Abstract
The cytochrome P450 (CYP) epoxygenase enzymes CYP2J and CYP2C catalyze the epoxidation of arachidonic acid to epoxyeicosatrienoic acids (EETs), which are rapidly hydrolyzed to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH). It is well-established that CYP epoxygenase-derived EETs possess potent vasodilatory effects; however, the cellular effects of EETs and their regulation of various inflammatory processes have become increasingly appreciated in recent years, suggesting that the role of this pathway in the cardiovascular system extends beyond the maintenance of vascular tone. In particular, CYP epoxygenase-derived EETs inhibit endothelial activation and leukocyte adhesion via attenuation of nuclear factor-kappaB activation, inhibit hemostasis, protect against myocardial ischemia-reperfusion injury, and promote endothelial cell survival via modulation of multiple cell signaling pathways. Thus, the CYP epoxygenase pathway is an emerging target for pharmacological manipulation to enhance the cardiovascular protective effects of EETs. This review will focus on the role of the CYP epoxygenase pathway in the regulation of cardiovascular inflammation, and 1) describe the functional impact of CYP epoxygenase-derived EET biosynthesis and sEH-mediated EET hydrolysis on key inflammatory process in the cardiovascular system, 2) discuss the potential relevance of this pathway to pathogenesis and treatment of cardiovascular disease, and 3) identify areas for future research.
Keywords: Inflammation, endothelial, cytochrome P450, CYP2J, CYP2C, epoxygenase, soluble epoxide hydrolase, epoxyeicosatrienoic acid, EET
1. Introduction
It is well-established that oxidative metabolism of arachidonic acid by cyclooxygenases (COX) and lipoxygenases (LOX) to biologically active eicosanoids plays a critical role in the regulation of various cellular and physiologic processes [1, 2]. However, it is less well-known that enzymes from the cytochrome P450 (CYP) system also stimulate the formation of various biologically active mediators and constitute a “3rd pathway” of arachidonic acid metabolism [3, 4]. The role of the CYP subfamily of enzyme in the hepatic metabolism of xenobiotics is well-recognized; however, certain CYPs also catalyze the oxidative metabolism of various endogenous substrates in extrahepatic tissue [5].
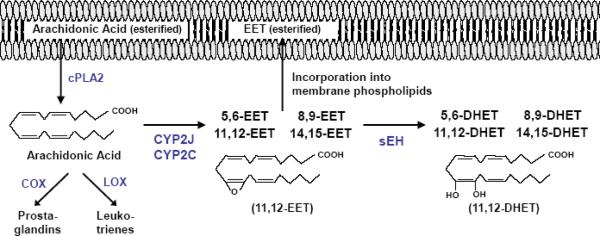
One important example in the cardiovascular system is the olefin epoxidation of arachidonic acid to four epoxyeicosatrienoic acid (EET) regioisomers (5,6-, 8,9-, 11,12-, 14,15-EET) by the CYP2C and CYP2J epoxygenase enzymes [4, 6]. Soluble epoxide hydrolase (sEH, Ephx2) rapidly hydrolyzes the EETs to their corresponding dihydroxyeicosatrienoic acid (DHET) metabolites [7], which, in general, are much less biologically active than EETs (Figure 1). EETs are also readily incorporated into cellular membranes via esterification to phospholipids for subsequent release by phospholipases [8]. Importantly, the CYP epoxygenases and sEH are expressed and metabolically active in various tissues and cell types, including endothelial cells and cardiomyocytes [6, 7, 9–11].
Figure 1. Overview of CYP Epoxygenase Pathway Metabolism.
Upon cPLA2 activation, arachidonic acid is released and oxidatively metabolized by cyclooxygenases (COX), lipoxygenases (LOX) and cytochromes P450 (CYP) into biologically active eicosanoids. This review focuses on the CYP epoxygenase pathway. Isoforms from the CYP2J and CYP2C subfamilies synthesize four epoxyeicosatrienoic acid (EET) regioisomers (11,12-EET is shown), which are subsequently incorporated into membrane phospholipids or rapidly hydrolyzed by soluble epoxide hydrolase (sEH) into their corresponding diol dihydroxyeicosatrienoic acid (DHET) metabolites (11,12-DHET is shown). The DHETs generally possess less potent biological activity than EETs.
It is well-established that EETs possess potent vasodilatory effects, which are mediated by smooth muscle cell hyperpolarization via the activation of calcium-sensitive potassium channels (BKCa++) and more pronounced in the presence of inhibition of prostacyclin and nitric oxide (NO) biosynthesis [12, 13]. Consequently, CYP-derived EETs are regarded as one of the primary endothelium-derived hyperpolarizing factors (EDHFs) [14]. The role of this pathway in blood pressure regulation has also been established, such that mice lacking sEH (Ephx2−/−) have significantly higher circulating EET levels and lower blood pressure compared with wild-type mice [15, 16], administration of a sEH inhibitor (sEHI) significantly lowers blood pressure in various rodent models of hypertension [17, 18], and Ephx2 is a susceptibility gene for hypertension-associated heart failure in rodents [19]. Importantly, sEHIs are currently in clinical development for the treatment of hypertension, with first-time-in-human studies initiated in November 2007.
However, the cellular effects of CYP-derived EETs and their regulation of various inflammatory processes have become increasingly appreciated in recent years, suggesting that the role of this pathway in the cardiovascular system extends beyond the maintenance of vascular tone. The primary objectives of this review are to 1) describe the functional impact of CYP epoxygenase-derived EET biosynthesis and sEH-mediated EET hydrolysis on key inflammatory process in the cardiovascular system, 2) discuss the potential relevance of this pathway to pathogenesis and treatment of cardiovascular disease, and 3) identify areas for future research.
2. Experimental strategies to potentiate CYP epoxygenase pathway function
Preclinical investigations evaluating the biological function of CYP-derived EETs in vitro have relied on exogenous administration of EETs, transfection/overexpression of CYP epoxygenases, and inhibition of sEH to potentiate the CYP epoxygenase pathway and characterize their anti-inflammatory properties. First, direct EET administration has enabled functional characterization of specific EET regio- and stereo-isomers; however, these epoxides are rapidly hydrolyzed by sEH to their less biologically active DHET metabolites, and consequently are not amenable to dosing in vivo. Second, overexpression the CYP2J or CYP2C epoxygenases by transfection increases cellular EET biosynthesis, and has enabled functional characterization of the human epoxygenase enzymes CYP2J2, CYP2C8 and CYP2C9 in in vitro and ex vivo models; however, experimental limitations with this approach also exist. Third, although EETs mediate many of their biological effects in a manner consistent with activation of a Gαs-coupled receptor, an “EET receptor” has not been cloned to date [20]. However, availability of the putative EET receptor antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) has proven instrumental in the functional characterization of the CYP epoxygenase pathway in vitro and ex vivo [21]. Fourth, pharmacological administration of a sEHI [17, 22] and deletion of the sEH gene (Ephx2−/− mice) [23, 24] significantly increase cellular EET levels in vitro and tissue and systemic EET levels in vivo. This pharmacological strategy has enabled investigators to characterize the anti-inflammatory properties of CYP-derived EETs in whole animal models, and is currently under investigation in Phase II clinical trials.
3. CYP epoxygenase pathway and the regulation of cardiovascular inflammation
3.1. Inhibition of endothelial activation and leukocyte adhesion
Endothelial activation, leukocyte-endothelial adhesion, and subsequent leukocyte transmigration across the endothelium is a primary event in the vascular inflammatory response and integral to the pathogenesis of cardiovascular diseases in humans, including atherosclerosis, heart failure and hypertension [25]. An early report observed that 14,15-EET administration enhanced adherence of U937 mononuclear cells to cultured endothelial cells [26]. Subsequent studies have demonstrated that CYP epoxygenase-derived EETs, in particular 11,12-EET, significantly attenuate endothelial activation and leukocyte-endothelial interactions in various model systems (Table 1) [11, 27–31].
Table 1.
Summary of the anti-inflammatory effects of CYP epoxygenase-derived EETs on the endothelium.
| CYP-derived EETs | Inflammatory Stimulus | Cell System / Tissue | Anti-inflammatory Phenotype | Mechanism | Reference |
|---|---|---|---|---|---|
| 11,12-EET | TNF-α | Human umbilical vein ECs | ↓ VCAM, ↓ ICAM, ↓ E-selectin expression | ↓ NF-κB activation | [11] |
| 11,12-EET | TNF-α | Mouse carotid arteries (in vivo) | ↓ VCAM expression, ↓ monocyte adhesion | - | [11] |
| 11,12-EET | TNF-α | Human umbilical vein ECs | - | ↓ NF-κB activation | [27] |
| 11,12-EET (and synthetic analogs) | TNF-α | Human saphenous vein ECs | ↓ VCAM expression | - | [28] |
| 5,6-EET, 8,9-EET, 11,12-EET, 14,15-EET, sEHI | TNF-α | Bovine aortic ECs | ↑ PPAR-γ activation | ↓ NF-κB activation | [29] |
| CYP2J2 expression, 8,9-EET | Hcy | Murine aortic ECs | ↓ MMP-9 activation | ↓ NF-κB activation | [30] |
| 5,6-EET, 8,9-EET, 11,12-EET, 14,15-EET | PMA | Bovine aortic ECs | ↓ PMN adherence ↑ PMN aggregation | - | [31] |
| 8,9-EET, 11,12-EET | None | Human dermal microvessel ECs | ↑ HO-1 expression and activity | - | [50] |
| CYP2C / 2J expression, 8,9-, 11,12-, 14,15-EET | None | Bovine aortic ECs | ↑ eNOS expression and activity | ↑ MAPK, ↑ PKC | [54] |
| 11,12-EET | LPS | Rat monocytes | ↓ PGE2 synthesis | - | [57] |
| 14,15-EET | Arachidonic acid | Murine brain microvessel SMCs | ↓ PGE2 synthesis | - | [59] |
| 11,12-EET | None | Bovine aortic ECs | ↓ t-PA expression | ↓ cAMP | [65] |
| CYP2C expression, 11,12-EET | ADP, Flow | Human umbilical vein ECs | ↓ P-selectin expression, ↓ platelet adhesion | ↑ membrane hyperpolarization | [68] |
| sEHI | AngII in ApoE−/− mice | Mouse aorta | ↓ ICAM, ↓ VCAM, ↓ IL-6 expression, ↓ macrophage infiltration | - | [103] |
Adenosine diphosphate: ADP; Cyclic adenosine monophosphate: cAMP; Homocysteine: Hcy; Phorbol myristate acetate: PMA; Polymorphonuclear cell: PMN; Smooth muscle cell: SMC.
3.1.1. Inhibition of NF-κB activation
Importantly, activation of nuclear factor-kappaB (NF-κB) is a central mediator of this process, which induces transcriptional up-regulation of endothelial cytokine, chemokine and cellular adhesion molecule (CAM) expression and drives the subsequent adherence of leukocytes to the endothelium [32]. Node et al. were the first to demonstrate that CYP-derived EETs possess potent anti-inflammatory properties in the vasculature via inhibition of NF-κB activation [11]. In cultured human umbilical vein endothelial cells (HUVECs), 11,12-EET (100 nM) significantly attenuated endothelial cell-surface expression of VCAM-1, E-selectin and ICAM-1 after stimulation with TNF-α (10 ng/mL). Importantly, these effects were independent of the EDHF properties, since BKCa++ channel inhibition with charybdotoxin or iberiotoxin did not alter the impact of 11,12-EET on TNF-α-induced VCAM-1 expression. Among the four EET regioisomers, 11,12-EET was the most potent inhibitor of VCAM-1 up-regulation, followed by 8,9-EET and 5,6-EET. Interestingly, 14,15-EET administration yielded no anti-inflammatory effect. Second, both transfection of the human CYP epoxygenase CYP2J2, which increased cellular EET biosynthesis, and exogenous administration of 11,12-EET significantly attenuated VCAM-1 promoter activation by TNF-α in a heterologous system. Third, intra-arterial infusion of 11-12-EET (100 ng/kg/min × 5.5-hours) attenuated VCAM-1 expression in isolated perfused murine carotid arteries 5-hours after intraperitoneal TNF-α (10 mcg/kg) administration, and significantly reduced TNF-α stimulated U937 mononuclear cell adhesion to isolated, perfused murine carotid arteries. Lastly, 11,12-EET significantly attenuated TNF-α stimulated IκB kinase (IKK) activity, inhibitor κB-α (IκB-α) degradation, and subsequent RelA translocation into endothelial cell nuclei, collectively demonstrating that CYP-derived EETs inhibit cytokine-induced endothelial activation and leukocyte adhesion via inhibition of NF-κB activation [11].
Subsequently, Fleming et al. confirmed that direct administration of 11,12-EET attenuated TNF-α induced NF-κB activation in HUVECs [27]. Falck et al. demonstrated that 11,12-EET, and a series of its structural analogues, inhibited TNF-α induced VCAM-1 expression in human saphenous vein endothelial cells (HSVEC) [28]. Moreover, all four EET regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EET) attenuated IκB-α degradation after TNF-α stimulation in bovine aortic endothelial cells (BAECs) [29], further demonstrating that EETs inhibit cytokine-mediated NF-κB activation in endothelial cells. A recent investigation in cultured mouse aortic endothelial cells (MAECs) observed that both transfection of the human CYP epoxygenase CYP2J2 and direct administration of 8,9-EET attenuated homocysteine (Hcy)-induced matrix metalloproteinase (MMP)-9 expression and activity, IκB-α degradation, RelA nuclear translocation, and NF-κB-DNA binding [30]. Direct 14,15-EET administration attenuated IκB-α degradation after TNF-α stimulation in a primary culture of human bronchi [33]. In cultured neonatal cardiomyocytes, administration of an sEHI inhibited angiotensin II (AngII)-stimulated RelA nuclear translocation [34].
Recent in vivo investigations have also demonstrated that inhibition of sEH-mediated EET hydrolysis significantly attenuate inflammatory responses in rodents. Induction of circulating cytokine (TNF-α, IL-6) and chemokine (MCP-5) concentrations, hepatic inducible nitric oxide synthase (iNOS) and COX-2 expression, and mortality by intraperitoneal LPS administration (10 mg/kg) was significantly attenuated in Ephx2−/− mice [23] and wild-type mice treated with a sEHI [22]. Similarly, sEHI administration protected rats from tobacco smoke-induced airway inflammation, including leukocyte infiltration into bronchial alveolar lavage fluid [35]. Although NF-κB activation was not directly measured in these studies, attenuation of LPS-mediated up-regulation of these inflammatory responses in vivo is consistent with inhibition of NF-κB activation. Moreover, sEHI treatment significantly attenuated myocardial IκB-α phosphorylation, IκB-α degradation, and nuclear RelA expression in mice 6-weeks after cardiac hypertrophy induced by thoracic aortic constriction [34]. Similarly, NF-κB activation in kidney and urinary excretion of monocyte chemoattractant protein (MCP)-1 was significantly attenuated in Ephx2−/− mice with deoxycorticosterone acetate plus high salt (DOCA-salt)-induced hypertension [16]. Collectively, these studies have demonstrated that CYP-derived EETs significantly attenuate pathologically relevant inflammatory responses in the cardiovascular system, including endothelial activation and leukocyte adhesion, which is mediated at least in part through inhibition NF-κB activation. However, future studies which further elucidate these effects in vivo, and the underlying mechanisms, are needed.
3.1.2. Activation of PPAR-α and PPAR-γ
The peroxisome proliferator-activated receptor (PPAR) transcription factors are members of the nuclear receptor superfamily which are activated by fatty acids and fatty acid derivatives, and are expressed in the vasculature and myocardium [36]. In addition to regulating lipid utilization, adipocyte differentiation and insulin sensitivity, PPAR-α and PPAR-γ elicit various anti-inflammatory effects upon activation including inhibition of NF-κB activation, CAM expression and leukocyte-endothelial adhesion [36–40]. Recent studies have demonstrated that all four EET and DHET regioisomers bind to the isolated ligand-binging domains, transactivate PPAR-α and PPAR-γ, induce PPAR/RXR heterodimer binding to a peroxisome proliferator response element (PPRE), and activate downstream PPAR-responsive gene expression in vitro [29, 41–44]. Interestingly, 14,15-DHET was the most potent transactivator of both PPAR-α and PPAR-γ [43, 44]. Moreover, further metabolism of EETs by CYP ω-hydroxylase (CYP4A) enzymes to their corresponding ω-alcohol metabolites also bind to and activate PPAR-α [41], demonstrating that downstream metabolic products may contribute to these effects.
Recent studies have demonstrated that PPAR-γ activation contributes to the anti-inflammatory effects of CYP-derived EETs. Liu et al. observed that phospholipase A2 (PLA2) and CYP epoxygenase inhibition attenuated laminar flow-activation of PPAR-γ [45], and laminar flow augmented cellular EET levels and decreased sEH mRNA in cultured BAECs [29]. Moreover, administration of the PPAR-γ antagonist GW96625 abolished the attenuation of TNF-α induced IκB-α degradation in BAECs produced by EET administration, suggesting that inhibition of NF-κB activation by EETs in endothelial cells may be mediated, at least in part, by PPAR-γ activation. However, this remains to be confirmed in vivo. Similar studies evaluating the contribution of PPAR-α activation to the anti-inflammatory effects of EETs are also necessary.
3.1.3. Increase HO-1 expression
Heme oxygenase (HO-1), the rate-limiting enzyme in the catabolism of heme, has important anti-oxidant, anti-inflammatory and vasodilatory properties in the vasculature [46–48]. The overlapping biological activities between EETs and the HO system have led researchers to evaluate the potential contribution of HO-1 to the properties of CYP-derived EETs in the cardiovascular system. Direct 11,12-EET administration increased HO-mediated carbon monoxide production by rat mesenteric arteries [49]. Moreover, HO inhibition abolished (8,9-, 11,12-EET) and partially inhibited (14,15-EET) BKCa++ mediated dilation of rat mesenteric arteries by EETs [49], demonstrating the contribution of HO activity to the vascular effects of EETs. Similarly, 8,9- and 11,12-EET administration significantly increased HO-1, but not HO-2, expression and activity in cultured endothelial cells and rat aortas [50]. Overexpression of HO-1 in rat kidney has been reported to suppress CYP expression and inhibit CYP epoxygenase metabolic activity [51], suggesting the potential presence of a feedback control mechanism [50]. However, the contribution of increased HO-1 expression to the anti-inflammatory effects of CYP-derived EETs has not been explored to date.
3.1.4. Increase eNOS expression
The impact of CYP-derived EETs on the regulation of vascular tone are most pronounced in the presence of endothelial nitric oxide synthase (eNOS) inhibition [12, 13], suggesting that these EDHFs serve as an important reserve system to NO. In fact, EET-mediated vasodilation is hypothesized to play a substantially larger role in the presence of established endothelial dysfunction and reduced NO availability, since NO inhibits CYP enzymatic activity [52] and attenuates the release of EDHF [53]. Moreover, CYP-derived EETs increased eNOS expression and NO biosynthesis in endothelial cells in vitro [54, 55], suggesting the presence of a functional interaction between these parallel pathways. This was first reported by Wang et al. in cultured BAECs incubated with EETs (50–200 nM) or transfected with CYP epoxygenase enzymes, which led to increased eNOS mRNA and protein expression and increased metabolic conversion of L-arginine to L-citrulline [54]. Although increased eNOS Thr495 phosphorylation, which inhibits eNOS activity, was also observed the net effect was an increase in eNOS metabolic activity [54]. These effects were mediated by activation of mitogen-activated protein kinase (MAPK) and protein kinase C (PKC) signaling [54]. Similarly, 11,12-EET has been reported to increase NOS activity in human platelets in vitro [56]. Although increased NO biosynthesis may contribute to the vasodilatory effects of EETs in certain vascular beds [55], the mechanistic contribution of increased eNOS expression and NO generation to the anti-inflammatory effects of EETs remains unclear and requires further investigation.
3.1.5. Decrease COX-2 mediated inflammatory responses
Multiple studies have demonstrated that CYP-derived EETs inhibit COX-2-mediated inflammatory responses via direct inhibition of COX-2 metabolic activity and/or attenuation of NF-κB-mediated up-regulation of COX-2 expression. Administration of 11,12-EET dose-dependently attenuated LPS-stimulated prostaglandin E2 (PGE2) production through inhibition of COX-2 activity, and pharmacological blockade of CYP epoxygenase activity has augmented LPS-induced synthesis of PGE2 in cultured rat monocytes [57, 58]. Similarly, 14,15-EET administration decreased PGE2 production in cultured murine brain microvascular smooth muscle cells without altering COX-1 or COX-2 expression [59]. Administration of a sEHI significantly attenuated LPS-mediated induction of hepatic COX-2 expression and circulating PGE2 levels in mice [22, 60]. Moreover, sEHI administration attenuated LPS-induced spinal COX-2 expression [61] and intracerebroventricular (ICV) injection of EETs attenuated LPS-induced fever [58] in rats in vivo, collectively demonstrating that potentiation of the CYP epoxygenase pathway attenuates COX-2 mediated inflammatory responses. These anti-inflammatory effects appear to occur via attenuation of NF-κB-mediated induction of COX-2 expression and competitive inhibition of COX-2 metabolic activity since EETs also are also weak substrates for COX enzymes [62, 63].
In contrast, Michaelis et al. observed that CYP2C9 overexpression increased COX-2 promoter activity, COX-2 expression, prostacyclin production and cAMP levels by approximately 2-fold in HUVECs under basal conditions, which were abolished by the CYP2C9-specific inhibitor sulfaphenazole [64]. Direct 11,12-EET administration also significantly increased COX-2 expression [64]. Importantly, these experiments were performed in the absence of an inflammatory stimulus. As described above, CYP-derived EETs have been shown to inhibit COX-2 expression and/or activity in the presence of inflammation. Further investigation remains necessary to elucidate the mechanism underlying these contradictory findings and characterize the functional interaction between COX-2 and the CYP epoxygenase pathway in the vasculature and myocardium in vivo.
3.2. Inhibition of hemostasis
Overexpression of CYP2J2 via transfection and direct 11,12-EET administration (100 nM) to BAECs significantly increased tissue plasminogen activator (t-PA) expression and activity via activation of the cAMP-dependent kinase PKA and independent of endothelial cell hyperpolarization [65]. No effect on plasminogen activator inhibitor (PAI)-1 was observed. In parallel, CYP2J2 overexpression and 11,12-EET increased intracellular cAMP levels, cAMP-response element (CRE) transactivation and Gαs GTP binding activity, further demonstrating that the anti-fibrinolytic effects of EETs were mediated by cAMP activation [65]. Moreover, inhibition of EET formation significantly attenuated thrombin-mediated t-PA release in human microvascular endothelial cells [66]. In suspended platelets in vitro, EETs (1–10 μM) also inhibited COX activity and arachidonic acid-induced platelet aggregation [67]. More recently, overexpression of the human epoxygenase CYP2C9 and direct EET administration hyperpolarized platelets (consistent with their EDHF properties), attenuated ADP-induced P-selectin expression, and inhibited platelet adhesion to endothelial cells in a membrane potential-dependent manner [68].
3.3. Protection against ischemia-reperfusion injury
Hypoxia-reoxygenation (H/R) or ischemia-reperfusion (I/R) injury induces inflammation through multiple mechanisms and results in vascular and myocardial dysfunction. A series of recent in vitro and in vivo studies have demonstrated that potentiation of the CYP epoxygenase pathway protects endothelial cells and cardiomyocytes from I/R-induced injury [69]. In cultured BAECs, overexpression of the human CYP2J2 epoxygenase and direct 11,12-EET administration significantly attenuated H/R-induced oxidative stress and endothelial cell injury [70]. Direct administration of EETs protected cardiomyocytes from H/R induced apoptosis in vitro through activation of PI3K/Akt signaling [71]. Similarly, in isolated murine pulmonary arteries and murine, rat and human myocardial tissue, H/R-induced apoptosis and injury was significantly attenuated by 11,12-EET administration, and in Ephx2−/− mice, via activation of PI3K/Akt signaling and ATP-sensitive potassium (KATP) channels [72].
Using the Langendorff isolated perfused heart model, post-ischemic recovery of contractile function was significantly improved and deleterious electrocardiographic changes (e.g., QT prolongation, ST elevation) and mitochondrial dysfunction (e.g., increased mitochondrial fragmentation, T-tubule swelling) were significantly attenuated in transgenic mice with cardiomyocyte-specific expression of the human CYP2J2 epoxygenase and with direct EET administration to wild-type mice [73–75]. A similar cardioprotective phenotype has also been observed in Ephx2−/− mice [24, 75]. Moreover, direct administration of EETs into the perfusate or treatment with a sEHI significantly reduced the infarct size and inhibited the myocardial remodeling after I/R injury in rodents and dogs [76–78]. Ephx2−/− mice exhibited an attenuated suppression of left-ventricular ejection fraction and induction of cardiac arrhythmias in AngII and thoracic aortic banding models of heart failure [19]. Recently, administration of an sEHI improved contractile function, decreased infarct size, decreased atrial and ventricular arrhythmias, and decreased induction of circulating cytokine and chemokine levels in mice 3-weeks after a surgically-induced myocardial infarction by coronary artery occlusion [79]. Importantly, administration of the CYP epoxygenase enzyme inhibitor (MS-PPOH) and EET receptor antagonist (14,15-EEZE) has abolished these phenotypes [24, 72–78] in multiple studies, demonstrating the direct contribution of CYP-derived EETs to cardioprotection.
These cardioprotective effects are mediated via activation of sarcolemmal and mitochondrial KATP channels and activation of p42/p44 MAPK, PI3K and PKA signaling [24, 72–75, 77, 78], which are well-characterized cardioprotective mechanisms [69]. More recently, up-regulation of b-type natriuretic peptide (BNP) expression and activation of natriuretic peptide receptor type-A (NPR-A) has been shown to mediate the cardioprotective effects of EETs after I/R injury, independent of PI3K [80]. Inhibition of sEH-mediated EET hydrolysis has also significantly reduced cerebral infarct volume in rodents after middle cerebral artery occlusion [81, 82], an established preclinical model of ischemic stroke. Collectively, these studies demonstrate that CYP-derived EETs offer substantial protection in preclinical models of I/R injury, in addition to their known vasodilatory effects, further substantiating their anti-inflammatory and protective properties in the cardiovascular system.
4. Vascular protective effects of the CYP epoxygenase pathway
4.1. Promotion of endothelial cell growth, survival, migration and angiogenesis
Recent studies have demonstrated that CYP-derived EETs promote endothelial cell survival through potent pro-mitogenic, pro-migratory, pro-angiogenic [64, 83–95] and anti-apoptotic [71, 72, 96–98] effects, which has been the subject of recent reviews [20, 99]. In addition to their direct pro-angiogenic effects, CYP derived EETs also contribute to hypoxia-[88] and vascular endothelial growth factor (VEGF)-mediated [93] stimulation of angiogenesis, further demonstrating their important role in this process. These pro-survival effects are mediated through activation of numerous cell signaling cascades in endothelial cells, including PI3K/Akt, ERK and p38 MAPK (Table 2). Most notably, CYP-derived EETs are potent activators of PI3K/Akt signaling, which is mediated via activation of the epidermal growth factor receptor (EGFR) [87, 90]. This leads to the downstream phosphorylation and inhibition of the forkhead transcription factors FOXO1 and FOXO3b, downregulation of the cyclin-dependent kinase inhibitor p27Kip1, and a subsequent increase in cyclin D1 expression [85]. CYP-derived EETs also increase endothelial cyclin D1 expression via increased MAPK phosphatase-1 activation and a subsequent decrease in JNK activity [84]. In parallel, induction of VEGF expression via signal transducer and activator of transcription (STAT)-3 activation [95] and induction of COX-2 expression via cAMP/PKA activation [64] have also been implicated in EET-induced angiogenesis. Although these signaling pathways are also important regulators of inflammatory responses, their contribution to the regulation of cardiovascular inflammation by CYP-derived EETs remain unclear and require further investigation, particularly in cardiovascular disease-specific in vivo models. Importantly, CYP-derived EETs have also been reported to promote tumor metastasis [94], consistent with their pro-survival and pro-angiogenic effects, while inhibition of CYP-derived EET formation has increased tumor cell apoptosis and decreased tumor growth and metastasis [98]. These deleterious consequences of increased CYP-derived EET formation require careful consideration and further investigation.
Table 2.
Summary of the key signaling pathways which mediate the pro-growth and pro-survival effects of CYP epoxygenase-derived EETs on endothelial cells.
| Signaling pathway | Endothelial cell system | Reference |
|---|---|---|
| ↑ PI3K/Akt | Human umbilical vein ECs | [85, 87, 92] |
| Murine pulmonary microvascular ECs | [89] | |
| Bovine aortic ECs | [90, 97] | |
| Human dermal microvascular ECs | [91] | |
| Human coronary artery ECs | [96] | |
| Human pulmonary microvascular ECs | [96] | |
| ↑ ERK | Porcine coronary artery ECs | [83] |
| Human coronary artery ECs | [83] | |
| Human umbilical vein ECs | [83, 84] | |
| Murine pulmonary microvascular ECs | [89] | |
| Bovine aortic ECs | [90, 97] | |
| ↑ p38 MAPK | Porcine coronary artery ECs | [83] |
| Human coronary artery ECs | [83] | |
| Human umbilical vein ECs | [84] | |
| Murine pulmonary microvascular ECs | [89] | |
| ↑ MAPK phosphatase-1 / ↓ JNK | Human umbilical vein ECs | [84] |
| ↑ cAMP/PKA | Human umbilical vein ECs | [64] |
Cyclic adenosine monophosphate: cAMP; Epidermal growth factor receptor: EGFR; Extracellular signal-regulated kinase: ERK; c-Jun N-terminal kinase: JNK; Mitogen-activated protein kinase: MAPK; Phosphatidylinositol 3-kinase: PI3K; Protein kinase A: PKA.
4.2 Attenuation of vascular remodeling
In contrast to endothelial cells, potentiation of the CYP epoxygenase pathway has yielded conflicting effects on the proliferation of vascular smooth muscle cells (VSMC) in vitro. In human aortic VSMCs, sEH inhibition and direct EET administration decreased platelet-derived growth factor (PDGF)-mediated stimulation of VSMC proliferation and cyclin D1 expression, independent of p42/p44 MAPK phosphorylation [100]. In addition, overexpression of the human CYP2J2 epoxygenase and direct 11,12-EET administration attenuated serum- and PDGF-induced migration of rat aortic VSMCs via increased EET-mediated intracellular cAMP generation and PKA activation [101]. These effects were independent of membrane hyperpolarization and COX activity. However, no significant effect on PDGT-induced VSMC proliferation was observed [101]. In contrast, 14,15-EET increased PDGF-induced proliferation in porcine aortic SMCs via inhibiting COX-mediated PGE2 synthesis [59]. Although these conflicting results suggest that inter-species differences in the anti-proliferative phenotype in VSMCs may exist, the mechanisms underlying the effects of CYP-derived EETs on VSMC proliferation remain poorly understood.
Interestingly, two recent investigations have reported that sEHI administration in the drinking water over 4 weeks significantly attenuated abdominal aortic aneurysm formation and atherosclerotic lesion area in apolipoprotein E deficient mice receiving subcutaneous AngII administration [102, 103]; however, sEHI treatment had no effect on ligation-induced remodeling of the carotid artery [103]. Atherosclerotic lesion area inversely correlated with plasma 11,12- and 14,15- EET:DHET ratios, which are biomarkers of sEH metabolic activity [102]. In addition, sEHI treatment downregulated pro-inflammatory gene expression in the aorta and circulating levels in serum, and reduced inflammatory cell infiltration into the vascular wall [103]. These data suggest that sEH-mediated EET hydrolysis may be important in the pathogenesis of atherosclerosis, beyond their vasodilatory effects, via the regulation of vascular inflammation. Importantly, future studies evaluating the effects of increased CYP epoxygenase-mediated EET biosynthesis and decreased sEH-mediated EET hydrolysis remain necessary to more fully understand the contribution of the CYP epoxygenase pathway to the pathogenesis of atherosclerosis, vascular inflammation and vascular remodeling in vivo, and further characterize the potential utility of therapies which modulate this pathway.
5. EET-independent effects of the CYP epoxygenase pathway and inflammation
5.1. CYP-mediated reactive oxygen species generation
Certain CYP enzymes are also a known source of pro-inflammatory reactive oxygen species (ROS), which enhance NF-κB activation and CAM expression [27, 104]. In addition to the biosynthesis of anti-inflammatory EETs, the CYP2C epoxygenases are also a significant source of ROS in vitro and in vivo [27, 105, 106]. For instance, despite increasing EET biosynthesis, overexpression of the human epoxygenase CYP2C9 increased ROS formation, NF-κB activation and VCAM-1 expression in endothelial cells in vitro [27]. Administration of the CYP2C-specific inhibitor sulfaphenazole decreased CYP2C-mediated ROS formation and abolished these pro-inflammatory effects [27]. Similarly, sulfaphenazole significantly attenuated myocardial injury and infarct size after I/R in rats [105], and enhanced endothelium-dependent vasodilator responses in patients with coronary artery disease (CAD) via suppression of ROS formation [106]. In contrast to the CYP2C epoxygenases, overexpression of human epoxygenase CYP2J2 decreased ROS formation in BAECs under conditions of enhanced oxidative stress [70]; although this anti-oxidant phenotype remains to be validated in other model systems. Therefore, the enzymatic source of EETs may also be a critical factor with respect to their net effect on inflammation, due to the potential for simultaneous ROS production. However, direct comparisons of CYP2J and CYP2C-mediated EET biosynthesis on inflammatory responses in vivo, and the underlying mechanisms, have not been explored and require further evaluation.
5.2. CYP epoxygenase metabolism of other fatty acids
In addition to arachidonic acid, linoleic acid is also metabolized by CYP epoxygenases to epoxyoctadecenoic acids (EpOMEs), which are subsequently hydrolyzed by sEH to dihydroxyoctadecenoic acids (DHOMEs). Importantly, plasma EpOME:DHOME ratios have been validated as sensitive in vivo biomarkers of sEH activity in Ephx2−/− mice [24], rodents treated with an sEHI [22, 35], and in humans carrying functionally relevant EPHX2 polymorphisms [107], such that higher EpOME:DHOME ratios are indicative of lower sEH metabolic activity. Interestingly, DHOMEs have been reported to elicit pro-inflammatory effects in pre-clinical models [108, 109]; however, it remains unclear whether decreased EpOME hydrolysis to DHOMEs, in addition to decreased EET hydrolysis, significantly contributes to the anti-inflammatory effects yielded by sEH inhibition.
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the 20 and 22-carbon ω-3 polyunsaturated fatty acid (PUFA) analogs of arachidonic acid, are also metabolized by CYP epoxygenase enzymes to epoxyeicosatetraenoic acids (EETeTr) and epoxydocasapentaenoic acids (EDP) respectively [110, 111]. Interestingly, 17,18-EETeTr has been reported to possess potent vasodilatory effects through activation of BKCa++ channels [111, 112], suggesting that CYP epoxygenase metabolism may contribute to the biological effects attributed to ω-3 PUFAs; however, the role of 17,18-EETeTr in the regulation of inflammation has not been studied to date. Future studies will be required to delineate the direct contribution of arachidonic acid-independent fatty acid metabolism to the regulation of cardiovascular inflammation by the CYP epoxygenase pathway.
6. Effect of inflammation on the CYP epoxygenase pathway
It is well-established that acute inflammation significantly alters hepatic CYP expression in vitro and in vivo [113–115]. In particular, cytokines suppress hepatic CYP expression in a pre-translational manner, and consequently decrease xenobiotic metabolism and clearance in preclinical models and humans [113–115]. Preclinical studies have also demonstrated that extrahepatic CYP2C and CYP2J epoxygenase expression are suppressed in response to acute inflammatory stimuli. For instance, pulmonary CYP2C11 and CYP2J4 expression were significantly suppressed in the cecal ligation and puncture (CLP) sepsis model in rats [116]. Similarly, pulmonary CYP2J4 protein expression [117] and CYP-dependent arachidonic acid metabolism [118] were suppressed in a rat model of Pseudomonas pneumonia, and renal CYP-mediated EET formation was suppressed by 81% in LPS-treated rats compared to saline-treated controls [119].
AngII also substantially impairs CYP epoxygenase pathway function in renal and vascular tissue. Subcutaneous AngII infusion for 2-weeks significantly increased renal cortical sEH protein expression in rats compared to untreated controls, which was paralleled by lower EET and higher DHET levels in urine [17]. Similarly, AngII treatment significantly induced sEH protein expression in HUVECs and BAECs in vitro and the aortic intima of rats in vivo [120]. These changes were prevented by treatment with losartan, suggesting that this effect was mediated by AT1 receptor activation [120]. The combination of AngII infusion and a high-salt diet significantly suppressed CYP2C and CYP2J and increased sEH protein expression in renal microvessels [121, 122]. Blockade of TNF-α with etanercept prevented these changes in CYP2C and sEH expression, while also reducing blood pressure, urinary protein and MCP-1 excretion, and renal leukocyte infiltration [121]. Moreover, in double transgenic rats (dTGR) which overexpress the human renin and angiotensinogen genes, renal CYP2C and CYP2J protein expression and EET formation was significantly lower compared to Sprague-Dawley control rats [123]. Treatment with fenofibrate, a PPAR-α activator, restored renal CYP2C protein expression and epoxygenase metabolic activity in dTGRs to the levels observed Sprague-Dawley rats, while also lowering blood pressure, renal inflammation and renal injury [124]. In obese Zucker rats, CYP2C and CYP2J expression was also significantly lower and sEH expression was higher in mesenteric arteries compared to Sprague-Dawley rats [125]. Similarly, renal tubule CYP2C protein expression and EET formation was significantly decreased in a rat model of high fat diet-induced hypertension; although, EET formation in renal microvessels remained unchanged in this model [126]. Lastly, sEH expression was induced in aorta, heart, and lung of mice chronically exposed to tobacco smoke [127].
These studies collectively demonstrate that various inflammatory stimuli, including activation of cytokines and the renin-angiotensin system, suppress CYP2C/2J and increase sEH expression, suggesting that alterations in CYP epoxygenase pathway expression and function may be an important consequence of inflammatory response in vivo. However, the specific underlying mechanisms remain unclear. Extensive studies in hepatocytes and liver tissue suggest that modulation of CYP expression by inflammatory stimuli is primarily transcription-dependent; although, identification of the specific transcription factors which mediate these alterations in CYP expression have yielded conflicting results and other mechanisms involving RNA stability and interactions with NO may also contribute [128–130]. Importantly, the functional implications of inflammation-induced changes in CYP and sEH expression on EET levels in the cardiovascular system in vivo requires further investigation, including studies in humans with cardiovascular disease.
7. Genetic variation and cardiovascular disease in humans
Genetic polymorphisms in EPHX2 and the human epoxygenases CYP2J2 and CYP2C8 with altered expression or function have been recently identified [131]. We and others have observed significant associations between these polymorphisms and risk of developing cardiovascular disease in which inflammation plays an integral pathological role, including CAD and ischemic stroke [131]. For example, a CYP2J2 proximal promoter polymorphism (−50G>T), which results in loss of a critical Sp1 transcription factor binding site, reduced CYP2J2 transcription and lowered plasma DHET levels, has been associated with significantly greater risk of CAD [132, 133]. In CYP2C8, the Arg139Lys/Lys399Arg (CYP2C8*3) variant results in lower CYP2C8 catalytic activity in vitro [134], and has been associated with higher risk of prevalent myocardial infarction [135] and incident CAD in cigarette smokers [136]. The EPHX2 Lys55Arg variant, which results in higher sEH-mediated EET hydrolysis in vitro [137], has been associated with higher apparent sEH activity in vivo and increased risk of incident CAD [107]. Associations between genetic variation in EPHX2 and coronary artery calcification, an established measure of subclinical atherosclerosis [138], and incident ischemic stroke events [139] have also been reported.
Collectively, these epidemiological analyses have demonstrated that genetic variation in the CYP epoxygenase pathway is associated with cardiovascular disease risk at the population level, suggesting that this pathway may also play an important role in the regulation of cardiovascular inflammation in humans. However, the functional contribution of these genetic variants to cardiovascular inflammation remains poorly understood and requires further study. Moreover, conflicting reports regarding the presence and strength of these relationships have been identified across studies, suggesting that these relationships are complex and likely most profound in certain subsets of the population [131]. Interestingly, we and others have reported that the association between CYP2J2, CYP2C8 and EPHX2 genetic variants and CAD risk are consistently most pronounced in cigarette smokers [107, 133, 136, 140], suggesting that the pathological impact of lower CYP-derived EETs may be greatest in those with underlying vascular dysfunction. Such an interaction would be consistent with the preclinical studies described above, since modulation of the CYP epoxygenase pathway appears to elicit its most substantial effects on cardiovascular function in the presence of a pathological stimulus (e.g., cytokine activation, I/R injury, etc.). Future studies evaluating the impact of genetic variation in the CYP epoxygenase pathway on prognosis in patients with established cardiovascular disease will offer important insight into this question, and potentially identify subsets of the population most likely to respond to therapies which modulate this pathway.
8. Summary and Future Directions
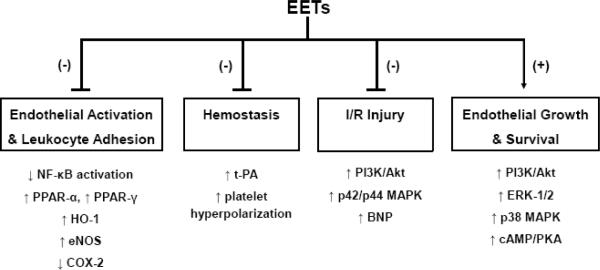
In recent years, the CYP epoxygenase pathway has been identified as an important regulator of cardiovascular inflammation in addition to its more well-established role in the regulation of vascular tone. The aforementioned preclinical studies have demonstrated that CYP epoxygenase-derived EETs act in a paracrine and/or autocrine manner to inhibit endothelial activation and leukocyte adhesion, inhibit hemostasis, attenuate myocardial I/R injury, and promote endothelial cell survival via modulation of multiple cell signaling pathways (Figure 2). Most notably, CYP-derived EETs attenuate NF-κB activation and promote activation of cAMP, PI3K/Akt, p42/p44 MAPK signaling in endothelial cells and cardiomyocytes. Although it remains unclear whether these effects are mediated via binding to a putative EET cell-surface and/or intracellular receptor, the collective preclinical evidence has demonstrated that potentiation of the CYP epoxygenase pathway elicits protection against various pathological stimuli in the cardiovascular system and has high potential for therapeutic application to various cardiovascular diseases.
Figure 2. Overview of the Cardiovascular Protective Effects of CYP Epoxygenase-Derived EETs.
A series of recent studies have demonstrated that CYP epoxygenase-derived EETs possess potent cardiovascular protective effects including inhibition of endothelial activation and leukocyte adhesion, inhibition of hemostasis, attenuation of myocardial and endothelial ischemia-reperfusion (I/R) injury, and promotion of endothelial cell survival.
Despite these advances, important questions remain which require further exploration. First, since most experiments to date have focused on the regulation of inflammatory responses in vitro, confirmation of these anti-inflammatory phenotypes and the underlying mechanisms in cardiovascular disease-relevant models in vivo remain necessary. Although the short half-life of EETs limit their bioavailability in vivo, the recent development of sEHIs conducive for in vivo dosing and transgenic/knockout rodent models have provided investigators the tools necessary to complete such studies. Second, identification of the putative EET receptor will enable the development of specific EET receptor agonists and antagonists, and substantially accelerate future research in this field. Third, further evaluation of interactions between the CYP epoxygenase pathway and parallel pathways known to regulate cardiovascular inflammation in vivo, including eicosanoid metabolism (e.g., COX-derived prostaglandins, LOX-derived leukotrienes) and non-eicosanoid metabolism (e.g., NO, renin-angiotensin system) pathways, remain necessary to more fully elucidate the net functional contribution of the CYP epoxygenase pathway to the pathogenesis and treatment of cardiovascular disease. Fourth, the potentially deleterious consequences of increasing CYP-derived EETs, most notably tumor development and metastasis, and the underlying mechanisms also require rigorous investigation. Lastly, clinical studies will ultimately be required to truly understand the functional role of the CYP epoxygenase pathway in the regulation of cardiovascular inflammation in humans, and define the efficacy and safety of potentiating CYP-derived EETs in patients with cardiovascular disease. Importantly, sEHIs are currently under investigation for hypertension in Phase II clinical trials, and provide substantial promise for translation of these preclinical findings into clinical trials.
Acknowledgements
The authors would like to acknowledge Dr. Cam Patterson for his helpful comments during the drafting of this manuscript. This work was supported by an American Foundation for Pharmaceutical Education Pre-doctoral Fellowship (K.N.T.), a Beginning Grant-in-Aid from the American Heart Association (C.R.L.), and grant R01GM088199 from the National Institute of General Medical Sciences (C.R.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement The authors have no conflicts of interest to disclose.
References
- [1].Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–82. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- [2].Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- [3].Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- [4].Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–62. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- [5].Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29:413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- [6].Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–8. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- [7].Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, et al. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276:14867–74. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- [8].Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- [9].Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem. 2004;52:447–54. doi: 10.1177/002215540405200403. [DOI] [PubMed] [Google Scholar]
- [10].Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–9. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- [13].Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–7. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- [14].Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–25. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- [15].Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–10. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- [16].Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, et al. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–8. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–4. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- [18].Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- [19].Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, et al. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40:529–37. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fleming I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007;82:60–7. doi: 10.1016/j.prostaglandins.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [21].Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–36. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- [22].Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–7. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, Jiang H, et al. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282:2891–8. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–50. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- [26].Pritchard KA, Jr., Tota RR, Stemerman MB, Wong PY. 14, 15-Epoxyeicosatrienoic acid promotes endothelial cell dependent adhesion of human monocytic tumor U937 cells. Biochem Biophys Res Commun. 1990;167:137–42. doi: 10.1016/0006-291x(90)91741-a. [DOI] [PubMed] [Google Scholar]
- [27].Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, et al. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- [28].Falck JR, Reddy LM, Reddy YK, Bondlela M, Krishna UM, Ji Y, et al. 11,12-epoxyeicosatrienoic acid (11,12-EET): structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett. 2003;13:4011–4. doi: 10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- [29].Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005;102:16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moshal KS, Zeldin DC, Sithu SD, Sen U, Tyagi N, Kumar M, et al. Cytochrome P450 (CYP) 2J2 gene transfection attenuates MMP-9 via inhibition of NF-kappabeta in hyperhomocysteinemia. J Cell Physiol. 2008;215:771–81. doi: 10.1002/jcp.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pratt PF, Rosolowsky M, Campbell WB. Effects of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–33. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- [32].de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- [33].Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: putative role of CPI-17. Am J Respir Cell Mol Biol. 2008;38:192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- [34].Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:18733–8. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci U S A. 2005;102:2186–91. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45:120–59. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- [37].Plutzky J. Medicine. PPARs as therapeutic targets: reverse cardiology? Science. 2003;302:406–7. doi: 10.1126/science.1091172. [DOI] [PubMed] [Google Scholar]
- [38].Wray J, Bishop-Bailey D. Epoxygenases and peroxisome proliferator-activated receptors in mammalian vascular biology. Exp Physiol. 2008;93:148–54. doi: 10.1113/expphysiol.2007.038612. [DOI] [PubMed] [Google Scholar]
- [39].Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–8. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- [40].Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, et al. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–81. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- [41].Cowart LA, Wei S, Hsu MH, Johnson EF, Krishna MU, Falck JR, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J Biol Chem. 2002;277:35105–12. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- [42].Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, et al. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther. 2005;314:260–70. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- [43].Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J, et al. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H55–63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- [44].Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35:1126–34. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- [45].Liu Y, Zhu Y, Rannou F, Lee TS, Formentin K, Zeng L, et al. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation. 2004;110:1128–33. doi: 10.1161/01.CIR.0000139850.08365.EC. [DOI] [PubMed] [Google Scholar]
- [46].Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, et al. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–11. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- [47].Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–82. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–90. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- [49].Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, et al. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- [50].Sacerdoti D, Colombrita C, Di Pascoli M, Schwartzman ML, Bolognesi M, Falck JR, et al. 11,12-epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–61. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [51].Abraham NG, Jiang S, Yang L, Zand BA, Laniado-Schwartzman M, Marji J, et al. Adenoviral vector-mediated transfer of human heme oxygenase in rats decreases renal heme-dependent arachidonic acid epoxygenase activity. J Pharmacol Exp Ther. 2000;293:494–500. [PubMed] [Google Scholar]
- [52].Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–23. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- [53].Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–7. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- [54].Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, et al. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther. 2003;307:753–64. doi: 10.1124/jpet.103.052787. [DOI] [PubMed] [Google Scholar]
- [55].Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- [56].Zhang L, Cui Y, Geng B, Zeng X, Tang C. 11,12-Epoxyeicosatrienoic acid activates the L-arginine/nitric oxide pathway in human platelets. Mol Cell Biochem. 2008;308:51–6. doi: 10.1007/s11010-007-9611-6. [DOI] [PubMed] [Google Scholar]
- [57].Kozak W, Aronoff DM, Boutaud O, Kozak A. 11,12-epoxyeicosatrienoic acid attenuates synthesis of prostaglandin E2 in rat monocytes stimulated with lipopolysaccharide. Exp Biol Med (Maywood) 2003;228:786–94. doi: 10.1177/15353702-0322807-03. [DOI] [PubMed] [Google Scholar]
- [58].Kozak W, Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci. 2004;9:3339–55. doi: 10.2741/1486. [DOI] [PubMed] [Google Scholar]
- [59].Fang X, Moore SA, Stoll LL, Rich G, Kaduce TL, Weintraub NL, et al. 14,15-Epoxyeicosatrienoic acid inhibits prostaglandin E2 production in vascular smooth muscle cells. Am J Physiol. 1998;275:H2113–21. doi: 10.1152/ajpheart.1998.275.6.H2113. [DOI] [PubMed] [Google Scholar]
- [60].Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Oliw EH. Metabolism of 5(6)-expoxyeicosatrienoic acid by ram seminal vesicles. Formation of novel prostaglandin E1 metabolites. Biochim Biophys Acta. 1984;793:408–15. doi: 10.1016/0005-2760(84)90256-x. [DOI] [PubMed] [Google Scholar]
- [63].Carroll MA, Balazy M, Margiotta P, Falck JR, McGiff JC. Renal vasodilator activity of 5,6-epoxyeicosatrienoic acid depends upon conversion by cyclooxygenase and release of prostaglandins. J Biol Chem. 1993;268:12260–6. [PubMed] [Google Scholar]
- [64].Michaelis UR, Falck JR, Schmidt R, Busse R, Fleming I. Cytochrome P4502C9-derived epoxyeicosatrienoic acids induce the expression of cyclooxygenase-2 in endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:321–6. doi: 10.1161/01.ATV.0000151648.58516.eb. [DOI] [PubMed] [Google Scholar]
- [65].Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, et al. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983–9. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- [66].Muldowney JA, 3rd, Painter CA, Sanders-Bush E, Brown NJ, Vaughan DE. Acute tissue-type plasminogen activator release in human microvascular endothelial cells: the roles of Galphaq, PLC-beta, IP3 and 5,6-epoxyeicosatrienoic acid. Thromb Haemost. 2007;97:263–71. [PubMed] [Google Scholar]
- [67].Fitzpatrick FA, Ennis MD, Baze ME, Wynalda MA, McGee JE, Liggett WF. Inhibition of cyclooxygenase activity and platelet aggregation by epoxyeicosatrienoic acids. Influence of stereochemistry. J Biol Chem. 1986;261:15334–8. [PubMed] [Google Scholar]
- [68].Krotz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, et al. Membrane-potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2004;24:595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- [69].Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–9. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, et al. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–20. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- [71].Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, et al. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724–35. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bodiga S, Zhang R, Jacobs DE, Larsen BT, Tampo A, Manthati VL, et al. Protective actions of epoxyeicosatrienoic acid: dual targeting of cardiovascular PI3K and KATP channels. J Mol Cell Cardiol. 2009;46:978–88. doi: 10.1016/j.yjmcc.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–14. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- [74].Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009;46:67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- [75].Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol. 2009;46:867–75. doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- [76].Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, et al. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemiareperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2128–34. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H537–42. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- [78].Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–91. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: Insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009 Aug 28; doi: 10.1016/j.yjmcc.2009.08.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chaudhary KR, Batchu SN, Das D, Suresh MR, Falck JR, Graves JP, et al. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc Res. 2009;83:362–70. doi: 10.1093/cvr/cvp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–8. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442:511–8. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- [84].Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277:15671–6. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- [85].Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–25. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- [86].Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, et al. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–24. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- [87].Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) Faseb J. 2003;17:770–2. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- [88].Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118:5489–98. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- [89].Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–46. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- [90].Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–32. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- [91].Zhang B, Cao H, Rao GN. Fibroblast growth factor-2 is a downstream mediator of phosphatidylinositol 3-kinase-Akt signaling in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. J Biol Chem. 2006;281:905–14. doi: 10.1074/jbc.M503945200. [DOI] [PubMed] [Google Scholar]
- [92].Webler AC, Popp R, Korff T, Michaelis UR, Urbich C, Busse R, et al. Cytochrome P450 2C9-induced angiogenesis is dependent on EphB4. Arterioscler Thromb Vasc Biol. 2008;28:1123–9. doi: 10.1161/ATVBAHA.107.161190. [DOI] [PubMed] [Google Scholar]
- [93].Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, et al. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295:C1292–301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res. 2007;67:6665–74. doi: 10.1158/0008-5472.CAN-06-3643. [DOI] [PubMed] [Google Scholar]
- [95].Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 2008;111:5581–91. doi: 10.1182/blood-2007-11-126680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, et al. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–31. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- [97].Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, et al. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol. 2007;293:H142–51. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen C, Li G, Liao W, Wu J, Liu L, Ma D, et al. Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J Pharmacol Exp Ther. 2009;329:908–18. doi: 10.1124/jpet.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Medhora M, Dhanasekaran A, Gruenloh SK, Dunn LK, Gabrilovich M, Falck JR, et al. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 2007;82:19–29. doi: 10.1016/j.prostaglandins.2006.05.025. [DOI] [PubMed] [Google Scholar]
- [100].Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2002;99:2222–7. doi: 10.1073/pnas.261710799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–7. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- [102].Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–23. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, et al. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29:1265–70. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- [104].Sasaki M, Ostanin D, Elrod JW, Oshima T, Jordan P, Itoh M, et al. TNF-alpha-induced endothelial cell adhesion molecule expression is cytochrome P-450 monooxygenase dependent. Am J Physiol Cell Physiol. 2003;284:C422–8. doi: 10.1152/ajpcell.00271.2002. [DOI] [PubMed] [Google Scholar]
- [105].Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, et al. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A. 2004;101:1321–6. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation. 2004;109:178–83. doi: 10.1161/01.CIR.0000105763.51286.7F. [DOI] [PubMed] [Google Scholar]
- [107].Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15:1640–9. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–6. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Slim R, Hammock BD, Toborek M, Robertson LW, Newman JW, Morisseau CH, et al. The role of methyl-linoleic acid epoxide and diol metabolites in the amplified toxicity of linoleic acid and polychlorinated biphenyls to vascular endothelial cells. Toxicol Appl Pharmacol. 2001;171:184–93. doi: 10.1006/taap.2001.9131. [DOI] [PubMed] [Google Scholar]
- [110].Barbosa-Sicard E, Markovic M, Honeck H, Christ B, Muller DN, Schunck WH. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem Biophys Res Commun. 2005;329:1275–81. doi: 10.1016/j.bbrc.2005.02.103. [DOI] [PubMed] [Google Scholar]
- [111].Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–76. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- [112].Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kargel E, Theuer J, et al. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39:609–13. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- [113].Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44:707–15. [PubMed] [Google Scholar]
- [114].Sewer MB, Koop DR, Morgan ET. Endotoxemia in rats is associated with induction of the P4504A subfamily and suppression of several other forms of cytochrome P450. Drug Metab Dispos. 1996;24:401–7. [PubMed] [Google Scholar]
- [115].Barclay TB, Peters JM, Sewer MB, Ferrari L, Gonzalez FJ, Morgan ET. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. J Pharmacol Exp Ther. 1999;290:1250–7. [PubMed] [Google Scholar]
- [116].Cui X, Wu R, Zhou M, Simms HH, Wang P. Differential expression of cytochrome P450 isoforms in the lungs of septic animals. Crit Care Med. 2004;32:1186–91. doi: 10.1097/01.ccm.0000124877.86743.37. [DOI] [PubMed] [Google Scholar]
- [117].Yaghi A, Bradbury JA, Zeldin DC, Mehta S, Bend JR, McCormack DG. Pulmonary cytochrome P-450 2J4 is reduced in a rat model of acute Pseudomonas pneumonia. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1099–105. doi: 10.1152/ajplung.00039.2003. [DOI] [PubMed] [Google Scholar]
- [118].Yaghi A, Bend JR, Webb CD, Zeldin DC, Weicker S, Mehta S, et al. Excess nitric oxide decreases cytochrome P-450 2J4 content and P-450-dependent arachidonic acid metabolism in lungs of rats with acute pneumonia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1260–7. doi: 10.1152/ajplung.00273.2003. [DOI] [PubMed] [Google Scholar]
- [119].Ogungbade GO, Akinsanmi LA, Jiang H, Oyekan AO. Role of epoxyeicosatrienoic acids in renal functional response to inhibition of NO production in the rat. Am J Physiol Renal Physiol. 2003;285:F955–64. doi: 10.1152/ajprenal.00092.2003. [DOI] [PubMed] [Google Scholar]
- [120].Ai D, Fu Y, Guo D, Tanaka H, Wang N, Tang C, et al. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:9018–23. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–62. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- [122].Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003;41:709–14. doi: 10.1161/01.HYP.0000047877.36743.FA. [DOI] [PubMed] [Google Scholar]
- [123].Kaergel E, Muller DN, Honeck H, Theuer J, Shagdarsuren E, Mullally A, et al. P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension. 2002;40:273–9. doi: 10.1161/01.hyp.0000029240.44253.5e. [DOI] [PubMed] [Google Scholar]
- [124].Muller DN, Theuer J, Shagdarsuren E, Kaergel E, Honeck H, Park JK, et al. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–32. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, et al. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R188–96. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- [126].Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, et al. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–9. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- [127].Maresh JG, Xu H, Jiang N, Gairola CG, Shohet RV. Tobacco smoke dysregulates endothelial vasoregulatory transcripts in vivo. Physiol Genomics. 2005;21:308–13. doi: 10.1152/physiolgenomics.00310.2004. [DOI] [PubMed] [Google Scholar]
- [128].Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–12. [PubMed] [Google Scholar]
- [129].Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36:205–16. doi: 10.1124/dmd.107.018747. [DOI] [PubMed] [Google Scholar]
- [130].Riddick DS, Lee C, Bhathena A, Timsit YE, Cheng PY, Morgan ET, et al. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab Dispos. 2004;32:367–75. doi: 10.1124/dmd.32.4.367. [DOI] [PubMed] [Google Scholar]
- [131].Theken KN, Lee CR. Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics. 2007;8:1369–83. doi: 10.2217/14622416.8.10.1369. [DOI] [PubMed] [Google Scholar]
- [132].Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, et al. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–6. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Liu PY, Li YH, Chao TH, Wu HL, Lin LJ, Tsai LM, et al. Synergistic effect of cytochrome P450 epoxygenase CYP2J2*7 polymorphism with smoking on the onset of premature myocardial infarction. Atherosclerosis. 2007;195:199–206. doi: 10.1016/j.atherosclerosis.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [134].Dai D, Zeldin DC, Blaisdell JA, Chanas B, Coulter SJ, Ghanayem BI, et al. Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics. 2001;11:597–607. doi: 10.1097/00008571-200110000-00006. [DOI] [PubMed] [Google Scholar]
- [135].Yasar U, Bennet AM, Eliasson E, Lundgren S, Wiman B, De Faire U, et al. Allelic variants of cytochromes P450 2C modify the risk for acute myocardial infarction. Pharmacogenetics. 2003;13:715–20. doi: 10.1097/00008571-200312000-00002. [DOI] [PubMed] [Google Scholar]
- [136].Lee CR, North KE, Bray MS, Couper DJ, Heiss G, Zeldin DC. CYP2J2 and CYP2C8 polymorphisms and coronary heart disease risk: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacogenet Genomics. 2007;17:349–58. doi: 10.1097/FPC.0b013e32809913ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, et al. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol. 2003;64:482–90. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- [138].Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2004;109:335–9. doi: 10.1161/01.CIR.0000109487.46725.02. [DOI] [PubMed] [Google Scholar]
- [139].Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, et al. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet. 2005;14:2829–37. doi: 10.1093/hmg/ddi315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Jr., Siscovick DS, et al. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190:26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]