Abstract
Cardiac cells are under constant, self-generated mechanical stress which can affect the differentiation of stem cells into cardiac myocytes, the development of differentiated cells and the maturation of cells in neonatal mammals. In this article, the effects of direct stretch, electrically induced beating and substrate elasticity on the behavior and development of cardiomyocytes are reviewed, with particular emphasis on the effects of substrate stiffness on cardiomyocyte maturation. In order to relate these observations to in-vivo mechanical conditions, we isolated the left ventricle of Black Swiss mice from embryonic day 13.5 through postnatal day 14 and measured the elastic modulus of the epicardium using atomic force microscope indentation. We found that the elastic modulus of the epicardium significantly changes at birth, from an embryonic value of 12 ± 4 kPa to a neonatal value of 39 ± 7 kPa. This change is in the range shown to significantly affect the development of neonatal cardiomyocytes.
Keywords: Cardiogenesis, rigidity, mouse, atomic force microscopy, mechanotransduction
Introduction
Cardiac tissue is subjected to dynamic mechanical stresses from very early development, without pause, for a person’s entire life. Portions of the heart experience shear stress that can be pulsatile, oscillatory or even turbulent at times. Much of the heart experiences both active stretching during filling and self-generated mechanical force during ejection. Because cardiomyocytes, or the contractile cells that comprise heart muscle, rhythmically contract, the stresses and strains that these cells experience are very sensitive to the mechanical properties of the surrounding cardiac tissue as well as the hydrostatic properties of the blood being pumped.
Cardiomyocytes respond to these mechanical forces, along with other possible signals, by proliferating, hypertrophying, and altering the heart geometry to enable the development of a fully-functioning four-chambered heart from a small heart tube, all while continuing to pump plasma and blood. Cardiomyocytes in the fully-formed heart continue to grow and mature even after birth. In the adult human heart, cells will hypertrophy or remodel in response to changing loads or conditions, sometimes in a compensatory fashion and sometimes pathologically, and the recent discoveries of some adult cardiac stem cell populations shows that the heart can often repair small areas even into adulthood.
In this article, we will review the mechanical sensitivity and mechanically-induced responses in cardiac cell differentiation, development and maturation. We will also present some original data on the changing mechanical properties of cardiac tissue during development and in ischemic regions of the adult heart.
Differentiation and cardiomyocyte development
Differentiation of a cardiac progenitor into a cardiomyocyte can be the first step in the development of a heart. Researchers have been able to generate cardiomyocytes from human embryonic stem cells (Boheler et al, 2002) and induced pluripotent stem cells (Mauritz et al, 2008; Narazaki et al, 2008) using a variety of chemical additives and culture techniques. Similar effects have also been observed in other stem cell sources not typically thought of as cardiomyogenic, including bone marrow-derived mesenchymal stem cells (MSC) (Fukuda, 2003; Xu et al, 2004; Yamada et al, 2007; Antonitsis et al, 2008), bone-marrow stromal cells (Makino et al, 1999), umbilical cord blood derived stem cells (Pereira et al, 2008) and adult cardiac stem cells (Bearzi et al, 2007; Smith et al, 2007). The true cardiogenic potential of MSC has been a matter of debate and several studies have found that MSC can express cardiac markers but not differentiate into functional cardiac cells (Jiang et al, 2002; Davani et al, 2003; Rose et al, 2008a). Many successful differentiation studies have involved direct co-culture of MSC and mature cardiomyocytes, and studies have noted that cardiac cells and stem cells can fuse in ways that make them difficult to distinguish from differentiated cells (Nygren et al, 2004; Rodic et al, 2004). Additionally, it has been noted that some successful techniques use MSC that have some degree of CD45 marker expression, which might indicate contamination with hematopoietic stem cells and those cells may be the only ones differentiating into cardiomyocytes (Rose et al, 2008b).
Studies have demonstrated that mechanical conditioning by direct stretching can affect cardiomyogenesis. The expression of cardiac differentiation markers sarcomeric α-actinin, MEF2c and GATA-4 increased in mouse embryonic stem cells after static stretching at 10% strain for 2 hours (Schmelter et al, 2006). It is interesting to note, however, that other studies have found that low frequency direct stretch of 10% strain for 10 cycles/minute could reduce differentiation of human embryonic stem cells, maintaining an undifferentiated state (Saha et al, 2006). Clearly complex mechanotransduction signaling can impact multiple systems and specifics of the cell culture and cell state may strongly affect the stem cell mechanotransductive response.
One study found that laminar shear flow of 10 dynes/cm2s over cultures of mouse embryonic stem cells resulted in an upregulation of expression of some cardiac markers, including MEF2c and sarcomeric α-actinin (Illi et al, 2005). Cultures of bone marrow-derived mesynchymal stem cells on substrates of various rigidity have found general myocyte marker expression increases when cells are plated on a substrates with a vary narrow range in elastic modulus, centered around 10kPa (Engler et al, 2006), though to our knowledge, no study has specifically demonstrated substrate stiffness effects on cardiomyogenesis from undifferentiated stem cells.
A larger amount of experimental data exists on the effects of mechanical stimulation on cells already partially differentiated toward the cardiac lineage and expressing cardiac markers. This could indicate that major mechanotransductive pathways are not expressed in pluripotent cells but are expressed and activated during the process of cardiogenesis. Cells hand-selected for beating colonies and expression of the cardiac markers cardiac α-myosin heavy chain (MHC), cardiac α-actin and the transcription factors GATA-4 and Nkx2.5 not only continued to increase expression of those markers when continuously stretched at 10% strain and 1.0 Hz for two weeks, but also formed cell-cell connections and synchronously beat both in culture and upon implantation onto infarcted rat hearts (Gwak et al, 2008). Furthermore, mouse embryonic stem cells genetically selected for expression of cardiac α-MHC showed a sensitivity to stretch frequency, increasing cardiac marker expression upon direct stretching at 3 Hz but decreasing expression at 1 Hz (Shimko and Claycomb, 2008).
Cardiomyocyte maturation
Cardiomyocytes will mature in the weeks following birth. This maturation can be viewed histologically through the appearance of well-defined sarcomeres (Lucchesi and Sweadner, 1991), marked cardiomyocyte hypertrophy (Li et al, 1996) and cell binucleation (Soonpaa et al, 1996). In addition, the mechanisms of calcium handling are altered in maturing cardiomyocytes, with extracellular calcium current through membrane channels accounting for most of the calcium transient in embryonic and neonatal cardiomyocytes, and calcium release from internal sarcomeric stores accounting for the calcium transient of adult cardiomyocytes (Gomez et al, 1994; Husse and Wussling, 1996). This change in calcium handling is concurrent with an increase in expression of the sarcoplasmic/endoplasmic calcium ATPase (SERCA2a) and the Ryanodine receptor (RyR), which acts as the sarcomeric calcium release channel (Lodish et al, 2000).
Direct stretch of cardiomyocytes can directly affect the activity of several ion channels and increase gap junction-mediated cell coupling, as reviewed in (Jacot et al, 2009). In maturing cardiomyocytes, experiments on neonatal rat atrial cells found that 13% biaxial strains results in differences in gene expression of specific potassium channels and currents, ultimately resulting in reduced action potential duration (Saygili et al, 2007; Rana et al, 2008). However, another group found that neonatal rat ventricular myocytes respond to anisotropic static stretch of 10 minutes by an increase in action potential duration (Zhang et al, 2008). The effect of both static and dynamic stretch to upregulate expression of connexin-43, which forms gap junctions that electrically couple cardiomyocytes, and the effective coupling of cells in response to stretch, has been very well documented (Wang et al, 2000; Zhuang et al, 2000; Shyu et al, 2001; Pimentel et al, 2002; Shanker et al, 2005; Yamada et al, 2005).
In addition to the role of mechanical factors in influencing the maturation and development of an aligned sarcomeric structure in neonatal cardiomyocytes, cell shape has also been shown to play a role. The use of geometric boundaries that force neonatal rat ventricular myocytes to spread into an elongated shape, similar to that of cardiomyocytes in vivo, leads to more sarcomeric alignment and clear axes of contraction (Bray et al, 2008; Parker et al, 2008).
Finally, in partially or wholly differentiated cells, electrical stimulation of the cells can induce beating, creating a dynamic loading of cell-matrix connections as well as portions of the cytoskeleton without the need for direct stretch. One group found that neonatal rat cardiomyocytes stimulated by an electric field in the direction of a micro-pattern-induced alignment increase cardiac elongation (Heidi Au et al, 2009) and that neonatal rat cardiomyocytes, cultured in a PEG-diacrylate gel with endothelial cells, were more excitable and expressed more connexin 43, which forms gap junctions to electrically couple the cells, when stimulated with electrical pulses during culture (Chiu et al, 2008).
The elastic modulus of the extracellular matrix surrounding cardiac cells has multiple possible methods of signaling. In other cell types, integrins binding to the extracellular matrix have been shown to develop more mature focal adhesions and mediate signaling pathways that affect complex cell behaviors when bound to a substrate with a higher elastic modulus (Beningo et al, 2001). Because neonatal cardiac cells will spread and elongate on a surface, and become more rounded in suspension, it is likely that this same integrin-mediated signaling plays a role in cardiomyocytes. In addition, a softer substrate, by definition, allows for a greater length of contraction for the same generated force, allowing for signaling mediated by contractile strain. Finally, the direct relationship between the force generated by a cardiomyocyte and its sarcomere length (Bluhm et al, 1995) links these two signaling pathways. A high degree of shortening can result in shorter sarcomeres (and faster shortening velocities), and therefore lower force on the connections to the matrix. Also, a high degree of spreading and prestress force generation can result in longer sarcomeres, and still higher force on the connections to the matrix.
Given these relationships, it is not surprising that studies have observed increasing cardiomyocyte force with increasing matrix stiffness. However, when neonatal rat cardiomyocytes were cultured for seven days on substrates of varying elasticity, they produced lower contractile force on substrates with elastic modulus of 25 kPa or above (Jacot et al, 2008). These cells on these stiffest gels appeared to lack the maturation seen in cells on the softer gels – they had fewer defined sarcomeres that spanned the width of the cell and they expressed less SERCA2a calcium pump, resulting in less stored calcium, lower calcium transients and therefore lower force. However, by inhibiting one pathway, the RhoA and Rho Kinase (ROCK) pathway that leads to the formation of actin stress fibers, these cells mature and generate more force than cells on softer substrates as expected.
Other groups have also detailed differences in cardiomyocyte behavior on different stiffness substrates in this same range of elastic modulus. Myocytes from chick embryos are more likely to beat and beat with a greater frequency when cultured on substrates of less than 10kPa (Engler et al, 2008). In an experiment on much softer materials, one study found that neonatal rat cardiomyocytes cultured on polyethylene glycol-based gels with a tensile modulus ranging between 8 and 1000 Pa were both more likely to beat and beat with greater frequency on the softest substrates (Shapira-Schweitzer and Seliktar, 2007).
Evaluating these results, and in particular relating specific response on a substrate of a specific elastic modulus to physiologic phenomena, requires detailed knowledge of these cellular environments in-vivo. One study used atomic force microscopy (AFM) to evaluate the elastic modulus of healthy and ischemic tissue in a rat heart. They found that the elastic modulus of healthy heart tissue was in the range of 10–15 kPa while the elastic modulus of ischemic tissue, created by left anterior descending (LAD) coronary artery ligation and evaluated at 14 days after surgery, increased to an elastic modulus around 50 kPa, with a linear transition zone of increasing modulus apparent at the edge of the infarct (Berry et al, 2006). Stress-strain relationships measured in developing chick embryos seem to indicate a stiffening of the developing heart tissue (Tobita et al, 2002), though specific measures of elastic modulus cannot be determined from these studies due to lack of resolution of ventricle geometry or thickness.
Additionally, one study found that neonatal human hearts have increased compliance (corresponding to a decreased elastic modulus) compared to adult human hearts, and suggested that this could be attributed to a higher ratio of Type I to Type IV collagen in the adult hearts (Marijianowski et al, 1994). Studies in chick embryos indicate initial production of Type IV collagen in early embryonic heart development, followed by a large increase in Type I collagen (Thompson et al, 1979). Furthermore, neonatal rat hearts show a drastic increase in collagen gene expression immediately following birth, as well as a change in the structural organization of collagen in the extracellular matrix (Carver et al, 1993). In order to effectively discuss maturation results in comparison to in vivo data, we have measured the elastic modulus of the left ventricles of embryonic and neonatal mice using AFM indentation.
Methods
For development studies, hearts were isolated from fetal and neonatal Black Swiss mice at embryonic day 13.5 and 16.5 (with birth at day 19) and at neonatal day 2, 5, 9 and 15. The time point of embryonic mice was verified by comparison with charts of Black Swiss mouse development. Hearts were opened and the left ventricle free wall was isolated. For ischemic studies, adult C57 mice were anesthetized and the left anterior descending coronary artery ligated with a suture, per previously described methods (Campbell et al, 2008). At 30 days post ischemia, the hearts were harvested and the ischemic portion of the left ventricle was dissected along with at least 5 mm of surrounding non-ischemic tissue. In both cases, this tissue was then pinned down at the edges by glass cover slips and double-sided tape, leaving a stripe of at least 1-mm of the epicardial surface uncovered. Effort was taken to ensure that the tissue was mounted in a relaxed state without significant prestress. In the ischemia model tissue, this strip of tissue included at least 1mm of non-ischemic and 1mm of ischemic tissue, clearly visible due to the thickness and color. This tissue was covered by room-temperature “arrest solution,” consisting of 24.9 mM NaHCO3, 1.2 mM KH2PO4, 11.1 mM Dextrose, 1.2 mM MgSO4, 18.8 mM KCl, 118 mM NaCl, 15 mM BDM, 1.0 mM CaCl2 and 10 units/mL Heparin, which prevented beating in the tissue. All animal studies were in accordance with University of California guidelines.
Tissue was mounted in an atomic force microscope (Bioscope, Veeco, Plainview, NY) and indented with a silicon nitride cantilever (DNP-10, Veeco) using the longest, and softest, of the four cantilevers on that tip. The spring constant for this tip was calculated as 0.062 N/m. Cantilevers were calibrated by indentation onto a second calibrated cantilever per supplier recommendation. Samples were indented with 2 μm total indentation length, adjusted to give approximately 1 μm indentation into the sample and 1 μm of travel prior to contact, at a frequency of 10 Hz. The indentation curves were allowed to stabilize before data was saved, and the tip was lifted from contact between each collected indentation curve. Each sample was indented in 7 locations laid out in a grid pattern spaced 10 μm apart, with 7 data curves collected per sample and the highest and lowest modulus discarded.
The average of the indentation and retraction curves was used to compute a single Young’s Modulus for each sample, in a method based on Domke & Radmacher (Domke and Radmacher, 1998). The elastic modulus was computed from a fit to the Hertzian contact solution (Sneddon, 1965). For all calculations, the Poisson’s ratio was assumed to be 0.45. The contact point used in the curve fit was computed as the height when a moving-average-smoothed derivative of the displacement curve crossed a threshold value (set to 1.0 for all measurements in this study). All computations and data fitting were performed using MATLAB v.7 (Mathworks, Inc., Natick, MA).
Results and Discussion
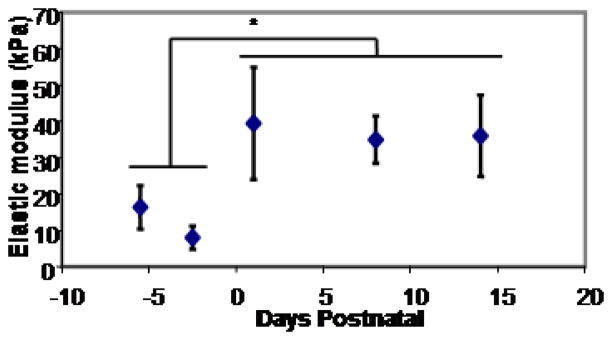
The elastic modulus calculated in samples of the developing mouse left ventricle tissue showed a clear discontinuity, and immediate increase in elastic modulus, at birth (Figure 1). The embryonic elastic modulus was found to be 12 ± 4 kPa and significantly increased to 39 ± 7 kPa in postnatal mouse ventricles (P<0.1). Interestingly, the embryonic ventricles had elastic moduli within the range previously found to produce neonatal myocytes with maximal contractile force, but neonatal ventricles were measured in the stiffness range that prevented maturation in cultured cells. This discrepancy could be an indication of the substantial differences between the 2-dimensional, in vitro cultures with a single bound matrix protein (collagen I in the case of the Jacot et al. study) used to evaluate these cells and the 3-dimensional in vivo environment incorporating a complex extracellular matrix containing many adhesion molecules along with proteoglycans, growth factors and various signaling molecules. Additionally, the elasticity of heart tissue is strongly anisotropic, while most hydrogel culture are isotropic and the technique of measuring elastic modulus by AFM indentation cannot distinguish an anisotropic modulus. Studies have found that stretch of aligned neonatal rat cardiomyocytes has a more drastic effect when cells are stretched tangential to their major axes than cells stretched along their major axis (Gopalan et al, 2003). Possibly, through similar mechanisms, the elastic modulus along a minor axis, and presumably more compliant, direction has a greater effect on the cells but cannot be easily measured using a standard AFM indentation method.
Figure 1.
The elastic modulus of the epicardial surface of sections of the LV from embryonic and neonatal Black Swiss mice as measured by AFM increased significantly between timepoints of E16.5 and P2, with birth at E19, from an average embryonic elastic modulus of 12 ± 4 kPa to a postnatal modulus of 39 ± 7 kPa per ANOVA followed by t-test comparison between embryonic and postnatal data (P<.01). Data points represent averages of 3 to 4 samples measured 7 times at 7 positions per sample, with the highest and lowest discarded. Error bars represent S.E.M.
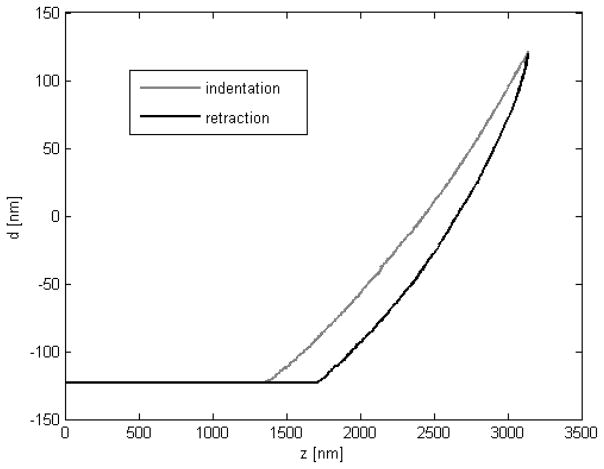
The indentation depth of 1μm was chosen to approximate the tissue elastic properties at a scale that might span the cell and matrix and indicate changes in both elastic components. Previous studies of cell stiffness have used indentation depths on the order of 100 nm (Mathur et al, 2001), while studies of bulk material properties have used indentation depths greater than 1 μm (Zaari et al, 2004). Finite element analysis has shown that AFM indentations approaching the order of 10% of the sample thickness can be heavily influenced by the underlying material (Costa and Yin, 1999) and, because native cardiomyocytes can be as thin as 10 μm along the short axis, we could expect both the cell and material to have an effect. In these studies, no dramatic change was observed at any portion of the force curve, and the curves match very well with a fit to Hertzian indentation (Figure 2).
Figure 2.
Sample indentation and retraction curve of the epicardium of an embryonic day 13 Black Swiss mouse. In this case the sample was indented nearly 1.5 μm and no clear transition between material properties was apparent. Typical samples in this study were indented only 1.0 μm. The approximately 300 nm difference between indentation and retraction is typical for all specimens in this study.
Titin, an intracellular protein necessary for sarcomeric structure, which provides passive restoring force to the cardiomyocyte and contributes significantly to the mechanical properties of the sarcomere (Granzier and Labeit, 2004), and could likely be responsible for apparent myocardial stiffening in developing mouse hearts. Studies have found that the ratio of the more compliant N2A titin isoform to the less compliant N2B titin isoform decreases rapidly over the first four days postnatal in mice (Lahmers et al, 2004). This results in an increased passive stiffness in the tissue when stretched beyond its slack length, and could explain changes in passive elastic modulus in these samples.
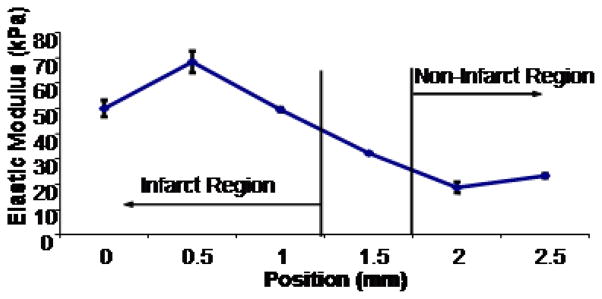
In the single post-ischemic ventricle measured, for comparison with earlier studies in rats by other groups, the elastic modulus in the non-infarct region was measured at 21 ± 2 kPa (Figure 3). This modulus increased gradually through a transition region covering approximately 1 mm to an average elastic modulus of 56 ± 6 kPa in the infarct region, which was significantly different from the non-infarct region (P<0.5). Both of these values are within the range of values found in a similar study in the rat heart (Berry et al, 2006).
Figure 3.
The elastic modulus of the epicardial surface of a section of the LV from one C57 mouse at 30 days post myocardial infarction induced by ligation of the left anterior descending coronary artery as measured by AFM was significantly higher in the ischemic region (56 +/− 6 kPa) than in the non-ischemic region (21 +/− 2 kPa) per ANOVA followed by t-test comparison between ischemic and non-ischemic regions (P<0.05). Data points represent averages of 7 measurements per position, with the highest and lowest discarded. Error bars represent standard deviation.
This tissue was mounted without stretch. We would expect stress to significantly alter tissue elasticity and the relationship between the degree of prestretch and the elastic modulus might indicate further alterations in mechanical behavior during development. We thus suggest examining the elastic modulus of stretched portions of developing cardiac materials as a future study.
As discussed in the introduction, rat cardiomyocytes mature rapidly in the two weeks following birth and there are many possible signals for this change. A change in substrate stiffness in in-vitro conditions seems to direct the maturation process (Jacot et al, 2008), and we have observed a dramatic change in stiffness in-vivo during this time. However, future experiments are necessary to show a direct relation.
Conclusion and Future Directions
Direct AFM indentation measurements of the left ventricle of developing mouse hearts show dramatic stiffening at birth. Further studies across the epicardium of an ischemic region reveals a transition area of stiffening tissue and a fourfold increase in elastic modulus in the infarct compared to surrounding healthy tissue. This confirms previous studies by another group in the rat.
Studies have observed significant effects of mechanotransduction, through cell stretching or varying the elasticity of the cell substrate, that impact progenitor cell differentiation into cardiac cells, development of those cells into functional myocytes, and maturation of those myocytes. Though some of these effects appear ubiquitous, such as the ability of many type of stretch over various times and frequencies to increase gap junction formation, most are highly dependent on the specific degree of stretch, frequency, range of elastic modulus or time period studies.
Many of the studies discussed in this manuscript describe new general phenomena relating mechanical factors to cardiomyocyte differentiation, development and maturation. In order to control for all other factors and change only the mechanical environment, experiments have, for the most part, been performed in-vitro with conditions that do not approximate the native environment. Thus, relating these phenomena back to specific events in cardiac development is very difficult. Additionally, the mechanistic rigor of protein or genetic signaling studies has, in general, not been applied to mechanical effects on cardiac cells and tissue. We would suggest that future research focus on defining the specific in-vivo mechanical environments, as we have attempted to do for this specific tissue in this study, as well as studying cell responses in environments that closely mimic the developing heart. An understanding of the specific mechanotransductive mechanisms involved in the early generation of cardiac cells, the specification of those cells and the formation of the specific structure and geometry of the heart could lead to many novel therapies for heart disease.
Acknowledgments
This work was supported by the National Institutes of Health (PPG, 5P01 HL46345-13 PIs: Knowlton & McCulloch). We thank Dayu Teng and Jerry Norwich of UCSD for AFM expertise, Job van der Loo of Eindhoven Technical University in The Netherlands for experimental assistance, Sylvia Evans of UCSD for the embryonic and neonatal Black Swiss mice and Andrew McCulloch of UCSD for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonitsis P, Ioannidou-Papagiannaki E, Kaidoglou A, Charokopos N, Kalogeridis A, Kouzi-Koliakou K, Kyriakopoulou I, Klonizakis I, Papakonstantinou C. Cardiomyogenic potential of human adult bone marrow mesenchymal stem cells in vitro. Thorac Cardiovasc Surg. 2008;56:77–82. doi: 10.1055/s-2007-989328. [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, McCulloch AD, Lew WY. Active force in rabbit ventricular myocytes. J Biomech. 1995;28:1119–22. doi: 10.1016/0021-9290(94)00018-y. [DOI] [PubMed] [Google Scholar]
- Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65:641–51. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PH, Hunt DL, Jones Y, Harwood F, Amiel D, Omens JH, McCulloch AD. Effects of biglycan deficiency on myocardial infarct structure and mechanics. Mol Cell Biomech. 2008;5:27–35. [PMC free article] [PubMed] [Google Scholar]
- Carver W, Terracio L, Borg TK. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat Rec. 1993;236:511–20. doi: 10.1002/ar.1092360311. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Iyer RK, King JP, Radisic M. Biphasic Electrical Field Stimulation Aids in Tissue Engineering of Multicell-Type Cardiac Organoids. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KD, Yin FC. Analysis of indentation: implications for measuring mechanical properties with atomic force microscopy. J Biomech Eng. 1999;121:462–71. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Herve P, Etievent JP, Kantelip JP. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- Domke J, Radmacher M. Measuring the elastic properties of thin polymeric films with the AFM. Langmuir. 1998;14:3320–3325. [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fukuda K. Use of adult marrow mesenchymal stem cells for regeneration of cardiomyocytes. Bone Marrow Transplant. 2003;32(Suppl 1):S25–7. doi: 10.1038/sj.bmt.1703940. [DOI] [PubMed] [Google Scholar]
- Gomez J-P, Potreau D, Raymond G. Intracellular calcium transients from newborn rat cardiomyocytes in primary culture. Cell Calcium. 1994;15:265–275. doi: 10.1016/0143-4160(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng. 2003;81:578–87. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–95. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, Yoo KJ, Putnam AJ, Kim BS. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29:844–56. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- Heidi Au HT, Cui B, Chu ZE, Veres T, Radisic M. Cell culture chips for simultaneous application of topographical and electrical cues enhance phenotype of cardiomyocytes. Lab Chip. 2009;9:564–75. doi: 10.1039/b810034a. [DOI] [PubMed] [Google Scholar]
- Husse B, Wussling M. Developmental changes of calcium transients and contractility during the cultivation of rat neonatal cardiomyocytes. Mol Cell Biochem. 1996;163–164:13–21. doi: 10.1007/BF00408636. [DOI] [PubMed] [Google Scholar]
- Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501–8. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- Jacot JG, McCulloch AD, Omens JH. Substrate Stiffness Affects the Functional Maturation of Neonatal Rat Ventricular Myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot JG, Raskin AM, Omens JH, McCulloch AD, Tung L. Mechanotransduction in cardiac and stem-cell derived cardiac cells. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity of the Heart. Springer; New York: 2009. p. 3. [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ Res. 2004;94:505–13. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–46. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. 4. WH Freeman and Company; New York: 2000. [Google Scholar]
- Lucchesi PA, Sweadner KJ. Postnatal changes in Na, K-ATPase isoform expression in rat cardiac ventricle. Conservation of biphasic ouabain affinity. J Biol Chem. 1991;266:9327–31. [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. 1994;23:1204–8. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Mathur AB, Collinsworth AM, Reichert WM, Kraus WE, Truskey GA. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech. 2001;34:1545–53. doi: 10.1016/s0021-9290(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, Yamanaka S, Yamashita JK. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- Parker KK, Tan J, Chen CS, Tung L. Myofibrillar architecture in engineered cardiac myocytes. Circ Res. 2008;103:340–2. doi: 10.1161/CIRCRESAHA.108.182469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira WC, Khushnooma I, Madkaikar M, Ghosh K. Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. J Tissue Eng Regen Med. 2008;2:394–399. doi: 10.1002/term.107. [DOI] [PubMed] [Google Scholar]
- Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90:671–7. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- Rana OR, Zobel C, Saygili E, Brixius K, Gramley F, Schimpf T, Mischke K, Frechen D, Knackstedt C, Schwinger RH, Schauerte P. A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am J Physiol Heart Circ Physiol. 2008;294:H532–40. doi: 10.1152/ajpheart.00649.2007. [DOI] [PubMed] [Google Scholar]
- Rodic N, Rutenberg MS, Terada N. Cell fusion and reprogramming: resolving our transdifferences. Trends Mol Med. 2004;10:93–6. doi: 10.1016/j.molmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008a;26:2884–92. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- Rose RA, Keating A, Backx PH. Do mesenchymal stromal cells transdifferentiate into functional cardiomyocytes? Circ Res. 2008b;103:e120. doi: 10.1161/CIRCRESAHA.108.186908. [DOI] [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–37. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- Saygili E, Rana OR, Reuter H, Frank K, Schwinger RH, Muller-Ehmsen J, Zobel C. Losartan prevents stretch-induced electrical remodeling in cultured atrial neonatal myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H2898–905. doi: 10.1152/ajpheart.00546.2006. [DOI] [PubMed] [Google Scholar]
- Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. Faseb J. 2006;20:1182–4. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- Shanker AJ, Yamada K, Green KG, Yamada KA, Saffitz JE. Matrix-protein-specific regulation of Cx43 expression in cardiac myocytes subjected to mechanical load. Circ Res. 2005;96:558–66. doi: 10.1161/01.RES.0000158964.42008.a2. [DOI] [PubMed] [Google Scholar]
- Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3:33–41. doi: 10.1016/j.actbio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu KG, Chen CC, Wang BW, Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:691–698. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- Sneddon I. The relation between load adn penetration in the axisymmetric Boussinesq problem for a punch of arbitraty profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–9. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- Thompson RP, Fitzharris TP, Denslow S, LeRoy EC. Collagen synthesis in the developing chick heart. Tex Rep Biol Med. 1979;39:305–19. [PubMed] [Google Scholar]
- Tobita K, Schroder EA, Tinney JP, Garrison JB, Keller BB. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am J Physiol Heart Circ Physiol. 2002;282:H2386–96. doi: 10.1152/ajpheart.00879.2001. [DOI] [PubMed] [Google Scholar]
- Wang TL, Tseng YZ, Chang H. Regulation of connexin 43 gene expression by cyclical mechanical stretch in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2000;267:551–7. doi: 10.1006/bbrc.1999.1988. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–31. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- Yamada K, Green K, Saffitz J. Distinct pathways regulate expression of cardiac electrical and mechanical junctional proteins in response to stretch. Circ Res. 2005;97:346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Sakurada K, Takeda Y, Gojo S, Umezawa A. Single-cell-derived mesenchymal stem cells overexpressing Csx/Nkx2.5 and GATA4 undergo the stochastic cardiomyogenic fate and behave like transient amplifying cells. Exp Cell Res. 2007;313:698–706. doi: 10.1016/j.yexcr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Zaari N, Rajagopalan P, Kim SK, Engler AJ, Wong JY. Photopolymerization in microfluidic gradient generators: Microscale control of substrate compliance to manipulate cell response. Advanced Materials. 2004;16:2133–2137. [Google Scholar]
- Zhang Y, Sekar RB, McCulloch AD, Tung L. Cell cultures as models of cardiac mechanoelectric feedback. Prog Biophys Mol Biol. 2008;97:367–82. doi: 10.1016/j.pbiomolbio.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Yamada KA, Saffitz JE, Kle’ber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87:316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]