Abstract
Objective:
To examine the electrophysiological and molecular properties of the transient outward current (Ito) in canine left ventricle using a novel Ito activator, NS5806.
Methods and Results:
Ito was measured in isolated epicardial (Epi), midmyocardial (Mid) and endocardial (Endo) cells using whole-cell patch-clamp techniques. NS5806 activation of Kv4.3 current was also studied in CHO-K1 cells and Xenopus laevis oocytes. In CHO-K1 cells co-transfected with Kv4.3 and KChIP2, NS5806 (10 μM) caused a 35 % increase in current amplitude and a marked slowing of current decay with τ increasing from 7.0±0.4 to 10.2±0.3 ms. In the absence of KChIP2, current decay was unaffected by NS5806. In ventricular myocytes, NS5806 increased Ito density by 80%, 82%, and 16% in Epi, Mid, and Endo myocytes, respectively (at +40 mV) and shifted steady-state inactivation to negative potentials. NS5806 also significantly slowed decay of Ito, increasing total charge to 227%, 192% and 83% of control in Epi, Mid and Endo cells, respectively (+40 mV, p<0.05). Quantification of Kv4.3 and KChIP2 mRNA in the 3 ventricular cell types revealed that levels of Kv4.3 message was uniform but those of KChIP2 were significantly greater in Epi and Mid cells. The KChIP2 gradient was confirmed at the protein level by Western blot.
Conclusions:
Our results suggest that NS5806 augments Ito by increasing current density and slowing decay and that both depend on the presence of KChIP2. Ito and its augmentation by NS5806 are greatest in Epi and Mid cells because KChIP2 levels are highest in these cell types.
Keywords: ventricular muscle, transient outward K+ channel, repolarization, heterogeneity
INTRODUCTION
Repolarization of the cardiac action potential is initiated and controlled by activation of a number of time- and voltage-dependent K+ currents. In dog heart at least four K+ currents play important roles in regulating the cardiac action potential duration: (i) a Ca2+-independent transient outward K+ current (Ito); (ii) an inwardly rectifying K+ current (IK1) and (iii) the rapid and slow forms of the delayed rectifier K+ current (IKr and IKs, respectively). An Ito has been identified in the myocardium of most mammalian species (for review see [1]). Ventricular epicardial (Epi) tissue has a more prominent Ito compared to endocardial (Endo) tissue [2-5]. Recently, it has been demonstrated that Ito can be modulated by several proteins such as K+-channel interacting protein (KChIP) [6,7], IRX [8], calcineurin/NFAT [9], DPP's [10] and various KCNE subunits [11,12].
Although an Ito gradient between Epi and Endo has been identified, the precise molecular identity of Ito in the canine ventricular myocardium remains unclear. It is generally believed that Kv4.3 channels comprise the majority of transient outward K+ channels in canine heart [13]. Previous studies have also identified KV1.4 and KV1.5 gene products in ventricular tissue [13,14]. However, the precise role of these alpha subunits and their contribution to canine Ito remains to be determined. Recent evidence also suggests that several β-subunits including KChIP2 can alter peak IKv4.3 density, slow decay of the current and accelerate recovery from inactivation[7]. However, the relative abundance of Kv4.3 and KChIP2 in canine ventricle remains controversial. Several studies suggest that Kv4.3 levels are uniform throughout the canine left ventricle and the gradient in Ito expression is due to a gradient in KChIP2 [15,16]. In contrast, another study found that KChIP2 protein was uniform throughout the left ventricle suggesting that Ito gradient in ventricle is not due to a gradient in KChIP2 levels [7]. Finally, Zicha et al. [17] found that both Kv4.3 and KChIP2 exhibit a transmural gradient, with Epi expression being greater than Endo expression in canine ventricle.
The present study compares the electrophysiological and molecular properties of the Ca2+-independent transient outward K+ currents in single myocytes isolated from the canine left ventricle. Results of our study indicate that the biophysical and molecular properties of Ito differ significantly in endocardial cells compared to midmyocardial (Mid) or epicardial cells. Analysis of the molecular subunits revealed that KChIP2 mRNA levels are lower in the endocardium, contributing to some of the observed biophysical differences. Furthermore, we found that the levels of putative Ito subunits Kv4.3 and Kv1.4 are equally distributed throughout the left ventricle. Utilizing the Ito activator, NS5806 [18], we confirm by pharmacological methods that KChIP2 is functionally important for Ito activation and that KChIP2 levels are lower in endocardial cells. Application of this Ito activator resulted in a significant increase in Ito, with the greatest effect observed in Epi and Mid cells. The regional variations in subunit contribution are responsible for the observed biophysical differences in magnitude and kinetics of Ito.
METHODS
Expression of Kv4.3 and KChIP2 in CHO-K1
Human (h) Kv4.3 (NM_172198) and hKChIP2.1 (NM_173192) were transiently expressed in CHO-K1 in a 1:3 molar ratio using Lipofectamine and Plus Reagent according to manufacturer's instruction (GIBCO, Invitrogen). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Substrate Department, University of Copenhagen, Denmark) supplemented with 10% fetal calf serum (GIBCO, Invitrogen) and 40 mg/L L-Proline at 37°C in 5% CO2.
Expression of Kv4.3 and KChIP2 in Xenopus laevis oocytes
Female Xenopus laevis frogs were anaesthetized with Tricain (2 g/l, Sigma) and ovarian lobes were removed. Oocytes were defolliculated enzymatically in 1% collagenase (Boehringer Mannheim) and 0.1% trypsin inhibitor (Sigma) in Kulori solution for 1 h followed by wash in Kulori containing 0.1% BSA (Sigma).
cRNA was prepared from hKv4.3 and hKChIP2.1 using the mMESSAGE mMACHINE T7 kit (Ambion). 50 nl cRNA was injected using a Nanoject microinjector (Drummond Scientific, Broomall, PA) in molar ratios of Kv4.3 and KChIP2. The concentration of Kv4.3 was kept constant at 0.1 ng. Oocytes were kept at 19°C and currents measured 2 days after injection.
Isolation of adult myocytes
Myocytes from Epi, Endo and Mid regions were prepared from canine hearts using techniques previously described [19,20]. Adult mongrel dogs were anesthetized with sodium pentobarbital (35 mg/kg i.v.), their hearts were rapidly removed and placed in nominally Ca2+-free Tyrode's solution. A wedge consisting of the left ventricular free wall was cannulated and perfused with nominally Ca2+-free Tyrode's solution containing 0.1% BSA for about 5 minutes. The wedge preparations were then subjected to enzyme digestion with the nominally Ca2+-free solution supplemented with 0.5 mg/ml collagenase (Type II, Worthington), 0.1 mg/ml protease (Type XIV, Sigma) and 1 mg/ml BSA for 8-12 minutes. After perfusion, thin slices of tissue from the Epi (<2 mm from the epicardial surface), Mid (about 5-7 mm from the epicardial surface), and Endo (<2 mm from the endocardial surface) were shaved from the wedge using a dermatome. The tissue slices were then placed in separate beakers minced and incubated in fresh buffer containing 0.5 mg/ml collagenase, 1 mg/ml BSA and agitated. The supernatant was filtered, centrifuged at 200 rpm for 2 minutes and the pellet containing the myocytes was stored in 0.5 mM Ca2+ HEPES buffer at room temperature.
Solutions
Kv4.3 currents in CHO-K1 was measured using an extracellular NaCl Ringer solution (mM): NaCl 140, KCl 4, CaCl2 2, MgCl2 1, HEPES 10, pH=7.4 adjusted with NaOH and an intracellular solution (mM): KCl 110, KOH/EDTA 31/10, CaCl2 5.17, MgCl2 1.42, HEPES 10, MgATP 4, pH=7.2 with KOH. Kulori solution contained (mM): NaCl 90, KCl 4, MgCl2 1, CaCl2 1, HEPES 5, pH=7.4 with NaOH. The nominally Ca2+-free dissecting buffer contained (mM): NaCl 129, KCl 5.4, MgSO4 2.0, NaH2PO4 0.9, glucose 5.5, NaHCO3 20 and was bubbled with 95% O2/5% CO2. Ventricular cells were superfused with HEPES buffer (mM): NaCl 126, KCl 5.4, MgCl2 1.0, CaCl2 2.0, HEPES 10, glucose 11, pH=7.4 with NaOH. The pipette solution consisted of (mM): K-aspartate 90, KCl 30, glucose 5.5, MgCl2 1.0, EGTA 5, MgATP 5, HEPES 5, NaCl 10, pH=7.2 with KOH.
Electrophysiology
Ito recordings from myocytes were performed as previously described [21]. All myocyte and CHO-K1 experiments were performed at 36°C. Voltage-clamp and conventional recordings were made using a MultiClamp 700A amplifier and MultiClamp Commander (Axon Instruments). Patch pipettes were fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburg, PA). Pipettes were pulled using a gravity puller (Narishige Corp) and the resistance ranged from 0.9-3 MΩ when filled with the internal solution. Cell capacitance was measured by applying −5 mV voltage steps. Electronic compensation of series resistance to 60-70% was applied. All analog signals were acquired at 10-25 kHz, filtered at 4-6 kHz, digitized with a Digidata 1322 converter (Axon Instruments) and stored using pClamp9 software.
Recordings from oocytes were performed using a two-electrode voltage-clamp amplifier (Dagan CA-1B; Chicago, IL). Borosilicate glass recording electrodes (Module Ohm, Denmark) were made using a DMZ-Universal Puller (Zeitz Instruments, Germany) and had a resistance of 0.5 to 1 MΩ when filled with 2 M KCl. Oocytes were superfused with Kulori solution and experiments performed at room temperature.
Analysis of mRNA Levels in the Left Ventricle
RNA preparation and cDNA synthesis
RNA was prepared from canine left ventricular tissue. Tissue samples were shaved using a dermatome and stored in an RNA stabilizing solution (RNAlater®-ICE, Ambion). Total RNA was purified from the homogenized tissue specimens (homogenizer Kinemtica, Buch & Holm, Switzerland) with Tri Reagent® (Sigma-Aldrich) according to the manufacturer's instructions. Total cDNA was synthesized from 2 μg total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystem) with random hexamer primers following the manufacturer's instructions.
Real-time PCR
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on the 7300 RT-PCR System (Applied Biosystem) and data were collected by SDS1.2 software. The selected genes were investigated using TaqMan assays (TaqMan® MGB probe and primers). The pre-designed gene expression assays from Applied Biosystems were as follows: cf02698011 (KCND3), cf02624497 (KCNIP2), cf02640342 (DPP10), cf02690512 (KCNE1), cf02625138 (KCNE2), cf02646775 (KCNE3), cf02650669 (KCNE4), cf02698674 (IRX5), cf02657295 (SMYD1), cf0263023048 (KCNA5), cf02659079 (β2-microglobulin), and cf02629556 (Cyclophilin B). The primers and probes targeting KCND2, KCNA4, KCNE5, DPP6, and HPRT were designed and synthesized by Applied Biosystems, following submission of intron spanning sequences using Primer Express 3.0 software. Thermal profile for all the real-time PCR reactions was as followed: 50°C (2 min), 95°C (10 min) and then 40 cycles with 95°C (15 sec) and 60°C (1 min).
Prior to the experiments, PCR efficiency for each assay was calculated using a standard curve constructed by plotting range of log cDNA input against Ct (threshold cycle) value. The slope of the plot was used to calculate the percentage amplification efficiency (PE). PE values ranged between 90% and 110% and an amplification efficiency of 2 (100%) per cycle has therefore been used in all the calculations. For each tissue specimen, each gene was quantified in triplicates. Three reference genes were tested: Cyclophilin B, HPRT, and β2-microglobulin. Quantification of these three genes gave almost similar result and Cyclophilin B was chosen for normalization. Normalized gene expression levels were calculated by the 2ΔΔCt method [22]. Delta cycle threshold (ΔCt) values were calculated by subtracting the Ct value of a target gene from the Ct value of Cyclophilin B for each sample. Values for the relative expression were obtained by using the following formula: 1/(2−ΔCt)*100.
Western blotting
Epi, Mid and Endo tissue from 5 dogs was snap-frozen in liquid nitrogen and stored in −80 C prior to protein isolation. Total membrane and cytosolic proteins were isolated using a method modified after Han et al. [23]. Briefly, the tissue (20-30 mg) was pulverized in liquid nitrogen and suspended in 500 μL of ice-cold TE buffer (containing Tris 20 mM, EDTA 1 mM supplemented with a cocktail of protease inhibitors: 10 μM 4-(2-aminoethyl)benzenesulphonyl fluoride, 0.2 μM leupeptin, 0.4 μM bestatin, 0.15 μM pepstatin A, 0.14 μM M E-64 and 8 pM aprotinin, all from Sigma). The tissue suspension was treated with 2% of Triton X-100 for 2 hours at 4°C, centrifuged at 15 000 g for 15 minutes at 4°C. The soluble fraction was retained and stored in −80 C prior to Western blotting. Protein concentration was measured (in triplicates for each sample) using Bradford method (DC Protein Assay, Sigma). Samples (50 μg/lane) were separated on precast polyacrylamide 4-15% SDS-PAGE gels (BioRad) at 100 V for 2 hours and transferred to hybond-P PVDF membranes (Amersham Biosciences, 0.45 μm) at 400 mA for 2 hours. Membranes were blocked with 5% non-fat milk in TBST (Tris 10 mM, NaCl 150 mM, 0.1% Tween 20, pH=7.4) for 1-2 hour at RT and then incubated overnight at 4°C in monoclonal antibody against KChIP2b (1:200, clone K60/73, obtained from NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the Department of Pharmacology, School of Medicine, University of California, Davis, CA 95616). The proteins were detected by HRP-conjugated donkey anti-mouse antibody (1/10000, Jackson Immunosearch Laboratories) and visualized by ECL staining (Supersignal West Pico Chemiluminescent detection system, Pierce). Immunoblots were exposed (for 5-10 minutes) on hyperfilm ECL (Amersham Biosciences). To assess equal loading, the membranes were stripped in Restore™ Western Blot Stripping Buffer (Pierce) for 20 minutes in RT and re-probed with a mouse anti-actin antibody (MAB1501, Chemicon International). Band density was quantified by Quantity-One software as Gaussian trace quantity. KChIP2 signal was detected as two bands with molecular weight between about 25-32 kDa.
Statistics
Pooled data are presented as Mean±SEM. Statistical analysis was performed using an ANOVA test followed by a Student-Newman-Keuls test or Student t-test, as appropriate, using SigmaStat software. Statistical analysis of RNA and protein expression was performed with a repeated measures ANOVA followed by a Tukey's post test. p<0.05 was considered statistically significant.
RESULTS
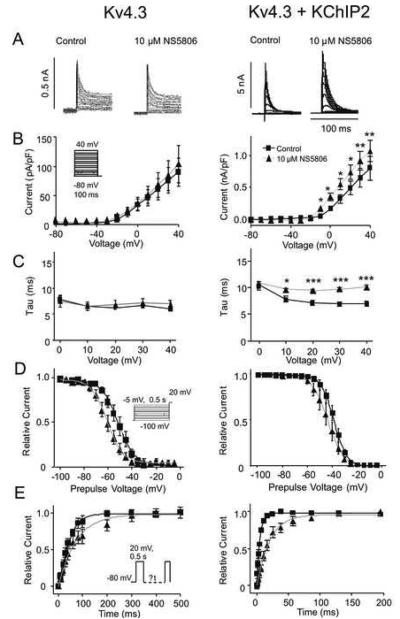
Our previous studies showed that NS5806 activated native Ito in canine ventricular tissue[18]. To address the mechanism of this activation, we tested the effect of the compound on Kv4.3 channels transiently expressed in CHO-K1 cells. As an initial basis of comparison, the enhancement of IKv4.3 by NS5806 (10 μM) was determined in the absence and presence of KChIP2 (Figure 1). Application of NS5806 to CHO-K1 cells expressing Kv4.3 and KChIP2 resulted in a 35 % increase in IKv4.3 peak current amplitude and a dramatic slowing in decay (τ), from 7.0±0.4 to 10.2±0.3 ms (Figure 1A-C). In contrast, application of NS5806 to CHO-K1 cells expressing only Kv4.3 did not change peak IKv4.3 (Figure 1A-B). The decay of the current was unaffected over a range of potentials (Figure 1C). These results suggest that NS5806 can be used to discriminate between currents mediated by KV4.3 alone and KV4.3 together with KChIP2 as the effect of NS5806 is dependent on the presence of KChIP2. We next evaluated the effect on steady state gating parameters using a prepulse-test pulse voltage clamp protocol (Figure 1D). Peak current following a 0.5 sec prepulse was normalized to the maximum current and plotted as a function of the prepulse voltage to obtain the availability of the channels and a Boltzmann function was fitted to the data. NS5806 caused a significant negative shift in the mid-inactivation voltage for KV4.3 from −51.6±0.8 to −60.7±0.8 and the respective slope factors were k=−6.8±0.7 and k=−6.4±0.7 (not significant). For KV4.3 in the presence of KChIP2, mid-inactivation significantly shifted from −38.6±0.1 to −43.6±0.3 mV and the respective slope factors were significantly different with k=−5.1±0.1 and k=−6.3±0.2 (Figure 1D). Recovery from inactivation was addressed by a double-pulse protocol (Figure 1E). For Kv4.3 channels the time constant (τ) was significantly slowed from τ =47.7±3.6 ms to 74.0±7.7 ms in the presence of NS5806. Similar results were found for Kv4.3 in the presence of KChIP2, where NS5806 slowed the recovery from τ=6.1±0.5 ms to 22.8±1.5ms.
Figure 1.
A: Representative traces of Kv4.3 currents recorded in the absence (left, n=6) and presence of KChIP2 (right, n=5-10) under control conditions and in presence of 10 μM NS5806. From a holding potential of −80 mV, cells were stepped to +40 mV in 10 mV increments. Panel B: Mean I-V relations for peak current density Panel C: Mean τ's in absence and presence of NS5806. Panel D: Voltage dependence of inactivation and Boltzmann curves showing mid-inactivation Panel E: Time-dependent recovery from inactivation was evaluated using a standard double pulse protocol from a holding of −80 mV.
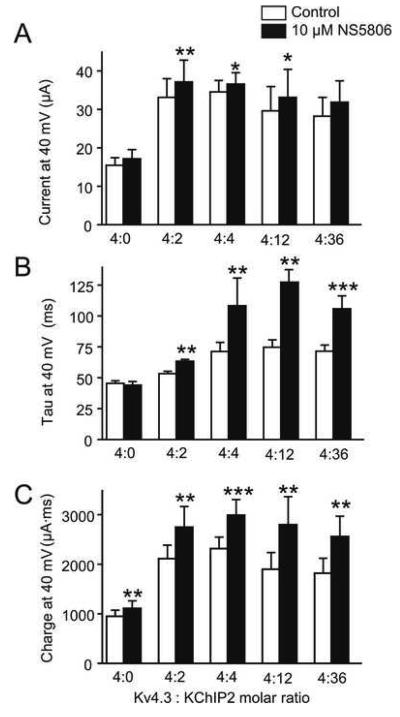
The enhancement of IKv4.3 as well as the slowing of IKv4.3. decay appeared to be dependent on KChIP2 and previous studies have suggested a differential distribution of KChIP2 levels across the canine left ventricle [15,16] In the next series of experiments, the effect of NS5806 on Xenopus laevis oocytes injected with different ratios of Kv4.3 and KChIP2 was evaluated (Figure 2). Application of N S5806 resulted in a significant increase in peak current by 12.1, 5.9 and 11.8% in channels with 4:2, 4:4, and 4:12 ratios respectively (Figure 2A) and resulted in a significant slowing of current decay only in the presence of KChIP2 (Figure 2B). This resulted in an increase in charge movement (Figure 2C). The effect of KChIP2 appeared to saturate at a 4:4 cRNA ratio.
Figure 2.
The effect of NS5806 on different ratios of Kv4.3 and KChIP2 expressed in Xenopus laevis oocytes. Mean data showing peak current (Panel A), decay (τ) of the current (Panel B) and total charge (Panel C). n=7-8 oocytes
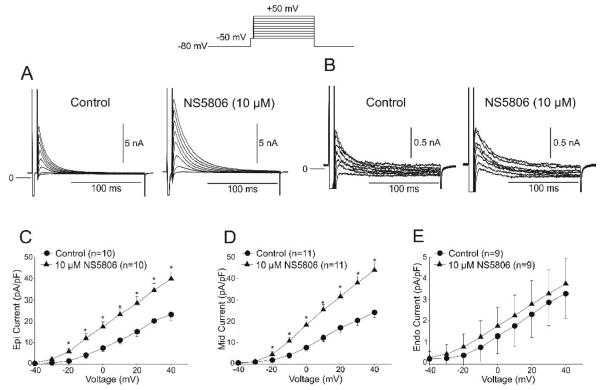
The data obtained on KV 4.3 expressed in CHO-K and Xenopus laevis oocytes could suggests that NS5806 can be used to discriminate whether KChIP2 is present in native Ito channels as KV4.3 current decay was slowed by NS5806 only when KChIP2 was co-expressed. Thus if KV4.3 and KChIP2 interact in ventricular cardiomyocytes we would expect an increase in current and a slowing of the decay. We next determined the effect of the Ito activator NS5806 in Epi Mid and Endo cardiomyocytes. The density of Ito was examined in the absence and presence of 10 μM NS5806. Cd2+ (300 μM) was added to the extracellular solution to block the calcium current (ICaL). Following a brief step to −50 mV to discharge sodium channels, voltage steps from −40 to +50 mV applied to all three cell types elicited fast activating and rapidly inactivating Ito currents. Representative currents measured in Epi and Endo are shown in Figure 3A-B. Application of NS5806 resulted in an increase in the magnitude of Ito as well as slowing the decay of the current. Analysis of the current-voltage (I-V) relation of peak Ito showed that the current density was significantly greater in Epi and Mid cells compared to Endo, as previously reported [3,17]. Application of 10 μM NS5806 significantly increased the peak current amplitude of Ito in Epi and Mid cells but had no effect in Endo cells (Figures 3C-E). Evaluation of Ito in ventricular myocytes showed that application of NS5806 increased the magnitude of current by 80%, 82%, and 16% in Epi, Mid, and Endo myocytes, respectively (at +40 mV).
Figure 3.
Representative Ito traces recorded from an Epi (Panel A) and an Endo cell (Panel B) under control conditions and in the presence of NS5806 (10 μM). The voltage clamp protocol is shown at the top of the figure and Cd2+ was present to block ICaL. Mean I-V relation for peak Ito from Epi (Panel C), Mid (Panel D) and Endo cells (Panel E) in absence and presence of NS5806 (10μM). The density of Ito was greater in Epi and Mid cells and application of NS5806 caused a substantial increase in the magnitude of Ito.
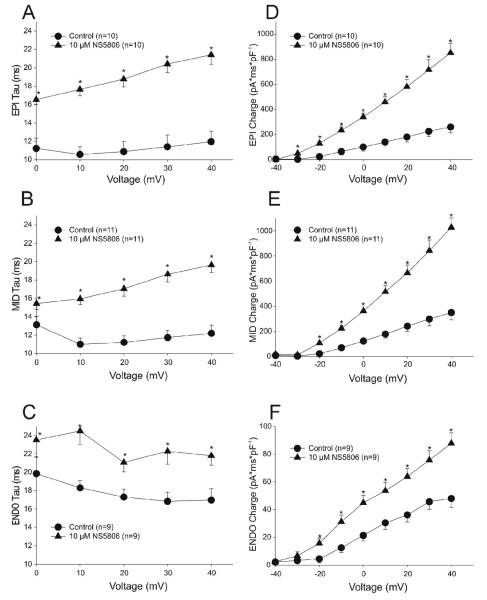
The time constant of decay (τ) of Ito following application of NS5806 was significantly slower in all 3 cell layers (Figure 4A-C). This slowing of Ito coupled with the increase in current magnitude resulted in a marked increase in total charge (assessed as area under the current trace, Figure 4D-F). While the area was significantly increased in all 3 cell layers, Epi and Mid cells showed the greatest effect. NS5806 increased total charge by 227%, 192% and 83% compared to control in Epi, Mid, and Endo cells respectively (at +40 mV, p<0.05).
Figure 4.
Ito decay as a function of voltage for Epi, Mid and Endo cells under control conditions and in the presence of NS5806 (10 μM) (Panels A-C). Panels D-F: Total Ito charge (area under the curve) was increased by NS5806 in all 3 cell layers.
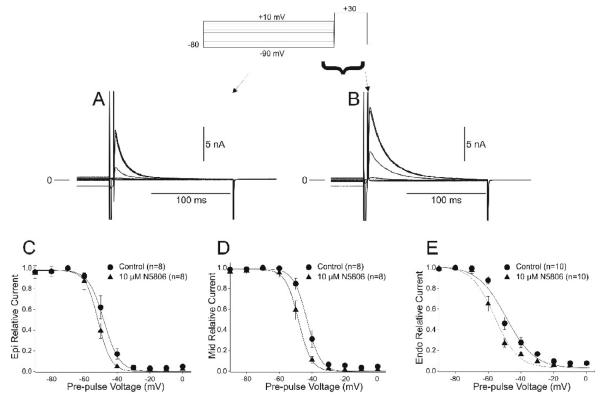
We next determined if the difference in current density between the three cell types was due to changes in steady state gating parameters. Steady state inactivation of Ito was evaluated using a prepulse-test pulse voltage clamp protocol (top of Figure 5) in presence of Cd2+. The peak current following a 2 sec prepulse was normalized to the maximum current and plotted as a function of the prepulse voltage to obtain the availability of the channels and a Boltzmann function was fitted to the data. In the absence of drug, the mid-inactivation voltage and slope factors were −46.0±1.0 mV, k=4.75±0.44 for Epi (Figure 5C), −43.7±0.8 mV, k=4.17±0.41 for Mid (Figure 5D) and −52.9±1.1, k=8.45±1.34 mV for Endo cells (Figure 5E). Application of NS5806 (10 μM) caused a significant shift in the mid-inactivation potential in all cell layers with mid-inactivation voltage and slope factors of −52.0±0.9 mV, k=4.18±0.37 for Epi (Figure 5C), −48.7±0.7 mV, k=4.04±0.59 for Mid (Figure 5D) and −56.3±0.8 mV, k=7.73±1.07 for Endo cells (Figure 5E). The mid-inactivation potential of Ito is significantly shifted to more negative potentials and this shift could not account for the increase in current magnitude observed in the presence of NS5806.
Figure 5.
Representative traces recorded under control conditions (Panel A) and after application of 10 μM NS5806 (Panel B) showing voltage dependence of inactivation of Ito. Boltzmann curves showing mid-inactivation voltages for Epi (Panel C), Mid (Panel D) and Endo (Panel E) cells in the absence and presence of drug.
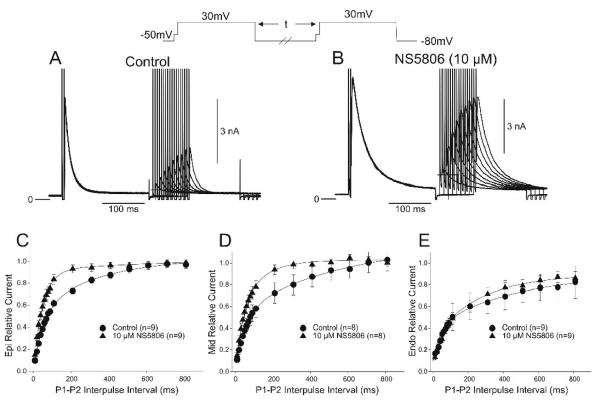
We next tested if Ito recovery from inactivation was different in the three ventricular cell layers. Representative traces of the frequency-dependent changes in Ito in the absence and presence of NS5806 are shown in Figure 6A-B. Reactivation of Ito at −80 mV for all three cell types showed a fast and a slow phase of recovery as follows: i) τ1=43.8±6.7 ms and τ2=256.6±18.9 ms for Epi cells, ii) τ1=39.7±8.3 ms and τ2=278.4±19.2 ms for Mid, and iii) τ1=75.2±8.6 ms and τ2=803.9±79.8 ms for Endo (Figure 6C-E). In addition, under control conditions (absence of NS5806) the recovery of Ito was much slower in Endo cells compared to Epi and Mid cells. In the presence of NS5806, the reactivation of Ito in Epi and Mid cells could be fit with a single exponential and was markedly faster with τ=55.6±1.7 ms for Epi and τ=71.3±2.8 ms for Mid (Figure 6C-D). However, in the presence of NS5806 reactivation time course of Ito in Endo cells was unchanged with τ1=87.2±8.2 ms and τ2=705.6±81.8 ms (Figure 6E).
Figure 6.
Representative traces recorded under control conditions (Panel A) and after 10 μM NS5806 (Panel B) showing recovery of Ito. Cd2+ was present to block ICaL. The recovery time-course of Ito recorded from Epi (Panel C), Mid (Panel D) and Endo (Panel E) cells in the absence and presence of drug.
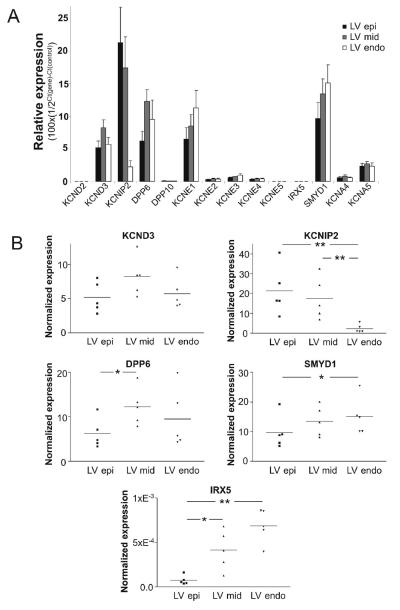
The differential effects of the Ito activator in the 3 ventricular cell layers could suggest that KChIP2 levels may be high in Epi and Mid cells and low in Endo tissue corresponding to the effects of NS5806 on Kv4.3 expressed with different ratios of KChIP2 (Figure 2). However, as KChIP2 slows Kv4.3 current decay and the decay of Endo Ito is slower than that of Epi and Mid (Figure 4), it seems unlikely that transmural differences in Ito kinetics, and response to NS5806 is solely due to differential KChIP2 expression. Other Kv channel subunits may contribute to Ito, in particular in Endo tissue. To test this hypothesis, the expression level of the genes encoding proteins suspected to mediate Ito as well as IKur was investigated (Figure 7). The relative mRNA expression levels of KCND2 and KCND3, encoding Kv4.2 and Kv4.3, respectively, showed an almost 10,000-fold higher expression of KCND3 as compared to KCND2, demonstrating that Kv4.3 is the predominant α-subunit. We also tested the expression of several ancillary subunits and found a high expression of KChIP2, DPP6 and KCNE1. DPP6 was found to be expressed in an approximately 100-fold higher level than DPP10. For the KCNE β-subunits, KCNE1 mRNA was expressed at a high level, KCNE2-4 in intermediate levels, and KCNE5 at a low level. The expression of 2 transcription factors, SMYD1 and IRX5, believed to be important for the Ito gradient in other species [24,25] was also investigated. SMYD1 (Bop) transcripts were found in a much higher abundance than IRX5 transcripts. KCNA4 (Kv1.4) and KCNA5 (Kv1.5) transcripts were found to be expressed in 10% and 30-50%, respectively, of the level of KCND3 (Kv4.3).
Figure 7.
qPCR of left ventricular canine tissue. Epi, Mid, and Endo tissue was isolated from 5 canine left ventricles. Taqman based assays (Applied Biosystems) were used to quantify mRNA of interest which was then normalized to cyclophilin B expression (Panel A). Dot plots of KCND3, KCNIP2, DPP6, IRX5, and SMYD1 expression (Panel B). The expression level values were obtained as described in materials and methods.
Transmural differences in the expression level were found for several genes (Figure 8). As reported previously, there was no gradient for KCND3 and a large gradient for KCNIP2 with higher expression in the EPI compared to the ENDO [15,16]. For DPP6 a difference in expression level was observed between Epi and Mid, with more transcript in the Mid. For both the transcription factors IRX5 and SMYD1 a higher expression level was found in Endo compared to the Epi consistent with suggested function as negative regulators of expression of Ito related genes.
Figure 8.
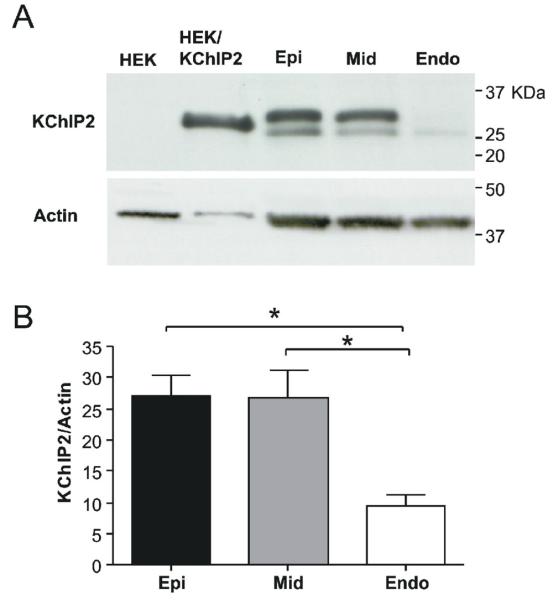
A: Transmural gradient of KChIP2 protein in canine left ventricle. Representative blot of KChIP2 expression in Endo-, Mid- and Epi. KChIP2 expressing and non-transfected HEK-293 cell lysates were included as controls. For Epi, Mid and Endo, two bands sized 25-32 kDa were consistently detected for all protein isolation methods tested. The same blot was re-probed with anti-actin antibody. Panel B: Both KChIP2 bands were quantified and KChIP2 expression normalized to actin expression. Tissue from 5 dogs was analysed.
The transmural gradient of KChIP2 transcript was paralleled at protein level as assessed by Western blotting (Figure 8). We repeatedly obtained two bands, using three other different methods of protein purification (data not shown). Antigenic peptide could not be obtained to assess the specificity of the KChIP2 signal but no unspecific bands were found in HEK-293 cell lysate. As the KChIP2 antibody was raised against a highly conserved region, the two bands could represent different KChIP2 splice variants as previously reported in cardiac tissue [26] and both bands were quantified (Figure 8).
DISCUSSION
Summary of Main Findings
In CHO-K1 cells and in Xenopus laevis oocytes, the effect on IKv4.3 decay by NS5806 was dependent on the presence of KChIP2. In the presence of KChIP2, application of NS5806 increased peak current and slowed current decay. This was reflected as a significant increase in total charge movement in the presence of KChIP2. In canine ventricular cells, Endo Ito was smaller, the decay significantly slower, mid-inactivation voltage was more negative and the recovery from inactivation substantially slower when compared to Mid and Epi Ito. Application of NS5806 caused an increase in the magnitude of Mid and Epi Ito but had no effect on Endo Ito magnitude. In all three layers, the decay of the current was slowed. In the presence od NS5806, recovery from inactivation was faster in Mid and Epi cells but reactivation of Endo Ito was unaffected. These observations could suggest that different subunits contribute to Endo Ito.
Analysis of mRNA expression levels of putative Ito subunits across the canine left ventricular wall revealed a differential distribution of KChIP2 levels with Endo tissue expressing the lowest levels. Kv4.3 levels appeared uniform and no other putative subunits exhibited a transmural gradient. These results suggest that a KChIP2 gradient is responsible for the Ito gradient observed in the canine left ventricle.
Molecular Identification of Ito in the Left Ventricle
The precise molecular identity of Ito in the canine ventricular myocardium remains unclear. It is generally believed that Kv4.3 channels comprise the majority of transient outward K+ channels in canine heart with lower levels of Kv1.4 also being identified [13,27]. Recent evidence suggests that several β-subunits including KChIP2 can alter peak IKv4.3 density, slow decay of the current and accelerate recovery from inactivation [7]. However, the relative abundance of Kv4.3 and KChIP2 in canine ventricle remains controversial. Our qPCR results confirm that the Ito gradient across the left ventricle is likely due to a KChIP2 gradient which was also confirmed at protein level. We also investigated if other putative Ito subunits exhibited a transmural gradient but found no significant differences. For KCNE1 and KCNE3 there was a tendency toward higher expression in Endo compared to Mid and Epi. In CHO-K1 cells, KCNE1 did not significantly affect Kv4.3 + KChIP2 current amplitude [12] whereas in HEK cells, KCNE1 has been demonstrated to increase Kv4.3 currents [28] and thus is an unlikely candidate responsible for the Ito gradient. KCNE3 has been demonstrated to inhibit Kv4.3±KChIP2 current [29,30] which is consistent with the smaller Ito in Endo.
We also observed the presence of Kv1.4 mRNA, in agreement with previous experiments [13]. Interestingly, results of our molecular analysis revealed the presence of Kv1.5 mRNA in higher amounts than that of Kv1.4 as previously reported [13]. While it has been shown that Kv1.5 is abundantly expressed in the atria and is thought to mediate IKur [31], recent studies have suggested that IKur is also present in ventricle [14]. If Kv1.4 and Kv1.5 contribute to Ito, then the relative contribution of Kv1.4 and Kv1.5 may be of greater importance in Endo where KChIP2 mRNA levels are low and the contribution of current generated by Kv4.3 smaller. In our study, the slower τ values as well as the slow recovery from inactivation observed in Endo support this.
Biophysical Analysis of Ito in the Left Ventricle
The electrophysiological properties of Kv4.3 channels are modulated by several β-subunits including KChIP2, which increases peak Kv4.3 current density, accelerates recovery from inactivation, and slows the decay (τ) of the current [16]. In the present study we demonstrated that the effect of NS5806 on heterologously expressed Kv4.3 channels is dependent on KChIP2. Epi and Mid cells had the highest KChIP2 levels and exhibited the largest Ito (Figure 2). Interestingly, the t of decay was faster in Epi and Mid than in Endo as also previously reported [5,32,33]. As KChIP2 slows Kv4.3 current decay, these findings may suggest that Endo Ito is not merely Kv4.3 in absence of KChIP2 and suggests that other subunits may contribute to Endo Ito. In further support of this notion is the observation that the decay of Ito in Epi, Mid and Endo was slowed by NS5806 suggesting that KChIP2 is a functional component of the Ito channels all three cell layers. The currents generated by Kv4.3 and KChIP2 channels did not recapitulate all the features of the native Ito. This is particularly evident when comparing the effect of NS5806 on recovery from inactivation. As also previous studies have demonstrated [5,32,33], the recovery from inactivation is much slower of Endo Ito than of Epi or Mid. The recovery of Epi and Mid Ito was accelerated by NS5806 whereas Endo Ito was unaffected. In contrast, NS5806 slowed recovery for heterologously expressed Kv4.3 independently of co-expression with KChIP2.
In agreement with previous publications, we found that KChIP2 increased Kv4.3 currents in both CHO-K1 cells and Xenopus laevis oocytes. The qPCR demonstrated a low abundance of KChIP2 in the endocardium and this would suggest less Kv4.3 mediated current. As the distribution of KV1.4 and KV1.5 message is uniform across the ventricular wall this would likely result in a greater relative contribution of current mediated by KV1.4 and KV1.5 channels to endocardial Ito. It is tempting to speculate that the relatively larger contribution from KV1.4 or KV1.5 channels to Endo Ito compared to Epi and Mid Ito accounts for the differences in current kinetics as well as response to NS5806.
In summary, we have identified a novel Ito activator that appears to have differential effect on IKv4.3 in the presence or absence of KChIP2. Application of this compound to canine ventricular cells resulted in a differential augmentation on Ito. Our results suggest that the KChIP2 gradient is mainly responsible for the Ito gradient observed in the canine left ventricle. Since Epi and Mid cells have the highest KChIP2 levels, application of NS5806 had the largest effect on the decay of Ito and produced the largest increase in Ito in those 2 cell layers.
ACKNOWLEDGEMENTS
We are grateful to Judy Hefferon, Art Iodice, Trine Christiansen and Bob Goodrow for excellent technical assistance.
FUNDING SOURCES
This work was supported by grants from the Carlsberg Foundation [2006010173 to KC]; the American Health Assistance Foundation [JMC]; the Danish National Research Foundation [SPO]; the National Institutes of Health [HL47678 to CA] and the Masons of New York State and Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Søren-Peter Olesen is consultant to NeuroSearch.
Reference List
- 1.Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: Physiological, pharmacological and clinical implications. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology. Section 2 The Cardiovascular System. Oxford University Press; New York: 2001. pp. 654–692. [Google Scholar]
- 2.Fedida D, Giles WR. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol. 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993;72:671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DL, Rasmusson RL, Qu YH, Strauss HC. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993;101:571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, et al. A Defect in the Kv Channel-Interacting Protein 2 (KChIP2) Gene Leads to a Complete Loss of I(to) and Confers Susceptibility to Ventricular Tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 7.Deschênes I, DiSilvestre D, Juang GJ, Wu RC, An WF, Tomaselli GF. Regulation of Kv4.3 current by KChIP2 splice variants: a component of native cardiac Ito? Circulation. 2002;106:423–429. doi: 10.1161/01.cir.0000025417.65658.b6. [DOI] [PubMed] [Google Scholar]
- 8.Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, et al. The homeodomain transcription factor Irx5 establishes the Mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res. 2004;94:1340–1350. doi: 10.1161/01.RES.0000128406.08418.34. [DOI] [PubMed] [Google Scholar]
- 10.Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative beta-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565:751–756. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001;88:1012–1019. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]
- 12.Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varro A, et al. Functional modulation of the transient outward current Ito by KCNE b-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res. 2006;71:695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Dixon EJ, Shi W, Wang H-S, McDonald C, Yu H, Wymore RS, et al. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar A, da Cunha DN, Lacombe VA, Zhou Q, Fox JJ, Hamlin RL, et al. The plateau outward current in canine ventricle, sensitive to 4-aminopyridine, is a constitutive contributor to ventricular repolarization. Br J Pharmacol. 2007;152:870–879. doi: 10.1038/sj.bjp.0707403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol. 2003;548:815–822. doi: 10.1113/jphysiol.2002.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, et al. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–748. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, et al. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81:686–694. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordeiro JM, Greene L, Heilmann C, Antzelevitch D, Antzelevitch C. Transmural heterogeneity of calcium activity and mechanical function in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2004;286:H1471–H1479. doi: 10.1152/ajpheart.00748.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cordeiro JM, Malone JE, Di Diego JM, Scornik FS, Aistrup GL, Antzelevitch C, et al. Cellular and subcellular alternans in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2007;293:H3506–H3516. doi: 10.1152/ajpheart.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumaine R, Cordeiro JM. Comparison of K(+) currents in cardiac Purkinje cells isolated from rabbit and dog. J Mol Cell Cardiol. 2007;42:378–389. doi: 10.1016/j.yjmcc.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Han W, Bao W, Wang Z, Nattel S. Comparison of ion-channel subunit expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- 24.Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, et al. The homeodomain transcription factor Irx5 establishes the Mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosati B, Grau F, McKinnon D. Regional variation in mRNA transcript abundance within the ventricular wall. J Mol Cell Cardiol. 2006;40:295–302. doi: 10.1016/j.yjmcc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Decher N, Uyguner O, Scherer CR, Karaman B, Yuksel-Apak M, Busch AE, et al. hKChIP2 is a functional modifier of hKv4.3 potassium channels: cloning and expression of a short hKChIP2 splice variant. Cardiovasc Res. 2001;52:255–264. doi: 10.1016/s0008-6363(01)00374-1. [DOI] [PubMed] [Google Scholar]
- 27.Akar FG, Wu RC, Deschênes I, Armoundas AA, Piacentino V, III, Houser SR, et al. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286:H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 28.Deschênes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 29.Lundby A, Olesen SP. KCNE3 is an inhibitory subunit of the Kv4.3 potassium channel. Biochem Biophys Res Commun. 2006;346:958–967. doi: 10.1016/j.bbrc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Delpón E, Cordeiro JM, Núñez L, Thomsen PEB, Guerchicoff A, Pollevick GD, et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, et al. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- 32.Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, et al. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]