Abstract
Objectives
Surgical ablation of ganglionated plexi (GP) has been proposed to increase the efficacy of the surgical treatment of atrial fibrillation (AF). This experimental canine study examined the electrophysiological attenuation and recovery of atrial vagal effects following GP ablation alone and combined with standard surgical lesion sets used to treat AF.
Methods
Dogs were divided into 3 groups: Group 1 (N=6) had focal ablation of the 4 major epicardial GP fat pads; Group 2 (N=6) had pulmonary vein isolation with GP ablation; and Group 3 (N=6) had posterior left atrial isolation with GP ablation. All fat pads were ablated. Sinus and atrioventricular (AV) interval changes during bilateral vagosympathetic trunk stimulation were examined before, after, and at four weeks post-ablation. Vagally induced effective refractory period (ERP) changes and mean QRST area changes (index of local innervation) were examined in 5 atrial regions.
Results
Sinus and AV interval changes and heart rate variability decreased immediately following ablation, but only sinus interval changes were restored significantly after 4 weeks in all groups. Ablation modified vagal effects on ERP or QRST area changed heterogeneously in Groups 1 and 2. In Group 3, regional vagal effects were attenuated extensively post-ablation in both atria. Posterior left atrial isolation with GP ablation incrementally denervated the atria. Chronically, vagal stimulation increased QRST area changes over control values in all groups. Heart rate variability was also assessed.
Conclusions
GP ablation significantly reduced vagal innervation to the atria. Restoration of vagal effects at 4 weeks suggested early atrial reinnervation.
Introduction
Parasympathetic tone has a profound effect on atrial electrophysiology. It results in a shortening of refractory periods without altering conduction.(1, 2) Combined with sympathetic tone, it facilitates triggered activity, which can be a factor in the induction of atrial fibrillation (AF).(3) Parasympathetic and sympathetic fibers enter the heart along the pulmonary veins, superior vena cava, and aorta.(4–6) They form synaptic junctions with other fibers in multiple ganglionated plexi (GP). These GP are part of a integrated network of nerves that form an intrinsic nervous system for the atria that modulates and controls the release of neural transmitters within the atria. In the canine atria there are 619±88 GP. However, four fat pads have been identified that contain a large concentration of GP. The four fat pads are located: 1) lateral to the right pulmonary veins (PRV); 2) below the inferior vena cava on the left atrium (IVC-LA); 3) at the junction of the superior vena cava and aorta (SVC-Ao); 4) and anterior to the left superior pulmonary vein (SLPV). (Figure 1) Stimulation of these fat pads slows heart rate and conduction in the AV node, and decreases refractory period in the atrial myocardium. (7) Stimulation of these fat pads in combination with sympathetic agonist can induce premature atrial beats and atrial fibrillation. Ablation of these fat pads has been shown to reduce the inducibility of AF.(8) However, selective ablation of only one of these fat pads can increase the vulnerability of the atria to AF by increasing the inhomogeneity of refractoriness.(9) Clinical studies using catheter or surgical approaches suggest that in combination a standard ablation approach, GP ablation increases the efficacy of the procedure.(10–13) It also has been proposed to GP ablation to surgical lesion sets in order to enhance results.(13) However, heart rate variability data suggests that reinnervation takes place following ablation of the GP.(10) In canine auto-transplantation, vagal reinnervation has been demonstrated functionally and histologically.(14) The data suggest that the innervation is remodeled inhomogenously. Reinnervation has also been demonstrated in humans following heart transplantation.(15–17) It is unclear what the acute and chronic effects are of GP ablation alone and combined with standard ablation lesions sets on regional atrial innervation. This study was designed to determine the acute and chronic electrophysiologic effects of GP ablation, GP ablation plus isolation of the left and right pulmonary veins (PVI), and GP ablation, PVI, and isolation of the intervening atria between the left and right PVs (Box).
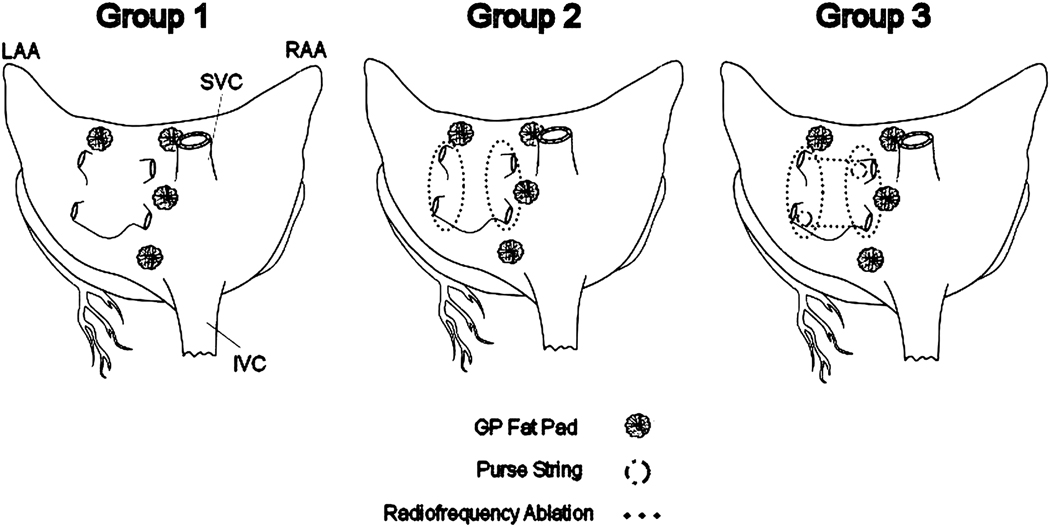
Figure 1.
Surgical ablation sets in each group. RAA, right atrial appendage; SVC, superior vena cavae; IVC, inferior vena cavae; LAA left atrial appendage.
Methods
Surgical Preparation
Eighteen adult mongrel dogs weighing between 25 and 30 kg were divided randomly into 3 groups. 1) Group 1 (n=6) had focal ablation of 4 epicardial GP fat pads located between the medial superior vena cava and the aortic root (SVC-Ao fat pad), at the junction of the right pulmonary vein and right atrium (RPV fat pad), at the inferior vena cava-left atrial junction (IVC-LA fat pad), from the left superior pulmonary vein (SLPV fat pad) 2) Group 2 (n=6) had PVI combined with GP ablation. 3) Group3 (n=6) had PVI and intervening tissue between the left and right pulmonary veins (Box) combined with GP ablation (Figure 1).
All animals were anesthetized with intravenous propofol (5 to 7 mg/kg), intubated with a cuffed endotracheal tube, and mechanically ventilated. An adequate level of anesthesia was maintained by inhaled isoflurane (1% to 3%). A limb-lead ECG was monitored. A femoral artery catheter was inserted to monitor systemic arterial pressure continuously. Arterial blood samples were drawn every 30 minutes to determine arterial oxygen tension, acid-base balance, and electrolyte levels‥
A median sternotomy was performed. A catheter was inserted into SVC through the internal mammary vein to monitor central venous pressure continuously. After the bilateral vagosympathetic trunks (VST) were dissected free, the pericardium was opened and three electrode templates containing 252 unipolar electrodes were sutured on the right and left atria. The electrode templates were approximated to the atria using suture passed through the tissue and template and then secured with a snare. This allowed the temporary removal and replacement of the templates in exactly the same location.
All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals”, published by the National Research Council (National Academy Press, 1996). The study protocol was approved by the Animal Studies Committee at Washington University School of Medicine.
Electrophysiologic Data Collection
Propranolol (0.2 mg/kg) was given prior to the collection of baseline electrophysiologic data. Bilateral VSTs were stimulated simultaneously using a pulse generator (Grass Instruments, Quincy, MA, USA), with a frequency of 10 Hz, amplitude of 10 V, and 5 ms pulse width duration. A bipolar pacing electrode was placed on the right and left atrial appendages. The right and left atrium were mapped with custom-made electrode templates containing 252 unipolar electrodes. The electrode templates were constructed from a form-fitting silicon elastomer (Specialty Silicone Fabricators, Paso Robles, CA, USA) that fit snugly on the entire atrial epicardium and contained 0.5 mm in diameter silver electrodes (Pacific Wire & Cable, Inc., Santa Ana, CA, USA). The interelectrode distance was 5 mm. The right atrial appendage (RAA) bipolar electrode was used to pace at a cycle length of 300 ms, with a 2 ms duration at twice the pacing threshold (mA). Atrial electrograms were recorded during 1) spontaneous normal sinus rhythm, 2) continuous pacing, 3) continuous pacing with VST stimulation, and 4) spontaneous normal sinus rhythm with VST stimulation. Data were acquired with a PC-based data acquisition and analysis system. The system included custom-programmed software capable of data acquisition, management, display, and analysis. Unipolar electrograms were recorded at a gain of 125 with a frequency response of 0.5 to 1000 Hz. Each channel was digitized at 2000 Hz with 12-bit resolution.
Programmed stimulation was applied to each electrode pair of the template on the RAA, inferior right atrium (IRA), left atrial appendage (LAA), inferior left atrium (ILA), and posterior left atrium (PLA) with and without VST stimulation. An eight-beat drive train (S1) at a basic cycle length of 300 ms was followed by premature extra stimulus (S2) at a coupling interval of 200 ms. The coupling interval of the premature stimulus was shortened by 5 ms increments. The longest coupling interval of the premature beat that failed to capture the atrium was determined as the local effective refractory period (ERP). All data were collected acutely (before and after the surgical ablation) and chronically (4 weeks after the initial operation) in each animal.
Fat Pad Stimulation
After data collection, high-frequency stimulation (1000 ppm, duration: 0.3 ms, 1–6 V) was applied to each fat pad using a bipolar hand held electrode (Max 10, AtriCure, West Chester, OH, USA) and pacer (PACE 203H, Oscar Inc.).(18) Sinus rate was monitored during stimulation. A 10% reduction in heart rate was used to indicate a vagal effect.
Surgical Ablation
In Group 1, the four epicardial fat pads were surgically removed, and the atrial tissue beneath the fat pad was focally ablated using the bipolar electrode that was used to stimulate the fat pad to create a transmural lesion. Each focal ablation time was set at 15 sec. In Groups 2 and 3, the RPV fat pad was completely dissected at the inter-atrial groove. Blunt dissection was then used to allow an umbilical tape to be passed around each pulmonary vein. A bipolar clamp ablation device (AtriCure, West Chester, OH, USA) was placed around the right and left pulmonary veins with a cuff of surrounding atrial tissue and ablated. GP ablation was confirmed by repeated stimulation.
In Group 3, a lesion was created between the right and left superior pulmonary veins and then between the right and left inferior pulmonary veins. These lesions were created by inserting the lower jaw of the clamp into the left atrium through a purse string suture. Following the ablations, the atrial tissue in the bilateral pulmonary veins and posterior wall of the left atrium was paced to make sure that the ablation created a transmural lesion with conduction block. After completing surgical ablation in each group, the electrode templates were reattached and electrophysiologic data were acquired. The chest was closed in layers, negative pressure was reestablished in the pleural cavity, and the animal was allowed to recover. Antibiotics were injected intramuscularly for 5 days postoperatively and analgesics were given as needed.
24-Hour Continuous ECG Recordings
All animals underwent 24 hour ECG recordings which were obtained preoperatively, and at 1, 7, 14, 21, and 27 days postoperatively using a 3-channnel digital recorder (IQmark, Brentwood, CA, USA). Heart rate variability (HRV) analysis was performed off-line. The ECG signal was sampled at 200 Hz. Premature beats, arrhythmias, electrical noise, or other aberrant ECG signals were excluded from the HRV analysis. Time-domain measures and power spectral analysis were performed. Time-domain HRV parameters were standard deviation of normal RR-intervals (SDNN), root mean square of standard deviation (rMSSD), and triangular index. Frequency-domain HRV parameters were high frequency power (HF:0.16–0.4 Hz) and low frequency power (LF:0.04–0.15 Hz).
Data Analysis
Spontaneous normal sinus intervals before and during vagal stimulation were measured. Atrioventricular intervals were calculated using data obtained during continuous pacing. Both interval changes were expressed as a percentage of lengthening (positive value) or shortening (negative value). ERP changes before and during vagal stimulation were expressed as percentages of baseline.
Atrial activation sequence data were analyzed using a computer program to determine the local activation times from unipolar tracings. The time of local activation was defined as the peak negative derivative of the major deflection of the unipolar complex. All electrograms were edited visually to verify accuracy of the computer-selected activation times. Computer-generated activation sequence maps were constructed from the recordings. Activation maps were displayed on a 3-dimensional surface model of the canine atrium.
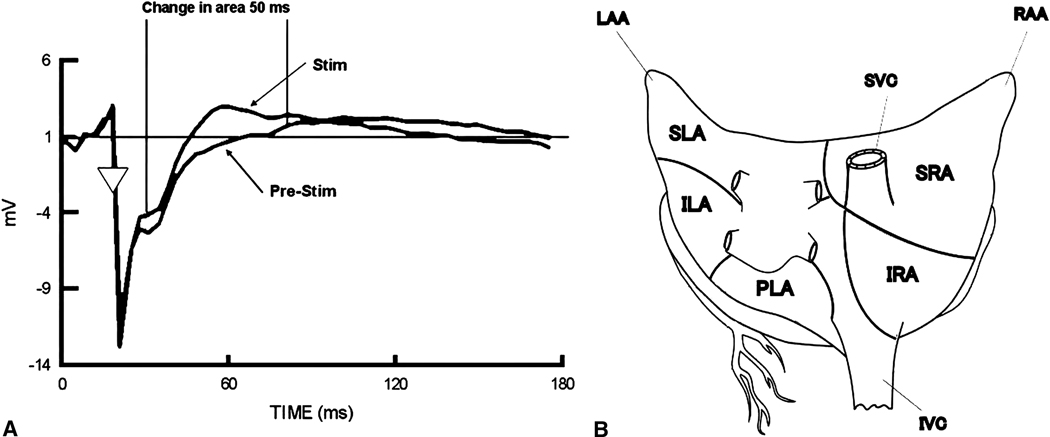
QRST area measurements of local electrograms have been used to detect changes in atrial electrical activity induced by stimulation of individual nerves.(19) The change in area of QRST deflection in the unipolar waveform is a reflection of changes in action potential duration, which is sensitive to sympathetic and parasympathetic stimulation. QRST area was determined using an isoelectric diastolic baseline calculated just after the pacing spike. The area calculation started 10 ms after the local activation and proceeded for 50 ms with VST stimulation (Figure 2A). The absolute value of the difference in the area under the curve in the paced and paced with stimulation data was calculated and normalized to the paced data. To determine the level of noise in the data (used as control value), two successive beats of paced data without stimulation were analyzed. Data from 252 sites were collected and divided into 5 anatomical regions consisting of the superior right atrium (SRA), inferior right atrium (RA), superior left atrium (SLA), inferior left atrium (ILA), and posterior left atrium (PLA). The mean QRST area change in each atrial region was then calculated (Figure 2B).
Figure 2.
A: Calculation of change in QRST area for unipolar electrographic analysis. Stim, Stimulation. B: Five atrial regions in QRST analysis. RAA, right atrial appendage; LAA, left atrial appendage; SVC, superior vena cavae; IVC, inferior vena cavae; SRA, superior right atrium; IRA, inferior right atrium; SLA, superior left atrium, ILA, inferior left atrium, PLA, posterior left atrium.
Statistical Analysis
All continuous values were expressed as mean±1 SE. All data were tested for normality (Schapiro-Wilks test) and equality of variance (Bartlett’s test). If needed, a log10 transform was performed, and the data were retested for normality and equality of variances. Analysis of variance was used to test the null hypothesis. The data over time (pre-ablation, post-ablation, 27 days after the ablation) within a group were compared with the repeated analysis of corvariance model with 1 factor. Post-hoc multiple comparisons were made with a Fishers LSD test or contrasts with a Dunn-Sidak correction. A significance level of 0.05 was considered statistically significant. All calculations were performed with the SPSS 11.5 statistical package (SPSS Inc., Chicago, IL, USA).
Results
Vagal GP Fat Pad Stimulation
Heart rate slowing was observed during stimulation of 33% (24/72) of fat pads. The RPV fat pad was the most consistent in reducing heart rate during stimulation. Heart rate slowed 77% (14/18) of the time during RPV fat pad stimulation by an average of 26.8±3.0 bpm (14–49%). Spontaneous AF was observed during stimulation of the IVC-LA fat pad in three animals. Five animals (27%) showed heart rate slowing during stimulation of the SVC-Ao fat pad and only two animals (11%) showed slowing during stimulation of the SLPV fat pad. Slowing of heart rate was eliminated after the fat pads were removed and the underlying atrial tissue was ablated.
Sinus and AV Interval Changes
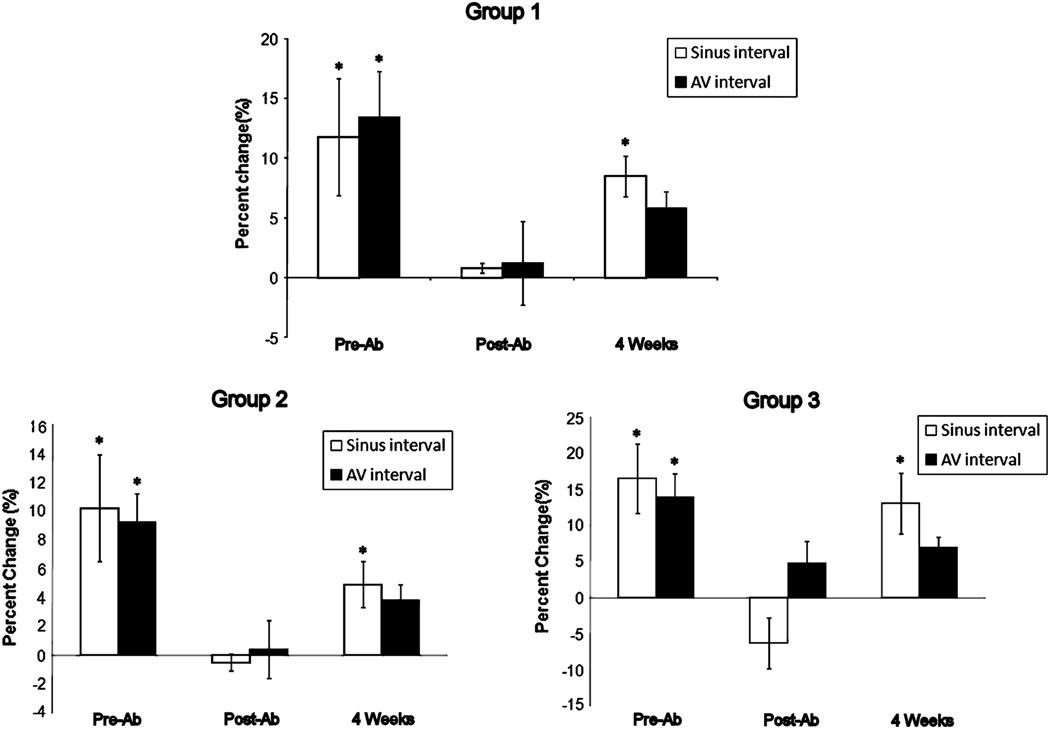
Postoperatively, ablation significantly decreased both sinus and AV interval changes during VST stimulation from control values in all groups (Figure 3) At four weeks, there was a partial restoration in heart rate during VST stimulation. Sinus interval changes were greater that post ablation values in all groups. (Group 1, Group 2, and Group 3; 8.5±1.7 vs 0.8±0.4, P=0.009, 4.9±1.6 vs −0.5±0.6, P=0.02, and 8.8±1.7 vs −0.2±0.6, P=0.047, respectively) (Figure 3). At four weeks, there was a partial restoration in the change in heart rate during VST stimulation. There were no significant differences in sinus (p=0.60) and AV interval changes (p=0.12) over time between the groups.
Figure 3.
Change in sinus and atrioventricular (AV) interval in Group A, B, and C. *P < 0.05 (for control vs VST stimulation)
Heart Rate Variability
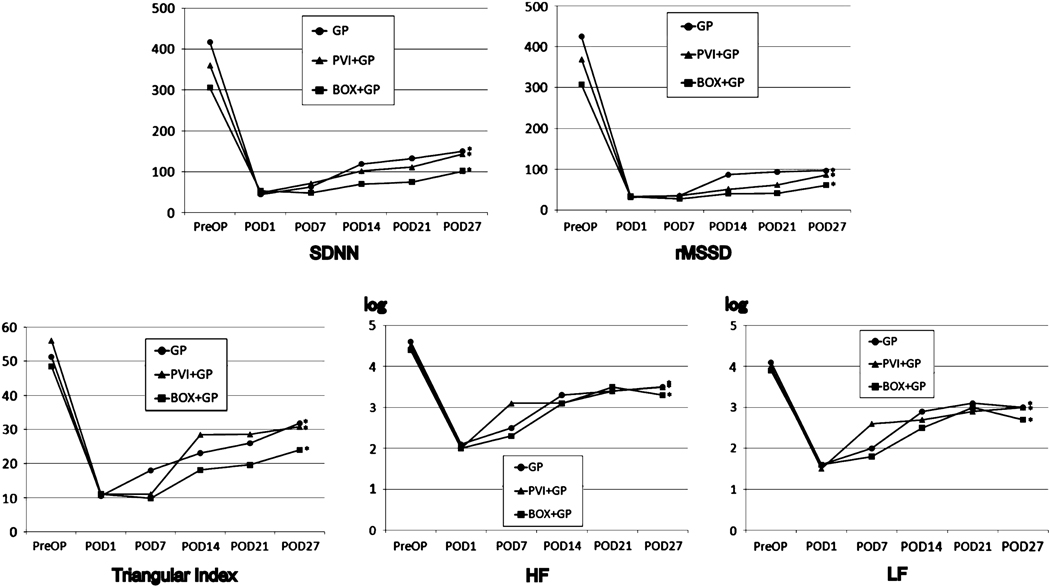
Figure 4 shows the change in heart rate variability (SDNN, RMSSD, Triangular Index, HF and LF) in all groups. Each group was similar to the others in that the value of every parameter significantly decreased immediately post-ablation (p<0.001) and remained low compared to pre-ablation values. (P<0.001) All measures of heart rate variability increased from POD1 to POD27. (p<0.007) There were no significant differences in any parameter of heart rate variability between the groups. (p>0.18).
Figure 4.
Change in heart rate variability. SDNN, standard deviation of normal RR intervals; rMSSD, root mean square of standard deviation; HF, high frequency power; LF, low frequency power.
Sinus Activation Mapping During Vagal Stimulation
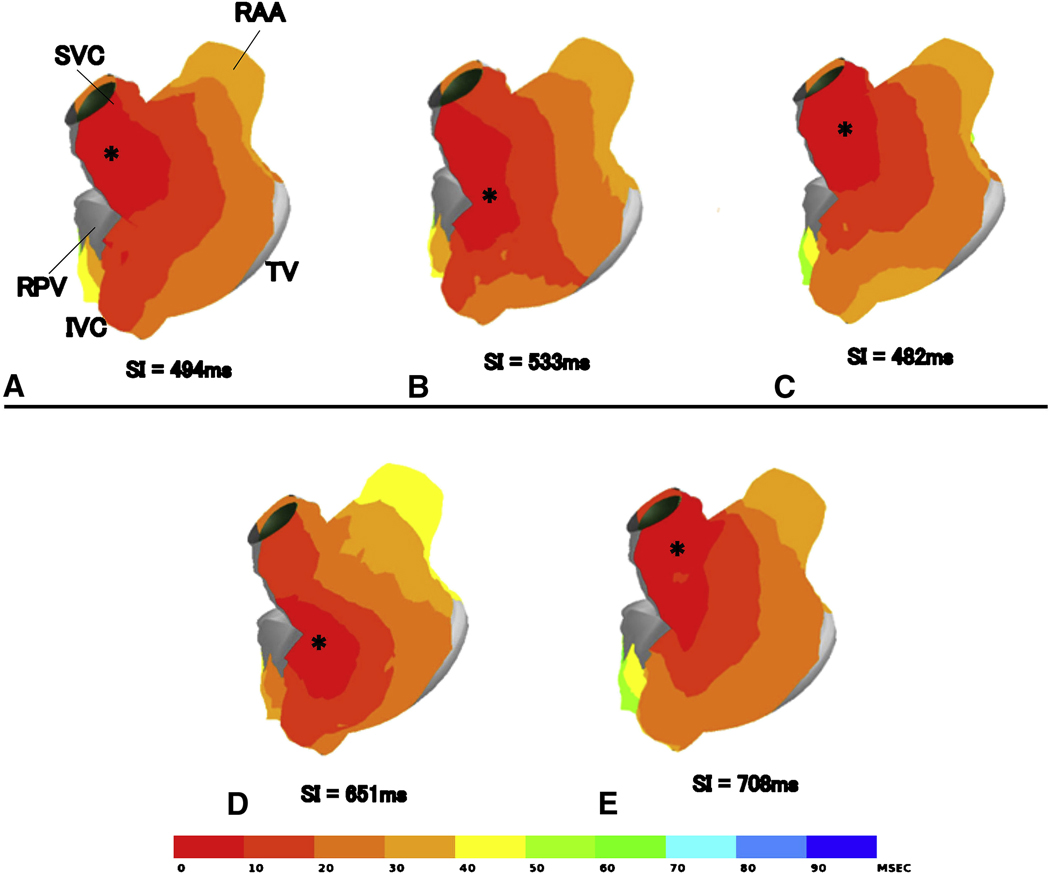
During control normal sinus rhythm, the earliest site of activation was observed at the cranial site of the sinus node at the juncture of the superior vena cava and right atrial appendage in most animals (17/18) (Figure 5A). Vagal stimulation produced a caudal shift of the earliest activation site in half of the animals in each group (Figure 5B). Two animals demonstrated multicentric activation, with two separate origins of activation. After the surgical ablation, the caudal shift of the earliest activation no longer occurred in Group 1 and Group 2 (Figure 5C). Two animals demonstrated a cranial shift of origin with shortening of the sinus interval (sinus interval change of −2.8 and −13 % respectively) in Group 3. Four weeks after the ablation, there were four animals (1 in Group 1, 1 in Group 2, 2 in Group 3) that demonstrated caudal or cranial shift with a prolonged sinus interval (sinus interval change of 6.1±1.3 %) (Figure 5E)
Figure 5.
Upper panel represents the atrial activation maps constructed from sinus rhythm without vagal stimulation (A), with vagal stimulation (B) pre-ablation, and sinus rhythm with vagal stimulation immediately post-ablation. Lower panel represents sinus activation maps without vagal stimulation (D) and with vagal stimulation (E) at 4 weeks in the same animal. The maps represent the lateral view in the right atrium. The asterisks indicate the earliest activation site in the right atrium. The atrial activation is shown with color coding at 10 ms increments. The numbers below the figure indicate the sinus interval time (ms) in the each activation. RAA, right atrial appendage; SVC, superior vena cavae; IVC, inferior vena cavae; TV, tricuspid valve; RPV, right pulmonary vein.
Mean QRST Area Change
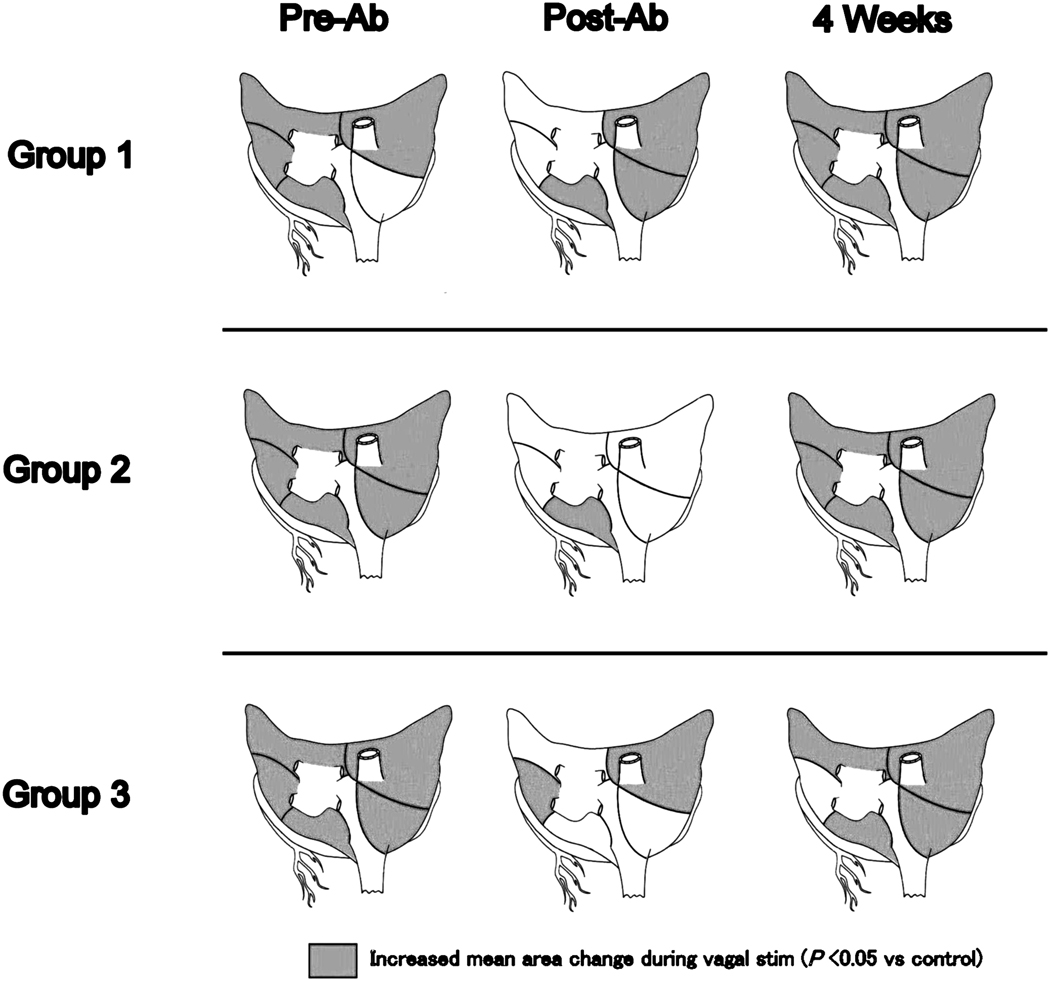
Figure 6 demonstrates the mean QRST area change in the five atrial regions in each group. A significant increase in the magnitude compared to the control (pre-stimulation value) is indicated by shading. Vagal stimulation led to QRST area changes greater than control values in all groups, except for the right inferior atrium in Group 1. Post-ablation, Group 1 showed no significant QRST area changes in the left superior and inferior atrium and increased area changes in the right inferior atrium. In Groups 2 and 3, vagal effects were extensively attenuated even in the right atrium. Group 2 showed no QRST area changes in the superior and inferior right atrium and the superior and inferior left atrium. In Group 3, area changes were absent in the inferior right atrium and the superior and posterior left atrium. Four weeks post-ablation, QRST area changes during vagal stimulation were present in all groups and in all regions compared to the post-ablation values except the left inferior atrium in Group 3 (P<0.05). In addition, vagal stimulation increased QRST area changes over pre-ablation values chronically in two regions in Group1 and 2 (Table 1)
Figure 6.
Mean QRST area change at 5 atrial regions pre-ablation, immediately post-ablation, and 4 weeks post-ablation. Significant increased area changes against control values was indicated by shading. (P<0.05)
Table 1.
QRST area change during vagal stimulation (4 weeks vs. pre-ablation).
| Resion | Group 1 | Group 2 | Group 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Ab | 4 Weeks | P-value | Pre-Ab | 4 Weeks | P-value | Pre-Ab | 4 Weeks | P-value | |
| SRA | 33.2±4.2 | 30.8±2.3 | 0.13 | 26.6±2.1 | 25.5±2.5 | 0.99 | 27.7±1.7 | 13.8±1.0 | <0.001 |
| IRA | 27.5±1.9 | 37.9±3.7 | 0.52 | 57.2±5.5 | 70.2±6.2 | <0.001 | 39.4±2.3 | 19.6±1.4 | <0.001 |
| SLA | 26.8±1.8 | 59.1±6.7 | 0.005 | 43.5±7.2 | 92.8±14.3 | <0.001 | 61.3±15.1 | 19.7±1.8 | <0.001 |
| ILA | 36.4±4.3 | 72.8±12.4 | 0.69 | 51.9±7.5 | 69.7±7.0 | 0.08 | 48.7±6.9 | 18.0±1.8 | <0.001 |
| PLA | 42.1±6.7 | 77.0±8.4 | 0.004 | 55.6±8.4 | 60.4±6.6 | 0.29 | 62.5±9.1 | 32.3±7.5 | <0.001 |
Change in Effective Refractory Period
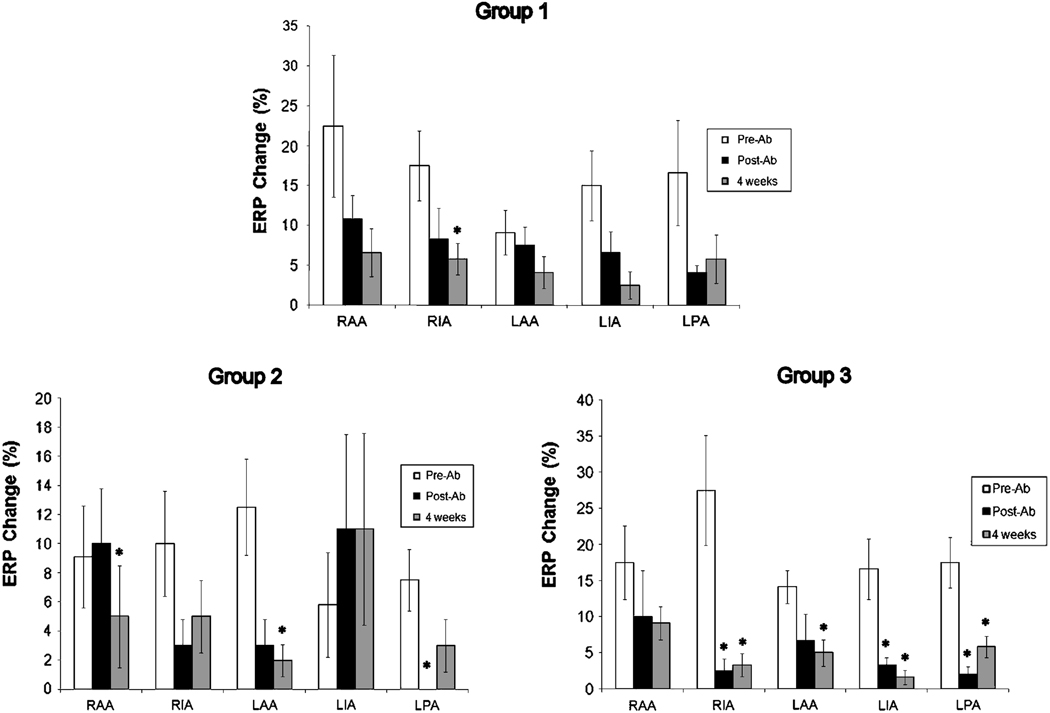
Figure 7 shows ERP changes during vagal stimulation pre-ablation, immediately post-ablation, and at 4 weeks post-ablation in each group. In Group1, ERP decreased in every region and remained low at 4 weeks postoperatively. However, there was no significant change in ERP in any atrial region immediately after ablation. The only significant change was an attenuated ERP change in the IRA at four weeks. (Pre-ablation vs. 4 weeks, 5.8±2.0% vs. 8.3±3.0%, P=0.013). In Group 2, ERP decreased in three atrial regions and increased in the RAA and LIA immediately post-ablation. A significantly smaller ERP change was observed in the RAA (pre-ablation vs. four weeks, 9.1±3.5% vs. 4.1±3.2%, P=0.041) and left atrial appendage (pre-ablation vs. four weeks, 12.5±3.3% vs. 1.6±1.0%, P=0.021). In Group 3, ablation decreased ERP in every region of the atria. At four weeks, these changes remained significantly low compared to pre-ablation in all regions except the RAA. (p=0.21)
Figure 7.
Change in effective refractory period (ERP) at 5 atrial regions in Group 1, 2, and 3. RAA, right atrial appendage; RIA, right inferior atrium; LAA, left atrial appendage; LIA, left inferior atrium; LPA, left posterior atrium. *P<0.05.
Discussion
The major finding of this study was that GP ablation, alone and combined with standard surgical ablation sets for atrial fibrillation, extensively reduced the effects of bilateral VST stimulation on not only the atrial conduction system, but also the atrial myocardium. However, within four weeks there was a return of parasympathetic effects on the sinus node and atrial myocardium. In addition, at four weeks, VST stimulation elicited a hyper-response in some areas of the atria compared to the control before ablation.
In all of the groups, the sinus and AV node response to VST stimulation was almost completely eliminated immediately after ablation. Heart rate variability also suggested that the normal neural tone in the intact animal was absent. The fact that there were no differences between any of the ablation groups suggest that the four GPs ablated in this study modulated most of all the functional neural input into the sinus and AV node. In a canine study by Lall et al, it was shown that the lesions of the Cox Maze IV or PVI without GP ablation only reduced parasympathetic and sympathetic effects in the sinus node and did not affect the AV node.(20) This suggests that the addition of the GP ablations increase the extensiveness of the denervation acutely.
The early recovery of neural function in the present study to the sinus node suggests that rapid parasympathetic reinnervation occurs in the canine. Recovery of the parasympathetic effects on the sinus node also were evident in the atrial activation mapping that showed a caudal or cranial shift of the earliest activation site during VST stimulation. Even though the AV nodal response to VST stimulation was not significant at four weeks, there was a trend toward a return of innervation. The lack of a complete return of parasympathetic effects to the AV node suggest that the reinnervation was not uniform. At four weeks, the parasympathetic effects to the AV node were still attenuated and it is unknown whether they would return to the preablation levels over a longer time period.
The effects of VST stimulation on the regional atrial myocardium, as measured by changes in the local QRST area of the unipolar electrogram, suggested that none of ablation sets completely eliminated the regional parasympathetic effects on atrial myocardium. Prior to ablation, this technique was able to demonstrate a change in local repolarization in almost all the regions in all of the groups. The addition of the surgical lesion sets to GP ablation increased the area of atrial myocardium that lost the parasympathetic effect on repolarization during VST stimulation. However, at four weeks the effects during VST stimulation returned in all three groups in virtually every region. The effects of VST stimulation on repolarization were greater at four weeks than during the preablation control in some of the regions and the extent of this increase was dependent on the lesion set. Only one region showed the hyper-response at four weeks in the GP ablation group (Gr 1), in the PVI group (Gr 2) two regions had a greater response, and in the Box lesion group (Gr 3), all five regions responded with a greater change. These data suggest that the return of the parasympathetic innervation was not only rapid, but was dependent on the ablation set. A possible mechanism for the hyper-response to vagal stimulation at four weeks is neural remodeling. Kaseda et al.(21) showed that vagal denervation in the canine atrium caused a supersensitive response to acetylcholine chronically, implying an increased number and/or density of muscarinic receptors. Sympathetic nerve sprouting and hyper-innervation also have been shown in the infarcted heart.(22) However, there have been no prior reports of hyper-reinnervation of the parasympathetic nerves in the atria. The ERP data was more ambiguous, although it suggested that ablation decreases the effects of VST stimulation. The variability in the data may be due to the time it takes to assess the ERP using the extra stimulus technique. The effects of VST stimulation may be attenuated during this time.
The data in the present study are similar to those of Oh et al.(23) who demonstrated that ablation of RPV and IVC-LA fat pads eliminated the parasympathetic effects on the sinus and AV nodes, and local refractory period acutely in the dog. However, they showed that sinus node, AV node, refractory periods, and inducibility of AF returned to preablation levels at four weeks. In canines, reinnervation occurred rapidly in our study similar to other studies. There is convincing evidence that this would also be the case in humans. Heart transplantation achieves total sympathetic and parasympathetic denervation.(15, 16) Sympathetic reinnervation is observed frequently after human heart transplantation, however parasympathetic reinnervation has been reported in only a few cases. Bernardi et al.(17) observed that the occurrence of vagal reinnervation depended on the type of surgery. They confirmed the presence of parasympathetic reinnervation in recent heart transplantation recipients (bi-caval technique), suggesting that interruption of parasympathetic fibers may regenerate similar to that observed with sympathetic fibers. In canine heart transplantation model it has been demonstrated histologically and functionally that vagal reinnervation occurred across the suture lines.(14) In patients who had a catheter PVI ablation and functional neural ablation, there was an attenuation in multiple heart rate variability parameters which returned to preablation levels after six months.(10)
Recently, there has been great interest in combining standard ablation lesion sets with GP ablation. Clinical studies have reported that GP ablation may increase the success rate for terminating and preventing AF in catheter ablation of AF. GP ablation effects appeared to last for 3–12 months.(10, 12) However, follow-up will be needed to determine if reinnervation increases the late failure rate.
GPs are mainly epicardial structures, but efferent fibers are located both subepicardialy, intramuscularly, and subendocardialy. Many surgical ablation devices are unable to ablate atrial tissue transmurally through the fat pad. Therefore, in the present study the fat pads were surgically removed to insure that ablations were transmural. The loss of a response to VST stimulation confirmed that the ablation was complete. Likewise, ablations applied to the endocardial surface during catheter ablation may be inadequate for ablating the nerve fibers that traverse the epicardium and fat pad.
The results of the present study suggest a cautionary approach to the addition of GP ablation accepted surgical lesions sets, such as the Cox-Maze procedure or PVI. This study convincingly demonstrated functional reinnervation over within a short time period. In patients with vagally mediated AF, reinnervation may recreate the original substrate for AF and there may be a recurrence. This is of particular concern if GP ablation alone is used as a treatment. A more important concern is that the reinnervation may be non-uniform, as was shown in the present study. This could create a new substrate for AF that did not originally exist in the patient. A well designed prospective randomized controlled trial, with long-term follow-up, will be required to determine if GP ablation alone, or in combination with another standard approach, is efficacious in the treatment of AF.
Study Limitations
There are quantitative differences in the number of ganglia and in the distribution of nerves between dogs and humans. (5) The number of nerves are less in dogs compared to humans, but in part, it is due to differences in atrial size. In the dog about 70% of all intrinsic ganglia supply the sinus node. However, in the human, more than half of the ganglia supply the AV node. Despite these differences, the innervation in humans and dogs is very similar. Caution should be used in extrapolating these findings to the clinical situation. While this study showed a return of parasympathetic effects, with direct thoracic nerve stimulation, we did not examine whether the intra-cardiac sympathetic and parasympathetic network returned to pre-ablation conditions.
Acknowledgments
This study was supported by NIH Grants R01 HL 032257, R01 HL085113 and T32 HL007776.
References
- 1.Schuessler RB, Bromberg BI, Boineau JP. Effect of neurotransmitters on the activation sequence of the isolated atrium. American Journal of Physiology. 1990;258(6 Pt 2):H1632–H1641. doi: 10.1152/ajpheart.1990.258.6.H1632. [DOI] [PubMed] [Google Scholar]
- 2.Schuessler RB, Grayson TM, Bromberg BI, Cox JL, Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circulation Research. 1992;71(5):1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 3.Tai CT, Chiou CW, Chen SA. Interaction between the autonomic nervous system and atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 2002 Jan;13(1):83–87. doi: 10.1046/j.1540-8167.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 4.Pauza DH, Pauziene N, Pakeltyte G, Stropus R. Comparative quantitative study of the intrinsic cardiac ganglia and neurons in the rat, guinea pig, dog and human as revealed by histochemical staining for acetylcholinesterase. Ann Anat. 2002 Mar;184(2):125–136. doi: 10.1016/S0940-9602(02)80005-X. [DOI] [PubMed] [Google Scholar]
- 5.Pauza DH, Skripka V, Pauziene N. Morphology of the intrinsic cardiac nervous system in the dog: a whole-mount study employing histochemical staining with acetylcholinesterase. Cells Tissues Organs. 2002;172(4):297–320. doi: 10.1159/000067198. [DOI] [PubMed] [Google Scholar]
- 6.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000 Aug 1;259(4):353–382. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007 Jul 3;50(1):61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 8.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000 Nov 28;102(22):2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 9.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J Cardiovasc Electrophysiol. 2002 Dec;13(12):1272–1279. doi: 10.1046/j.1540-8167.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 10.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 Jan 27;109(3):327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 11.Saad EB, Saliba WI, Marrouche NF, Natale A. Pulmonary vein firing triggering atrial fibrillation after open heart surgery.[see comment] Journal of Cardiovascular Electrophysiology. 2002;13(12):1300–1302. doi: 10.1046/j.1540-8167.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- 12.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006 Aug 29;114(9):876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 13.Mehall JR, Kohut RM, Jr, Schneeberger EW, Taketani T, Merrill WH, Wolf RK. Intraoperative epicardial electrophysiologic mapping and isolation of autonomic ganglionic plexi. Ann Thorac Surg. 2007 Feb;83(2):538–541. doi: 10.1016/j.athoracsur.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DA, Thompson GW, Ardell JL, McCraty R, Stevenson RS, Sangalang VE, et al. The heart reinnervates after transplantation. Ann Thorac Surg. 2000 Jun;69(6):1769–1781. doi: 10.1016/s0003-4975(00)01240-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991 Apr;83(4):1210–1220. doi: 10.1161/01.cir.83.4.1210. [DOI] [PubMed] [Google Scholar]
- 16.Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993 Sep;88(3):1110–1118. doi: 10.1161/01.cir.88.3.1110. [DOI] [PubMed] [Google Scholar]
- 17.Bernardi L, Valenti C, Wdowczyck-Szulc J, Frey AW, Rinaldi M, Spadacini G, et al. Influence of type of surgery on the occurrence of parasympathetic reinnervation after cardiac transplantation. Circulation. 1998 Apr 14;97(14):1368–1374. doi: 10.1161/01.cir.97.14.1368. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto S, Voeller RK, Melby SJ, Lall SC, Chang NL, Schuessler RB, et al. Surgical ablation for atrial fibrillation: the efficacy of a novel bipolar pen device in the cardioplegically arrested and beating heart. J Thorac Cardiovasc Surg. 2008 Nov;136(5):1295–1301. doi: 10.1016/j.jtcvs.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regional distribution of atrial electrical changes induced by stimulation of extracardiac and intracardiac neural elements. J Thorac Cardiovasc Surg. 1995 Feb;109(2):377–388. doi: 10.1016/S0022-5223(95)70400-0. [DOI] [PubMed] [Google Scholar]
- 20.Lall SC, Foyil KV, Sakamoto S, Voeller RK, Boineau JP, Damiano RJ, Jr, et al. Pulmonary vein isolation and the Cox maze procedure only partially denervate the atrium. J Thorac Cardiovasc Surg. 2008 Apr;135(4):894–900. doi: 10.1016/j.jtcvs.2007.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaseda S, Zipes DP. Supersensitivity to acetylcholine of canine sinus and AV nodes after parasympathetic denervation. Am J Physiol. 1988 Sep;255(3 Pt 2):H534–H539. doi: 10.1152/ajpheart.1988.255.3.H534. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004 Jul 9;95(1):76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 23.Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006 Jun;3(6):701–708. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]