Abstract
With continued development and improvement of tissue engineering therapies for small articular lesions, increased attention is being focused on the challenge of engineering partial or whole synovial joints. Joint-scale constructs could have applications in the treatment of large areas of articular damage or in biological arthroplasty of severely degenerate joints. This review considers the roles of shape, loading and motion in synovial joint mechanobiology and their incorporation into the design, fabrication, and testing of engineered partial or whole joints. Incidence of degeneration, degree of impairment, and efficacy of current treatments are critical factors in choosing a target for joint bioengineering. The form and function of native joints may guide the design of engineered joint-scale constructs with respect to size, shape, and maturity. Fabrication challenges for joint-scale engineering include controlling chemo-mechano-biological microenvironments to promote the development and growth of multiple tissues with integrated interfaces or lubricated surfaces into anatomical shapes, and joint-scale bioreactors which nurture and stimulate the tissue with loading and motion. Finally, evaluation of load-bearing and tribological properties can range from tissue to joint scale and can focus on biological structure at present or after adaptation.
Keywords: synovial joint, articular cartilage, tissue engineering, shape, motion
1. INTRODUCTION
Healthy synovial joints facilitate efficient and pain-free skeletal articulation. Within synovial joints, articular cartilage covers the ends of long bones and acts as a low friction, wear resistant, bearing surface in the presence of a lubricious synovial fluid. Extrasynovial ligaments and, when present, intrasynovial ligaments and menisci provide stability and help define range of motion. However, the biomechanical functions of joint tissues can be compromised by trauma or disease such as osteoarthritis. Because the intrinsic regeneration of normal articular surfaces is limited and present medical therapies are palliative and not disease-modifying (Buckwalter and Mankin,1998), surgery is often performed to treat pain and dysfunction.
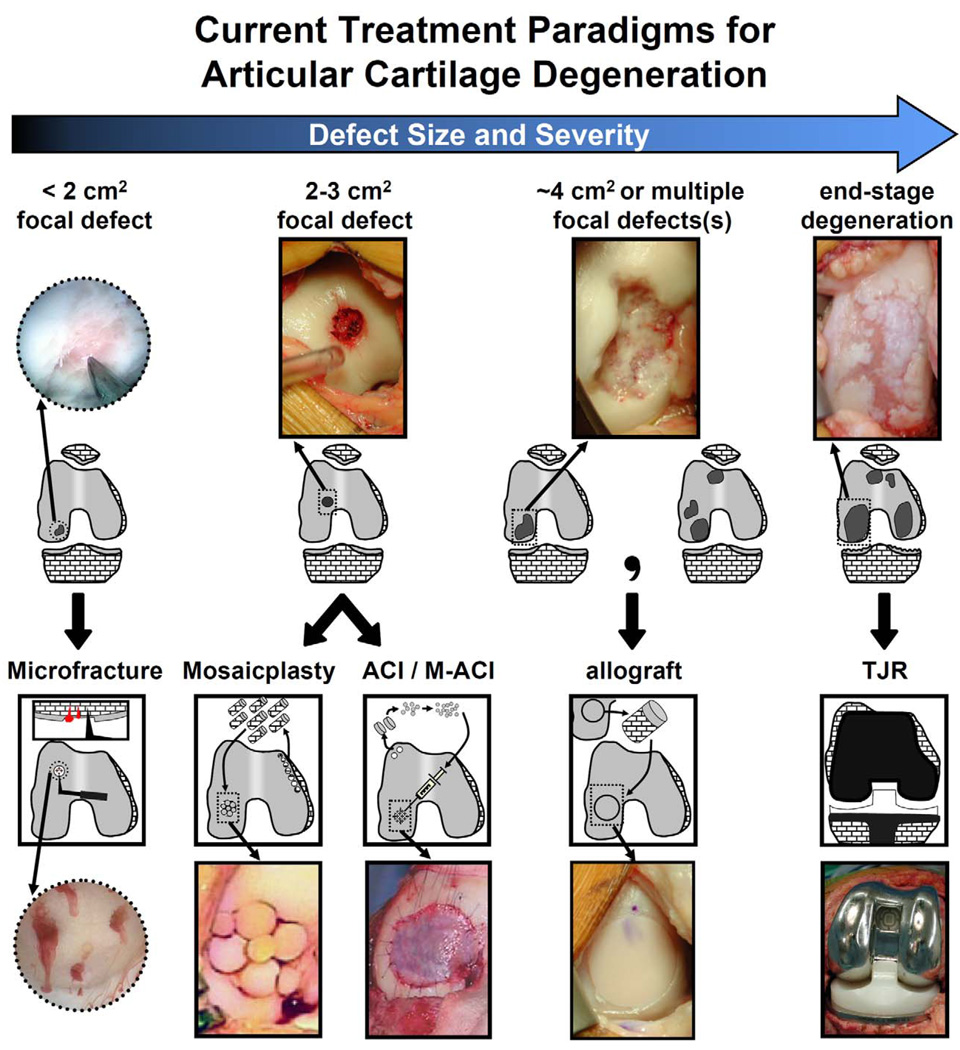
The current surgical treatment paradigm for damaged articular surfaces depends on lesion size and severity (Fig. 1). Microfracture is commonly used for focal cartilage defects <2cm2 to stimulate reparative mesenchymal stem cells from subchondral bone to form a fibrocartilage repair tissue (Buckwalter and Mankin, 1998, Steadman, et al., 2002). Osteochondral autograft transplantation or autologous chondrocyte implantation (ACI) are used for 2–3cm2 defects, and osteochondral allografts are used for large (∼4cm2) or multiple lesions (Brittberg, 1999, Bugbee, 2004, Hangody, et al., 2001). For severe degeneration, joint resurfacing or replacement is typically prescribed.
Figure 1.
Current treatment modalities for articular surface damage of varying size and severity. Focal defects (<1 cm2, arthroscopy image) treated with microfracture. Focal defects (1–2 cm2) treated with autograft mosaicplasty or (matrix-assisted) autologous chondrocyte implantation (ACI/M-ACI). Single or multiple large (2–4 cm2) defects treated with allografts. End-stage osteoarthritic degeneration treated by total joint replacement. Microfracture photo is reprinted with permission from Elsevier (Steinwachs, et al., 2008). Mosaicplasty and ACI photos are reprinted with permission from The Journal of Bone and Joint Surgery, Inc. (Hangody, et al., 2004, Jones and Peterson, 2006).
While these treatments are evolving, limitations continue to spur the development of new therapies. Drawbacks include the quality and consistency of repair tissue with microfracture and ACI (Buckwalter and Mow, 1992, Minas, 2001), osteochondral graft availability and chondrocyte viability with allogenic grafts (Schachar, et al., 1999, Vangsness, et al., 2003), and wear and loosening of prostheses (Bauer and Schils, 1999, Revell, 2008). Tissue engineering therapies are attractive for their potential to restore the biological and mechanical functions of joints. As treatments for small articular defects improve, focus is shifting to the larger-scale paradigm of bioengineering partial or whole joints in complex shapes. Such biological joints could ultimately allow for replacement of large areas of joint damage by providing transplantable anatomically-shaped grafts that function and adapt under physiological joint loading and motion.
This paper provides a forward-looking view of scientific and engineering challenges along the path to creation of partial or whole synovial joints, with particular emphasis on the roles of shape and motion in joint mechanobiology. Design considerations include biomedical need and biological-biomechanical targets. Solution development includes component identification, assembly, cultivation, and testing.
2. SYNOVIAL JOINT GEOMETRY, MOTION, AND CARTILAGE CONTACT
The native structure and function of synovial joints in the body provide design goals for joint engineering. Surface geometries, whole-joint motions, and relative motions of cartilage surfaces give rise to the spectrum of native joints. The complex shapes and motions of normal and engineered joints can be quantified.
2.A. Synovial Joint Geometry
The surface geometry of joints affects joint congruence and contact mechanics. Atypical geometries predispose joints to certain diseases through altered biomechanics (Beck, et al., 2005, Colombo, et al., 2008, Daniel, et al., 2005, Ganz, et al., 2003, Noble, et al., 2003). The geometries of synovial joints can be generalized into five shapes according to predominant curvatures of articulating surfaces (Fig. 2).
Figure 2.
Synovial joint classifications based on geometry and examples.
Planar joints have a flat or slightly curved surface geometry. Cylindrical joints have surfaces that are curved in one direction and flat in the orthogonal direction. Spherical (or “ball-and-socket”) joints, have approximately constant radius of surface curvature. Elliptical joints have radii of curvature that differ in orthogonal directions. Saddle joints have an articulating surface that is concave in one direction and convex in an orthogonal direction.
2.B. Joint and Cartilage Surface Motion
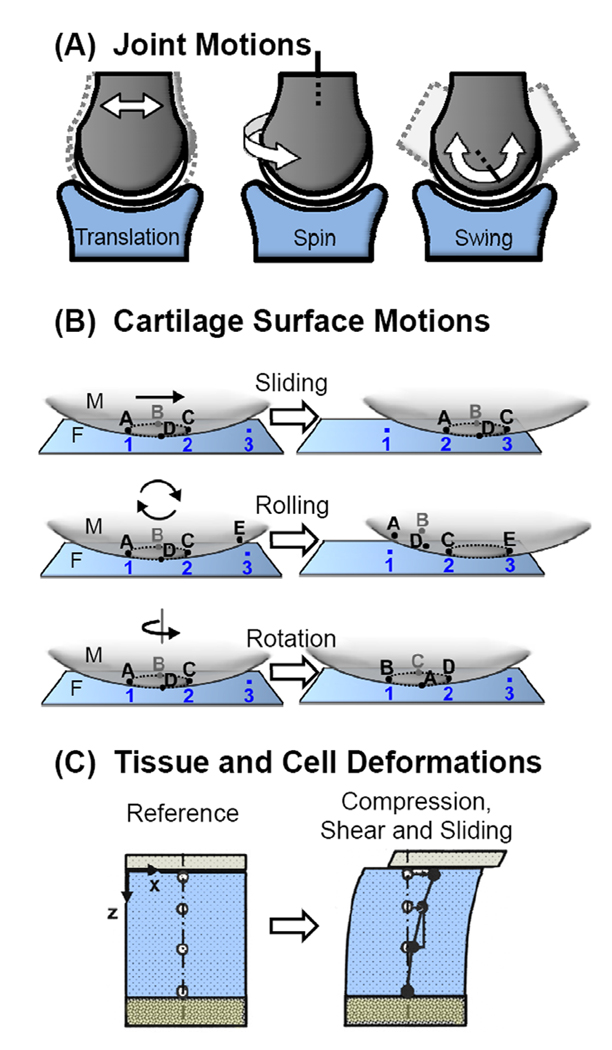
Cartilage-capped long bones can articulate against an opposing surface in three basic motions (Fig. 3A). Translation results in a change in position along an arbitrary axis. Spin motion involves rotation about a longitudinal axis of bone and occurs with internal and external rotation of the limbs. Swing motion is defined as rotation about an orthogonal axis and occurs during flexion, extension, adduction, and abduction. These motions are limited by the presence of ligaments, joint capsules, and muscles, and dependent on the geometry of the opposing surface. The combination of these three basic motions leads to the complex kinematics of the body.
Figure 3.
Schematic of the types of A) whole-joint motions and B) cartilage surface contact motions. C) Tracking of tissue and cell deformations due to joint loading.
Relative motions between two cartilage surfaces in contact can be decomposed into sliding, rotation, and rolling movements (Fig. 3B). as illustrated for a simple joint viewed as a fixed (F) and a moving (M) surface. Sliding occurs when the contact points of F remain the same while the contact points of M change. Rolling occurs when there is a one-to-one correspondence between the points on F and M (i.e. each point in F always comes into contact with the same corresponding point on M within one revolution). During rotation, the same points on F and M are involved in the articulation, while the relative positions between F and M points change. Combinations of sliding and rolling, sliding and rotation, and rotation and rolling result in changes of F and M contact points as well as their correspondences. While all types of motion do not occur physiologically in all joints, examples of each occur to certain degrees in various joints.
2.C. Quantitative Shape Analysis of Joints
Analysis of joint surfaces allows definition of local and global shape features with a limited number of parameters. Various laboratory (photogrammetry, stereophotogrammetry, laser scanning) and clinical (radiography, arthrography, B-mode ultrasound, computed tomography, magnetic resonance imaging) methods have been used to acquire surface data at various resolutions. Data can be fit to mathematical representations of joint geometry such as bicubic patches, B-splines, and thin-plate splines (Ateshian and Eckstein, 2005, Ateshian, et al., 1991, Gu, et al., 2008). Joint shape descriptors include mean and Gaussian curvatures calculated at points along the surface. Alternative representations are from 2D and 3D statistical shape modeling of biological structures (Cootes, et al., 1995, Lorenz, 2000). Accurate and efficient methods of surface quantification will facilitate the design of shaped grafts for joint repair.
2.D. Joint Motion Analysis and Cartilage Contact Mechanics
Quantification of joint mechanics and cartilage surface interactions provides constraints and boundary conditions for theoretical models as well as design targets for the movement of bioengineered joints. Traditionally, optical, magnetic, or optoelectronic systems have been used to track surface motions (Khumsap, et al., 2004, Wilson, et al., 2009), while 2D (biplanar radiography, fluoroscopy) and 3D (CT and MRI) scanning methods have been used to estimate joint-scale forces and moments (Freeman and Pinskerova, 2005, Muhle, et al., 1999, You, et al., 2001). The advancement of experimental and computational methods has allowed for estimation of contact areas and surface deformation during joint motion.
Such information from joint-scale analyses can be used with experimental and theoretical analyses at the cell and tissue scale in order to elucidate tissue deformations and surface interactions at a finer scale during the relative motion of two contacting cartilage surfaces (Fig. 3C) (Gratz and Sah, 2008). Compressive (Schinagl, et al., 1997) and shear properties (Buckley, et al., 2008, Wong, et al., 2008) of cartilage tissue vary with depth from the articular surface. In turn, tissue-scale cartilage deformation has been related to local stresses and strains at the cellular level both experimentally using high resolution microscopy (Guilak, et al., 1995, Szafranski, et al., 2004) and theoretically using multi-scale biphasic finite element models (Guilak and Mow, 2000), yielding estimates of fluid flow and strain amplification in the pericellular matrix (PCM) around chondrocytes (Alexopoulos, et al., 2005, Gupta and Haut Donahue, 2006). Features of chondrocyte deformation and load appear to regulate chondrocyte matrix metabolism and membrane transport (Huselstein, et al., 2008, Lammi, 2004). Further development of multi-scale methods for describing and analyzing motions and forces in natural joint geometries will improve the understanding of mechanobiology of native and engineered cartilage during development, homeostasis, and degeneration.
3. DESIGN CONSIDERATIONS FOR JOINT-SCALE TISSUE ENGINEERING
The shift to designing joint-scale osteochondral constructs will be driven by consideration of biomedical need and by the customization of size, maturity, and shape.
3.A. Biomedical Need
The biomedical need for a large-scale tissue engineered construct for an individual joint is dictated by the incidence and prevalence of degeneration, severity of functional impairment, and efficacy of current treatments. For example, the prevalence of osteoarthritis is higher in the knee than the ankle (Brown, et al., 2006, Cushnaghan and Dieppe, 1991); however, both joints are important in mobility during daily activities, and the general effectiveness of treatment of osteoarthritis by joint replacement is poor for the ankle relative to the knee (Clifford and Mallon, 2005). In addition, with the predicted increase in osteoarthritis prevalence to almost epidemic levels associated with increasing longevity in the general population (Lawrence, et al., 2008), there are clear needs for therapies before, or instead of, traditional total joint replacement to avoid difficult revision surgeries and improve biological and kinematic joint function. This is especially true for young patients with advanced osteoarthritis (Dorr, et al., 1994). Thus, the greatest impact of biological joint replacement will be in joints afflicted with osteoarthritis at a high incidence, and with a major impact on quality of life and an unmet clinical need.
3.B. Graft Size and Maturity
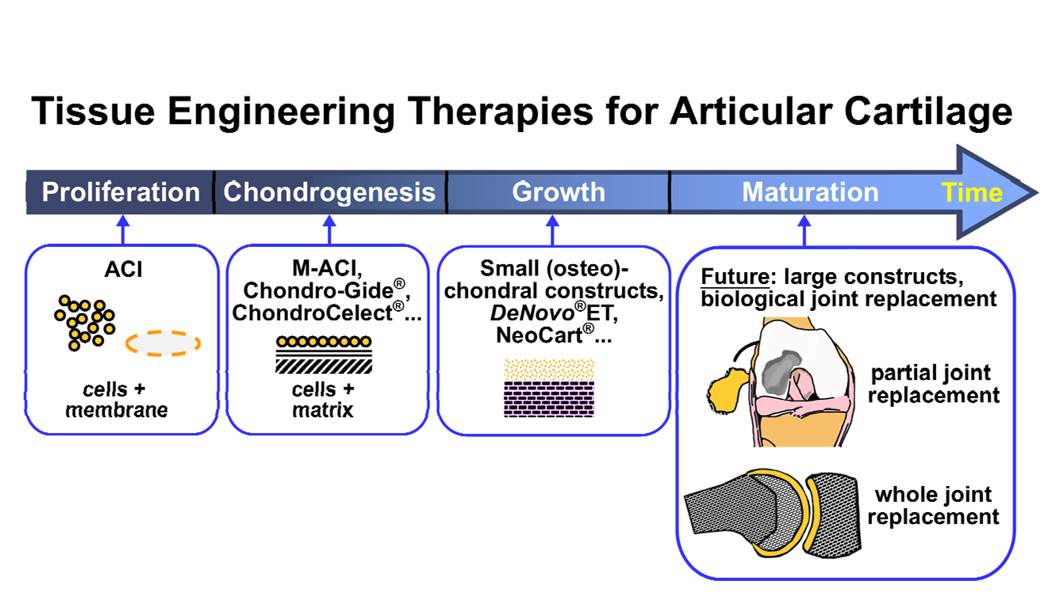
The extent and severity of joint degeneration dictate the size and load-bearing capabilities of an engineered graft. For cell-based cartilage repair therapies that recapitulate normal development and growth, tissue formation progresses from cell isolation and proliferation to chondrogenesis, tissue expansion, and tissue maturation (Fig. 4). Most current therapeutic tissue engineering treatments are immature compared to native tissue and intended primarily for relatively small defects. However, existing allograft and tissue engineering treatments are shifting toward larger defects. Constructs for biological hemi-, unicompartmental, or total joint arthroplasty will benefit from being more mature in terms of biomechanical function in order not only to contribute effectively to load-bearing but also to minimize rehabilitation time in vivo. Biomechanically mature grafts may also help restore the mechanical environment of the joint from a chronically abnormal state, contributing to further degeneration, to a healthier one. It remains to be determined how mature joint-scale constructs should be at the time of implantation into particular lesions.
Figure 4.
Developmental progression of biomimetic tissue engineering therapies for articular cartilage repair. Current therapies using ACI, M-ACI, and small chondral and osteochondral constructs, are advancing incrementally towards larger, more phenotypically stable and mature tissues at the time of implantation. Future engineered partial or whole-joint constructs may require or benefit from further in vitro maturation of tissues. Chondro-Gide®, ChondroCelect®, DeNovo®ET, and NeoCart® are products of Geistlich Pharma AG (Wolhusen, Switzerland), TiGenix (Leuven, Belgium), ISTO Technologies, Inc. (St. Louis, Missouri), and Histogenics Corporation (Waltham, Massachusetts), respectively.
3.C. Graft Shape
In joint-scale engineering, both a custom-fit shape and a “shoe-size” approach to shape may be appropriate for the implant. Traditionally, joint arthroplasty has been performed with limited sizes of off-the-shelf, modular implants because of their reliability and high costs associated with customization (Dennis and Lynch, 2005, Laskin, 1976). However, the range of available implant sizes has increased in recent years to allow for customization as anatomical variations associated with race, stature, and gender are being elucidated (Weng and Fitzgerald, 2006). Joint shape-matched implants (Zimmer Gender Solutions Knee™, Warsaw, IN) target women and specific patients (ConforMIS, Burlington, MA). Patient-specific surgical guides and instruments allow surgeons to make cuts and select implants that match the normal anatomy of each patient (OtisMed, Alameda, CA; Biomet, Warsaw, IN; Depuy, Warsaw, IN). In joint-scale engineering, customization would allow surgeons to treat unique joint geometries and may be useful for partial resurfacing surgeries, where the opposing articulating surface is left intact. On the other hand, a “shoe-size” approach would simplify the process of construct creation and may be more applicable for total joint arthroplasties, where both joint surfaces are replaced.
4. TISSUE ENGINEERING STRATEGIES FOR PARTIAL AND WHOLE JOINTS
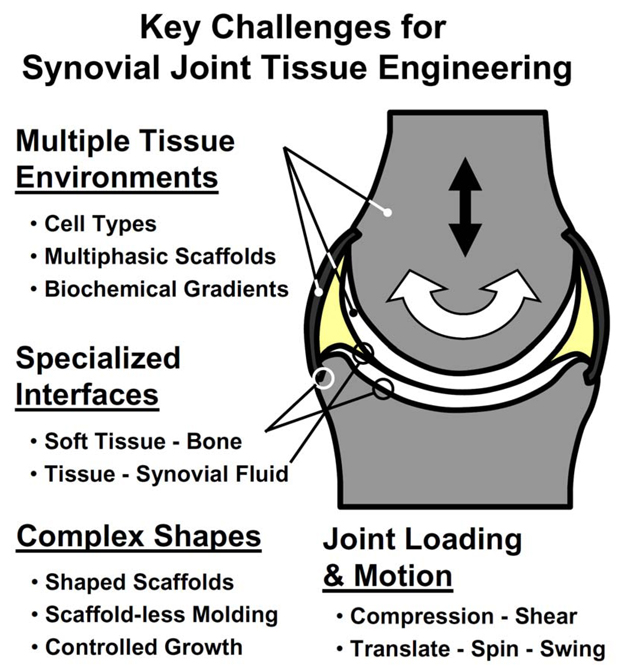
Advances in tissue engineering of cartilage (Klein, et al., 2009), bone (Khan, et al., 2008), meniscus (Sweigart and Athanasiou, 2001), and ligament (Woo, et al., 2004) serve as the foundations for generating partial or whole joints. Critical additional areas for scientific investigation and engineering development include the creation of multi-tissue units, specialized tissue interfaces, complex tissue shapes, and bioreactors capable of applying loads and motion to joints (Fig. 5).
Figure 5.
Key challenges for advancing tissue engineering of partial or whole synovial joints include creating multiple tissue environments, specialized interfaces, complex tissue shapes, and bioreactors for the application of joint loading and motion.
4.A. Engineering Environments for Multiple Tissues
Several repair strategies for dysfunctional synovial joints use the common approach of transplanting functional units consisting of soft tissue (cartilage, ligament, or meniscus) attached to bone. Compared to soft-tissue grafts alone, multi-tissue grafts can offer enhanced integration between graft and host through strong fixation and bone-to-bone healing (Bugbee, 2004, Cole, et al., 2003, Schoderbek, et al., 2007). Such grafts restore bulk tissues and preserve the specialized interfaces between soft tissues and bone.
To engineer a multi-tissue unit such as an osteochondral construct, distinct local environments are needed for promoting tissue-specific formation (Hwang, et al., 2009). The available cell sources can dictate the use of multiple cell types or a single population of stem cells capable of differentiation into multiple tissues. Within a single construct, regulation of distinct cell functions is achieved through spatially heterogeneous biochemical or physical stimuli. Gradients of biochemical factors can be produced by tethering and diffusion (Wang, et al., 2009), while multiphasic scaffolds can present distinct physical cues for chondrogenesis and osteogenenesis (Tampieri, et al., 2008). Porous osteoconductive scaffolds have also been widely investigated as substrates supporting the formation of cartilage by chondrocyte encapsulation in hydrogel (Buschmann, et al., 1992, Lima, et al., 2008) or scaffold-free techniques (Allan, et al., 2007, Masuda and Sah, 2005).
4.B. Engineering Integrated Tissue Interfaces
Functional integration of engineered tissues within a construct may benefit from additional understanding of the physical, chemical, and biological properties found at native tissue interfaces. Recent studies have focused on quantifying components of the structure, composition, and function of the calcified cartilage interface between articular cartilage and subchondral bone (Ferguson, et al., 2003, Hwang, et al., 2008), the fibrocartilage interface between ACL and bone (Moffat, et al., 2008, Wang, et al., 2006), and the ligament-fibrocartilage interface between meniscus and bone (Maes and Haut Donahue, 2006, Villegas, et al., 2008). Co-culture studies using chondrocytes, osteoblasts, fibroblasts, and mesenchymal stem cells are elucidating the biological crosstalk between neighboring tissues (Gerstenfeld, et al., 2003, Jiang, et al., 2005, Nakaoka, et al., 2006, Sanchez, et al., 2005, Wang and Lu, 2006). These studies elucidate concepts that may serve as biomimetic strategies to reproduce functional interfaces in engineered grafts and provide benchmarks against which the properties of engineered interfaces can be compared.
Physical integration of engineered tissues to form a functional unit can be achieved from initial assembly of components, as in a biphasic scaffold, or else by separate culture of tissues with subsequent bonding by sutures, fibrin glue, and other methods (Martin, et al., 2007, O'shea and Miao, 2008). While these strategies may yield physical integration, engineering design principles are be needed to recreate the specialized features found in native interfaces (Yang and Temenoff, 2009). Making use of specific cellular phenotypes, such as the ability of deep zone chondrocytes to initiate matrix calcification, can facilitate the biomimetic formation of a zone of calcified cartilage in osteochondral constructs (Allan, et al., 2007). Analogously, larger scaffolds support transitional fibrocartilage phenotypes for bridging the ACL-bone interface (Spalazzi, et al., 2006).
4.C. Engineering Lubricated Tissue Surfaces
Within the synovial joint, interfaces occur not only between two adjacent tissues but also between tissues and the synovial fluid compartment with specialized lubricated tissue surfaces. The tissue-fluid interface is critical to the low friction, wear resistant properties of a synovial joint. Lubricant molecules, such as hyaluronan (HA) and proteoglycan 4 (PRG4), are secreted into the synovial space by the surrounding tissues including synovium, articular cartilage, menisci, and intra-articular ligaments (Jay, et al., 2000, Schumacher, et al., 1999, Schumacher, et al., 2005, Smith and Ghosh, 1987, Sun, et al., 2006). Articular cartilage constructs which actively secrete PRG4 at the superficial surface were generated by stratifying zonal populations of chondrocytes (Klein, et al., 2003). Lubricant secretion from engineered tissues may be further modulated by biomechanical and biochemical stimuli (Grad, et al., 2005, Khalafi, et al., 2007, Schmidt, et al., 2008). Functionally lubricious fluid mimicking synovial fluid containing HA and PRG4 has also been generated in a bioreactor through biomimetic co-culture of synoviocytes and chondrocytes (Blewis, et al., 2009).
4.D. Forming Anatomical Shapes
When formed into appropriate anatomical shapes, osteochondral constructs and other engineered functional units may become useful as grafts for joint repair or parts for constructing whole bioengineered joints. Several techniques exist for imparting anatomical contours to tissue constructs. Most scaffold materials can be cast, milled, printed, or otherwise formed into anatomical shapes to serve as positive templates for tissue growth. Hydrogels with encapsulated cells are typically conducive to shaping by injection into molds and polymerization in situ. Anatomical geometries have been created with scaffold-free approaches by seeding primary or alginate-recovered chondrocytes at high density into molds (Aufderheide and Athanasiou, 2007, Han, et al., 2008). Anatomical imaging data, obtained from MRI or CT, can serve as the inputs for computer-aided machining or solid freeform fabrication of scaffolds or molds with high shape fidelity (Ballyns, et al., 2008, Feinberg, et al., 2001). However, the shape fidelity of a construct may diminish with subsequent tissue growth or scaffold degradation. Growth may need to be controlled or predicted a priori with the aid of mechanobiological growth models (Davol, et al., 2008, Heegaard, et al., 1999). Tissue shape may also be modulated in vitro through physical or biochemical means as demonstrated by the contouring of immature cartilage (Williams, et al., 2007) and the lengthening of ligaments and tendons (Esther, et al., 2008, Wood, et al., 2003).
The application of shaping techniques is evident in examples of anatomically-shaped (hemi-)joint fabrication. These include phalanges formed with solvent-cast synthetic polymer scaffolds in combination with periosteum and isolated chondrocytes (Isogai, et al., 1999, Landis, et al., 2005, Sedrakyan, et al., 2006), patella formed from a milled trabecular bone substrate with a molded chondrocyte-agarose layer (Hung, et al., 2003), and mandibular condyle formed from molded PEG hydrogel with stratified layers of encapsulated stem cells (Alhadlaq, et al., 2004). Whole-joint bioengineering has been attempted by apposition of engineered distal and middle phalanges with a joint capsule formed by a tenocyte-seeded PGA mesh (Isogai, et al., 1999, Landis, et al., 2005), and resurfacing devitalized chick knees with chondrocyte-seeded collagen sponges (Warden, et al., 2004, Zaleske, et al., 2003). In these whole-joint studies, anti-adhesion sheets of silicone or ePTFE were used to prevent fusion of opposing articular surfaces during in vitro or subcutaneous in vivo culture.
4.E. Bioreactors for Joint Loading and Motion
The tissues which comprise a joint are typically mechanosensitive, altering their biological activities and, in turn, their biochemical and physical properties, in response to the local physical environment (Allori, et al., 2008, Grodzinsky, et al., 2000, Guilak, et al., 1997). In addition to biochemical stimuli and nutrient and waste exchange, many bioreactors provide specific mechanical cues to guide the growth, remodeling, and maturation of engineered musculoskeletal tissues (Vunjak-Novakovic, et al., 2005, Williams, et al., 2008). Physical stimuli may be transmitted through the fluid medium in bioreactors producing hydrostatic pressure gradients or fluid flow across or through a construct. Mechanical stimuli may also be applied directly to constructs in the form of static or dynamic tension, compression, or shear.
While simplified loading regimes may facilitate the development of models and an understanding of mechanobiological processes, bioreactor designs have been evolving to incorporate complex loading and motion, with opposing surfaces that compress, shear, and slide. One approach is to apply loading and traction to a tissue surface, such as that achieved when a chondral tissue pin is articulated against a ceramic hip prosthesis which can undergo compression and biaxial rotation (Wimmer, et al., 2004). Certain types of applied articulation modulate chondrocyte gene expression, including that of PRG4 lubricant and cartilage oligomeric matrix protein, in a velocity dependent manner (Grad, et al., 2006, Wimmer, et al., 2009). A different approach is to apply loading to joint components in a biomimetic configuration. When a continuous passive motion device was transformed into a whole-joint bioreactor, PRG4 synthesis by articular chondrocytes in knees joints was upregulated in a manner dependent on surface loading and motion (Nugent-Derfus, et al., 2007). These studies suggest that mechanical conditioning of engineered joints may affect the the tribological properties of the bathing medium and synovial interfaces, which in turn would affect the local mechanobiological environment of the cells within articulated tissues.
5. MECHANICAL TESTING AND ANALYSIS OF SYNOVIAL JOINTS
The success of a tissue or graft for joint repair may depend on its load-bearing properties and its ability to survive within a demanding joint environment. A variety of mechanical properties can be evaluated for a tissue or graft at scale levels ranging from tissue-level to intact joints. The time scale and conditions over which properties are evaluated may address properties at steady-state or evolving under the influence of mechanobiological stimuli.
5.A. Load-Bearing Properties of Articular Cartilage
Mechanical testing is often performed on localized regions of a joint or on excised tissue samples to simplify experimental configurations and analyses. With appropriate boundary conditions and with assumptions about sample (in)homogeneity and (an)isotropy, material properties can be estimated. Indentation testing, for example, has been widely used to characterize the health and function of cartilage because it is relatively easy to perform, nondestructive measure, and interpretable in terms of structural (Kempson, et al., 1971) or material biomechanical properties (Mow and Huiskes, 2005). Indentation measures are sensitive to the structure and composition of the tissue (Jurvelin, et al., 1988, Lu, et al., 2004) and, therefore, can reflect cartilage degeneration (Bae, et al., 2003, Froimson, et al., 1997, Kempson, et al., 1971) and repair (Messner and Gillquist, 1993, Wakitani, et al., 1989), as well as tissue edges and interfaces (Bae, et al., 2004, Smith and Mansour, 2000). Compressive and tensile testing methods have also been used to assess the structural and material properties of cartilaginous repair tissue. Compressive testing of engineered tissue before implantation (Bastiaansen-Jenniskens, et al., 2008, Lebourg, et al., 2008, Obradovic, et al., 2001, Pfeiffer, et al., 2008, Spiller, et al., 2008) and at the time of retrieval (Ando, et al., 2007, Chu, et al., 1997) reflect load-bearing properties while tensile properties of repair tissue between an implant and host cartilage reflect the degree of integrative repair (Gratz, et al., 2006). These tests, however, utilize excised tissue samples of specified or measured geometry for calculation of material properties.
5.B. Assessment of Tribological Properties
In the synovial joint, cartilage slides against cartilage, meniscus, synovium, and ligament, and lubrication of these tissues by synovial fluid appears critical to reducing friction and wear. Friction is the resistance to motion between two bodies in contact. The friction coefficient is the ratio of the forces transverse (frictional) and normal to the contact area (Ateshian and Mow, 2005, Swanson, 1979). Wear is the progressive loss of the bearing substance from a body in contact with another body as a result of mechanical action, whether through relative sliding or cyclic loading. A wear coefficient can be defined as the ratio of the volume of material lost to the load normal to the contact and to the total sliding distance (Stachowiak and Batchelor, 2005). In vivo, wear can be estimated from serial MRI scans (Mccann, et al., 2009). In vitro, wear volumes for a hydrated tissue such as cartilage can be estimated from the amount of collagen or proteoglycan released into the test solution and thereby account for tissue swelling that occurs with OA (Maroudas, 1976). Cartilage surface roughening is another index of wear, visualized classically as India ink up-take on the tissue surface (Meachim, 1972) and quantified through digital imaging methods (Chang, et al., 1997) or estimated by surface profilometry to determine surface roughness (Northwood, et al., 2007).
5.C. Tissue-Scale Tribological Testing
A variety of in vitro lubrication tests apply linear translation or rotation to excised tissue samples (Fig. 6A,B). At this scale, tissues are isolated from their surroundings, sample geometry is simplified from that of the joint, and fluid depressurization paths and characteristic times can be controlled. A variety of lubrication regimes are generally operative during linear translation of two deforming surfaces, while the boundary lubrication regime is predominantly operative with rotational motion, since the same two surfaces remain in contact (Bowden and Tabor, 1950, Malcom, 1976). Cartilage has been tested in contact with artificial materials (Forster and Fisher, 1996, Radin, et al., 1971, Wang and Ateshian, 1997) or with cartilage (Davis, et al., 1979, Katta, et al., 2009, Malcom, 1976, Schmidt and Sah, 2007). Such tests have been used to evaluate the lubricity of synovial fluid in health and disease, as well as to assess the friction-reducing capabilities of putative lubricating molecules (Davis, et al., 1979, Forster and Fisher, 1996, Schmidt, et al., 2007). Engineered cartilage tissue exhibits friction coefficients that decrease with increasing maturation time and increasing water content (Morita, et al., 2006, Plainfosse, et al., 2007).
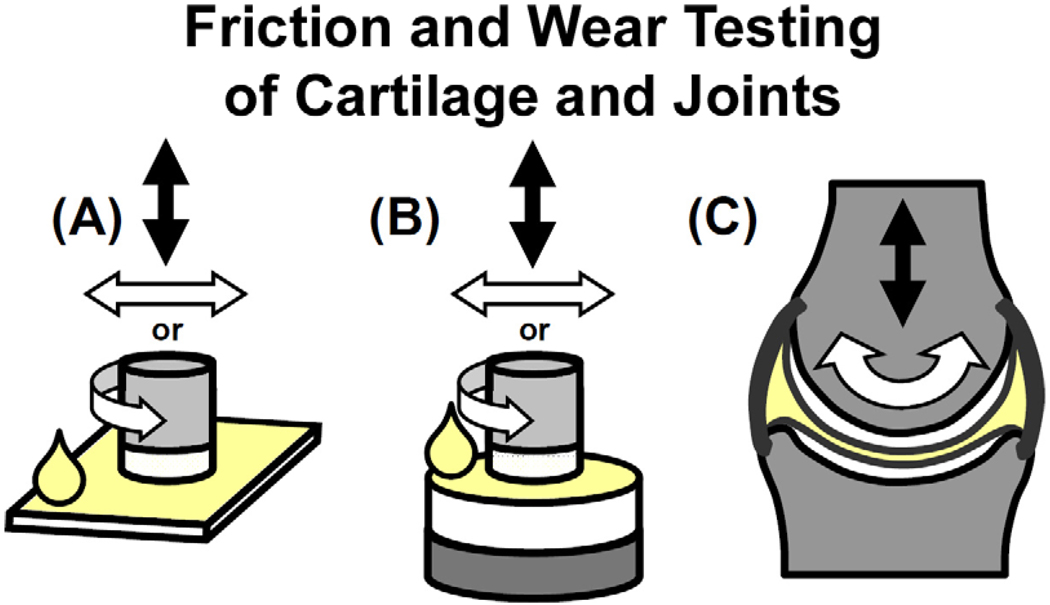
Figure 6.
Configurations for friction and wear testing of lubricated cartilage with varying complexity and physiological relevance. Friction and wear tests can be performed with small samples of cartilage in apposition to (A) a material or (B) cartilage, or with (C) whole-joint testing systems. Lubricant solutions within these systems may be varied to reflect various physiological states.
Analogous tests assess the wear of cartilage against a variety of counter-surfaces. Cartilage wear has been analyzed with counter-surfaces of polished stainless steel plates (Berrien, et al., 2000, Lipshitz and Glimcher, 1979, Owellen, 1997) and polymers used for tissue engineering applications (Katta, et al., 2007, Kobayashi and Oka, 2004), as well as cartilage with various lubricants (Temple, et al., 2007). Wear testing of engineered tissues may be useful for material and fluid characterization, with the advantage of well-defined sample geometries and fluid depressurization times.
5.D. Joint-Scale Tribological Testing
Whole-joint systems for testing lubrication function offer the advantage of tissue geometries and loading patterns that may be physiological (Fig. 6C) and thereby indicative of in vivo articulation. A whole joint testing system, such as a continuous passive motion device, allows evaluation of the ability of a defect repair strategy to withstand joint motion (Drobnic, et al., 2006) or to quantitatively evaluate the joint friction or wear resulting from a repair strategy. Intact joints have been tested, either in passive or active pendulum instruments or with robotic arms to assess the effects of experimentally-induced cartilage degeneration (Jay, et al., 2007, Obara, et al., 1997) and synovial fluid lubrication (Linn, 1967) on the frictional properties between two joint surfaces. The large contact areas, however, reflect lubrication mediated by fluid pressure and the complex geometry may require complex motion and loading patterns (Frank, et al., 1984). In addition, the presence of ligaments, menisci, and synovium may modulate the lubrication mechanics, and complicate interpretation of the contribution of cartilage-on-cartilage contact. Unicompartmental knee joint testing systems have also been used to assess the friction between cartilage surfaces with and without a meniscus (Mccann, et al., 2009) and between cartilage and metal surfaces (Mccann, et al., 2009).
Articular cartilage wear has also been assessed using an actively-loaded pendulum-style instrument on partial and whole joints. In ex vivo studies of whole bovine metatarsophalangeal joints, very mild disruption of the articular surface appeared after >1×106 cycles of articulation under static load and was accelerated with a superimposed impact load (Radin and Paul, 1971). Cartilage wear can be modulated by treatments simulating the degradation of cartilage and the stiffening of cartilage or bone (Radin, et al., 1982). Other studies using a unicompartmental pendulum system to articulate a femoral condyle against a tibial plateau (Mccann, et al., 2009) or other metal plate (Mccann, et al., 2009) have induced wear after only 3600 cycles of dynamic loading. Such results may indicate that the unicompartmental test system may be useful as an accelerated test system of tissue structure and lubrication function; or they may reflect abnormal loading patterns and a lack of biological response. As joint-scale tissue engineering strategies evolve, evaluation of the ability to articulate with little friction between and low wearing of the bearing surfaces, under physiologically-motivated loads and motions, will be increasingly important.
6. CONCLUSION
Anatomically-shaped, engineered synovial joints that could adapt to the functional demands of the host environment may have enormous clinical applicability in the future, especially for the treatment of large and extensively damaged or degenerated articular surfaces. Engineering tissues at this scale poses exciting challenges in design, assembly, culture, and testing. Success will require not only the biological understanding, quantitative characterization, and bioengineering analysis of multiple components and environmental cues, but also thorough analyses of the role that joint shape and motion play in mechanobiology. With the advancement in fields such as imaging, biomaterials, and biomechanics, therapeutically-useful engineered joints are “the shape of things to come.”
Acknowledgments
This work was supported by grants from the National Institutes of Health, the National Science Foundation, and the Howard Hughes Medical Institute through the HHMI Professors Program (to UCSD for RLS). Additional individual support was received through NSF Graduate Fellowships (to EFC and GMW) and a NIH F31 Predoctoral Fellowship (to GMW). We thank Jennifer Hwang for suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None
REFERENCES
- 1.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Alford JW, Cole BJ. Cartilage restoration, part 2: Techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 3.Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A, Mao JJ. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 4.Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13:167–177. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 5.Allori AC, Sailon AM, Pan JH, Warren SM. Biological basis of bone formation, remodeling, and repair-part iii: Biomechanical forces. Tissue Eng Part B Rev. 2008;14:285–293. doi: 10.1089/ten.teb.2008.0084. [DOI] [PubMed] [Google Scholar]
- 6.Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28:5462–5470. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Ateshian GA, Eckstein F. Quantitative anatomy and imaging of diarthrodial joint articular layers. In: Mow VC, Huiskes R, editors. Basic orthopaedic biomechanics and mechano-biology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 409–446. [Google Scholar]
- 8.Ateshian GA, Mow VC. Friction, lubrication, and wear of articular cartilage and diarthrodial joints. In: Mow VC, Huiskes R, editors. Basic orthopaedic biomechanics and mechano-biology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 447–494. [Google Scholar]
- 9.Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech. 1991;24:761–776. doi: 10.1016/0021-9290(91)90340-s. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–2205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- 11.Bae WC, Law AW, Amiel D, Sah RL. Sensitivity of indentation testing to step-off edges and interface integrity in cartilage repair. Ann Biomed Eng. 2004;32:360–369. doi: 10.1023/b:abme.0000017553.01798.12. [DOI] [PubMed] [Google Scholar]
- 12.Bae WC, Temple MM, Amiel D, Coutts RD, Niederauer GG, Sah RL. Indentation testing of human cartilage: Sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382–3394. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 13.Ballyns JJ, Gleghorn JP, Niebrzydowski V, Rawlinson JJ, Potter HG, Maher SA, Wright TM, Bonassar LJ. Image-guided tissue engineering of anatomically shaped implants via mri and micro-ct using injection molding. Tissue Eng Part A. 2008;14:1195–1202. doi: 10.1089/ten.tea.2007.0186. [DOI] [PubMed] [Google Scholar]
- 14.Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, Verhaar JA, van Osch GJ, Degroot J. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008;16:359–366. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Bauer TW, Schils J. The pathology of total joint arthroplasty.Ii. Mechanisms of implant failure. Skeletal Radiol. 1999;28:483–497. doi: 10.1007/s002560050552. [DOI] [PubMed] [Google Scholar]
- 16.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: Femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 17.Berrien LS, Furey MJ, Veit HP. Tribological study of joint pathology. Crit Rev Biomed Eng. 2000;28:103–108. doi: 10.1615/critrevbiomedeng.v28.i12.170. [DOI] [PubMed] [Google Scholar]
- 18.Blewis ME, Lao BJ, Jadin K, McCarty WJ, Hwang J, Antonacci JM, Bugbee WD, Firestein GS, Sah RL. Biomimetic bioengineering of synovial fluid: A bioreactor for a functional lubricant solution. Trans Orthop Res Soc. 2009;34:121. [Google Scholar]
- 19.Bowden FP, Tabor D. The friction and lubrication of solids. New York: Oxford University Press; 1950. [Google Scholar]
- 20.Brittberg M. Autologous chondrocyte transplantation. Clin Orthop Rel Res. 1999;367S:147–155. doi: 10.1097/00003086-199910001-00016. [DOI] [PubMed] [Google Scholar]
- 21.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 22.Buckley MR, Gleghorn JP, Bonassar LJ, Cohen I. Mapping the depth dependence of shear properties in articular cartilage. J Biomech. 2008;41:2430–2437. doi: 10.1016/j.jbiomech.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Buckwalter JA, Mankin HJ. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 24.Buckwalter JA, Mow VC. Cartilage repair in osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, editors. Osteoarthritis: Diagnosis and medical/surgical management. Philadelphia: W.B. Saunders Co; 1992. pp. 71–107. [Google Scholar]
- 25.Bugbee WD. Osteochondral allograft transplantation. In: Cole BJ, Malek MM, editors. Articular cartilage lesions: A practical guide to assessment and treatment. New York: Springer; 2004. pp. 82–94. [Google Scholar]
- 26.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 27.Chang DG, Iverson EP, Schinagl RM, Sonoda M, Amiel D, Coutts RD, Sah RL. Quantitation and localization of cartilage degeneration following the induction of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1997;5:357–372. doi: 10.1016/s1063-4584(97)80039-8. [DOI] [PubMed] [Google Scholar]
- 28.Chu CR, Dounchis JS, Yoshioka M, Sah RL, Coutts RD, Amiel D. Osteochondral repair using perichondrial cells: A one year study in rabbits. Clin Orthop Rel Res. 1997;340:220–229. doi: 10.1097/00003086-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 29.Clifford PE, Mallon WJ. Sports after total joint replacement. Clin Sports Med. 2005;24:175–186. doi: 10.1016/j.csm.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Cole BJ, Carter TR, Rodeo SA. Allograft meniscal transplantation: Background, techniques, and results. Instr Course Lect. 2003;52:383–396. [PubMed] [Google Scholar]
- 31.Colombo V, Palla S, Gallo LM. Temporomandibular joint loading patterns related to joint morphology: A theoretical study. Cells Tissues Organs. 2008;187:295–306. doi: 10.1159/000113408. [DOI] [PubMed] [Google Scholar]
- 32.Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models - their training and application. Computer Vision and Image Understanding. 1995;61:38–59. [Google Scholar]
- 33.Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis. 1991;50:8–13. doi: 10.1136/ard.50.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel M, Iglic A, Kralj-Iglic V. The shape of acetabular cartilage optimizes hip contact stress distribution. J Anat. 2005;207:85–91. doi: 10.1111/j.1469-7580.2005.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis WHJ, Lee SL, Sokoloff L. A proposed model of boundary lubrication by synovial fluid: Structure of boundary water. J Biomech Eng. 1979;101:185–192. [Google Scholar]
- 36.Davol A, Bingham MS, Sah RL, Klisch SM. A nonlinear finite element model of cartilage growth. Biomech Model Mechanobiol. 2008;7:295–307. doi: 10.1007/s10237-007-0098-6. [DOI] [PubMed] [Google Scholar]
- 37.Dennis DA, Lynch CB. Stability advantages of a modular total hip system. Orthopedics. 2005;28:s1049–S1052. doi: 10.3928/0147-7447-20050902-09. [DOI] [PubMed] [Google Scholar]
- 38.Dorr LD, Kane TJ, 3rd, Conaty JP. Long-term results of cemented total hip arthroplasty in patients 45 years old or younger. A 16-year follow-up study. J Arthroplasty. 1994;9:453–456. doi: 10.1016/0883-5403(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 39.Drobnic M, Radosavljevic D, Ravnik D, Pavlovcic V, Hribernik M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee. Osteoarthritis Cartilage. 2006;14:337–344. doi: 10.1016/j.joca.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Esther RJ, Creighton RA, Draeger RW, Weinhold PS, Dahners LE. Effect of nkisk on tendon lengthening: An in vivo model for various clinically applicable dosing regimens. J Orthop Res. 2008;26:971–976. doi: 10.1002/jor.20594. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg SE, Hollister SJ, Halloran JW, Chu TM, Krebsbach PH. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 2001;169:309–321. doi: 10.1159/000047896. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203:191–202. doi: 10.1046/j.1469-7580.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forster H, Fisher J. The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng [H] 1996;210:109–119. doi: 10.1243/PIME_PROC_1996_210_399_02. [DOI] [PubMed] [Google Scholar]
- 44.Frank C, Akeson WH, Woo SL, Amiel D, Coutts RD. Physiology and therapeutic value of passive joint motion. Clin Orthop Relat Res. 1984:113–125. [PubMed] [Google Scholar]
- 45.Freeman MA, Pinskerova V. The movement of the normal tibio-femoral joint. J Biomech. 2005;38:197–208. doi: 10.1016/j.jbiomech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Froimson M, Ratcliffe A, Gardner T, Mow V. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage. 1997;5:377–386. doi: 10.1016/s1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- 47.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 48.Gerstenfeld LC, Barnes GL, Shea CM, Einhorn TA. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connect Tissue Res. 2003;44 Suppl 1:85–91. [PubMed] [Google Scholar]
- 49.Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249–256. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 50.Grad S, Lee CR, Wimmer MA, Alini M. Chondrocyte gene expression under applied surface motion. Biorheology. 2006;43:259–269. [PubMed] [Google Scholar]
- 51.Gratz KR, Sah RL. Experimental measurement and quantification of frictional contact between biological surfaces experiencing large deformation and slip. J Biomech. 2008;41:1333–1340. doi: 10.1016/j.jbiomech.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gratz KR, Wong VW, Chen AC, Fortier LA, Nixon AJ, Sah RL. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: Tensile modulus of repair tissue and integration with host cartilage. J Biomech. 2006;39:138–146. doi: 10.1016/j.jbiomech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 54.Gu D, Chen Y, Dai K, Zhang S, Yuan J. The shape of the acetabular cartilage surface: A geometric morphometric study using three-dimensional scanning. Med Eng Phys. 2008;30:1024–1031. doi: 10.1016/j.medengphy.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Guilak F, Mow VC. The mechanical environment of the chondrocyte: A biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 56.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 57.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic orthopaedic biomechanics. New York: Raven Press; 1997. pp. 179–207. [Google Scholar]
- 58.Gupta T, Haut Donahue LT. Role of cell location and morphology in the mechanical environment around meniscal cells. Acta Biomater. 2006;2:483–492. doi: 10.1016/j.actbio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Han EH, Bae WC, Hsieh-Bonassera ND, Wong VW, Schumacher BL, Gortz S, Masuda K, Bugbee WD, Sah RL. Shaped, stratified, scaffold-free grafts for articular cartilage defects. Clin Orthop Relat Res. 2008;466:1912–1920. doi: 10.1007/s11999-008-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hangody L, Feczko P, Bartha L, Bodo G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Rel Res. 2001:328–336. doi: 10.1097/00003086-200110001-00030. [DOI] [PubMed] [Google Scholar]
- 61.Hangody L, Rathonyi GK, Z D, Vasarhelyi G, Fules P, Modis L. Autologous osteochondral mosaicplasty. J Bone Joint Surg. 2004;86-A Supplement 1:65–72. [PubMed] [Google Scholar]
- 62.Heegaard JH, Beaupre GS, Carter DR. Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J Orthop Res. 1999;17:509–517. doi: 10.1002/jor.1100170408. [DOI] [PubMed] [Google Scholar]
- 63.Hung CT, Lima EG, Mauck RL, Taki E, LeRoux MA, Lu HH, Stark RG, Guo XE, Ateshian GA. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853–1864. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 64.Huselstein C, Netter P, de Isla N, Wang Y, Gillet P, Decot V, Muller S, Bensoussan D, Stoltz JF. Mechanobiology, chondrocyte and cartilage. Biomed Mater Eng. 2008;18:213–220. [PubMed] [Google Scholar]
- 65.Hwang J, Bae WC, Shieu W, Lewis CW, Bugbee WD, Sah RL. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 2008;58:3831–3842. doi: 10.1002/art.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang J, Görtz S, Sah RL, Bugbee WD. Osteochondral graft transfer - techniques, outcomes, and the future. US Musculoskeletal Review. 2009;3:75–80. [Google Scholar]
- 67.Isogai N, Landis W, Kim TH, Gerstenfeld LC, Upton J, Vacanti JP. Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am. 1999;81-A:306–316. doi: 10.2106/00004623-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Jay GD, Britt DE, Cha D-J. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 69.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, Elsaid K, Kim KS, Cui Y, Warman ML. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–770. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 71.Jones DG, Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88:2502–2520. doi: 10.2106/00004623-200611000-00025. [DOI] [PubMed] [Google Scholar]
- 72.Jurvelin J, Saamanen AM, Arokoski J, Helminen HJ, Kiviranta I, Tammi M. Biomechanical properties of the canine knee articular cartilage as related to matrix proteoglycans and collagen. Eng Med. 1988;17:157–162. doi: 10.1243/emed_jour_1988_017_042_02. [DOI] [PubMed] [Google Scholar]
- 73.Katta J, Jin Z, Ingham E, Fisher J. Effect of nominal stress on the long term friction, deformation and wear of native and glycosaminoglycan deficient articular cartilage. Osteoarthritis Cartilage. 2009;17:662–668. doi: 10.1016/j.joca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Katta JK, Marcolongo M, Lowman A, Mansmann KA. Friction and wear behavior of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for articular cartilage replacement. J Biomed Mater Res A. 2007;83:471–479. doi: 10.1002/jbm.a.31238. [DOI] [PubMed] [Google Scholar]
- 75.Kempson GE, Spivey CJ, Swanson SA, Freeman MA. Patterns of cartilage stiffness on normal and degenerate human femoral heads. J Biomech. 1971;4:597–609. doi: 10.1016/0021-9290(71)90049-2. [DOI] [PubMed] [Google Scholar]
- 76.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (szp) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 77.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: Material and matrix considerations. J Bone Joint Surg Am. 2008;90 Suppl 1:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 78.Khumsap S, Lanovaz JL, Clayton HM. Three-dimensional kinematic analysis of horses with induced tarsal synovitis. Equine Vet J. 2004;36:659–663. doi: 10.2746/0425164044848073. [DOI] [PubMed] [Google Scholar]
- 79.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, Thonar EJ, Sah RL. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi M, Oka M. Characterization of a polyvinyl alcohol-hydrogel artificial articular cartilage prepared by injection molding. J Biomater Sci Polym Ed. 2004;15:741–751. doi: 10.1163/156856204774196135. [DOI] [PubMed] [Google Scholar]
- 82.Lammi MJ. Current perspectives on cartilage and chondrocyte mechanobiology. Biorheology. 2004;41:593–596. [PubMed] [Google Scholar]
- 83.Landis WJ, Jacquet R, Hillyer J, Lowder E, Yanke A, Siperko L, Asamura S, Kusuhara H, Enjo M, Chubinskaya S, Potter K, Isogai N. Design and assessment of a tissue-engineered model of human phalanges and a small joint. Orthod Craniofac Res. 2005;8:303–312. doi: 10.1111/j.1601-6343.2005.00353.x. [DOI] [PubMed] [Google Scholar]
- 84.Laskin RS. Modular total knee-replacement arthroplasty. A review of eighty-nine patients. J Bone Joint Surg Am. 1976;58:766–773. [PubMed] [Google Scholar]
- 85.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. Part ii. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lebourg M, Sabater Serra R, Mas Estelles J, Hernandez Sanchez F, Gomez Ribelles JL, Suay Anton J. Biodegradable polycaprolactone scaffold with controlled porosity obtained by modified particle-leaching technique. J Mater Sci Mater Med. 2008;19:2047–2053. doi: 10.1007/s10856-007-3282-4. [DOI] [PubMed] [Google Scholar]
- 87.Lima EG, Grace Chao PH, Ateshian GA, Bal BS, Cook JL, Vunjak-Novakovic G, Hung CT. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008;29:4292–4299. doi: 10.1016/j.biomaterials.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Linn FC. Lubrication of animal joints I. The arthrotripsometer. J Bone Joint Surg Am. 1967;49-A:1079–1098. [PubMed] [Google Scholar]
- 89.Lipshitz H, Glimcher MJ. In vitro studies of the wear of articular cartilage Ii. Characteristics of the wear of articular cartilage when worn against stainless steel plates having characterized surfaces. Wear. 1979;52:297–339. [Google Scholar]
- 90.Lorenz C. Generation of point-based 3d statistical shape models for anatomical objects. Comput Vis Image Underst. 2000;77:175–191. [Google Scholar]
- 91.Lu XL, Sun DD, Guo XE, Chen FH, Lai WM, Mow VC. Indentation determined mechanoelectrochemical properties and fixed charge density of articular cartilage. Ann Biomed Eng. 2004;32:370–379. doi: 10.1023/b:abme.0000017534.06921.24. [DOI] [PubMed] [Google Scholar]
- 92.Maes JA, Haut Donahue TL. Time dependent properties of bovine meniscal attachments: Stress relaxation and creep. J Biomech. 2006;39:3055–3061. doi: 10.1016/j.jbiomech.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 93.Malcom LL. An experimental investigation of the frictional and deformational responses of articular cartilage interfaces to static and dynamic loading, PhD thesis. San Diego: University of California; 1976. [Google Scholar]
- 94.Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 95.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Masuda K, Sah RL. Tissue engineering of articular cartilage. In: Vunjak-Novakovic G, Freshney RI, editors. Culture of cells for tissue engineering. New York: John Wiley & Sons; 2005. pp. 157–190. [Google Scholar]
- 97.McCann L, Ingham E, Jin Z, Fisher J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 98.McCann L, Ingham E, Jin Z, Fisher J. An investigation of the effect of conformity of knee hemiarthroplasty designs on contact stress, friction and degeneration of articular cartilage: A tribological study. J Biomech. 2009 doi: 10.1016/j.jbiomech.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 99.Meachim G. Light microscopy of indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972;31:457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Messner K, Gillquist J. Synthetic implants for the repair of osteochondral defects of the medial femoral condyle: A biomechanical and histological evaluation in the rabbit knee. Biomaterials. 1993;14:513–521. doi: 10.1016/0142-9612(93)90240-3. [DOI] [PubMed] [Google Scholar]
- 101.Minas T. Autologous chondrocyte transplantation for focal chondral defects of the knee. Clin Orthop Rel Res. 2001;391S:349–361. doi: 10.1097/00003086-200110001-00032. [DOI] [PubMed] [Google Scholar]
- 102.Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, Hung CT, Lu HH. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105:7947–7952. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morita Y, Tomita N, Aoki H, Sonobe M, Wakitani S, Tamada Y, Suguro T, Ikeuchi K. Frictional properties of regenerated cartilage in vitro. J Biomech. 2006;39:103–109. doi: 10.1016/j.jbiomech.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 104.Mow VC, Huiskes R. Basic orthopaedic biomechanics and mechano-biology. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 105.Muhle C, Brossmann J, Heller M. Kinematic ct and mr imaging of the patellofemoral joint. Eur Radiol. 1999;9:508–518. doi: 10.1007/s003300050702. [DOI] [PubMed] [Google Scholar]
- 106.Nakaoka R, Hsiong SX, Mooney DJ. Regulation of chondrocyte differentiation level via co-culture with osteoblasts. Tissue Eng. 2006;12:2425–2433. doi: 10.1089/ten.2006.12.2425. [DOI] [PubMed] [Google Scholar]
- 107.Noble PC, Kamaric E, Sugano N, Matsubara M, Harada Y, Ohzono K, Paravic V. Three-dimensional shape of the dysplastic femur: Implications for thr. Clin Orthop Relat Res. 2003:27–40. [PubMed] [Google Scholar]
- 108.Northwood E, Fisher J, Kowalski R. Investigation of the friction and surface degradation of innovative chondroplasty materials against articular cartilage. Proc Inst Mech Eng [H] 2007;221:263–279. doi: 10.1243/09544119JEIM178. [DOI] [PubMed] [Google Scholar]
- 109.Nugent-Derfus GE, Takara T, O'Neill JK, Cahill SB, Gortz S, Pong T, Inoue H, Aneloski NM, Wang WW, Vega KI, Klein TJ, Hsieh-Bonassera ND, Bae WC, Burke JD, Bugbee WD, Sah RL. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of prg4. Osteoarthritis Cartilage. 2007;15:566–574. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O'Shea TM, Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev. 2008;14:447–464. doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 111.Obara T, Mabuchi K, Iso T, Yamaguchi T. Increased friction of animal joints by experimental degeneration and recovery by addition of hyaluronic acid. Clin Biomech (Bristol, Avon) 1997;12:246–252. doi: 10.1016/s0268-0033(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 112.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 113.Owellen MC. Biotribology: The effect of lubricant and load on articular cartilage wear and friction. PhD thesis. Blacksburg: Virginia Polytechnic Institute and State University; 1997. [Google Scholar]
- 114.Pfeiffer E, Vickers SM, Frank E, Grodzinsky AJ, Spector M. The effects of glycosaminoglycan content on the compressive modulus of cartilage engineered in type ii collagen scaffolds. Osteoarthritis Cartilage. 2008;16:1237–1244. doi: 10.1016/j.joca.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Plainfosse M, Hatton PV, Crawford A, Jin ZM, Fisher J. Influence of the extracellular matrix on the frictional properties of tissue-engineered cartilage. Biochem Soc Trans. 2007;35:677–679. doi: 10.1042/BST0350677. [DOI] [PubMed] [Google Scholar]
- 116.Radin EL, Paul IL. Response of joints to impact loading. I. In vitro wear. Arthritis Rheum. 1971;14:356–362. doi: 10.1002/art.1780140306. [DOI] [PubMed] [Google Scholar]
- 117.Radin EL, Paul IL, Swann DA, Schottstaedt ES. Lubrication of synovial membrane. Ann Rheum Dis. 1971;30:322–325. doi: 10.1136/ard.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radin EL, Swann DA, Paul IL, McGrath PJ. Factors influencing articular cartilage wear in vitro. Arthritis Rheum. 1982;25:974–980. doi: 10.1002/art.1780250810. [DOI] [PubMed] [Google Scholar]
- 119.Revell PA. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J R Soc Interface. 2008;5:1263–1278. doi: 10.1098/rsif.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005;13:988–997. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 121.Schachar NS, Novak K, Muldrew K, Zernicke RF, McGann LE. Articular cartilage joint surface reconstruction techniques. J Orthop Sci. 1999;4:457–461. doi: 10.1007/s007760050130. [DOI] [PubMed] [Google Scholar]
- 122.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL. Differential regulation of proteoglycan 4 metabolism in cartilage by il-1α, igf-i, and tgf-β1. Osteoarthritis Cartilage. 2008;16:90–97. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 124.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 125.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 126.Schoderbek RJ, Jr., Treme GP, Miller MD. Bone-patella tendon-bone autograft anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:525–547. doi: 10.1016/j.csm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 127.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cdna sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 128.Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL. Proteoglycan 4 (prg4) synthesis and immunolocalization in bovine meniscus. J Orthop Res. 2005;23:562–568. doi: 10.1016/j.orthres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 129.Sedrakyan S, Zhou ZY, Perin L, Leach K, Mooney D, Kim TH. Tissue engineering of a small hand phalanx with a porously casted polylactic acid-polyglycolic acid copolymer. Tissue Eng. 2006;12:2675–2683. doi: 10.1089/ten.2006.12.2675. [DOI] [PubMed] [Google Scholar]
- 130.Smith CL, Mansour JM. Indentation of an osteochondral repair: Sensitivity to experimental variables and boundary conditions. J Biomech. 2000;33:1507–1511. doi: 10.1016/s0021-9290(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 131.Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–122. doi: 10.1007/BF00270463. [DOI] [PubMed] [Google Scholar]
- 132.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 2006;12:3497–3508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 133.Spiller KL, Laurencin SJ, Charlton D, Maher SA, Lowman AM. Superporous hydrogels for cartilage repair: Evaluation of the morphological and mechanical properties. Acta Biomater. 2008;4:17–25. doi: 10.1016/j.actbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 134.Stachowiak GW, Batchelor AW. Engineering tribology. Burlington: Elsevier Butterworth-Heinemann; 2005. [Google Scholar]
- 135.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: Surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170–176. [PubMed] [Google Scholar]
- 136.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin Orthop Rel Res. 2001:362–369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 137.Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39 Suppl 1:S26–S31. doi: 10.1016/j.injury.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 138.Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- 139.Swanson SAV. Friction, wear, and lubrication. In: Freeman MAR, editor. Adult articular cartilage. England: Pitman Medical, Tunbridge Wells; 1979. pp. 415–460. [Google Scholar]
- 140.Sweigart MA, Athanasiou KA. Towards tissue engineering of the knee meniscus. Tissue Eng. 2001;7:111–129. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- 141.Szafranski JD, Grodzinsky AJ, Burger E, Gaschen V, Hung HH, Hunziker EB. Chondrocyte mechanotransduction: Effects of compression on deformation of intracellular organelles and relevance to cellular biosynthesis. Osteoarthritis Cartilage. 2004;12:937–946. doi: 10.1016/j.joca.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 142.Tampieri A, Sandri M, Landi E, Pressato D, Francioli S, Quarto R, Martin I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials. 2008;29:3539–3546. doi: 10.1016/j.biomaterials.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 143.Temple MM, Nguyen Q, Moy TM, Cardenas CO, Nguyen TD, Bugbee WD, Wong VW, Sah RL. Synovial fluid and cartilage degeneration modulate in vitro wear of articulating cartilage. Trans Orthop Res Soc. 2007;32:611. [Google Scholar]
- 144.Vangsness CT, Jr., Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: Tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474–481. doi: 10.1177/03635465030310032701. [DOI] [PubMed] [Google Scholar]
- 145.Villegas DF, Hansen TA, Liu DF, Donahue TL. A quantitative study of the microstructure and biochemistry of the medial meniscal horn attachments. Ann Biomed Eng. 2008;36:123–131. doi: 10.1007/s10439-007-9403-x. [DOI] [PubMed] [Google Scholar]
- 146.Vunjak-Novakovic G, Meinel L, Altman G, Kaplan D. Bioreactor cultivation of osteochondral grafts. Orthod Craniofac Res. 2005;8:209–218. doi: 10.1111/j.1601-6343.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 147.Wakitani S, Kimura T, Hirooka A, Ochi T, Yoneda M, Yasui N, Owaki H, Ono K. Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg Br. 1989;71-B:74–80. doi: 10.1302/0301-620X.71B1.2915011. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Ateshian GA. The normal stress effect and equilibrium friction coefficient of articular cartilage under steady frictional shear. J Biomech. 1997;30:771–776. doi: 10.1016/s0021-9290(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 149.Wang IE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–1755. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 150.Wang IN, Lu HH. Role of cell-cell interactions on the regeneration of soft tissue-to-bone interface; Conf Proc IEEE Eng Med Biol Soc; 2006. pp. 783–786. [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Warden S, Zaleske DJ, Glowacki J. Fate of a chimeric joint construct in an ectopic site in scid mice. Cell Transplant. 2004;13:161–168. doi: 10.3727/000000004773301843. [DOI] [PubMed] [Google Scholar]
- 153.Weng HH, Fitzgerald J. Current issues in joint replacement surgery. Curr Opin Rheumatol. 2006;18:163–169. doi: 10.1097/01.bor.0000209428.69528.96. [DOI] [PubMed] [Google Scholar]
- 154.Williams GM, Klisch SM, Sah RL. Bioengineering cartilage growth, maturation, and form. Pediatr Res. 2008;63:527–534. doi: 10.1203/PDR.0b013e31816b4fe5. [DOI] [PubMed] [Google Scholar]
- 155.Williams GM, Lin JW, Sah RL. Cartilage reshaping via in vitro mechanical loading. Tissue Eng. 2007;13:2903–2911. doi: 10.1089/ten.2007.0053. [DOI] [PubMed] [Google Scholar]
- 156.Wilson DR, McWalter EJ, Johnston JD. The measurement of joint mechanics and their role in osteoarthritis genesis and progression. Med Clin North Am. 2009;93:67–82. doi: 10.1016/j.mcna.2008.08.004. x. [DOI] [PubMed] [Google Scholar]
- 157.Wimmer MA, Alini M, Grad S. The effect of sliding velocity on chondrocytes activity in 3d scaffolds. J Biomech. 2009;42:424–429. doi: 10.1016/j.jbiomech.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 158.Wimmer MA, Grad S, Kaup T, Hanni M, Schneider E, Gogolewski S, Alini M. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004;10:1436–1445. doi: 10.1089/ten.2004.10.1436. [DOI] [PubMed] [Google Scholar]
- 159.Wong BL, Bae WC, Chun J, Gratz KR, Sah RL. Biomechanics of cartilage articulation: Effects of lubrication and degeneration on shear deformation. Arthritis Rheum. 2008;58:2065–2074. doi: 10.1002/art.23548. [DOI] [PubMed] [Google Scholar]
- 160.Woo SL, Jia F, Zou L, Gabriel MT. Functional tissue engineering for ligament healing: Potential of antisense gene therapy. Ann Biomed Eng. 2004;32:342–351. doi: 10.1023/b:abme.0000017551.93144.1a. [DOI] [PubMed] [Google Scholar]
- 161.Wood ML, Luthin WN, Lester GE, Dahners LE. Tendon creep is potentiated by nkisk and relaxin which produce collagen fiber sliding. Iowa Orthop J. 2003;23:75–79. [PMC free article] [PubMed] [Google Scholar]
- 162.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009 doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.You BM, Siy P, Anderst W, Tashman S. In vivo measurement of 3-d skeletal kinematics from sequences of biplane radiographs: Application to knee kinematics. IEEE Trans Med Imaging. 2001;20:514–525. doi: 10.1109/42.929617. [DOI] [PubMed] [Google Scholar]
- 164.Zaleske D, Peretti G, Allemann F, Strongin D, MacLean R, Yates KE, Glowacki J. Engineering a joint: A chimeric construct with bovine chondrocytes in a devitalized chick knee. Tissue Eng. 2003;9:949–956. doi: 10.1089/107632703322495592. [DOI] [PubMed] [Google Scholar]