Abstract
Exposure to cocaine during the fetal period can produce significant lasting changes in the structure and function of the brain. Cocaine exerts its effects on the developing brain by blocking monoamine transporters and impairing monoamine receptor signaling. Dopamine is a major central target of cocaine. In a mouse model, we show that cocaine exposure from embryonic day 8 (E8) to E14 produces significant reduction in dopamine transporter activity, attenuation of dopamine D1-receptor function and upregulation of dopamine D2-receptor function. Cocaine’s effects on the D1-receptor are at the level of protein expression as well as activity. The cocaine exposure also produces significant increases in basal cAMP levels in the striatum and cerebral cortex. The increase in the basal cAMP levels was independent of dopamine receptor activity. In contrast, blocking the adenosine A2a receptor downregulated of the basal cAMP levels in the cocaine-exposed brain to physiological levels, suggesting the involvement of adenosine receptors in mediating cocaine’s effects on the embryonic brain. In support of this suggestion, we found that the cocaine exposure downregulated adenosine transporter function. We also found that dopamine D2- and adenosine A2a-receptors antagonize each other’s function in the embryonic brain in a manner consistent with their interactions in the mature brain. Thus, our data show that prenatal cocaine exposure produces direct effects on both the dopamine and adenosine systems. Furthermore, the dopamine D2 and adenosine A2a receptor interactions in the embryonic brain discovered in this study unveil a novel substrate for cocaine’s effects on the developing brain.
Introduction
Cocaine exposure during the fetal period can lead to lasting impairment of neurological function (Chasnoff et al., 1989a; Chasnoff et al., 1989b; Chiriboga et al., 1993; Chiriboga et al., 2009; Delaney-Black et al., 1996; Eyler et al., 2009; Kosofsky and Wilkins, 1998). Cocaine exerts its effects by blocking the activity of monoamine transporters. Central actions of cocaine are believed to be mainly due to blockade of the dopamine transporter, the resulting decrease in dopamine re-uptake at the synapse and increase in extracellular dopamine levels (Bhide, 2009; Meyer et al., 1993; Ritz et al., 1990; Ritz et al., 1987). Persistent increases in extracellular dopamine levels can impair pre- and pos-synaptic receptor activity by impairing receptor - G protein coupling mechanisms (Zhen et al., 2001). Since cocaine in the maternal circulation can penetrate the placental and fetal blood-brain barriers, and since dopamine, dopamine transporter and dopamine receptors are present in the fetal brain, cocaine from the maternal circulation can disrupt dopaminergic signaling mechanisms in the fetal brain (Akbari et al., 1992; Jones et al., 2000; Kosofsky et al., 1994; Levitt et al., 1997; Mayes, 1999; Meyer et al., 1993; Wang et al., 1995b).
Cocaine can interfere with dopaminergic signaling in the mature brain via direct actions on the dopaminergic system as well as indirectly via its effects on the adenosine receptor (Shen et al., 2008; Soria et al., 2006). Dopamine and adenosine receptors engage in antagonistic interactions that play significant roles in the regulation of motor and cognitive functions (Fuxe et al., 2007; Schwarzschild et al., 2006). Whether dopamine-adenosine interactions occur in the embryonic brain or whether cocaine can affect the adenosine system of the embryonic brain has remained unclear.
We report that administration of cocaine to pregnant mice from 8th to 14th day of pregnancy [embryonic day 8 (E8) to E14; equivalent to first trimester of human gestation] not only impairs dopamine receptor signaling but also adenosine receptor signaling in the brain. Cocaine’s effects on the dopaminergic system involve attenuation of D1-receptor signaling and enhancement of D2-receptor signaling. Cocaine’s effects on the adenosine system of the embryonic brain involve reduction in extracellular adenosine uptake and increase in extracellular adenosine levels. We also show that antagonistic interactions between dopamine D2- and adenosine A2a-receptors occur in the embryonic brain. Therefore, cocaine likely produces its effects on brain development by directly affecting the dopamine and adenosine signaling mechanisms and also by impairing dopamine-adenosine interactions.
Material and Methods
Animals
Timed-pregnant Swiss-Webster mice were obtained from Charles River Laboratories (Wilmington, MA). A transplacental cocaine exposure paradigm described previously (Kosofsky et al., 1994; Wilkins et al., 1998) was used to expose mouse embryos to cocaine twice daily from the morning of embryonic day 8 (E8; day of conception = E0) to the evening of E14, inclusive. At the beginning of the experiment, pregnant dams of comparable weight were assigned to cocaine (40 mg/kg/day) or saline control groups. The dams were handled for 5 min each morning, beginning on the 6th day of pregnancy (corresponding to E6). From E8 to E14, cocaine was injected subcutaneously to the cocaine group twice daily (7 AM and 7 PM; 20 mg/kg/injection; total daily dose = 40 mg/kg). Dams in saline control group received subcutaneous saline injections (same volume as the cocaine injection to the cocaine dams), twice daily, from E8 to E14 at the same time that the dams in the cocaine group received their cocaine injections. The dams were singly housed in a temperature and humidity controlled environment, on a 12 hour light/dark cycle with food and water available ad libitum. The dams were anesthetized on E15 (Ketamine, 50 mg/kg body weight and Xylazine, 10 mg/kg body weight, i.p.) at 9 AM and the embryos were removed by hysterectomy for further analyses. The age of each embryo was ascertained by examination of the external morphological features (Kaufman, 1992; Theiler, 1972). Embryos (or entire litters) that did not fulfill the criteria for E15 were discarded from this analysis. All of the experimental procedures were in full compliance with institutional guidelines and the NIH Guide for the Care and Use of Laboratory Animals.
Cyclic AMP assay
The embryonic brains were dissected in sterile, cold phosphate buffered saline using a dissection microscope. From each brain, the frontal cortex (approximately the rostral third of the cerebral wall) and the striatal primordium (ganglionic eminence and the striatal differentiating fields) were micro-dissected and collected. Samples of each brain region pooled from both right and left hemispheres were cut into 2mm2 segments and incubated for 10 min at 37 °C in minimal essential medium buffered with 20mM HEPES at pH 7.3 and containing 100µM ascorbic acid, 100µM pargyline and 0.5mM Rolipram (a phosphodiesterase inhibitor). The various drugs described in the Results section were added to the medium at concentrations stated in the Results section and the tissue suspension was incubated for 30 min at room temperature. The reaction was stopped by adding 10% TCA (final concentration). The cAMP was purified and assayed by methods described previously (de Mello et al., 1982; Gilman, 1970; Matsuzawa and Nirenberg, 1975). The cAMP levels were normalized to protein levels, assayed by the Lowry method.
Western Blot
Samples of the frontal cortex and striatum were collected from E15 brains, as described above and also from control adult (P60) brains. The samples were homogenized with RIPA buffer with a proteinase inhibitor cocktail. Protein concentration was estimated with the Bradford method. Samples were diluted in buffer composed of 10% glycerol (v/v), 1% h-mercaptoethanol, 3% SDS, and 62.5mM Tris base and boiled for 5 min. Approximately 30µg of protein from each sample was electrophoresed in 10% SDS–PAGE and transferred to nitrocellulose membranes (ECL-Hybond). Then, the membranes were washed with Tween 20 Tris-buffered saline (TTBS) and blocked for 1.5 h with 5% skim milk in TTBS. The membranes were incubated with the anti-D1 receptor antibody (1:1000 in TTBS; Sigma) overnight at 4°C, rinsed in TTBS and incubated with anti-rat peroxidase conjugated secondary antibody for 45 minutes at room temperature. Following three washes in TTBS (10 min each), the immunoreaction was detected with ECL kit (Amersham). Blots were reprobed with anti-actin antibody (1:1000 in TTBS, Santa Cruz) for 2 hr at room temperature, rinsed in TTBS and incubated with anti-mouse peroxidase conjugated secondary antibody for 45 min at room temperature. Following three TTBS washes (10 min each), the immunoreaction was detected with the ECL kit. Band intensities were analyzed by using Quantity One 4–6 software (Bio-Rad Laboratories Inc)
Immunohistochemistry
Fresh frozen embryonic brains were sectioned in the coronal plane in a cryostat at 15mµ thickness. The sections were blocked for 30 minutes with 1% BSA and incubated with the anti D1 receptor antibody (dilution 1:1000) overnight at 4°C. The sections were washed 3 times with phosphate buffered saline and incubated with a fluorescent anti-rat secondary antibody (1:400) for 40 minutes at room temperature. Following 3 more rinses with PBS, the sections were stained with propidium iodide solution to label cell nuclei and mounted with Gelmount.
[3H]-Dopamine uptake
Samples of the E15 frontal cortex and striatum were incubated for 1 h in 1 ml of MEM containing 1µCi [3H]-dopamine (2.4 × 10−6M) buffered at pH 7.4 with 20mM HEPES at 37°C or 8°C (to block dopamine active transport). Then, the medium was removed and the tissue washed six times with 2 ml cold PBS. This procedure was sufficient to wash out the radioactivity not taken up by the tissue. After the washes, 1ml water was added to the tissue to disrupt the cells. Following successive freeze-thaw cycles, cellular radioactivity was assayed using a scintillation counter. The protein content was assayed by the Lowry method.
[3H]-Adenosine uptake
The same procedure as above was used except that the tissue was incubated for 1 h in 1 ml of MEM containing 1µCi [3H]-adenosine (1.7 × 10−6M), instead of [3H]-dopamine.
We were concerned that any residual cocaine in the tissue samples may have an impact on the neurotransmitter uptake assays. The last cocaine administration to the pregnant dams had occurred 12 hr prior to the removal of the embryos for tissue dissection, minimizing the likelihood that high concentration of cocaine would remain in the tissue after dissection. To minimize the impact of any residual cocaine, the striatal and cortical tissue samples were washed in culture medium 3 times prior to exposure to [3H]-dopamine or [3H]-adenosine. The concentration of the any residual cocaine, if present at all, would not have been higher than that found in the intact brain just before the tissues were dissected.
Results
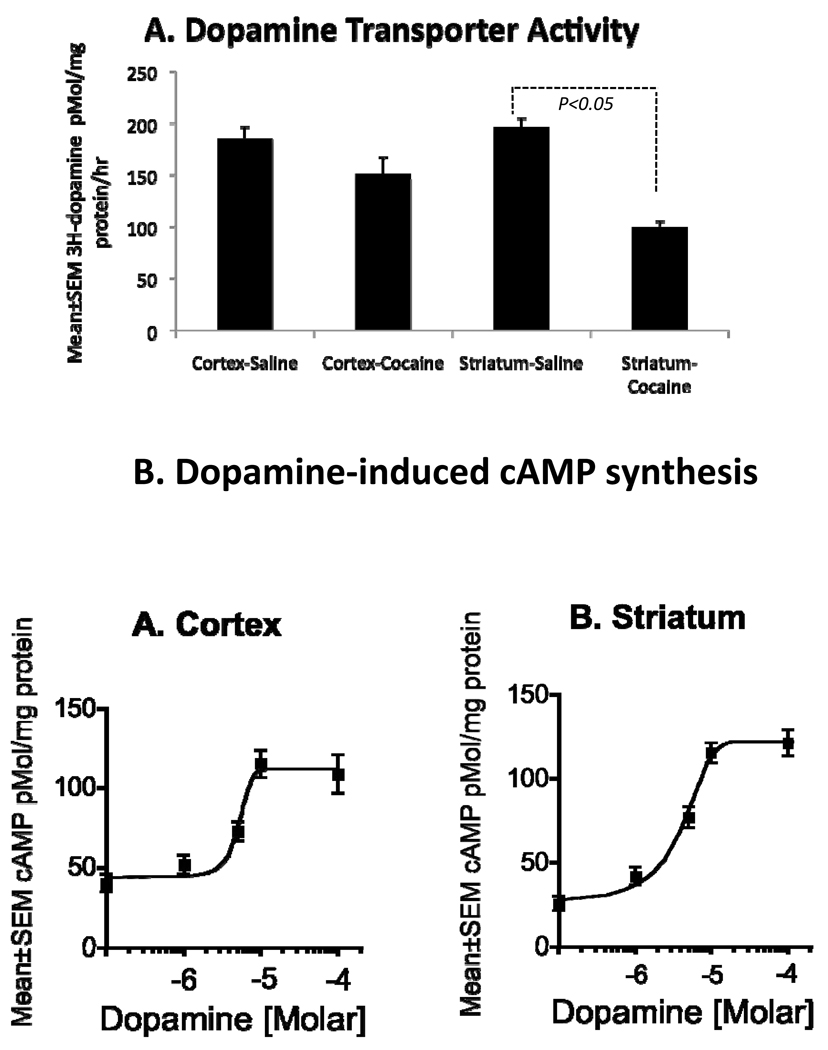
We used 3H-dopamine uptake to assay dopamine transporter function in the striatum and cerebral cortex from saline- or cocaine-exposed E15 mice. We found a significant decrease in 3H-dopamine uptake in the striatum (~50%; p<0.001) of the cocaine-exposed embryos compared to saline-exposed embryos (Fig. 1A) indicating attenuation of dopamine transporter function and increased extracellular dopamine. The cocaine exposure did not produce statistically significant changes in 3H-dopamine uptake in the cerebral cortex (~18% decrease in cocaine-exposed cortex; p = 0.06; Fig. 1A).
Figure 1.
A. Cocaine exposure significantly reduces uptake of exogenous 3H-dopamine in explants of E15 mouse striatum. Cocaine does not produce significant changes in this measurement in explants of E15 cerebral cortex. B. Dopamine produces dose-dependent increases in cAMP levels in explants of cerebral cortex and striatum obtained from E15 mice. Maximal cAMP levels are induced at a dopamine concentration of 10µM (10−5M) in both tissue samples.
To determine whether dopamine can alter intracellular cAMP levels at E15, we incubated homogenized samples of striatum or cerebral cortex from saline-exposed E15 mice with dopamine (1, 5, 10 or 100 µM) for 30 minutes in the presence of ascorbic acid (an anti-oxidant) and pargyline (a monoamine oxidase B inhibitor). Dopamine increased intracellular cAMP levels in a dose-dependent manner in the striatum as well as the cerebral cortex (Fig. 1B). The cAMP levels reached plateau at 10µm concentration in both the samples (Fig 1B).
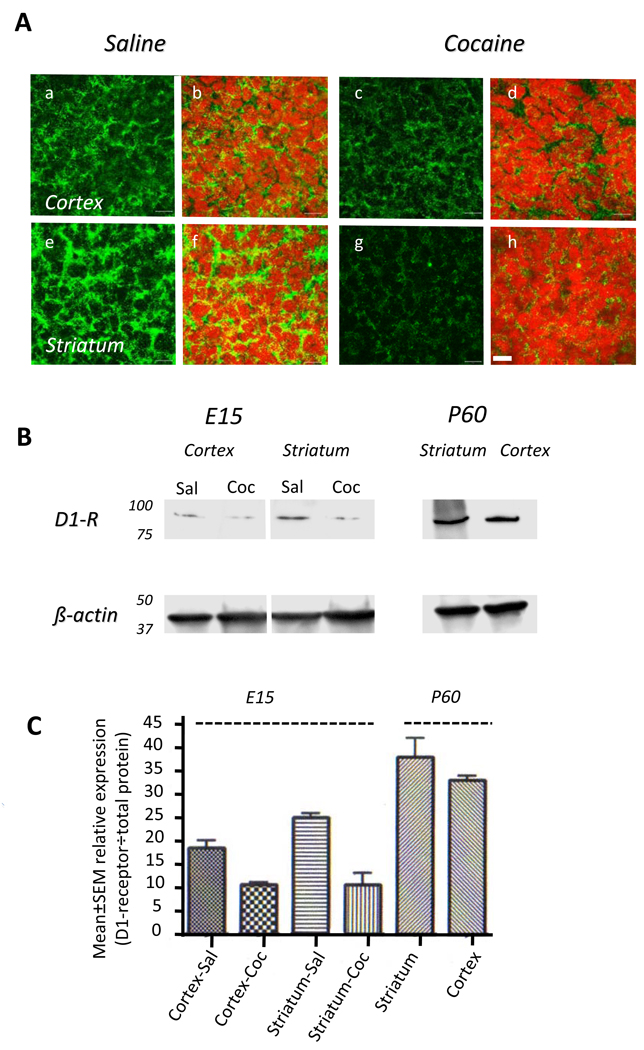
Next we examined D1-receptor expression in the striatum and cerebral cortex of saline- or cocaine-exposed E15 mice by immunohistochemistry. Punctate, membrane-bound and cytoplasmic labeling was found in the dorsal cerebral wall (future cerebral cortex) as well as in the striatum of the basal forebrain in both groups of mice (Fig. 2A). However, the intensity of the labeling was dramatically reduced in the cocaine-exposed embryonic brain in both the regions (Fig. 2A). Western blot confirmed that D1-receptor protein expression was significantly reduced in the cerebral cortex and striatum of the cocaine-exposed embryos (Fig. 2B, C). Since specificities of commercially available antibodies to the D1-receptor protein can vary, we used samples of the cortex and striatum from normal adult mice along with the embryonic brain samples in the western blot so that the data from the saline-exposed embryos and adults could be compared to our earlier data on developmental expression of the D1-receptor in striatal membrane preparations (Araki et al., 2007). In agreement with our earlier findings (Araki et al., 2007), the expression of the D1-receptor in the embryo was significantly lower compared to that in the adult, in both cocaine- or saline-exposed groups (Fig. 2B,C).
Figure 2.
Dopamine D1-receptor expression in the saline- or cocaine-exposed E15 mouse brain. A. Immunohistochemistry reveals D1-receptor labeling (green) in cryostat sections of the cerebral cortex (a–d) and striatum (e–h). Nuclei are labeled with propidium iodide (red). The D1-receptor immunoreactivity is localized to the cytoplasm and it is significantly reduced in the cocaine exposed cerebral cortex (c,d) and striatum (g,h) compared to the saline exposed cerebral cortex (a,b) and striatum (e,g). B. Western blot reveals reductions in D1-receptor protein in the cerebral cortex and striatal extracts from an E15 cocaine-exposed embryo (Coc) compared to age-matched saline-exposed embryo (Sal). To verify antibody specificity, samples of striatum and cortex from postnatal day 60 (P60) mice were analyzed in parallel experiments. Protein loading was standardized by using β-actin. C. Quantitative analysis of band intensities shows reduced intensities in the cocaine-exposed samples compared to the saline-exposed control samples. The significantly higher expression in the P60 samples compared to the E15 samples is also evident.
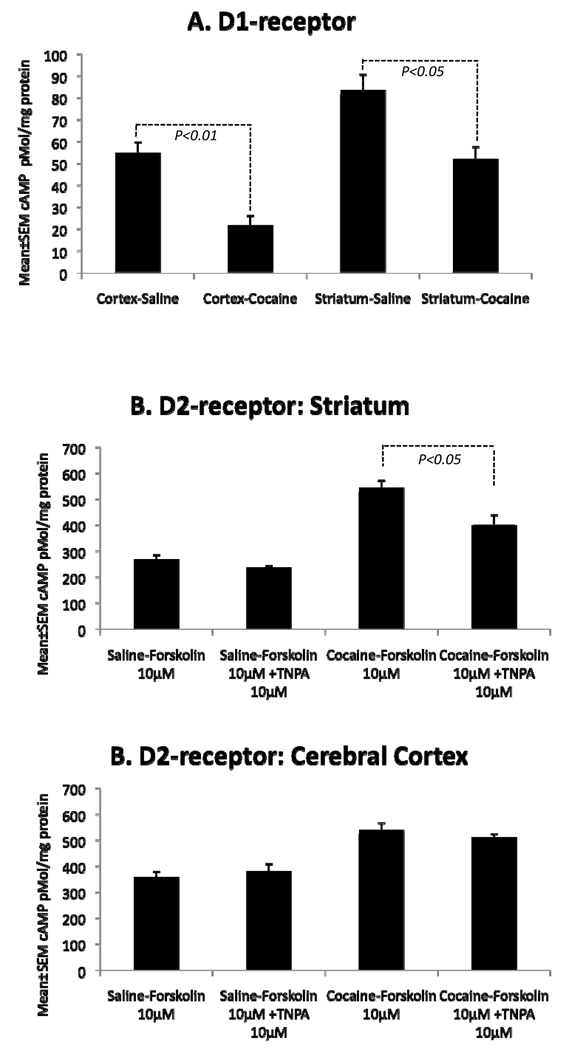
To assay D1-receptor function directly, we exposed homogenized samples of the striatum or cerebral cortex to the selective D1-receptor agonist SKF81297 and measured intracellular cAMP levels. Although SKF81297 increased cAMP levels in tissue samples from both saline- and cocaine-exposed embryos, the net increase in cAMP levels (stimulated – basal) was significantly smaller in the cocaine-exposed striatum (~38%; p=0.01) and cortex (~60%; p=0.003) compared to the same regions in the saline-exposed brains (Fig. 3). These data show that dopamine D1-receptor function is significantly attenuated in the cocaine-exposed embryonic striatum and cerebral cortex.
Figure 3.
Dopamine receptor activity was examined by using cAMP synthesis assay in samples of the cerebral cortex and striatum from saline – or cocaine-exposed E15 embryos. Net change in cAMP levels is illustrated [net increase = (basal – stimulated)]. A. D1-receptor agonist SKF 81297 induced significantly smaller net increases in cAMP levels in both the brain regions of the cocaine-exposed embryo compared to the saline-exposed embryo. B. The D2-receptor agonist TNPA did not produce significant changes in forskolin-stimulated cAMP levels in the striatum of saline-exposed E15 embryos but produced significant decreases in this measurement in the striatum of the cocaine-exposed embryos. C. TNPA did not produce significant changes in forskolin-induced cAMP levels in the cerebral cortex of saline- or cocaine-exposed E15 embryos.
Next, we examined the effects of cocaine exposure on D2 receptor expression and function. The western blot or immunohistochemistry did not yield conclusive data in then embryonic brain samples (e.g. the negative controls showed staining that resembled that in the positive controls). Therefore, we abandoned analysis of D2 receptor expression and focused on function by cAMP synthesis assay. D2 receptor is coupled to Gi/o proteins, which inhibit adenylyl cyclase and decrease intracellular cAMP levels. To assay D2 receptor function, we initially exposed homogenates of the striatum or cerebral cortex from saline- or cocaine-exposed embryos to forskolin (10µM), which increased the cAMP levels in all the samples, as expected (Fig. 3B,C). In separate experiments, we exposed the samples to forskolin plus the D2 receptor agonist 11-trihydroxy-N-propyl-noraporphine hydrobromide (TNPA; 10 or 100 µM). TNPA did not produce significant changes in the forskolin-induced cAMP levels in the striatum or cortex of saline-exposed embryos, suggesting that D2-receptor activity in these brain regions was extremely low (Fig. 3B,C). However, TNPA (100 µM) induced significant reduction in forskolin-induced cAMP levels in the striatum of cocaine-exposed embryos (Fig. 3B), suggesting increased D2-receptor activity in the cocaine-exposed striatum. TNPA did not induce significant changes in this measurement in the cerebral cortical samples from the cocaine-exposed embryos (Fig. 3C. These data show that the cocaine exposure produces significant increases in D2 receptor activity in the striatum but does not affect D2-receptor activity in the cerebral cortex.
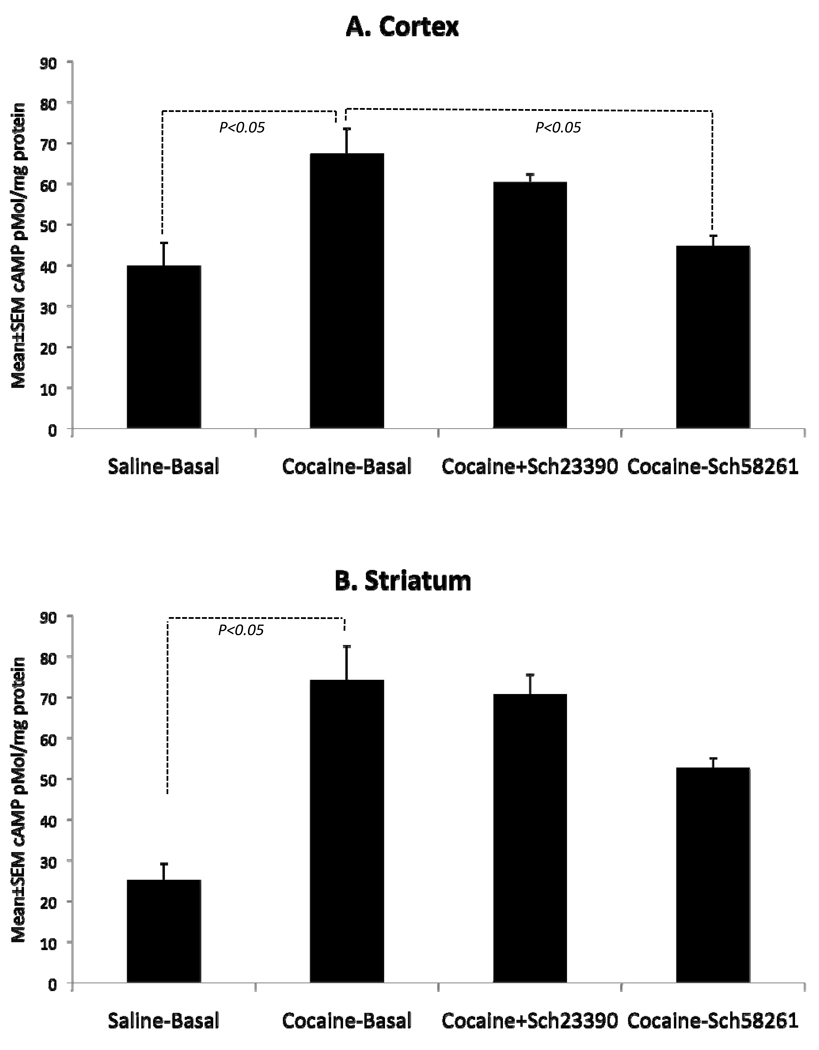
In the course of these experiments, we discovered that basal (un-stimulated) cAMP levels were significantly higher in the cortex and striatum of cocaine-exposed embryos compared to the saline-exposed embryos (Fig. 4). This seemed paradoxical to us because decreased D1-receptor activity and increased D2-receptor activity in the cocaine-exposed brains can be expected to decrease basal cAMP levels rather than increasing it. Since activation of the D1-receptor is one mechanism by which cAMP levels can increase, we examined if blocking the D1-receptor using Schering 23390, a selective D1-receptor antagonist, could decrease the basal cAMP levels. We found that Schering 23390 did not affect basal cAMP levels in either brain region of the cocaine-exposed embryo (Fig. 4). Thus, the increased basal cAMP levels could not be explained based on dopaminergic signaling mechanisms alone.
Figure 4.
Basal cAMP level and its modulation by dopamine D1- and adenosine A2a receptor antagonists in samples of the cerebral cortex (A) and striatum of E15 saline- or cocaine-exposed embryos. Basal (unstimulated) cAMP levels are significantly elevated in the cerebral cortex and striatum of cocaine-exposed embryos compared to the saline-exposed controls. A2a receptor antagonist Schering 58261 decreases the basal levels in the cerebral cortex of the cocaine-exposed embryos such that the levels are not statistically different from the basal levels seen in the saline-exposed controls. The D1-receptor antagonist Schering 23390 does not affect the basal levels in the cortex. Neither Schering58261 nor Schering 23390 has statistically significant effects on basal cAMP levels in the cocaine-exposed striatum.
The striatum is enriched in adenosine receptors. Activation of the adenosine A2a receptors, which are positively coupled to adenylyl cyclase via Gs protein, increases intracellular cAMP levels. A2a receptor mRNA is expressed in the rodent striatum early in embryonic period and striatal neurons express A2a mRNA soon after becoming postmitotic (Weaver, 1993). A2a receptors mediate structural and behavioral changes induced by exposure to psychostimulants including cocaine (Chen et al., 2000; Marcellino et al., 2007). We examined whether A2a receptors could mediate the increase in basal cAMP levels in the cocaine exposed embryonic brain.
We found that A2a receptor antagonist Schering 58261 produced decreases in the basal cAMP levels in the cocaine-exposed E15 cerebral cortex and striatum (Fig. 4). The decrease was statistically significant in the cerebral cortex but not the striatum. Schering 58261 did not produce significant changes in basal cAMP levels in saline-exposed E15 cerebral cortex or striatum (Mean±SEM cAMP levels in pMol/mg protein: Cortex basal = 39.83±5.72; Cortex Schering 58261 = 35.66±1.73; striatum basal striatum basal = 25.17±4.01; striatum Schering 58261 = 30.33±1.73; t-tests, p>0.05).
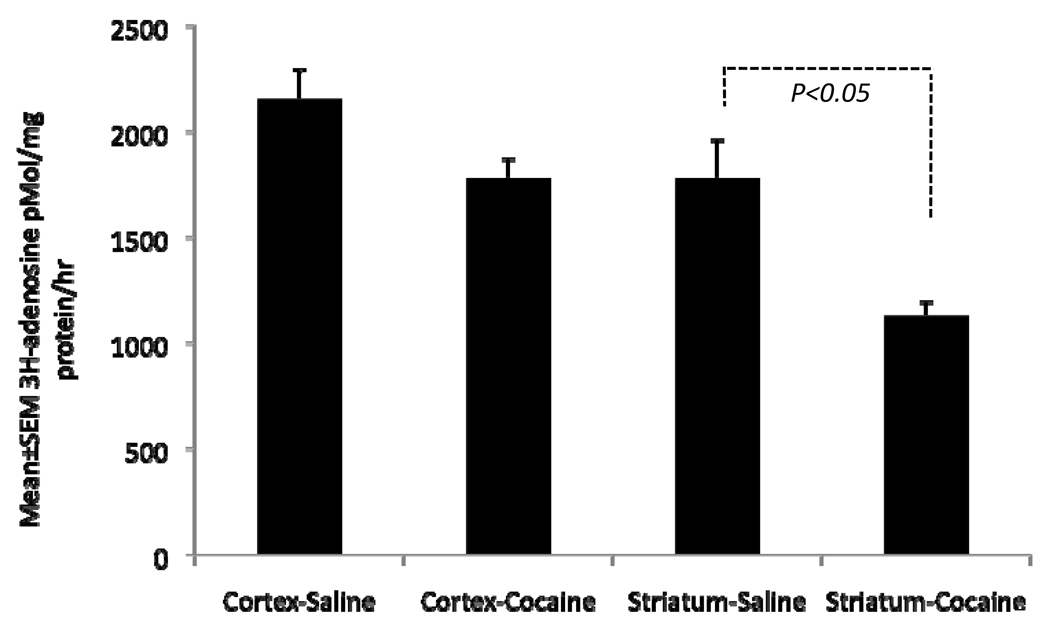
Since adenosine receptor signaling appeared to be altered by the cocaine exposure, next we examined whether adenosine uptake is altered in the cocaine exposed embryonic brain by assaying 3H-adenosine uptake. We found that adenosine uptake was reduced significantly in the cocaine-exposed striatum (by ~ 37%; p<0.05; Fig. 5). Although adenosine uptake was also reduced in the cerebral cortex of the cocaine-exposed embryo, the reduction was not statistically significant (~17% reduction, p=0.05; Fig. 7). These data show that cocaine exposure interferes with adenosine uptake, presumably by blocking the activity of the adenosine transporter.
Figure 5.
Exogenous 3H-adenosine uptake in the cerebral cortex and striatum of E15 saline- or cocaine-exposed E15 embryos. The uptake was significantly reduced in the striatum but not the cortex of the cocaine-exposed embryo.
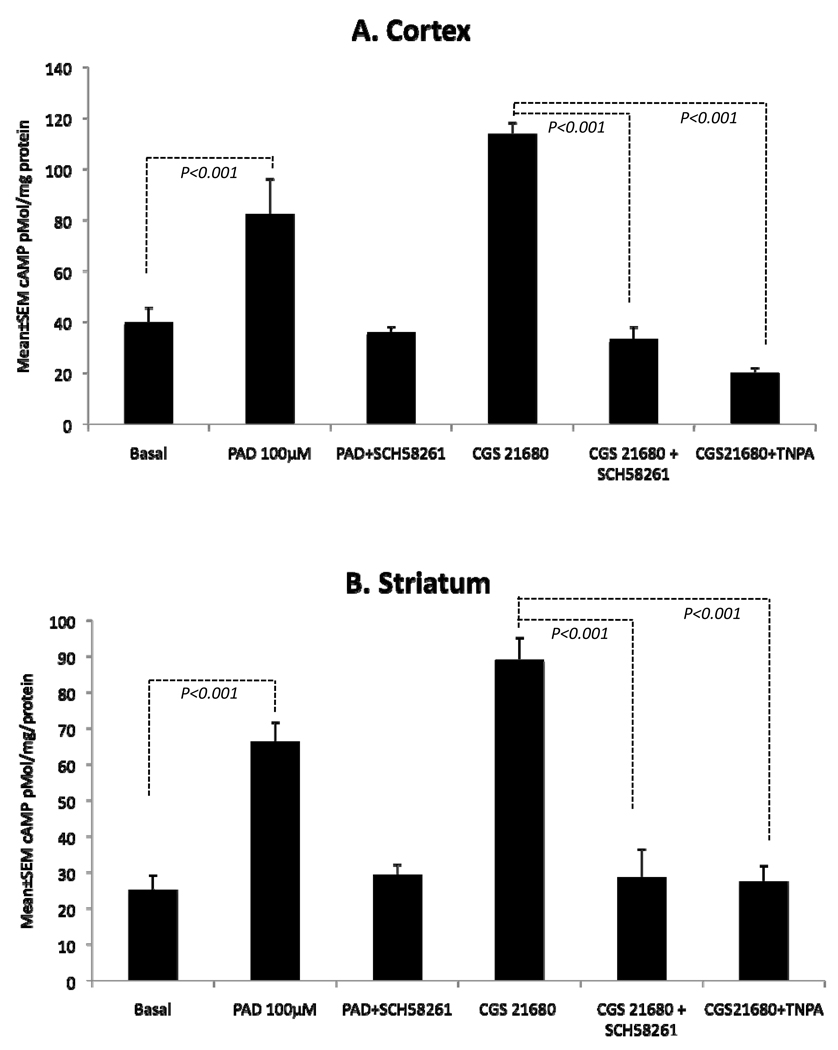
Dopamine D2 receptor and adenosine A2a receptor have antagonistic functions in the mature striatum (Ferre et al., 2007; Fuxe et al., 2007; Schwarzschild et al., 2006). We examined if similar antagonism existed in the embryonic striatum in terms of effects on cAMP synthesis. Initially, we established that periodate-oxidized adenosine (PAD; 100µM) increased cAMP synthesis in the E15 striatum (~163%; p<0.001) and cerebral cortex (~106%; p<0.001; Fig. 6). The selective A2a antagonist Schering 58261 fully blocked the PAD-induced increase in cAMP (Fig. 6) suggesting involvement of A2a receptor in the cAMP synthesis. A selective A2a agonist CGS21680 also increased cAMP levels in the E15 striatum (~253%; p<0.001) and cerebral cortex (~185%; p<0.001) (Fig. 6). The selective A2a antagonist Schering 58261 fully blocked the CGS21680-induced increase in cAMP (Fig. 6) further confirming the involvement of the A2a receptor.
Figure 6.
Dopamine D2- and adenosine A2a-receptor interactions in the cerebral cortex (A) and striatum (B) of E15 saline-exposed (control) embryos. Periodate-oxidized adenosine (PAD) significantly increased cAMP levels in both the cerebral cortex and the striatum. PAD-induced increase in cAMP was blocked in the presence of the A2a antagonist Schering58261 in both the regions. The selective A2a agonist CGS21680 produced significant increases in cAMP levels and the increase was blocked by the A2a antagonist Schering21680 as well as by the D2-receptor agonist TNPA in both the brain regions.
Finally, we examined if dopamine D2 and adenosine A2a receptors produced antagonistic effects in the embryonic brain as in the mature brain. When we incubated samples of the E15 striatum and cerebral cortex with the selective A2a agonist CGS21680 plus the D2 receptor agonist TNPA, the CGS21680-induced increase in cAMP levels was blocked in both tissue samples (Fig. 6). Thus, activation of the D2 receptor blocked the action of the A2a receptor with regard to cAMP synthesis. The blockade of CGS21680-induced cAMP synthesis produced by TNPA was comparable to the blockade of this parameter by the selective A2a antagonist Schering 58261 (Fig. 6), suggesting antagonistic actions between the D2 and A2a receptors in the fetal brain.
Discussion
Our data show that prenatal cocaine exposure influences dopamine and adenosine signaling in the striatum and cerebral cortex. Moreover, the data show that dopamine D2 and adenosine A2a receptors antagonize each other’s actions in the embryonic brain. Therefore, cocaine likely exerts its effects on the developing brain by directly interfering with dopamine and adenosine receptor signaling as well as via impairment of dopamine D2 and adenosine A2a receptor interactions.
A number of earlier studies showed that cocaine blocks dopamine transporter function in the embryonic brain and interferes with dopamine D1-receptor signaling by uncoupling the receptor from its G protein partner (Friedman et al., 1996; Simansky and Kachelries, 1996; Unterwald et al., 2003; Wang et al., 1995a). The attenuation of the dopamine D1-receptor signaling has been demonstrated using receptor agonist-induced cAMP synthesis assays (Unterwald et al., 1993). Our data confirm these earlier findings by showing reduced uptake of exogenous dopamine in the cocaine-exposed E15 brain, which reflects reduced dopamine transporter function (Fig. 1), and reduced cAMP synthesis following activation of the dopamine D1-receptor (Fig. 3), which indicates attenuation of D1-receptor function. We also show that the cocaine exposure decreases expression of the D1-receptor protein in the embryonic brain (Fig. 2). Thus, cocaine’s effects on the D1-receptor in the embryonic brain may occur both at the level of protein expression and receptor G-protein coupling.
Both D1- and D2-receptors are expressed in the embryonic brain (Araki et al., 2007; Ohtani et al., 2003) and both may be vulnerable to cocaine’s effects. However, the effects of cocaine exposure on the dopamine D2-receptor in the embryonic brain had not been well characterized. Our data show that D2 receptor activity is upregulated in the E15 striatum following the cocaine exposure (Fig. 3B). Thus, in the embryonic striatum of the cocaine-exposed mice the overall dopaminergic tone is in favor of the D2-receptor because not only is its activity upregulated but that of the D1-receptor is significantly downregulated. Since, dopamine D1- and D2-receptors produce opposite effects on developmental events such as neurogenesis (Ohtani et al., 2003; Popolo et al., 2004) and neuronal migration (Crandall et al., 2007) and since dopamine’s overall effects on neurogenesis and neuronal migration in the embryonic striatum are D1-lke effects (Crandall et al., 2007; Ohtani et al., 2003; Popolo et al., 2004), cocaine exposure likely reverses dopamine’s physiological effects in favor of the D2-receptor. Further support for cocaine-induced upregulation of D2-like effects in the embryonic brain is derived from our studies of GABA neuron migration from the striatum to the cerebral cortex in the embryonic brain. We found that cocaine produced significant decreases in the migration of GABA neurons in the same manner as activation of the D2-receptor in control embryos or knockout of the D1-receptor in a mouse model (Crandall et al., 2004; Crandall et al., 2007).
An unexpected finding in the present study was that cocaine exposure led to significant increases in basal (unstimulated) cAMP levels in the embryonic striatum and cortex (Fig. 4). It was unexpected because the D1-receptor, which is positively coupled to adenylyl cyclase and whose activation leads to increases in cAMP (Monsma et al., 1990; Robinson and Caron, 1996; Schinelli et al., 1994) is downregulated in the cocaine-exposed striatum whereas D2-receptor, which is negatively coupled to adenylyl cyclase and whose activity decreases cAMP (Monsma et al., 1990; Robinson and Caron, 1996; Schinelli et al., 1994) is upregulated. Neither complete blockage of the D1-receptor nor further activation of the D2-receptor reduced the basal cAMP to physiological levels seen in the saline controls. However, blocking the adenosine A2a receptors decreased the basal cAMP levels in the cocaine-exposed cerebral cortex to the levels seen in the control cortex. We did not see a similar effect in the saline-exposed cerebral cortex. This led us to consider whether cocaine can modulate adenosine receptor signaling in the embryonic brain.
Indeed, cocaine exposure reduced adenosine uptake in the embryonic striatum (Fig. 5) reflecting attenuation of adenosine transporter function and suggesting direct effects of cocaine on the adenosine system of the embryonic brain. In the mature striatum adenosine and dopamine receptors heterodimerize and modulate each other’s function by antagonistic mechanisms (Ferre et al., 2007; Fuxe et al., 2007; Fuxe et al., 2008). Adenosine receptors are expressed in the embryonic rodent brain early in development (Shearman and Weaver, 2001; Weaver, 1993). However, whether the D2 and A2a receptors interact with each other in the embryonic brain was not known. Our data show that dopamine D2- and adenosine A2a receptors antagonize each other’s effects on cAMP synthesis (Fig. 6) suggesting similar interactions between the two receptors in the embryonic and mature striatum. Our finding that D2-A2a interactions occur in the embryonic striatum unveils dopamine-adenosine receptor interactions as a novel substrate for cocaine’s effects on the developing brain.
We found that blocking the A2a receptor led to significant reductions in basal cAMP levels in the cocaine-exposed cortex but not the striatum. One possibility for the lack of significant cAMP reductions in the striatum is that a higher concentration of the A2a antagonist Sch58261 than that used here may be needed to completely block A2a receptors because striatal A2a receptor expression is higher than that in the cortex. An earlier study (Unterwald et al., 2003) found significant reductions in basal cAMP levels in the striatum of E18 mice exposed to cocaine from E10 to E17. However, in that study theophylline, an antagonist of adenosine receptors (Bishnoi et al., 2007) was used to block phopshodiesterase activity in the cAMP assays (Unterwald et al., 2003). Therefore, it is likely that in that study also basal cAMP levels in the cocaine-exposed brain were increased as a result of cocaine’s effects on the adenosine receptor and/or dopamine-adenosine interactions.
In summary, our data show that prenatal cocaine exposure produces direct effects on both the dopamine and adenosine systems. It attenuates dopamine D1-receptor function and enhances the D2-receptor function thereby tilting the physiological D1-D2 balance in favor of the D2-receptor. Dopamine D2 and adenosine A2a receptors produce antagonistic effects on each other in the embryonic brain. The D2-A2a interactions offer an additional substrate for cocaine’s actions on the developing brain.
Acknowledgements
Supported by USPHS grants RO1DA020796 and P30NS045776 to PGB and a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil to RCCK. We gratefully acknowledge advice and assistance from our colleagues Deirdre McCarthy, Jia-Qian Ren and John Sims in the course of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Akbari HM, Kramer HK, Whitaker-Azmitia PM, Spear LP, Azmitia EC. Prenatal cocaine exposure disrupts the development of the serotonergic system. Brain Res. 1992;572:57–63. doi: 10.1016/0006-8993(92)90450-n. [DOI] [PubMed] [Google Scholar]
- Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: A review of current concepts Seminars Cell. Dev Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Bishnoi M, Chopra K, Kulkarni SK. Theophylline, adenosine receptor antagonist prevents behavioral, biochemical and neurochemical changes associated with an animal model of tardive dyskinesia. Pharmacol Rep. 2007;59:181–191. [PubMed] [Google Scholar]
- Chasnoff IJ, Griffith DR, MacGregor S, Dirkes K, Burns KA. Temporal patterns of cocaine use in pregnancy. Perinatal outcome. Journal of American Medical Association. 1989a;261:1741–1744. [PubMed] [Google Scholar]
- Chasnoff IJ, Lewis DE, Griffith DR, Willey S. Cocaine and pregnancy: clinical and toxicological implications for the neonate. Clin Chem. 1989b;35:1276–1278. [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Bateman DA, Brust JC, Hauser WA. Neurologic findings in neonates with intrauterine cocaine exposure. Pediatr Neurol. 1993;9:115–119. doi: 10.1016/0887-8994(93)90045-e. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Starr D, Kuhn L, Wasserman GA. Prenatal cocaine exposure and prolonged focus attention. Poor infant information processing ability or precocious maturation of attentional systems? Dev Neurosci. 2009;31:149–158. doi: 10.1159/000207502. [DOI] [PubMed] [Google Scholar]
- Crandall JE, Hackett HE, Tobet SA, Kosofsky BE, Bhide PG. Cocaine exposure decreases GABA neuron migration from the ganglionic eminence to the cerebral cortex in embryonic mice. Cerebral Cortex. 2004;14:665–675. doi: 10.1093/cercor/bhh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J. Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mello MC, Ventura AL, Paes de Carvalho R, Klein WL, de Mello FG. Regulation of dopamine- and adenosine-dependent adenylate cyclase systems of chicken embryo retina cells in culture. Proc Natl Acad Sci U S A. 1982;79:5708–5712. doi: 10.1073/pnas.79.18.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ostrea E, Jr, Romero A, Baker D, Tagle MT, Nordstrom-Klee B, Silvestre MA, Angelilli ML, Hack C, Long J. Prenatal cocaine and neonatal outcome: evaluation of dose-response relationship. Pediatrics. 1996;98:735–740. [PubMed] [Google Scholar]
- Eyler FD, Warner TD, Behnke M, Hou W, Wobie K, Garvan CW. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31:121–136. doi: 10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends in Neurosciences. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Friedman E, Yadin E, Wang HY. Effect of prenatal cocaine on dopamine receptor-G protein coupling in mesocortical regions of the rabbit brain. Neuroscience. 1996;70:739–747. doi: 10.1016/s0306-4522(96)83011-9. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Rivera A, Diaz-Cabiale Z, Filip M, Gago B, Roberts DC, Langel U, Genedani S, Ferraro L, de la Calle A, Narvaez J, Tanganelli S, Woods A, Agnati LF. Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev. 2008;58:415–452. doi: 10.1016/j.brainresrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Gilman AG. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970;67:305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LB, Stanwood GD, Reinoso BS, Washington RA, Wang HY, Friedman E, Levitt P. In utero cocaine-induced dysfunction of dopamine D1 receptor signaling and abnormal differentiation of cerebral cortical neurons. J Neurosci. 2000;20:4606–4614. doi: 10.1523/JNEUROSCI.20-12-04606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. New York: Academic Press; 1992. [Google Scholar]
- Kosofsky BE, Wilkins AS. A mouse model of transplacental cocaine exposure. Clinical implications for exposed infants and children. Ann.NY Acad.Sci. 1998;846:248–261. [PubMed] [Google Scholar]
- Kosofsky BE, Wilkins AS, Gressens P, Evrard P. Transplacental cocaine exposure: A mouse model demonstrating neuroanatomic and behavioral abnormalities. J Child Neurol. 1994;9:234–241. doi: 10.1177/088307389400900303. [DOI] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Roberts DC, Navarro G, Filip M, Agnati L, Lluis C, Franco R, Fuxe K. Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Res. 2007;1143:208–220. doi: 10.1016/j.brainres.2007.01.079. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H, Nirenberg M. Receptor-mediated shifts in cGMP and cAMP levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975;72:3472–3476. doi: 10.1073/pnas.72.9.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shearman LP, Collins LM, Maguire RL. Cocaine binding sites in fetal rat brain: implications for prenatal cocaine action. Psychopharmacology. 1993;112:445–451. doi: 10.1007/BF02244892. [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc.Natl.Acad.Sci.USA. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG. Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci. 2003;23:2840–2850. doi: 10.1523/JNEUROSCI.23-07-02840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Chimeric D2/D3 dopamine receptors efficiently inhibit adenylyl cyclase in HEk 293 cells. J.Neurochem. 1996;67:212–219. doi: 10.1046/j.1471-4159.1996.67010212.x. [DOI] [PubMed] [Google Scholar]
- Schinelli S, Paolillo M, Corona Gl. Opposing actions of D1- and D2-dopamine receptors on arachidonic acid release and cyclic AMP production in striatal neurons. J.Neurochem. 1994;62:944–949. doi: 10.1046/j.1471-4159.1994.62030944.x. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen J-F, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends in Neurosciences. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Weaver DR. Distinct pharmacological mechanisms leading to c-fos gene expression in the fetal suprachiasmatic nucleus. J Biol Rhythms. 2001;16:531–540. doi: 10.1177/074873001129002222. [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simansky K, Kachelries WJ. Prenatal exposure to cocaine selectively disrupts motor responses to D-amphetamine in young and mature rabbits. Neuropharmacology. 1996;35:71–78. doi: 10.1016/0028-3908(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Theiler K. The House Mouse. Development and Normal Stages from Fertilization to 4 Weeks of Age. Berlin: Springer; 1972. [Google Scholar]
- Unterwald EM, Cox BM, Kreek MJ, Cote TE, Izenwasser S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse. 1993;15:33–38. doi: 10.1002/syn.890150104. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Ivkovic S, Cuntapay M, Stroppolo A, Guinea B, Ehrlich ME. Prenatal exposure to cocaine decreases adenylyl cyclase activity in embryonic mouse striatum. Brain Res Dev Brain Res. 2003;147:67–75. doi: 10.1016/s0165-3806(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Wang HY, Runyan S, Yadin E, Friedman E. Prenatal exposure to cocaine selectively reduces D1 dopamine receptor-mediated activation of striatal Gs proteins. J Pharmacol Exp Ther. 1995a;273:492–498. [PubMed] [Google Scholar]
- Wang X-H, Levitt P, Grayson DR, Murphy EH. Intrauterine cocaine exposure of rabbits: Persistent elevation of GABA-immunoreactive neurons in anterior cingulate cortex but not visual cortex. Brain Res. 1995b;689:32–46. doi: 10.1016/0006-8993(95)00528-x. [DOI] [PubMed] [Google Scholar]
- Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res. 1993;20:313–327. doi: 10.1016/0169-328x(93)90058-w. [DOI] [PubMed] [Google Scholar]
- Wilkins AS, Genova LM, Posten W, Kosofsky BE. Transplacental cocaine exposure 1: A rodent model. Neurotoxicol Teratol. 1998;20:215–226. doi: 10.1016/s0892-0362(97)00125-6. [DOI] [PubMed] [Google Scholar]
- Zhen X, Torres C, Wang HY, Friedman E. Prenatal exposure to cocaine disrupts D1A dopamine receptor function via selective inhibition of protein phosphatase 1 pathway in rabbit frontal cortex. J Neurosci. 2001;21:9160–9167. doi: 10.1523/JNEUROSCI.21-23-09160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]