Abstract
In humans, taurine (2-aminoethanesulfonic acid) is mainly obtained from diet. Despite the fact that the health effects of taurine are largely unknown, taurine has become a popular supplement and ingredient in energy drinks in recent years. Evidence from mechanistic and animal studies has shown that the main biological actions of taurine include its ability to conjugate bile acids, regulate blood pressure (BP), and act as a potent antioxidant and anti-inflammatory agent. These actions suggest that high levels of taurine may be protective against coronary heart disease (CHD). However, data from epidemiologic and intervention studies in humans are limited. We review what is known about taurine’s metabolism, its transportation in the body, its food sources, and evidence of its effect on cardiovascular health from in vitro, animal, and epidemiologic studies. We also discuss shortcomings of the human studies that need to be addressed in the future. The identification of taurine as a preventive factor for CHD may be of great public health importance.
Keywords: Taurine, coronary heart disease, cholesterol, blood pressure, antioxidant
Introduction
Coronary heart disease (CHD) has decreased in the US as a result of preventive measures such as smoking cessation and treatment of hypertension, dyslipidemia, diabetes mellitus, and obesity. However, CHD remains the single largest killer of American men and women, with an estimate of 8.7 million US men and 7.3 million US women affected by CHD in 2005 (1). Identification of new factors that may help reduce incidence of CHD could have an important public health impact.
Diet can influence heart health, and recent evidence strongly supports the idea that beneficial dietary factors such as fruits, vegetables, legumes, whole grains, and vegetable oils should be consumed adequately. In humans, diet is the main source of taurine (2-aminoethanesulfonic acid), a sulfur-containing molecule (Figure 1). Smaller amounts of taurine are also synthesized endogenously in the liver from methionine and cysteine. Taurine exists freely in cytosol and is most abundant in the heart, retina, developing brain and blood (Table 1). Today, taurine is a key ingredient in “energy” drinks such as Red Bull (1000 mg), Monster (2000 mg), and Rockstar (3000 mg), although there is no evidence of taurine’s effects on physical activity. The high concentration of taurine in these popular drinks, however, underscores the importance of evaluating the potential health implications of taurine. We review the taurine content of different foods and the metabolism and transport of taurine in the body. We examine evidence from in vitro, animal and human studies on the potential of taurine in protecting against CHD. We also discuss the shortcomings of previous human studies and suggest future directions.
Figure 1. Taurine Structure.
Table 1. Taurine Concentrations in Various Tissues.
| Tissue Type | Taurine Concentration* |
|---|---|
| Human | |
| Brain | |
| Developing | 4-20 μmol/g (40) |
| Adult | 1-9 μmol/g (40) |
| Heart | 6 μmol/g (41); 15-25 μmol/g (42) |
| Liver | 2 μmol/g (41) |
| Skeletal muscles | 5 μmol/g (41) |
| Retina | 30-40 μmol/g (41) |
| Plasma | 50-80 μmol/L (41, 42); 100 μmol/L (43) |
| Leukocytes & platelets | 13-17 μmol/L (44); 10-50 μmol/L (43) |
| Rat | |
| Brain | 3 μmol/g (41); 5 μmol/g (45, 46) |
| Heart | 20 (46); 30 μmol/g (47) |
| Liver | 3 μmol/g (41); 4 μmol/g (45, 46) |
| Skeletal muscles | 7μmol/g (41); 16 μmol/g (45) |
| Retina | 27 μmol/g (41); 50 μmol/g (47) |
| Plasma | 360 μmol/L (45); 450 μmol/L (41) |
| Kidney | 7 μmol/g (46); 9 μmol/g (45) |
Units for some values have been changed from those originally published for uniformity
Taurine metabolism and transport in humans
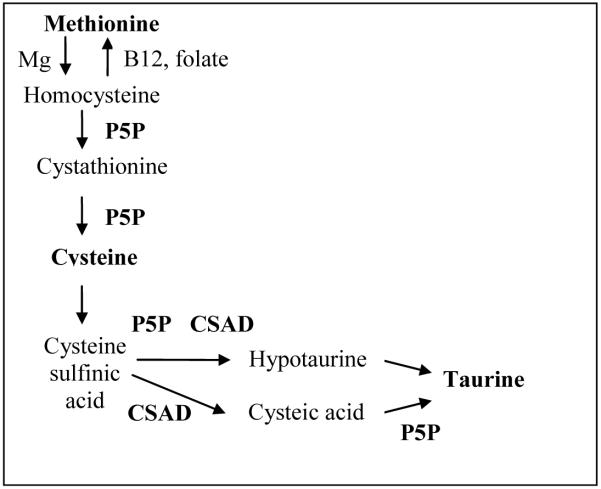
Synthesis of taurine begins in the liver with a magnesium-catalyzed methylation of methionine to form homocysteine, a process which can be reversed by the vitamin B12 and the folate dependent enzyme methionine synthetase (Figure 2 adapted from (2)). Next, homocysteine donates its sulfur group to form cystathionine and under the influence of pyridoxal-5′phosphate (P5P) cystathionine is broken down to cysteine. Cysteine, catalyzed by cysteine deoxygenase, combines with dioxygen to become cysteine sulfinic acid, which is then decarboxylated by cysteine sulfinic acid decarboxylase (CSAD) and P5P to hypotaurine. Hypotaurine is oxidized to taurine by hypotaurine dehydrogenase. Alternatively, taurine is formed following the oxidation of cysteine sulfinic acid to cysteic acid and the decarboxylation of cysteic acid by P5P (3).
Figure 2. Synthesis of Taurine.
(adapted from (2))
Humans have a low level of CSAD, and, therefore, obtain most of their taurine from foods (4). Taurine obtained from food is absorbed by the small intestine. After absorption, carrier-mediated active transport in the brush border membrane moves taurine to enterocytes, which deliver it to the portal vein (5). Taurine is then transported to the liver and released into circulation and can then enter cells via the taurine transporter (TauT), which in turn responds to the concentration of taurine in cells (6). A high concentration of taurine downregulates TauT, and taurine is excreted from the body in urine. Conversely, when the taurine concentration is low, TauT is upregulated and taurine is reabsorbed into circulation through the renal tubules in the kidney.
Taurine level in foods
The mean content of taurine in selected foods is shown in Table 2. Overall, low amounts of taurine are found in dairy, such as ice cream and cow’s milk. The highest amounts of taurine can be found in shellfish, especially scallops, mussels, and clams. High amounts of taurine can also be found in the dark meat of turkey and chicken, and turkey bologna. Cooking has been shown to have no adverse effect on taurine levels (7), and taurine values from the same food sources are fairly consistent across different studies. The mean daily taurine intake for adult human non-vegetarians has been estimated between 40 and 400 mg (8), typically falling closer to the lower end of the range. However, the amount of taurine bioavailable in humans after consuming foods containing taurine is not known. In human trauma patients, a dose-response was found between taurine given intravenously at 0-50 mg/kg and the amount of taurine in serum (9).
Table 2. Taurine Amounts in Foods.
| Food | Method of Preparation | Mean Taurine Content mg/100g* (SEM)† |
|---|---|---|
| Beef | Raw | 43.1 (7.6) (48); 46.3 (4.6) (49) |
| Broiled | 38.4 (9.7) (48) | |
| Chicken dark meat | Raw | 82.6 (4.6) (49); 169.6 (37.4) (48) |
| Broiled | 199.1(27.4) (48) | |
| Chicken light meat | Raw | 17.5 (0.4) (49); 17.8 (3.3) (48) |
| Broiled | 14.5 (3.9) (48) | |
| Turkey dark meat | Raw | 306 (69) (48) |
| Roasted | 299.6 (52.2) (48) | |
| Turkey light meat | Raw | 29.5 (6.9) (48) |
| Roasted | 11.1 (1.1) (48) | |
| Veal | Raw | 39.8 (12.5) (48) |
| Broiled | 46.7 (10.3) (48) | |
| Pork, loin | Raw | 50.1 (3.8) (49); 61.2 (10.6) (48) |
| Roasted | 56.8 (11.5) (48) | |
| Lamb dark meat | Raw | 43.8 (4.1) (49); 47 (50) |
| Ham, picnic | Baked | 49.8 (5.8) (48) |
| Salami, cotto beef | Cured | 59.2 (7.8) (48) |
| Bologna, pork/beef | Cured | 31.4 (4) (48) |
| Bologna, turkey | Cured | 122.7 (5.3) (48) |
| Tuna, albacore | Canned | 41.5 (12.8) (48) |
| Tuna, chunk light | 39 (11.8) (48) | |
| White fish | Raw | 113.9 (13) (49); 151.2 (22.9) (48) |
| Cooked | 172.1 (53.6) (48) | |
| Shrimp, small | Cooked | 10.5 (1.4) (48) |
| Shrimp, medium | Raw | 39.4 (12.8) (48); 155.2 (3.8) (49) |
| Mussels | Raw | 655.4 (72) (48) |
| Oysters | Fresh | 70 (50); 396.7 (29) (48) |
| Cod | Frozen | 31 (50) |
| Clams | Raw | 240 (50); 513.1 (50.1) (49); 520.7 (97.4) (48) |
| Canned | 152 (50) | |
| Octopus | Raw | 388 (12.5) (49) |
| Scallop | Raw | 827.7 (15.4) (48) |
| Squid | Raw | 356.7 (95) (48) |
| Cow’s milk | Unprocessed | <0.5 (7) |
| 3.5% fat, whole | 2.4 (0.3) (48) | |
| 2.0% fat, low fat | 2.3 (0.2) (48) | |
| 0.5%, non-fat | 2.5 (0.3) (48) | |
| Nonfat, dried | 7.0 (48) | |
| Yogurt, low-fat plain | 3.3 (0.5) (48) | |
| Yogurt, low-fat peach | 7.8 (0.9) (48) | |
| Ice cream/vanilla | 1.9 (48) | |
| Pasteurized milk | 6 (50) |
Units for some values in Table 2 have been adapted from those previously published for uniformity
SEM = standard error of the mean
Mechanisms of taurine protection against CHD
We review data from in vitro, animal, and limited human studies of the ability of taurine to conjugate bile acids, regulate blood pressure (BP), and reduce oxidative stress and inflammation.
Lipid detoxification
In vitro and animal studies
Taurine’s main function in the body is the conjugation of cholesterol into bile acids, changing cholesterol’s solubility and enabling its excretion. This process can be accelerated through the upregulation of 7-alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in the production of bile acids (10). Taurine has been shown to have time- and dose-response effects on CYP7A1 mRNA levels in Hep G2 cells (human hepatoblastoma cells used to study cholesterol function). In these cells, the level of CYP7A1 mRNA increased with increasing concentrations of taurine (2, 10 and 20 mmol/L) both in the presence and absence of 0.2 mmol/L cholesterol. Furthermore, the expression of CYP7A1 was significantly greater 4 hours after taurine treatment than in cells without taurine treatment, and continued to significantly increase at 24 and 48 hours (11), suggesting that the effect of taurine may be sustained.
The cholesterol profiles of rats, mice, hamsters, guinea pigs, and rabbits have all been shown to be affected by taurine. For example, taurine supplementation of 0.25 - 50g/kg for two weeks led to significant dose-dependent attenuation in the increase of serum cholesterol in Wistar rats fed a diet high in cholesterol compared to a group fed a high cholesterol diet without supplementation. This effect has been attributed to an increased level of CYP7A1 mRNA in the liver observed in the taurine supplemented group (12).
Taurine may also decrease cholesterol levels through an upregulation of the hepatic low density lipoprotein receptor (LDLR) and/or through an improvement in the binding of LDL to LDLR. Golden Syrian hamsters fed a high fat diet supplemented with 1% taurine for two weeks compared to unsupplemented hamsters had significantly reduced serum total cholesterol (317 versus 543 mg/dL) and LDL+VLDL cholesterol (213 versus 460 mg/dL). Radiolabelled LDL tracers in the body revealed that taurine upregulated the activity of LDLR, increased LDL uptake by the liver, and increased LDL turnover in blood (13). C57BL/6 mice fed a high fat diet supplemented with 1% taurine for one month showed a significant decrease, compared to control mice fed a high fat diet without taurine, in total serum cholesterol (126 versus 181 mg/dL, respectively) and LDL+VLDL cholesterol (70 versus 120 mg/dL, respectively). However, liver LDLR protein levels measured by Western blot showed no difference between the two groups (14). Additional studies are needed to clarify taurine’s role in regulating LDL.
Human studies
The effects of taurine on lipid levels were examined in several small randomized trials (Table 3). A single-blind study of 22 healthy male Japanese volunteers between the ages of 18-29 (15) examined the effects of 6 g/day of taurine supplementation versus placebo on participants’ lipid profiles. Volunteers were placed on a three-week diet designed to increase their cholesterol levels. The control group had a statistically significant increase in total cholesterol, LDL cholesterol, and LDL, while the corresponding increases of the taurine supplemented group were smaller and not statistically significant. However, it is unknown whether the beneficial effects of taurine would only be seen among individuals with high-fat diet.
Table 3. Human Studies Assessing the Association of Taurine with Heart Disease and CHD Risk Factors.
| Citation | Country | Study Design | Dosage | Sample Size & Characteristics | Age (years) | Endpoint | Findings |
|---|---|---|---|---|---|---|---|
| Mizushima, 1997 (26) | Japan & Brazil | Cross-sectional | N/A | 433 Japanese in Japan 269 Japanese in Brazil | 45-59 | Hypercholesterolemia Hypertension | Hypercholesterolemia prevalence*: Men: 5.8% in Japan vs. 28.3% in Brazil† Women: 19.0% in Japan vs. 22.1% in Brazil‡ Hypertension prevalence*: Men: 20.0% in Japan vs. 26.7% in Brazil‡ Women: 14.0% in Japan vs. 32.0% in Brazil† |
| Liu, 2001 (25) | China | Cross-sectional | N/A | 775 Han 510 Uygur 204 Kazak 125 Tibetan | 49-54 | Partial correlation coefficient of taurine excretion with BP |
Han: SBP r = -0.06‡;DBP r = -0.12§ Uygur: SBP r = -0.01‡; DBP r = -0.09‡ Kazak: SBP r = -0.09‡; DBP r = -0.04‡Tibetan: SBP r and DBP r = -0.25§ |
| Yamori, 2001 (36) | 16 countries | Ecologic | N/A | 1,352 males 1,382 females | 48-56 | Urinary taurine excretion vs. age-adjusted IHD mortality rates | Men, β = -0.38 per 100,000/μmol/day* Women, β = -0.15 per 100,000/μmol/day* |
| Yamori, 2006 (51) | 16 countries | Ecologic | N/A | 2,462 males | 45-74 | Urinary taurine excretion vs. age-adjusted IHD mortality rates | Men, β = -0.3 per 100,000/μmol/day* |
| (Taurine vs. Placebo) | |||||||
| Fujita, 1987 (27) | Japan | RCT | 6g taurine or placebo/day for 1 wk | 19 borderline hypertensives | 20-25 | Δ SBP Δ DBP Δ Epinephrine | Δ SBP = -9.0 vs. -2.7 mmHg Δ DBP = -4.1 vs. -1.2 mmHg Δ Epinephrine = -14.6 vs. -1.9 pg/ml† |
| Milei, 1992 (35) | Argentina | RCT | 5g taurine or placebo 1-3 hrs before CABG | 12 patients with stable angina | 30-60 | Δ Oxidative stress | Ratio of reperfusion and preischemic sample means = 1.12 vs. 2.45* |
| Mizushima, 1996 (15) | Japan | RCT | High cholesterol diet with 6g taurine or placebo/day for 3 wks | 22 male volunteers | 18-29 | Δ Total cholesterol Δ LDL-cholesterol Δ LDL Δ Norepinephrine |
Δ Total cholesterol = 22.1 vs. 25.4 mg/dl† Δ LDL-C = 6.7 vs. 17.1 mg/dl† Δ LDL = 28.1 vs. 43.9 mg/dl† Δ Norepinephrine = 7 vs. 35 μg/day§ |
| Zhang, 2004 (16) | China | RCT | 3g taurine or placebo/day for 7 wks | 30 overweight or obese college students | 18-23 | Δ Triglyceride Δ Total cholesterol Δ HDL-C Δ Atherogenic ‡ |
Δ Triglyceride = -8.12 vs. +3.09 mg/dL § ** Δ Total cholesterol = -9.67 vs. 0 mg/dL † ** Δ HDL-C = +3.09 vs. -0.39mg/dL † ** Δ Atherogenic index = -0.45 vs. -0.08†† |
Hycholesterolemia and hypertension prevalences are weighted averages calculated from original article
P < 0.001
Not significant
P < 0.05
P < 0.01
P < 0.05
P < 0.001
No significant change
Atherogenic index = [(TC – HDL-C)/HDL-C]
P < 0.05
Converted from mmol/L to mg/dL
P < 0.01
In a double-blind randomized study of 30 overweight or obese college students (body mass index [BMI] ≥ 25), who received either 3 g/day of taurine supplementation or placebo for seven weeks, average changes in lipid levels over time in the treatment group were compared with those in the placebo group. At baseline, there were no differences in any parameters between the two groups. After seven weeks of supplementation, plasma triglycerides decreased by 8 mg/dL in the taurine supplemented group, and increased by 3 mg/dL in the placebo group. These changes were statistically significantly different between the two groups (p = 0.04). Additionally, the atherogenic index [(total cholesterol - HDL cholesterol)/HDL cholesterol] was reduced in the taurine supplemented group (2.75 to 2.30) after seven weeks, and this reduction was statistically significantly different from the changes in the placebo group (2.91 to 2.99). Changes in other measures such as total cholesterol and HDL-cholesterol were not statistically different between the two groups (16). These findings suggest that taurine may reduce triglyceride levels; however, the study’s limitations, including small sample size, with only 15 participants in each arm, short length of supplementation, and baseline health status of the participants, call for future large studies to confirm the results.
Effects on BP
In vitro and animal studies
The main mechanism through which taurine may decrease BP is thought to be the attenuation of angiotensin II signaling, which causes vasoconstriction and consequently increases BP (17). Taurine may also reduce BP through enhancement of the kinin-kallikrein system in the kidney that causes vasodilation (18). Taurine may also lower BP by decreasing levels of epinephrine (which increases heart rate) and norepinephrine (which causes vasoconstriction). In hypertensive rats supplemented with 1.5% taurine in drinking water for eight weeks, the mean plasma norepinephrine level in the taurine supplemented rats was 383 pg/mL, significantly lower than in the control rats (615 pg/mL). There was also a significant difference between the mean epinephrine level in the taurine supplemented rats (232 pg/mL) compared to the control group (892 pg/mL) (19).
Taurine supplementation effectively controlled high BP in the most common animal models of hypertension including: spontaneously hypertensive rats (SHR) (19), deoxycorticosterone acetate-salt rats (DOCA-salt /Sprangue-Dawley) (20), salt-sensitive Dahl-S rats (21), renovascular hypertensive rats (22), and hyperinsulinemic rats (Wistar) (23). For example, hypertension in SHR and SHR stroke-prone (SHR-SP) rats was significantly attenuated by the addition of 3% taurine to the drinking water. After 72 days of the experiment, the difference in BP between the control group and the SHR-SP group was 30 mmHg (24).
Human studies
Analyses from the WHO Cardiovascular Diseases and Alimentary Comparison (WHO-CARDIAC), a multi-center cross-sectional study, have suggested an inverse correlation between urinary excretion of taurine and BP (Table 3, (25)). After adjusting for age, sex and potassium levels, a study of different ethnic Chinese populations found a significant inverse correlation between 24 hour taurine excretion and diastolic BP in 755 Han participants and a significant inverse correlation between 24 hour taurine excretion and both diastolic and systolic BP in 125 Tibetan participants. The Uygur or the Kazak populations showed small, nonsignificant negative correlations between 24 hour taurine excretion and both systolic and diastolic BP (25). One major limitation of the study is its cross-sectional design in which taurine and BP status were measured at the same time, making it difficult to know which temporally preceded the other. Potential confounders that are related to both taurine level and BP were not considered in the study. In addition, population-specific factors that led to differences in the correlations among ethnic groups were not investigated.
An inverse correlation between BP and taurine excretion has also been seen in Japanese immigrants in Brazil (26). In this cross-sectional study, a population based-sample of 433 middle-aged Japanese in Shimane and Okinawa, Japan, and 269 Japanese immigrates from Shimane and Okinawa to Brazil showed that native Japanese had a significantly greater urinary excretion of taurine compared to Japanese immigrants in Brazil. This observation was consistent with a gradient in the prevalence of hypertension and hypercholesterolemia with lower prevalence in the Japanese living in Japan compared to the Japanese immigrants living in Brazil (26), suggesting the environment and not genetics as the source of the prevalence gradients, including a possible role of taurine intake. Although the study acknowledged differences in diet, it did not discuss other lifestyle differences between the two groups. Since this study used prevalence rather than incidence data, the temporal sequence of events could not be established.
In a double-blind, placebo-controlled trial of 19 borderline hypertensive patients between the ages of 20 and 25 (27), 6g of taurine supplementation/day significantly decreased systolic and diastolic BP over time, whereas in the placebo group BP did not change significantly. Furthermore, plasma epinephrine levels in the taurine treatment group decreased significantly but remained at a similar level in the placebo group. Norepinephrine levels decreased non-significantly in both the taurine supplemented and placebo groups (27). Although the results suggest protective effects of taurine, statistical testing was not conducted to directly compare longitudinal changes in the treatment and placebo groups. Other limitations of the study are its small sample size, with only ≤ 10 participants in each of the study groups, short length of supplementation (7 days), and the fact that participants had preexisting borderline hypertension, limiting the generalizability of the results to healthy individuals. Nevertheless, the findings on norepinephrine were consistent to the previously discussed study by Mizushima et al (15), in which urinary norepinephrine levels increased significantly in individuals given a high cholesterol diet without taurine supplementation but did not change significantly in individuals fed a high cholesterol diet supplemented with taurine.
Anti-oxidation and anti-inflammation
In vitro and animal studies
Atherosclerosis is recognized as a chronic inflammatory process resulting from oxidation and oxygen radicals. Oxidant activity, measured by thiobarbituric acid reactive substances (TBARS), was significantly less in the plasma of male Wistar rats fed a high fat diet supplemented with 50 mg/kg/day taurine for six months (1.6 nmol/ml) compared to rats fed a high fat diet without taurine supplementation (2.4 nmol/ml) (28). Serum TBARS were also significantly lower in apolipoprotein E-deficient mice after 2% taurine supplementation for 12 weeks (8.6 nmol/mL), compared to mice without taurine supplementation (11.1nmol/mL) (29).
Taurine is also known to react with hypochlorous acid (HOCl), a powerful oxidant, to create a more stable taurine chloramine (TauCl) in vivo to block the production of proinflammatory cytokines. For example, 0.4 mM TauCl added to adherent leukocytes taken from healthy volunteers and activated with lipopolysacharides (LPS) significantly reduced the amount of interleukin-6 (IL-6) produced. In murine peritoneal neutrophils with acute inflammation activated by recombinant interferon-γ (INF-γ) and LPS, TauCl in concentrations ranging from 0.03-0.3 mmol/L significantly inhibited the production of IL-6 in a dose-dependent manner (30).
The adhesion of circulating leukocytes to endothelial cells and their transendothelial migration is an initiating step of atherosclerosis (31). The expression of intracellular adhesion molecule-1 (ICAM-1), which mediates cell-cell adhesion, was decreased by taurine in Sprague-Dawley rats with impaired reactive oxygen species (ROS) scavenging capability. Taurine given intravenously at 200 mg/kg for 5 days before the induction of inflammation prevented a significant increase in the expression of ICAM-1 in the post-capillary venular (high endothelial cell region) (32).
The production of tumor necrosis factor-α (TNF-α), an important pro-inflammatory cytokine, has been shown to be downregulated by taurolidine, a derivative of taurine. Taurolidine blocked the production of TNF-α by 50-90% in human peripheral blood mononuclear cells from healthy donors stimulated by LPS and INF-γ (33). Additionally, the amount of TNF-α released from mouse macrophage-like RAW 264.7 cells was reduced in a dose-dependent manner by TauCl given in concentrations ranging from 0.2 - 1 mmol/L (34).
Human studies
Taurine’s antioxidant activity in humans has received little attention. In a placebo-controlled trial of 12 patients with stable angina (35), intravenous infusion of 5g taurine one to three hours before coronary artery bypass surgery reduced the level of lipoperoxidation products, an indicator of ROS, during reperfusion (restoration of blood flow). The mean oxidative stress ratio comparing reperfusion to pre-operative biopsy samples was 1.12 in the taurine pretreated group versus 2.45 in the placebo group (35). Larger studies are needed to evaluate the effect of taurine in healthy individuals.
Human studies of taurine and heart disease
Table 3 includes two other human studies of the association between taurine and heart disease. The WHO-CARDIAC study (36, 37), which recruited random samples of men and women 48 to 56 years of age from 24 study centers in 16 countries, investigated the ecological correlation between dietary factors and ischemic heart disease (IHD). As expected, urinary taurine levels were highest in Japanese men (2,180.6 μmol/day) and women (1,590.0 μmol/day), who had the greatest seafood consumption, and lowest in Canadian men (191.6 μmol/day) and Russian women (127.5 μmol/day). A significant inverse correlation was found between the group-level median value of urinary taurine excretion and age-adjusted IHD mortality rates in the study areas, both in men and women. The associations remained significant after adjustment for serum total cholesterol, BMI and urinary sodium to potassium excretion ratios (36). A separate analysis of the male participants in the 16 countries found age-adjusted IHD mortality rates in the area populations to be significantly negatively associated with the average urinary taurine excretion after adjusting for group means of BMI, total cholesterol, urinary sodium/potassium ratio, polyunsaturated fatty acids, and polyunsaturated fatty acids/saturated fatty acids ratio(37). However, the findings of this study are subject to ecologic fallacy because it is unknown whether the individuals who died of IHD actually had low levels of urinary taurine excretion, and adjustment of group means of potential confounders may not address the confounding effects at the individual level. Also, other potential confounders related to both CHD and taurine intake such as smoking status, physical activity, and socioeconomic status were not considered. Additionally, urinary taurine levels are unstable and may be highly dependent on daily food consumption which may be influenced by seasonal changes in food availability.
Discussion
In summary, animal and in vitro studies have provided insights into the mechanisms by which taurine can improve lipid profile, lower BP, and act as an antioxidant and anti-inflammation agent, suggesting great potential of taurine in improving the profile of cardiovascular risk factors and reducing occurrences of cardiovascular disease. A few small clinical trials and observational studies in humans have also suggested short-term benefits of taurine supplementation on lipid and BP profiles.
The data from existing human studies indicate that taurine may confer substantial benefits in reducing the risk of CHD on the population level. For instance, based on a meta-analysis of individual data for one million adults in 61 prospective studies, a 2 mmHg lower usual SBP would decrease stroke mortality by 10% and IHD or other vascular cause mortality by 7% in the middle aged (38). A clinical trial of taurine supplementation showed that taurine reduced blood pressure 6 mmHg more than placebo (27). However, several limitations of the existing studies should be considered, including: 1) ecologic study design with analyses based on group-level data; 2) small sample sizes in the randomized clinical trials, with fewer than 30 participants in all of them; 3) characteristics of the study populations, including subjects with existing CHD, hypertension, or obesity; and 4) short-term duration of taurine supplementation in the randomized clinical trials (≤ 2 months); and 5) lack of information on potential confounders in observational studies. Future observational epidemiologic studies that address these limitations are needed to evaluate long-term human health effects of taurine on BP, cholesterol profile, and other risk factors for CHD.
Currently no prospective epidemiologic studies have been conducted to investigate taurine’s possible association with CHD incidence. One of the reasons for this could be the lack of a reliable measure of long-term taurine level. Use of questionnaires to estimate taurine dietary intake is difficult because the content of taurine differs appreciably by type of seafood and cut of meat, posing a challenge in calculating taurine intake from diet questionnaires. Biochemical measurements of taurine reflecting an “internal dose” would be more accurate. However, it is important to evaluate to what extent the level measured in urine or blood samples fluctuates over time before using these measurements in large epidemiologic studies. In addition, lifestyle or other nutritional factors that may be related to both taurine levels and cardiovascular outcomes are largely unknown. These data are needed to support the validity of findings in epidemiologic studies of taurine and CHD.
Although no minimum level of intake with adverse effect has been set for taurine, a recent risk assessment study designated the upper level of taurine supplementation at 3 g per day. This assessment was based on toxicological evidence from a review of all human clinical trials with taurine supplementation (39). The only adverse effects noted after consuming a 3 g dose of taurine were gastrointestinal disturbances. It should be noted that the minimum dose used in the existing trials was 3 g/day, much greater than the usual intake of taurine from diet (< 0.4 g/day). However, an inverse association between taurine and CHD-related outcomes has been reported in ecologic studies without taurine supplementation, suggesting that potential beneficial effects of taurine may exist at lower levels. Future studies are needed to evaluate the full dose-response relationship between taurine intake and CHD-related outcomes. Although some “energy drinks” contain high levels of taurine (> 1 g/serving), they also contain high amounts of caffeine and other ingredients; therefore, health effects relating to their use should be evaluated separately.
The relationship between dietary sources of taurine and the biochemical availability of taurine in the human body await research investigation. For example, knowledge of the specific equation relating food intake to serum level of taurine would be useful if taurine has preventive effects. In addition to taurine, fish and shellfish may contain other nutrients or environmental contaminants that may influence heart health, including cholesterol, omega-3 fatty acids, mercury, PCBs (polychlorinated biphenyls), and dioxins. Understanding taurine’s role in CHD etiology may help improve current dietary guidelines for CHD. Further research will be needed to evaluate whether taurine is beneficial for subgroups in the population with high risk of CHD, or those who do not or cannot regularly consume meat or seafood.
In conclusion, considering the in vitro, animal, and human studies reviewed, there are several plausible mechanisms through which taurine may decrease the risk of CHD. However, the evidence from epidemiologic studies is limited due to the shortcomings in study design, sample size, and the characteristics of study populations. Nutritional studies of dietary sources of taurine and the biochemical availability of taurine, as well as epidemiologic studies using CHD or CHD risk factors as endpoints are needed to provide more definitive answers about the influence of long-term taurine levels on preclinical and clinical CHD outcomes.
Acknowledgments
This research was supported by U.S. grants: NIH grants ES000260, CA16087, CA098661 and American Heart Association grant 0835569D.
Footnotes
This research was supported by U.S. grants: NIH grants ES000260, CA16087, CA098661 and American Heart Association grant 0835569D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AHA . Heart disease and stroke statistics - 2008 update at a glance. American Heart Association; Dallas, Texas: 2008. pp. 1–43. [Google Scholar]
- 2.Birdsall TC. Therapeutic applications of taurine. Alt Med Rev. 1998;3:128–36. [PubMed] [Google Scholar]
- 3.Huxtable RJ. Expanding the circle 1975-1999: Sulfur biochemistry and insights on the biological functions of taurine. Advances in experimental medicine and biology. 2000;483:1–25. [PubMed] [Google Scholar]
- 4.Schuller-Levis G, Park E. Is taurine a biomarker? Adv Clin Chem. 2006;41:1–21. doi: 10.1016/S0065-2423(05)41001-X. [DOI] [PubMed] [Google Scholar]
- 5.O’Flaherty L, Stapleton PP, Redmond HP, Bouchier-Hayes DJ. Intestinal taurine transport: a review. Eur J Clin Invest. 1997;27:873–80. doi: 10.1046/j.1365-2362.1997.2000747.x. [DOI] [PubMed] [Google Scholar]
- 6.Tappaz ML. Taurine biosynthetic enzymes and taurine transporter: molecular identification and regulations. Neurochem Res. 2004;29:83–96. doi: 10.1023/b:nere.0000010436.44223.f8. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton PP, Charles RP, Redmond HP, BouchierHayes DJ. Taurine and human nutrition. Clin Nutr. 1997;26:103–8. doi: 10.1016/s0261-5614(97)80234-8. [DOI] [PubMed] [Google Scholar]
- 8.Finnegan D. The health effects of stimulant drinks. British Nutrition Foundation Nutrition Bulletin. 2003;28:147–55. [Google Scholar]
- 9.Paauw JD, Davis AT. Taurine supplementation at three different dosages and its effects on trauma patients. Am J Clin Nutr. 1994;60:203–6. doi: 10.1093/ajcn/60.2.203. [DOI] [PubMed] [Google Scholar]
- 10.Yamori Y, Murakami S, Ikeda K, Nara Y. Fish and lifestyle-related disease prevention: Experimental and epidemiological evidence for anti-atherogenic potential of taurine. Clin Exp Pharmacol Physiol Suppl. 2004;31:S20–3. doi: 10.1111/j.1440-1681.2004.04122.x. [DOI] [PubMed] [Google Scholar]
- 11.Lam NV, Chen W, Suruga K, Nishimura N, Goda T, Yokogoshi H. Enhancing effect of taurine on CYP7A1 mRNA expression in Hep G2 cells. Amino Acids. 2006;30:43–48. doi: 10.1007/s00726-005-0244-3. [DOI] [PubMed] [Google Scholar]
- 12.Yokogoshi H, Mochizuki H, Nanami K, Hida Y, Miyachi F, Oda H. Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. Journal of Nutrition. 1999;129:1705–1712. doi: 10.1093/jn/129.9.1705. [DOI] [PubMed] [Google Scholar]
- 13.Murakami S, Kondo Y, Toda Y, et al. Effect of taurine on cholesterol metabolism in hamsters: up-regulation of low density lipoprotein (LDL) receptor by taurine. Life Sci. 2002;70:2355–66. doi: 10.1016/s0024-3205(02)01507-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Matuda K, Nishimura N, Yokogoshi H. The effect of taurine on cholesterol degradation in mice fed a high-cholesterol diet. Life Sci. 2004;74:1889–98. doi: 10.1016/j.lfs.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Bi L, Fang JH, et al. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–271. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer SW, Lombardini JB, Azuma J. Interaction between the actions of taurine and angiotensin II. Amino Acids. 2000;18:305–318. doi: 10.1007/pl00010320. [DOI] [PubMed] [Google Scholar]
- 18.Kohashi N, Okabayashi Y, Hama J, Katori R. Decreased urinary taurine in essential hypertension. Prog Clin Biol Res. 1983;125:73–87. [PubMed] [Google Scholar]
- 19.Yamamoto J, Akabane S, Yoshimi H, Nakai M, Ikeda M. Effects of taurine on stress-evoked hemodynamic and plasma catecholamine changes in spontaneously hypertensive rats. Hypertension. 1985;7:913–22. doi: 10.1161/01.hyp.7.6.913. [DOI] [PubMed] [Google Scholar]
- 20.Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest. 1988;82:993–7. doi: 10.1172/JCI113709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ideishi M, Miura S, Sakai T, Sasaguri M, Misumi Y, Arakawa K. Taurine amplifies renal kallikrein and prevents salt-induced hypertension in Dahl rats. J Hypertens. 1994;12:653–61. [PubMed] [Google Scholar]
- 22.Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- 23.Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can. J. Physiol. Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- 24.Nara Y, Yamori Y, Lovenberg W. Effect of dietary taurine on blood pressure in spontaneously hypertensive rats. Biochem. Pharmacol. 1978;27:2689–92. doi: 10.1016/0006-2952(78)90043-6. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Liu L, Ding J, et al. Ethnic and environmental differences in various markers of dietary intake and blodd pressure among Chinese Han and three other minority peoples of China: Results from the WHO cardiovascular diseases and alimentary comparison (CARDIAC) study. Hepertens Res. 2001;24:315–322. doi: 10.1291/hypres.24.315. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima S, Moriguchi EH, Ishikawa P, et al. Fish intake and cardiovascular risk among middle-aged Japanese in Japan and Brazil. J. Cardiovasc. Risk. 1997;4:191–199. [PubMed] [Google Scholar]
- 27.Fujita T, Katsuyuki A, Noda H, Yasushi I, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–32. doi: 10.1161/01.cir.75.3.525. [DOI] [PubMed] [Google Scholar]
- 28.Sethupathy S, Elanchezhiyan C, Vasudevan K, Rajagopal G. Antiatherogenic effect of taurine in high fat diet fed rats. Indian J Exp Biol. 2002;40:1169–72. [PubMed] [Google Scholar]
- 29.Kondo Y, Toda Y, Kitajima H, et al. Taurine inhibits development of atherosclerotic lesions in apolipoprotein E-deficient mice. Clin Exp Pharmacol Physiol. 2001;28:809–15. doi: 10.1046/j.1440-1681.2001.03527.x. [DOI] [PubMed] [Google Scholar]
- 30.Marcinkiewicz J, Grabowska A, Bereta J, Bryniarski K, Nowak B. Taurine chloramine down-regulates the generation of murine neutrophil inflammatory mediators. Immunopharmacology. 1998;40:27–38. doi: 10.1016/s0162-3109(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 31.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;326:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 32.Casey RG, Gang C, Joyce M, Bouchier-Hayes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J Vasc Res. 2007;44:31–39. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 33.Bedrosian I, Duane Sofia R, Wolff SM, Dinarello CA. Tauroline, an analogue of the amino acid taurine, suppresses interleukin 1 and tumor necrosis factor synthesis in human peripheral blood mononuclear cells. Cytokine. 1991;3:568–575. doi: 10.1016/1043-4666(91)90483-t. [DOI] [PubMed] [Google Scholar]
- 34.Park E, Quinn MR, Wright CE, Schuller-Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J. Leukoc. Biol. 1993;54:119–124. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- 35.Milei J, Ferreira R, Llesuy S, Forcada P, Covarrubias J, Boveris A. Redution of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Am Heart J. 1992;123:339–45. doi: 10.1016/0002-8703(92)90644-b. [DOI] [PubMed] [Google Scholar]
- 36.Yamori Y, Liu L, Ikeda K, et al. Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: Results from the WHO-CARDIAC Study. Hypertens Res. 2001;24:453–457. doi: 10.1291/hypres.24.453. [DOI] [PubMed] [Google Scholar]
- 37.Yamori Y, Liu L, Mizushima S, Ikeda K, Nara Y. Male cardiovascular mortality and dietary markers in 25 population samples of 16 countries. J Hypertens. 2006;24:1499–1505. doi: 10.1097/01.hjh.0000239284.12691.2e. [DOI] [PubMed] [Google Scholar]
- 38.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 39.Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul Toxicol Pharmacol. 2008;50:376–99. doi: 10.1016/j.yrtph.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Sturman JA, Rassin DK, Gaull G. Taurine in the development of the central nervous system. In: Babeau A, Huxtable RJ, editors. Taurine and Neurological Disorders. Raven; New York: 1978. pp. 47–91. [Google Scholar]
- 41.Hayes KC, Sturman JA. Taurine in metabolism. Annu Rev. 1981;1:401–25. doi: 10.1146/annurev.nu.01.070181.002153. [DOI] [PubMed] [Google Scholar]
- 42.Sole MJ, Jeejeebhoy KN. Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr Opin Clin Nutr Metab Care. 2000;3:417–24. doi: 10.1097/00075197-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 44.Learn DB, Fried VA, Thomas EL. Taurine and hypotaurine content of human leukocytes. J Leukoc Biol. 1990;48:174–82. [PubMed] [Google Scholar]
- 45.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–40S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 46.Spaeth DG, Schneider DL, Sarett HP. Taurine synthesis, concentration, and bile salt conjugation in rat, guinea pig, and rabbit. Proc Soc Exp Biol Med. 1974;147:855–8. doi: 10.3181/00379727-147-38455. [DOI] [PubMed] [Google Scholar]
- 47.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 48.Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. J Parenter Enteral Nutr. 1990;14:183–8. doi: 10.1177/0148607190014002183. [DOI] [PubMed] [Google Scholar]
- 49.Pasantes-Morales H, Quesada O, Alcocer L, Sánchez Olea R. Taurine content in foods. Nutr Rep Int. 1989;40:793–801. [Google Scholar]
- 50.Lourenço R, Camilo ME. Taurine: a conditionally essential amino acid in humans? An Overview in health and disease. Nutr Hosp. 2002;17:262–70. [PubMed] [Google Scholar]
- 51.Yamori Y, Liu L, Mizushima S, Ikeda K, Nara Y. Male cardiovascular mortality and dietary markers in 25 population samples of 16 countries. Journal of Hypertension. 2006;24:1499–1505. doi: 10.1097/01.hjh.0000239284.12691.2e. [DOI] [PubMed] [Google Scholar]