Abstract
BACKGROUND
Classically, cardiac sarcolemmal KATP channels have been thought to be composed of Kir6.2 (KCNJ11) and SUR2A (ABCC9) subunits. However, the evidence is strong that SUR1 (sulfonylurea receptor type 1, ABCC8) subunits are also expressed in the heart and that they play a significant functional role in the atria.
METHODS
To examine this further, we have assessed the effects of isotype-specific potassium channel-opening drugs, diazoxide (specific to SUR1>SUR2A) and pinacidil (SUR2A>SUR1), in intact hearts from wild type mice (WT, n=6), SUR1−/− (n=6), and Kir6.2−/− mice (n=5). Action potential durations (APDs) in both atria and ventricles were estimated by optical mapping of the posterior surface of Langendorff-perfused hearts. To confirm the atrial effect of both openers, isolated atrial preparations were mapped in both WT (n=4) and SUR1−/− (n=3) mice. The glass microelectrode technique was also used to validate optical action potentials.
RESULTS
In WT hearts, diazoxide (300 µM) decreased APD in atria (from 33.8±1.9 ms to 24.2±1.1 ms, p<0.001) but was without effect in ventricles (APD 60.0±7.6 ms vs 60.8±7.5 ms, respectively, NS), consistent with an atrial-specific role for SUR1. The absence of SUR1 resulted in loss of efficacy of diazoxide in SUR1−/− atria (APD 36.8±1.9 ms vs 36.8±2.8 ms, respectively, NS). In contrast, pinacidil (300 µM) significantly decreased ventricular APD in both WT and SUR1−/− hearts (from 60.0±7.6 ms to 29.8±3.5 ms in WT, p<0.001; and from 63.5±2.1 ms to 24.8±3.8 ms in SUR1−/−, p<0.001), but did not decrease atrial APD in either WT or SUR1−/− hearts. Glibenclamide (10µM) reversed the effect of pinacidil in ventricles and restored APD to control values. The absence of Kir6.2 subunits in Kir6.2−/− hearts resulted in loss of efficacy of both openers (APD 47.2±2.2 ms vs 47.6±2.1 ms and 50.8±2.4 ms, and 90.6±5.7 ms vs 93.2±6.5 ms and 117.3±6.4 ms, for atria and ventricle in control versus diazoxide and pinacidil, respectively).
CONCLUSION
Collectively, these results indicate that in the same mouse heart, significant differential KATP pharmacology in atria and ventricles, resulting from SUR1 predominance in forming the atrial channel, leads to differential effects of potassium channel openers on APD in the two chambers.
INTRODUCTION
Sarcolemmal ATP-sensitive potassium (KATP) channels are prominently expressed throughout the heart [1, 2]. In pathophysiological conditions, clear roles for KATP channels have been identified, including their participation arrhythmogenesis [3, 4], and protection from the contractile impairment following ischemia-reperfusion [5–8]. At the molecular level, KATP channels are understood to be multisubunit protein complexes, and classically, cardiac sarcolemmal KATP channels have been thought to be primarily composed of Kir6.2 (inward-rectifier potassium channel 6.2, KCNJ11) and SUR2A (sulfonylurea receptor type 2A, ABCC9) subunits [9, 10][11], together with other regulatory proteins (creatine kinase, GAPDH, and others) [12–15]. However, the evidence is strong that SUR1 (sulfonylurea receptor type 1, ABCC8) subunits are also expressed in the heart and that they play a significant functional role, especially in the atria [16–18]. Recently, we demonstrated that the SUR1 subunit is strongly expressed in the mouse atrium and that atrial sarcolemmal KATP requires SUR1 for functional channel expression.[18]
The SUR subunit determines the specificity and selectivity of KATP agonists and antagonists [19]. Assessment of the functional effects of KATP agonists has been routinely exploited to infer the physiological outcome of KATP activation. A critical assumption in these experiments is that diazoxide does not affect sarcolemmal KATP channels. Because channel structure is regionally distinct, sarcolemmal KATP channels in the intact mouse heart are likely to exhibit chamber-specific pharmacology. In isolated cells, it has been shown that atrial KATP is more sensitive to diazoxide (specific to SUR1>SUR2A) than pinacidil (SUR2A>SUR1)[19], whereas ventricular KATP has the opposite specificity to the potassium channel opening drugs [18]. How and whether this differential pharmacology in isolated cells is manifest in the intact heart is unclear, and to examine this, we have simultaneously monitored the effects of diazoxide and pinacidil on action potential duration (APD) in atria and ventricles of intact hearts from wild type (WT) mice and mice with deletions of SUR1 (SUR1−/−) and Kir6.2 (Kir6.2−/−). Diazoxide shortened the action potential in atria, but not in ventricles, of WT hearts, but was without effect in both atria and ventricles in SUR1−/− hearts. Conversely, pinacidil significantly decreased ventricular APD in both WT and SUR1−/−, but was without effect on atrial APD in both lines of mice. Knock-out of Kir6.2 resulted in loss of efficacy of both openers, in both atria and ventricles.
MATERIALS AND METHODS
Generation and care of genetically modified mice
All procedures complied with the standards for the care and use of animal subjects as stated in the Guide of the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996) and protocols were approved by the Animal Studies Committee at Washington University School of Medicine. Data were obtained from adult (aged 11 to 20 weeks) wild type mice (WT), sulfonylurea receptor type 1 knock-out mice (SUR1−/−) and Kir6.2 knock-out mice (Kir6.2−/−). The generation of SUR1−/− and Kir6.2−/− mice has been described elsewhere [20, 21]. Separate groups of mice were studied as (1) whole heart preparations (WT, n=6; SUR1−/−, n=6; and Kir6.2−/−, n=5) and (2) isolated atrial preparations (WT, n=4; and SUR1−/−, n=3).
Isolated heart preparations
The isolated heart preparation was performed as described previously [22]. Briefly, a Langendorff perfusion protocol modified for murine heart was used. Mice were anesthetized using a mixture of Ketamine and Xalazine, with 100 Units of Heparin. After mid-sternal incision, the heart was removed and placed in oxygenated (95% O2, 5% CO2) constant-temperature (37±1°C), modified Tyrode solution of the following composition: 128.2 mM NaCl, 4.7 mM KCl, 1.19 NaH2PO4, 1.05 mM MgCl2, 1.3 mM CaCl2, 20.0 mM NaHCO3, and 11.1 mM glucose (pH=7.35±0.05). While bathed in the same solution, lung, thymus, and fat tissue were dissected and removed. A short section of aorta was attached to a custom made 21-gauge cannula. After cannulation, the heart was superfused and retrogradely perfused with Tyrode solution passed through the 5-µm filter (Millipore, Billerica, USA) and warmed (37°C) using a water jacket and circulator (ThermoNESLAB EX7, Newtown, USA). Perfusion was performed using a peristaltic pump (Peri-Star, WPI, Sarasota, USA) under constant aortic pressure of 60 to 80 mm Hg measured by pressure-amplifier (TBM4M, WPI, Sarasota, USA). Cannulation was followed by (1) preparation of the intact heart, or (2) isolated atria for image analysis.
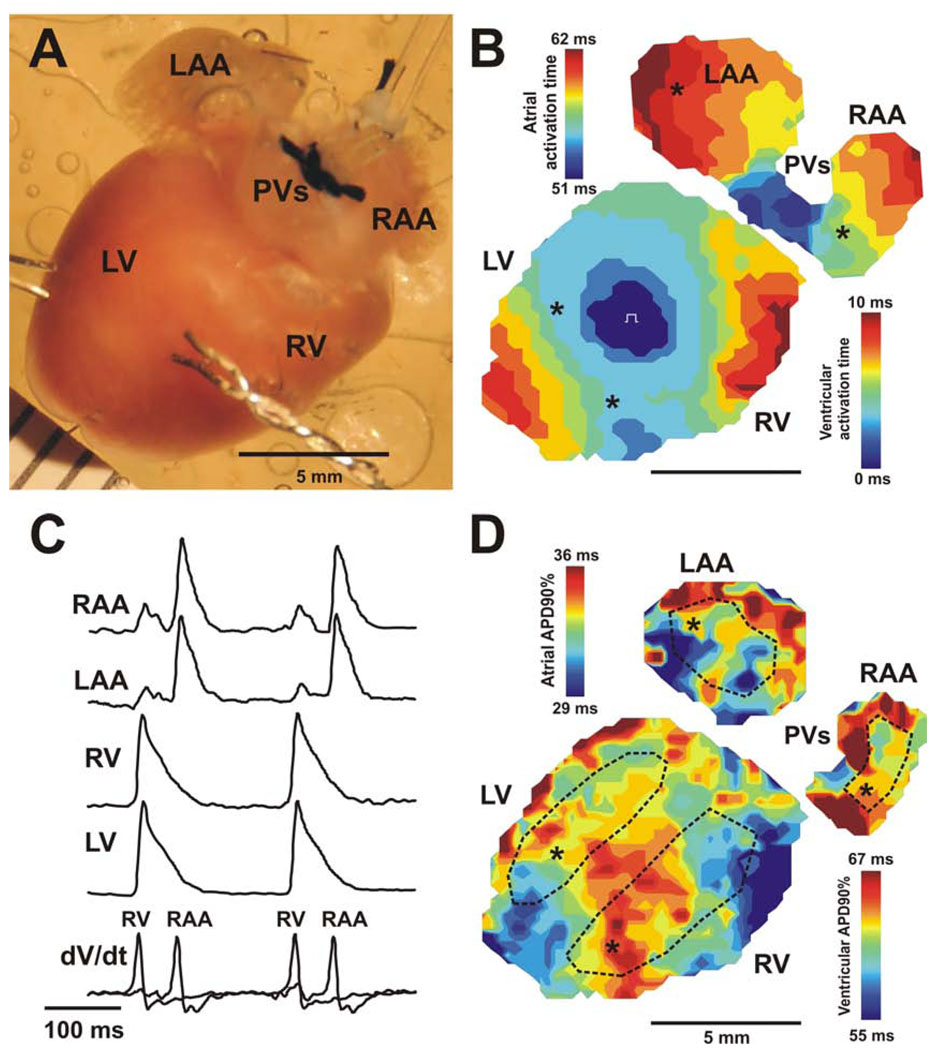
A photograph of the intact mouse heart preparation is shown on Figure 1A. The isolated heart was pinned at the apex to the Sylgard bottom of the chamber to prevent stream-induced movement. The right and left atrial appendages were stretched and pinned to flatten them and allow optical measurements from maximal surface of the atria. A small silicon tube, fixed by silk to the nearby connective tissue, was inserted into the left ventricle through the pulmonary vein, left atria, and tricuspid valve to prevent solution congestion and subsequent ischemia after suppression of ventricular contractions. This also prevented acidification of the perfusion solution and development of ischemia in the left ventricle, which otherwise developed without this precaution.
Figure 1. Optical mapping of isolated mouse heart.
Action potential duration in both atria and ventricles were simultaneously estimated by optical mapping of the posterior surface of Langendorff-perfused mouse hearts. A photograph of the murine heart during a typical experiment is presented on panel A. Panel B illustrates activation maps of both atria and ventricles during electrical pacing of the center of the right ventricle free wall. Two color time scales for atrial and ventricular activation are presented, with 41 ms atrio-ventricular delay between the end of atrial activation and the beginning of ventricular activation. In panel C, recordings of optical action potentials from different regions of the heart (marked by asterisks) as well as corresponding derivatives (dV/dt) of optical signals used for activation map reconstruction are presented. Panel D illustrates action potential duration distribution maps of both atria and ventricles from the same heart. Dotted lines show areas selected for action potential duration estimation in each heart chamber. RA and LA – the right and left atrium; RV and LV – the right and left ventricle; PVs – pulmonary veins.
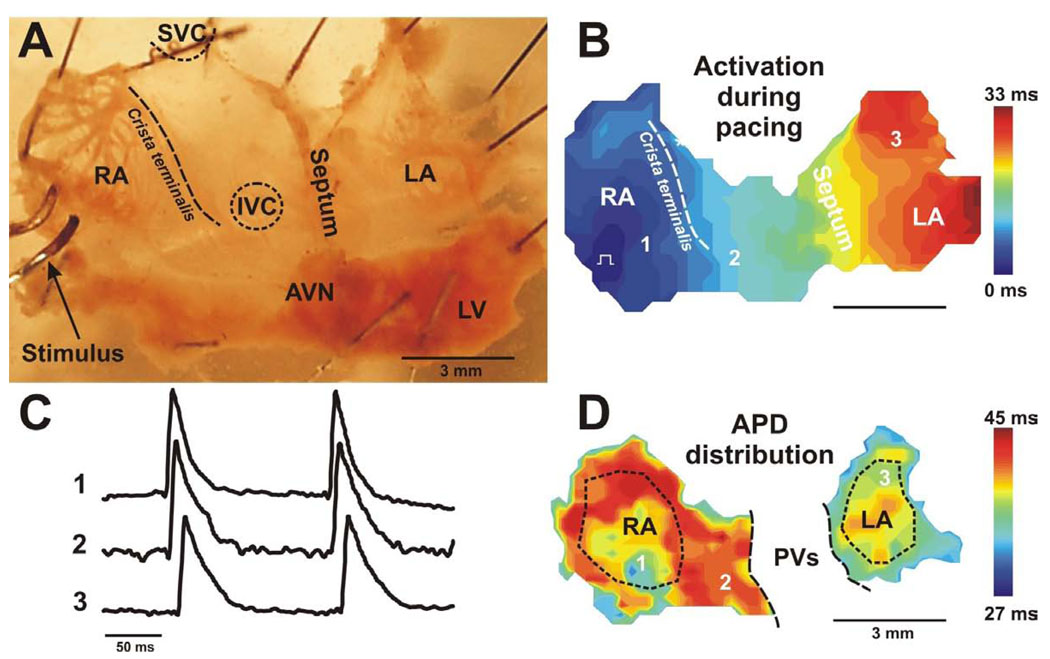
For the isolated atrial preparation (Fig. 5A), the heart was cannulated as described above, ventricles were dissected away, and the atria were stretched and then pinned to the bottom of a Sylgard-coated chamber and superfused with Tyrode solution at a constant rate of ~8 ml/min. Both left and right atria (LA and RA, correspondingly) as well as the atrioventricular junction (AVJ) were accessible in this preparation. A rim of ventricular tissue was preserved, to pin the preparation and prevent damage to the atria. The medial limb of the crista terminalis was cut to open the RA appendage, and the pacing electrode was located on the edge of the RA appendage. The interatrial septal tissue was partially removed to reduce the scattering from out of focus tissue. These procedures did not result in any irregularities in spontaneous rhythm.
Figure 5. Optical mapping of the isolated mouse atria preparation.
(A) Photograph of a typical preparation consisting of isolated adult mouse atria is shown. The preparation is oriented so that the right atrium is on the left side of the image. Dashed line marks location of crista terminalis. (B) Representative example of atrial activation map obtained during right atria pacing. (C) Optical recordings from different atrial sites labeled on maps. (D) Representative example of action potential duration (APD) distribution map reconstructed for the same preparation as presented on panel A and B. Dotted lines show areas selected for action potential duration estimation in RA and LA regions. The pulmonary veins (PVs) region was excluded from APD analysis. RA and LA – right and left atrium; SVC and IVC – superior and inferior vena cava; LV – left ventricle; AVN – atrioventricular node.
The excitation-contraction uncoupler blebbistatin (10 µM, Tocris Bioscience, USA) was used to prevent the effects of motion artifacts on the APD estimation. We showed in our previous studies in different species (dog, rabbit, rat, and human) that blebbistatin does not change action potential morphology or conduction properties [23]. In addition, we used microelectrode recordings to validate the optical signal. In this case, the application of blebbistatin induced a transient (5–10 min) increase of cycle length of spontaneous rhythm (data not shown).
Imaging system
Coronary perfused hearts were stained by perfusion with voltage-sensitive dye (RH-237, 5 µL of 1 mg/mL dimethyl sulfoxide, DMSO in Tyrode solution), for 5–7 minutes. Isolated atrial preparations were stained by direct application of the dye dissolved in the bathing solution. Staining by RH-237 did not induce a significant change of cycle length, as typically observed for di-4-ANNEPS.
Excitation light (530/540 nm) was generated by a 250-W xenon arc lamp with a constant-current, low-noise, power supply (Oriel Instruments, Stratford, CT). The light was passed through a heat filter, a shutter, and excitation filter (530/40 nm). A flexible light guide directed the band-pass-filtered light onto the preparation, and a shutter was used to ensure that the preparation was exposed to light only during image acquisition. The fluorescent light emitted from the preparation was long-pass (>650 nm) filtered using an edge pass filter (Thorlabs, NJ) before reaching the camera. Emitted light was directed towards a MiCAM Ultima-L CMOS camera (SciMedia, CA) with high spatial (100×100 pixels, 230±20 µm per pixel) and temporal (1,000–3,000 frames/sec) resolution. The acquired fluorescent signal was digitized, amplified, and visualized using custom software (SciMedia, CA).
Experimental protocols
After isolation and cannulation, motion suppression, and dye staining, preparations were equilibrated for 5–10 min before imaging. Then, control measurements during both spontaneous rhythm and ventricular pacing were made. To estimate the effects of stimulation of different types of sulfonylurea receptors, we tested two potassium channel-opening drugs: diazoxide (specificity for SUR1 > SUR2A), and pinacidil (specificity for SUR2A > SUR1)[19]. Diazoxide or pinacidil (300 µM) was delivered through both perfusion and superfusion solutions, and was applied for 20–25 min to reach steady-state effect. After optical measurements, hearts were washed out for 30 min before delivery of a second test compound. After washout, additional staining as well as additional injection of blebbistatin was performed if needed based on the quality of the optical signal. Following application of diazoxide and/or pinacidil, a selective blocker of KATP channels, glibenclamide (10 µM), was added to the solution to reverse the effect of channel openers. This protocol was applied for both intact heart and isolated atrial preparations.
Data processing
A customized Matlab-based computer program [24] was used to analyze optical signals. First, the signals were filtered using a low-pass Butterworth filter at 200 Hz. All optical action potentials were then averaged and normalized to a range from −85 to 15 mV. Finally, action potential duration at 90% of repolarization (APD90) and maximum upstroke derivative (dV/dtmax) were calculated for each action potential using the normalized optical signal and its derivatives. Activation maps were constructed from activation times, which were determined from the dV/dtmax calculations.
To estimate the changes of APD over the atria and ventricles, we selected several regions corresponding to recognized anatomic features (Fig.1D, dotted areas) and computed summary statistics for the APDs within each selected region. Four regions used were: 1) the RA, the right atrial tissue anterior to the sulcus terminalis (this area contains the trabeculated part of the right atrial wall referred to as the “right atrial free wall”); 2) the LA, the trabeculated left atrial tissue anterior to the narrow junction separating the appendage from the smooth-walled part of the left atrium receiving the pulmonary veins; 3) the RV, the right ventricular tissue located from the right of the interventricular septum on the posterior view; and 4) the LV, the left ventricular tissue located from the left of the interventricular septum on the posterior view.
Microelectrode study
The glass microelectrode technique was used to validate optical action potentials. The isolated atrial preparation was used as for optical mapping. Transmembrane potentials were recorded at 5 kHz by conventional glass microelectrodes filled with 3 M KCl-filled glass microelectrodes with 15- to 25 MΩ resistance as described before [25].
Statistical analysis
Values are expressed as means ± SEM. Hypothesis testing was carried out using an unpaired student t-test and chi-squared analysis with Yates correction. A value of p<0.05 was considered statistically significant.
RESULTS
Differential potassium channel opener - sensitivity of APD in intact mouse heart
Figure 1 shows a representative example of the activation pattern of an intact Langendorff-perfused WT heart obtained during simultaneous mapping of both atria and ventricles during continuous ventricular pacing at a cycle length of 200 msecs. The atrial activation scale begins after an AV delay with the first breakthrough, detected by the imaging system (Fig. 1B). During ventricular pacing, activation originated at the RV free wall and spread through the ventricles. Following 10 ms of ventricular activation and 41 ms AV delay, the excitation emerged in the right atria near the inferior vena cava and activated both atria within 11 ms. AV-delay was easy to define using optical action potentials (OAP) recordings. It should be noted that the APD in left ventricle was significantly longer, as has recently been reported by others [26], although no such differences were observed between the atria. The small amplitude ventricular OAPs detectable within the optical traces recorded from RA and LA epicardium are due to light scattering from ventricles (Fig.1C)[24]. For APD analysis, we selected atrial OAPs without significant ventricular signals that could affect APD estimation.
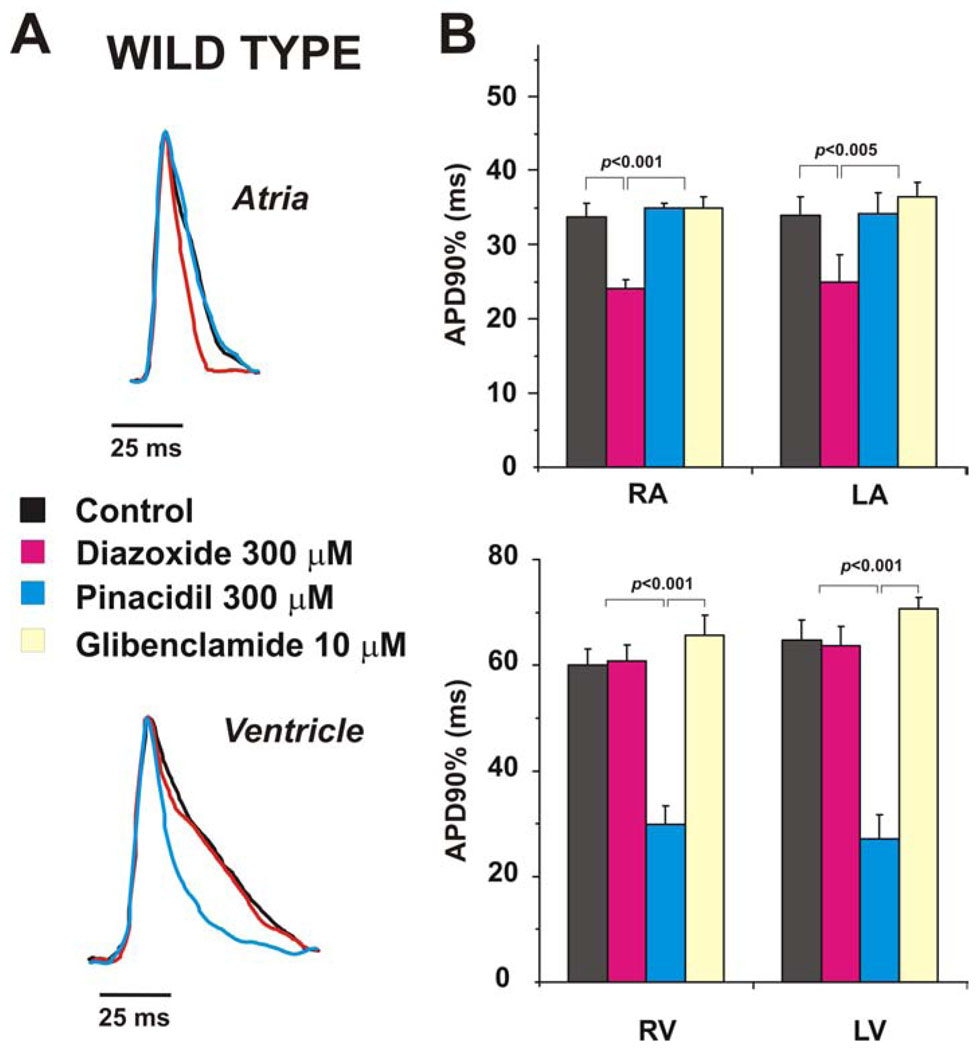
Representative examples of superimposed OAPs in control and after application of drugs are shown in Fig.2A. In WT hearts, diazoxide (300 µM) significantly (p<0.001) decreased APD in the atria but was without effect in the ventricles. In contrast, pinacidil (300 µM) did not change APD in the atria but dramatically decreased APD in ventricles (Fig.2), the effect of pinacidil being statistically greater in left, than in right ventricle (APD decreasing by 56.3±4.2% vs 49.4±3.4% in LV and RV, respectively, p<0.05). Similar results were obtained in preliminary experiments (not shown) in free running hearts. In these hearts, the spontaneous heart rate was decreased slightly by both diazoxide (from control 282 ± 28 beats/min to 272 ± 26 beats/min, n=6) and by pinacidil (from control 295 ± 13 beats/min to 270 ± 31 beats/min, n=4). Treatment by glibenclamide (10µM) reversed the effect of pinacidil in ventricles and prolonged APD back to control values (Fig.2B). Taken together, the data demonstrate heterogeneity of SUR-selective channel opener action in the murine heart further supporting, and consistent with, the conclusion that the molecular make-up of sarcolemmal KATP channels in the atrium and ventricle is distinct.
Figure 2. Effect of KATP channel openers in wild type mouse.
(A) Superimposed recordings of optical action potentials measured in atria (top) and ventricles (bottom) in the presence or absence of KATP channel opening drugs. (B) Summary data from experiments as in panel A (n = 6). * - p<0.001.
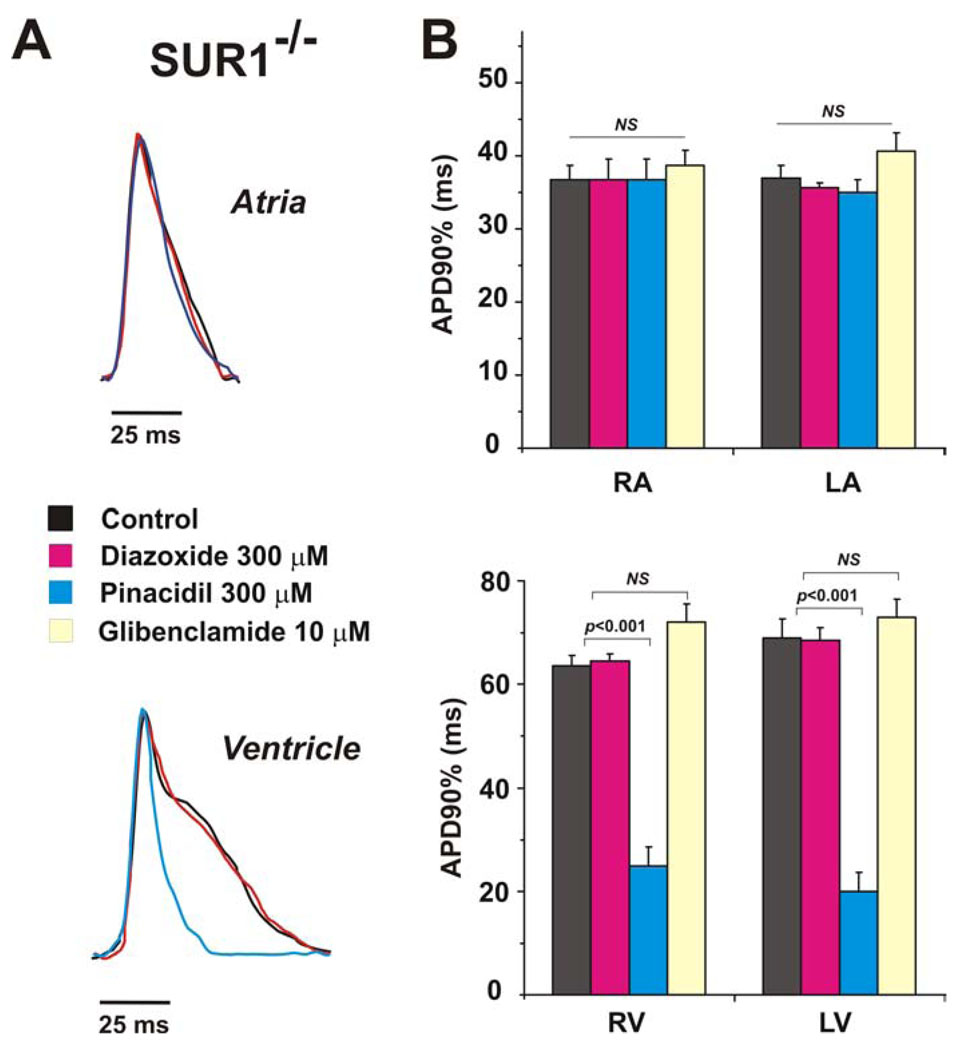
In contrast to WT, diazoxide (300 µM) had no effect on APD in atria or ventricles of SUR1−/− hearts (Fig. 3), while pinacidil (300 µM) decreased ventricular APD to the same extent as WT, and did not change atrial APD (Fig.3). As in WT hearts, the left ventricular APD was significantly longer and the effect of pinacidil was significantly greater in left, than in SUR1−/− right ventricle (APD decreasing by 62.3±12.0% vs. 50.9±4.1% in left and right ventricles respectively, p<0.05). Treatment by glibenclamide (10µM) again reversed the effect of pinacidil in ventricles and prolonged APD to control values. Interestingly, the APD in both atria and ventricles of SUR1−/− hearts was significantly longer (p<0.05) than in WT hearts.
Figure 3. Effect of KATP channel openers in SUR−/− mouse.
(A) Superimposed recordings of optical action potentials measured in atria (top) and ventricles (bottom) in the presence or absence of KATP channel opening drugs. (B) Summary data from experiments as in panel A (n = 6). * - p< 0.001.
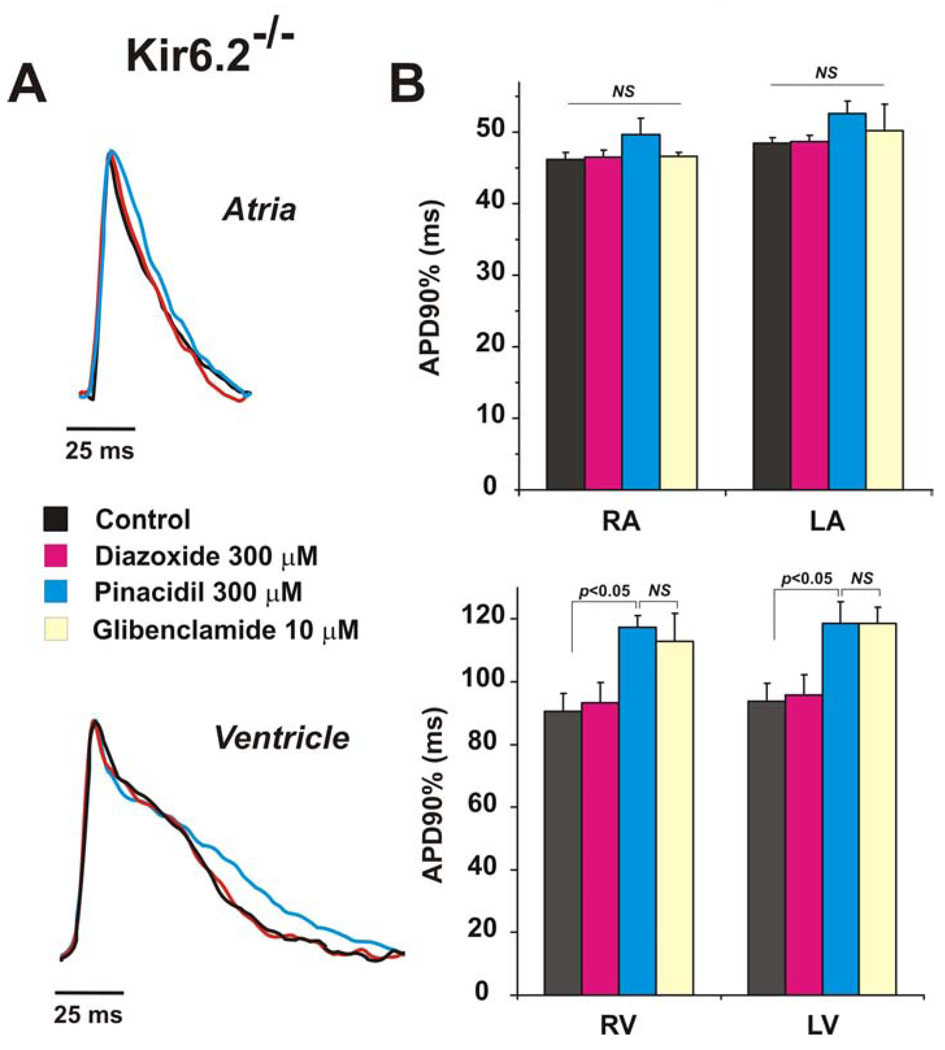
While the data above support the conclusion that diazoxide and pinacidil exert effects directly on the cardiac sarcolemmal KATP channel, it is possible that effects of diazoxide and pinacidil on vascular KATP indirectly influence cardiac APD. To rule this out, we examined the effects of diazoxide and pinacidil on hearts from Kir6.2−/− animals that lack cardiac, but not vascular, KATP channels[ 5]. Consistent with a direct effect of the channel openers on cardiac sarcolemmal channels, the absence of the Kir6.2 subunit resulted in loss of efficacy of both diazoxide and pinacidil in all heart chambers (Fig. 4). Moreover, the APD in both atria and ventricles of Kir6.2−/− hearts was quite dramatically longer (p<0.01) than in WT or SUR1−/− hearts. It is also notable that pinacidil significantly prolonged APD in Kir6.2−/− hearts. This effect was statistically significant in ventricles and was not eliminated by glibenclamide, which may indicate a non-specific inhibitory action of pinacidil on other K+ currents [27].
Figure 4. Effect of KATP channel openers in Kir6.2−/− mouse.
(A) Superimposed recordings of optical action potentials measured in atria (top) and ventricles (bottom) in the presence or absence of KATP channel opening drugs. (B) Summary data from experiments as in panel A (n = 5). * - p<0.001.
Effects of potassium channel openers on action potential in isolated mouse atria
As noted above, scattering of ventricular OAP signal could potentially interfere with estimations of the atrial APD. Although carefully selected OAPs in which scattering was minimal were chosen for analysis, we sought to confirm the effect of KATP openers in isolated atrial preparations, using fluorescent imagining as well as microelectrodes, where spurious light scattering is not a problem. Fig.5B shows an typical example of atrial activation during electrical pacing. To characterize APD, we selected two regions (marked by dotted lines) within the preparation that excluded the pulmonary vein area, because of the low signal-to-noise ratio in this region. A typical control pattern of APD distribution in the atria preparation is presented in Fig.5D. Atrial signals were of good quality without motion and scattering artifacts except at the edges of the preparations, which were excluded from analysis. The APDs were shorter in the atrial appendages than in the posterior walls. Also, the sinus node area had prolonged APDs compared to the atrial tissue, which was the apparent basis for significant APD heterogeneity within each atrium. Furthermore, we observed a non-significant inter-atrial gradient of APD, such that APDs were slightly longer in the right than the left atria.
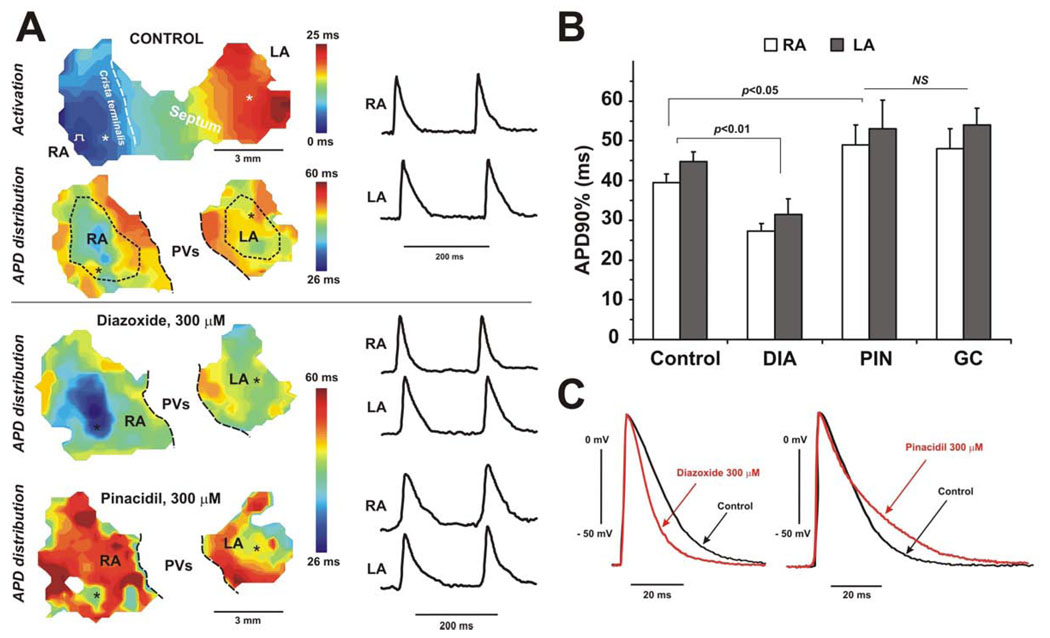
Figure 6 and Figure 7 demonstrate the effect of both openers on APD in isolated mouse atria preparations. Representative examples of APD distribution in WT and SUR1−/− hearts are shown in Fig.6A and Fig.7A, respectively. The summary data for diazoxide and pinacidil effect in WT and SUR1−/− hearts are shown in Fig.6B and Fig.7B, respectively. Diazoxide shortened APDs throughout the WT atria, but again had no effect on APDs in SUR1−/− atria. Microelectrode results confirmed data obtained optically (Fig.6C and 7C) showing the same degree of APD shortening for WT group and no effect in SUR1−/− mice. Compared to data from the whole heart experiment (Fig.2), diazoxide showed the same effect: 28±4% in RA and 27±5% in LA versus 31±4% in RA and 30±6% in LA, in whole heart and isolated atria preparations respectively.
Figure 6. Effect of KATP channel openers on the APD in WT isolated atria.
(A), APD distribution through the atria under control conditions, and after perfusion of diazoxide (300 µM), and pinacidil (300 µM). APD distribution color time scales are presented in the same time range for all maps. The atrial activation map is shown in control conditions. APDs were measured during right atria pacing with cycle length of 200 ms. Dotted lines show areas selected for APD estimation in RA and LA regions. The pulmonary veins (PVs) region was excluded from APD analysis. For each APD map, recordings of optical action potentials are presented from selected areas of right (RA) and left (LA) atria (marked by asterisks). (B) Summary of the effect of diazoxide (DIA, 300 µM), pinacidil (PIN, 300 µM), and glibenclamide (GC, 10 µM) on the APD in RA and LA in WT mice (n=4). (C). Microelectrode recordings of action potentials obtained during control conditions and after perfusion of diazoxide (left panel) or pinacidil (right panel).
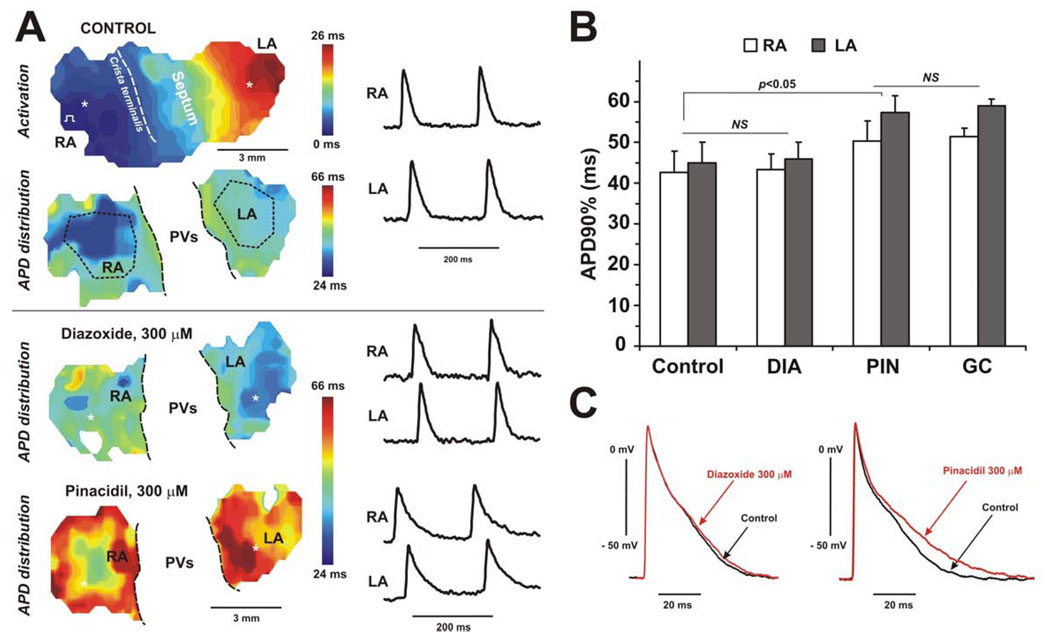
Figure 7. Effect of KATP channel openers on the APD in SUR1−/− isolated atria.
(A) APD distribution through the atria under control conditions, and after perfusion of diazoxide (300 µM), and pinacidil (300 µM). All abbreviations are the same as on the Figure 6. (B) Summary effect of diazoxide (DIA, 300 µM), pinacidil (PIN, 300 µM), and glibenclamide (GC, 10 µM) on the APD in RA and LA in SUR1−/− mice (n=3). (C) Microelectrode recordings of action potentials obtained during control conditions and after perfusion of diazoxide (left panel) or pinacidil (right panel).
Also confirming the findings in the intact heart (Fig.2–3), pinacidil did not decrease, but significantly prolonged APD in both WT and SUR1−/− atria (Fig.6–7). As observed for Kir6.2−/− hearts, this effect was statistically significant (p<0.05) and also was not eliminated by glibenclamide, again consistent with a non-specific inhibitory action of pinacidil on other K+ currents[27]. Microelectrode recordings confirmed this effect and revealed the lengthening of repolarization phase 3 (Fig.6C and 7C).
DISCUSSION
Chamber specificity of KATP channel structure and pharmacology
Since the original descriptions of the cloning and expressed properties of different SUR isoforms, it has been commonly accepted that cardiac sarcolemmal KATP channels are formed by the co-assembly of SUR2A and Kir6.2 subunits, together with other regulatory proteins (creatine kinase, GAPDH, and others)[12–15]. This dogma has been reinforced by the demonstration that KATP channel activity is essentially absent in isolated ventricular myocytes from both Kir6.2−/− and SUR2−/− animals [9]. However, we have recently demonstrated that the structural basis of sarcolemmal KATP channels in isolated murine atrial and ventricular myocytes is distinct: biochemical and functional data clearly show that SUR1 is strongly expressed in the WT atrium and is required for functional channel expression in atria, but is not involved in normal ventricular channel expression [18]. On the other hand, SUR2A is clearly involved in the generation of normal ventricular KATP [11], but its role in the generation of the atrial channel has not been systematically examined. The regionally distinct KATP channel structure gives rise to differential potassium channel opener sensitivity in isolated mouse atrial and ventricular myocytes: in excised membrane patches, atrial KATP is activated by the SUR1-specific opener diazoxide but not the SUR2-specific pinacidil, whereas ventricular KATP is activated by pinacidil, but not diazoxide [18].
Relatively few previous studies have examined differential activation of atrial and ventricular KATP, although Baertschi and colleagues have performed several comparative studies of KATP activation in neonatal rat atrial and ventricular myocytes [28–30]. These studies reveal differential diazoxide sensitivity in atria versus ventricle, consistent with our own findings. One early study of rabbit and guinea pig myocytes also revealed that although the potassium channel openers pinacidil and lemakalim (BRL38227, structurally similar to cromakalim, also a SUR2-specific activator [31]) activated KATP in ventricular cells, they reduced atrial Ito, without activating KATP [32], again consistent with our findings for pinacidil action on the atrial action potential. Together with good evidence that KATP channels in rat atrial and ventricular myocytes exhibit similar distinctions in their response to potassium channel openers [28, 30], these rabbit and guinea pig myocyte findings [32] lead us to conclude that differential molecular make-up – and hence differential pharmacology - of atrial and ventricular myocyte KATP channels is a common property of rodent hearts. Systematic study of the role of specific gene products in generating functional channel variants by examination of gene knockouts is not possible in larger animals, but there is evidence in canine heart for direct effects of diazoxide on Purkinje fibers [33]. Our own preliminary data on human heart samples also indicate similar mixed sensitivities to pinacidil and diazoxide in both intact atria and ventricles [34], and in isolated myocytes (Zhang, H.X., Kurata, H.K. and Nichols, C.G., unpublished.), and so extrapolation of this conclusion to hearts from other species must be made with caution.
Sub-regional KATP channel structure and pharmacology?
In the present study, we did not systematically examine the effects of diazoxide or pinacidil on the pacemaker activity of free running hearts, although preliminary studies suggested that heart rate was not significantly affected by either drug (data not shown). KATP is clearly present in the mouse sino-atrial node (SAN) [35], although the density may be considerably lower than in myocytes, since presumably maximal activation with dinitropheno-induced metabolic inhibition only caused ~2x increase in cycle length [35], potentially explaining our inability to resolve pinacidil effects in the present study on SAN cycle length. More significantly, these channels were activated by pinacidil [35], as are KATP channels in rabbit SAN [36], counter to the suggestion that SUR1 encodes KATP throughout the atrium, and suggesting that pacemaker KATP make-up may involve SUR2. Patch-clamp studies cannot exhaustively examine cells from all sub-regions of the cardiac chambers, and important local variations of subunit expression might be present, and may cause more complex pharmacologic activation patterns in the intact tissue. Nevertheless, the present studies demonstrate (1) that diazoxide-induced action potential shortening in atria is uniform (Fig.6–7), (2) the same specific activation patterns are observed in the intact heart (Fig.2–Fig 4), and (3) that the SUR1 subunit is responsible for this atrial specific diazoxide sensitivity, giving rise to differential changes in APD in atria versus ventricles when simultaneously treated in the intact heart.
Physiological and clinical implications
The present studies confirm the relevance of a very specific SUR distribution in mouse atria versus ventricles, but reveal additional actions of potassium channel openers. Surprisingly, pinacidil prolonged APD in Kir6.2−/− hearts, significantly so in the left and right ventricles (Fig. 4). As this effect was not eliminated by glibenclamide, it may indicate a non-specific action of pinacidil on the Ito or IK1 currents [27, 32, 37]. Kir6.2−/− hearts, moreover, reveal marked action potential prolongation in both atria and ventricles, compared to WT. This might suggest a basal KATP activation in WT hearts, but since glibenclamide caused only minimal action potential prolongation in WT hearts [38] (Fig. 2), this seems unlikely and suggests that action potential prolongation in Kir6.2−/− hearts reflects some additional remodeling [39, 40].
Activation of KATP channels will tend to shorten the action potential and reduce the refractory period, which may induce or exacerbate re-entrant arrhythmias such as atrial fibrillation [41]. There are reports that both rat and mouse atrial KATP activates more readily in the intact cell than does ventricular KATP [18, 30], consistent with SUR1 being more sensitive to metabolic activation than SUR2A-based channels [42]. Thus it may be expected that atrial KATP channel will activate more readily than ventricular under the relevant physiological or pathophysiological conditions. The functional consequence of this is not apparent, although consistent with the prediction, we consistently observe delayed atrio-ventricular conduction only in mice that overexpress SUR1, but not SUR2A, in cardiac myocytes [43]. At this juncture, the physiological relevance of differential make-up of atrial and ventricular KATP channels remains unclear, and will require further study.
Acknowledgments
This work was supported by NIH grant HL95010 to CGN and HL85369 to IRE. We are grateful to Drs. Susumu Seino and Mark Magnuson for Kir6.2−/− and SUR1−/− mice, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
REFERENCES
- 1.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005 Jun;38(6):937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 3.Billman GE. The cardiac sarcolemmal ATP-sensitive potassium channel as a novel target for anti-arrhythmic therapy. Pharmacology & Therapeutics. 2008;120(1):54–70. doi: 10.1016/j.pharmthera.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, et al. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[DeltaN30,K185Q] in the heart. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H836–H845. doi: 10.1152/ajpheart.00011.2007. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88(6):570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 6.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, et al. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. American Journal of Physiology - Heart & Circulatory Physiology. 2003;284(6):2106–2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006 Aug 1;15(15):2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Kane GC, Behfar A, Liu X-K, Dyer RB, Faustino RS, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. The Journal of Physiology. 2006 December;577(3):1053–1065. doi: 10.1113/jphysiol.2006.119511. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83(11):1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16(5):1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 11.Shi N-Q, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. Journal of Molecular and Cellular Cardiology. 2005;39(1):51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, et al. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J Biol Chem. 2005 Nov 18;280(46):38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford RM, Budas GR, Jovanovic S, Ranki HJ, Wilson TJ, Davies AM, et al. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO J. 2002 Aug 1;21(15):3936–3948. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. ASEB J. 2002 Jan;16(1):102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal K(ATP) channel. EMBO Rep. 2005 Sep;6(9):848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elrod JW, Harrell M, Flagg TP, Gundewar S, Magnuson MA, Nichols CG, et al. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008 Mar 18;117(11):1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- 17.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, et al. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5(1):1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, et al. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ Res. 2008 Dec 5;103(12):1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Ren G, O'Rourke B, Marban E, Seharaseyon J. Pharmacological Comparison of Native Mitochondrial KATP Channels with Molecularly Defined Surface KATP Channels. Mol Pharmacol. 2001 February 1;59(2):225–230. 2001. [PubMed] [Google Scholar]
- 20.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002 Oct 4;277(40):37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, et al. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95(18):10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glukhov AV, Fedorov VV, Mohler PJ, Anderson ME, Efimov IR. Functional anatomy of the murine sinus node: Evidence from high-resolution optical mapping. AJP. 2009 doi: 10.1152/ajpheart.00756.2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007 May;4(5):619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 24.Fedorov VV, Kostecki G, Hemphill M, Efimov IR. Atria are more susceptible to electroporation than ventricles: implications for atrial stunning, shock-induced arrhythmia and defibrillation failure. Heart Rhythm. 2008 Apr;5(4):593–604. doi: 10.1016/j.hrthm.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedorov VV, Li L, Glukhov A, Shishkina I, Aliev RR, Mikheeva T, et al. Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: possible role of Cx43 and Cx45 up-regulation. Heart Rhythm. 2005 Sep;2(9):966–975. doi: 10.1016/j.hrthm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Waldeyer C, Fabritz L, Fortmueller L, Gerss J, Damke D, Blana A, et al. Regional, age-dependent, and genotype-dependent differences in ventricular action potential duration and activation time in 410 Langendorff-perfused mouse hearts. Basic Res Cardiol. 2009 Mar 14; doi: 10.1007/s00395-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 27.Honore E, Lazdunski M. Two different types of channels are targets for potassium channel openers in Xenopus oocytes. Febs Letters. 1991;287(1–2):75–79. doi: 10.1016/0014-5793(91)80019-y. [DOI] [PubMed] [Google Scholar]
- 28.Baron A, van BL, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circulation Research. 1999;85(8):707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- 29.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead Transcription Factors Coordinate Expression of Myocardial KATP Channel Subunits and Energy Metabolism. Circ Res. 2008 February 1;102(2):e20–e35. doi: 10.1161/CIRCRESAHA.107.166744. 2008. [DOI] [PubMed] [Google Scholar]
- 30.Poitry S, van Bever L, Coppex F, Roatti A, Baertschi AJ. Differential sensitivity of atrial and ventricular K(ATP) channels to metabolic inhibition. Cardiovasc Res. 2003 Feb;57(2):468–476. doi: 10.1016/s0008-6363(02)00715-0. [DOI] [PubMed] [Google Scholar]
- 31.Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of K(ATP) channel isoforms are required for selective interaction with K(+) channel openers. J Biol Chem. 2000;275(2):717–720. doi: 10.1074/jbc.275.2.717. [DOI] [PubMed] [Google Scholar]
- 32.Ogbaghebriel A, Shrier A. Differential responsiveness of atrial and ventricular myocytes to potassium channel openers. J Cardiovasc Pharmacol. 1995 Jan;25(1):65–74. doi: 10.1097/00005344-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Mull KP, Debnam Q, Kabir SM, Bhattacharyya ML. Role of action potential shortening in the prevention of arrhythmias in canine cardiac tissue. Clin Exp Pharmacol Physiol. 1999 Dec;26(12):964–969. doi: 10.1046/j.1440-1681.1999.03169.x. [DOI] [PubMed] [Google Scholar]
- 34.Fedorov VV, Glukhov AV, Chang R, Kostecki G, Schuessler RB, Nichols CG, et al. KATP channel openers diazoxide and pinacidil induce reentrant arrhythmias in both atria and ventricles of myopathic human hearts. Heart Rhythm. 2009 May; doi: 10.1016/j.yjmcc.2011.04.016. Proceedings of Scientific Sessions 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuzaki K, Sato T, Miki T, Seino S, Nakaya H. Role of sarcolemmal ATP-sensitive K+ channels in the regulation of sinoatrial node automaticity: an evaluation using Kir6.2-deficient mice. The Journal of Physiology. 2008 June;586(11):2767–2778. doi: 10.1113/jphysiol.2007.148932. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han X, Light PE, Giles WR, French RJ. Identification and properties of an ATP-sensitive K+ current in rabbit sino-atrial node pacemaker cells. J Physiol. 1996 Jan 15;490(Pt 2):337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogbaghebriel A, Shrier A. Inhibition of metabolism abolishes transient outward current in rabbit atrial myocytes. Am J Physiol. 1994 Jan;266(1 Pt 2):H182–H190. doi: 10.1152/ajpheart.1994.266.1.H182. [DOI] [PubMed] [Google Scholar]
- 38.Faivre JF, Findlay I. Effects of tolbutamide, glibenclamide and diazoxide upon action potentials recorded from rat ventricular muscle. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 39.Flagg TP, Charpentier F, Manning-Fox J, Remedi MS, Enkvetchakul D, Lopatin A, et al. Remodeling of excitation-contraction coupling in transgenic mice expressing ATP-insensitive sarcolemmal KATP channels. American Journal of Physiology - Heart & Circulatory Physiology. 2004;286(4):H1361–H1369. doi: 10.1152/ajpheart.00676.2003. [DOI] [PubMed] [Google Scholar]
- 40.Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circ Res. 2001 Nov 23;89(11):944–956. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- 41.Nattel S, Li D, Yue L. Basic mechanisms of atrial fibrillation--very new insights into very old ideas. Annu Rev Physiol. 2000;62:51–77. doi: 10.1146/annurev.physiol.62.1.51. [DOI] [PubMed] [Google Scholar]
- 42.Masia R, Enkvetchakul D, Nichols C. Differential nucleotide regulation of K(ATP) channels by SUR1 and SUR2A. Journal of Molecular & Cellular Cardiology. 2005;39:491–501. doi: 10.1016/j.yjmcc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Flagg TP, Remedi MS, Masia R, McLerie M, Lopatin A, Nichols C. Transgenic overexpression of SUR1 in the heart exerts dominant negative effects on sarcolemmal K ATP. Journal of Molecular & Cellular Cardiology. 2005;39:647–656. doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]