example, in cortical neurons, BDNF prevented cell death due to DNA damage by activating the ERK pathway (Hetman et al., 1999). ERK is also the major pathway that is responsible for BDNF-induced cerebellar neuron survival (Bonni et al., 1999). On the other hand, the PI-3K/Akt pathway is the major mediator for the prosurvival effects of BDNF in the SH-SY5Y neuroblastoma cell line (Encinas et al., 1999) as well as for the protective effects of BDNF against cortical neuronal death induced by serum withdrawal (Hetman et al., 1999). There are also conditions in which both ERK and PI-3K pathways are indispensable for BDNF protection (Almeida et al., 2005; Nguyen et al., 2009; Sun et al., 2008). With regard to apoptosis induced by NMDAR blockade during brain development, Hansen et al (2004) reported that exogenous BDNF inhibited MK-801-induced apoptosis, though the underlying mechanisms were not studied. However, these authors did report that the expression of constitutively active Ras enhanced ERK activity and prevented MK-801induced apoptosis, suggesting a role of the Ras/ERK pathway. In agreement with that report, we observed that BDNF prevented PCP-induced inhibition of ERK activity (Fig. 3A). Furthermore, blocking ERK activation with PD98059 abrogated BDNF protection (Fig. 2A). These data provided strong support for the involvement of ERK in protection by BDNF. On the other hand, we also found that BDNF prevented PCP-induced inhibition of the PI-3K/Akt pathways (Fig. 3B). In support of the involvement of this pathway, inhibitors of either PI-3K or Akt largely attenuated BDNF protection (Fig. 2A and 2B). Interestingly, we previously demonstrated that the protection by lithium against PCP-induced apoptosis also requires activation of both the ERK and PI-3K/Akt pathways (Xia et al., 2008). Considering that PCP treatment inhibits both ERK and PI-3K pathways, and these two pathways are simultaneously involved in the pro-survival effects of NMDAR activation (Almeida et al., 2005), we postulate that activation of both pathways is essential and necessary to rescue cell death in developing brains caused by NMDAR blockade.
A major goal of this study was to elucidate the relationship between the PI-3K/Akt and ERK pathways involved in BDNF protection. As shown in Fig. 3, chemical inhibition of PI-3K activation with LY294002 specifically decreased phosphorylation of Akt, while it had no effect on the phosphorylation of ERK. On the other hand, inhibition of ERK with PD98059 inhibited ERK phosphorylation, but had no effect on Akt phosphorylation. These data indicate that though both the ERK and PI-3K/Akt pathways are involved, they are activated by BDNF independently. This result is contradictory to the finding in hippocampal neurons that there is a crosstalk between the two pathways activated by BDNF to prevent glutamate-induced cell death (Almeida et al., 2005). In that study, the PI-3K inhibitor LY294002 blocked BDNF-induced ERK activation. There is a previous report showing that protection of BDNF against hypoxic toxicity in cortical neurons also involves both PI-3K and MAPK pathways, but in that study, no interaction between the two pathways was found (Sun et al., 2008). That is, LY294002 did not affect BDNF-induced ERK phosphorylation and the ERK inhibitor U0126 did not affect BDNF-induced Akt phosphorylation. It is possible that cell type and the specific nature of the insult determine not only the involvement of the diverse signaling pathways, but also their relationship to BDNF. In our cortical slice model, the ERK and PI-3K/Akt pathways are independently activated by BDNF.
This independent activation and simultaneous contribution of the PI3-K/Akt and ERK pathways to the protection afforded by BDNF against PCP-evoked apoptosis may be due to a common mechanism that is downstream of the two pathways. Indeed, we previously observed that GSK-3β inhibitor AR-A0144418 prevented PCP-induced cell death without altering the inhibitory effect of PCP on ERK and Akt activity and therefore have proposed that GSK-3β is the most likely candidate of the common mechanism (Xia et al., 2008). GSK-3β has been demonstrated to be important for neuronal apoptosis and to be crucial for PI-3K-mediated neuronal survival (Hetman et al., 2000) and in this study, we observed that LY294002 prevented BDNF-evoked GSK-3β phosphorylation at serine 9, while PD98059 did not. Importantly, however, concurrent exposure to PD98059 and LY294002 caused significantly greater inhibition of BDNF-evoked phosphorylation of GSK-3β at serine 9 than did LY294002 alone. These data imply that the ERK pathway may act as a regulator for PI-3K/Akt inhibition of GSK-3β activity and provides support for our hypothesis that GSK-3β is the key downstream target that mediates the anti-apoptotic effects of activating the PI-3K/Akt and ERK pathways. The mechanism by which ERK regulates the activity of GSK-3β in our model is unclear. It has been reported that ERK activation protects cortical neurons from GSK-3β activation-induced apoptosis through an unknown mechanism that is independent of serine 9 phosphorylation (Habas et al., 2006; Hetman et al., 2002). Recently, in the HepG2 cell line, it was reported that ERK phosphorylates GSK-3β at the threonine 43 residue, which in turn facilitated its consequent phosphorylation by other kinases at serine 9 (Ding et al., 2005). Although we did not determine the possible site on which ERK might phosphorylate GSK-3β, it is quite possible that in this model, ERK might regulate GSK-3β activity by phosphorylating other GSK-3β residues that would facilitate its phosphorylation at serine 9 by PI-3K/Akt.
CREB has been shown to be the key mediator for BDNF-mediated cell survival (Finkbeiner, 2000; Finkbeiner et al., 1997). Muting the transcriptional activity of CREB impaired BDNF protection (Bonni et al., 1999; Lee et al., 2009). CREB is phosphorylated at serine 133 and therefore activated by various kinases, including ERK, Akt and GSK-3β (Bonni et al., 1999; Du and Montminy, 1998; Salas et al., 2003). In accordance with reports from other groups (Hansen et al., 2004), we found that blocking the NMDAR with PCP during brain development decreased CREB-ser-133 phosphorylation time-dependently. We also observed that BDNF pretreatment restored CREB ser-133 phosphorylation back to its normal level. The GSK-3β inhibitor, AR-A014418, mimicked BDNF in that it prevented PCP-induced CREB dephosphorylation. Considering that BDNF inhibited PCP-induced GSK-3β activation (Fig. 3), we postulate that BDNF may increase CREB activity by inhibiting GSK-3β activity.
Taken together, this study demonstrated that BDNF prevents PCP-induced neuronal apoptosis in developing brain by activating the PI-3K/Akt and ERK pathways in parallel. In turn, the PI-3K/Akt and ERK pathways may act cooperatively to suppress GSK-3β activity. It is proposed that suppression of GSK-3β activity by BDNF may underlie BDNF-induced CREB phosphorylation and protection against PCP-induced apoptosis.
Acknowledgments
This work was supported by NIH grant 1RO1DA-02073-27 Ike Extension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Johnson KM. Atypical anti-schizophrenic drugs prevent changes in cortical N-methyl-D-aspartate receptors and behavior following sub-chronic phencyclidine administration in developing rat pups. Pharmacol Biochem Behav. 2008;90:569–577. doi: 10.1016/j.pbb.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R. Trophic effect of glutamate. Curr Top Med Chem. 2006;6:961–968. doi: 10.2174/156802606777323700. [DOI] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Jhamandas A, Aujla H, Xue L, Dagnone RV, Boegman RJ, Jhamandas K. Neonatal exposure to the glutamate receptor antagonist MK-801: effects on locomotor activity and pre-pulse inhibition before and after sexual maturity in rats. Neurotox Res. 2002;4:477–488. doi: 10.1080/10298420290031414. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Encinas M, Iglesias M, Llecha N, Comella JX. Extracellular-regulated kinases and phosphatidylinositol 3-kinase are involved in brain-derived neurotrophic factor-mediated survival and neuritogenesis of the neuroblastoma cell line SH-SY5Y. J Neurochem. 1999;73:1409–1421. doi: 10.1046/j.1471-4159.1999.0731409.x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: A Major Mediator of Neuronal Neurotrophin Responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J Neurosci Res. 2003;72:622–628. doi: 10.1002/jnr.10609. [DOI] [PubMed] [Google Scholar]
- Habas A, Kharebava G, Szatmari E, Hetman M. NMDA neuroprotection against a phosphatidylinositol-3 kinase inhibitor, LY294002 by NR2B-mediated suppression of glycogen synthase kinase-3beta-induced apoptosis. J Neurochem. 2006;96:335–348. doi: 10.1111/j.1471-4159.2005.03543.x. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, Ikonomidou C. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16:440–453. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Hsuan SL, Habas A, Higgins MJ, Xia Z. ERK1/2 antagonizes glycogen synthase kinase-3beta-induced apoptosis in cortical neurons. J Biol Chem. 2002;277:49577–49584. doi: 10.1074/jbc.M111227200. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- Lee B, Cao R, Choi YS, Cho HY, Rhee AD, Hah CK, Hoyt KR, Obrietan K. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–1265. doi: 10.1111/j.1471-4159.2008.05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3beta signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- Marc L, Anissa AD, Roberto G, Lawrence K, Robert I. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biological psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by NGF and BDNF against neurotoxin-exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3-kinase and MAPK pathways. Neurochem Res. 2009;34:942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas TR, Reddy SA, Clifford JL, Davis RJ, Kikuchi A, Lippman SM, Menter DG. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J Biol Chem. 2003;278:41338–41346. doi: 10.1074/jbc.M302972200. [DOI] [PubMed] [Google Scholar]
- Semba J, Wakuta M, Suhara T. Different effects of chronic phencyclidine on brain-derived neurotrophic factor in neonatal and adult rat brains. Addict Biol. 2006;11:126–130. doi: 10.1111/j.1369-1600.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A stereotaxic atlas of the developing rat brain. University of California Press; Berkeley: 1970. [Google Scholar]
- Sun X, Zhou H, Luo X, Li S, Yu D, Hua J, Mu D, Mao M. Neuroprotection of brain-derived neurotrophic factor against hypoxic injury in vitro requires activation of extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Int J Dev Neurosci. 2008;26:363–370. doi: 10.1016/j.ijdevneu.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Väisänen J, Saarelainen T, Koponen E, Castrén E. Altered trkB neurotrophin receptor activation does not influence the N-methyl--aspartate receptor antagonist-mediated neurotoxicity in mouse posterior cingulate cortex. Neuroscience Letters. 2003;350:1–4. doi: 10.1016/s0304-3940(03)00744-4. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. Differential effects of acute and subchronic administration on phencyclidine-induced neurodegeneration in the perinatal rat. J Neurosci Res. 2005;81:284–292. doi: 10.1002/jnr.20559. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology. 2007;32:1178–1194. doi: 10.1038/sj.npp.1301202. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Lithium protection of phencyclidine-induced neurotoxicity in developing brain: the role of phosphatidylinositol-3 kinase/Akt and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathways. J Pharmacol Exp Ther. 2008;326:838–848. doi: 10.1124/jpet.107.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang QG, Li C, Zhang GY. Subtoxic N-methyl-D-aspartate delayed neuronal death in ischemic brain injury through TrkB receptor- and calmodulin-mediated PI-3K/Akt pathway activation. Hippocampus. 2007;17:525–537. doi: 10.1002/hipo.20289. [DOI] [PubMed] [Google Scholar]
- Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]