Abstract
Alcoholism is a complex disorder that represents an important contributor to health problems worldwide and that is difficult to encompass with a single preclinical model. Additionally, alcohol (ethanol) influences the function of many neurotransmitter systems, with the interaction at γ-aminobutyric acidA (GABAA) receptors being integral for ethanol's reinforcing and several withdrawal-related effects. Given that some steroid derivatives exert rapid membrane actions as potent positive modulators of GABAA receptors and exhibit a similar pharmacological profile to that of ethanol, studies in the laboratory manipulated GABAergic steroid levels and determined the impact on ethanol's rewarding- and withdrawal-related effects. Manipulations focused on the progesterone metabolite allopregnanolone (ALLO), since it is the most potent endogenous GABAergic steroid identified. The underlying hypothesis is that fluctuations in GABAergic steroid levels (and the resultant change in GABAergic inhibitory tone) alter sensitivity to ethanol, leading to changes in the positive motivational or withdrawal-related effects of ethanol. This review describes results that emphasize sex differences in the effects of ALLO and the manipulation of its biosynthesis on alcohol reward- versus withdrawal-related behaviors, with females being less sensitive to the modulatory effects of ALLO on ethanol-drinking behaviors but more sensitive to some steroid manipulations on withdrawal-related behaviors. These findings imply the existence of sex differences in the sensitivity of GABAA receptors to GABAergic steroids within circuits relevant to alcohol reward versus withdrawal. Thus, sex differences in the modulation of GABAergic neurosteroids may be an important consideration in understanding and developing therapeutic interventions in alcoholics.
Keywords: neurosteroid, ethanol, C57BL/6 mice, self-administration, lickometer, reinstatement, finasteride, gonadectomy, adrenalectomy, handling-induced convulsions
Introduction
The present review describes results that were presented at a recent symposium entitled, “Sex-Specific Therapeutic Strategies Based on Neuroactive Steroids: In Search for Innovative Tools for Neuroprotection”. Certainly, the importance of sex and the impact of gonadal steroids on brain function and behavior are being increasingly recognized (e.g., Young & Becker, 2009). Given the sex differences in human disease, it is desirable to consider sex differences in preclinical models. Sex differences in ethanol intake are well-documented in rodents, with intake in females higher than that in males (e.g., Belknap et al., 1993; Chester et al., 2008; Finn et al., 2004b; Lancaster et al., 1996; Yoneyama et al., 2008). Sex-dependent sensitivity to other drugs also occurs, with females generally being more sensitive to the rewarding effects of drug than males (see review by Carroll et al., 2004). For example, female rats acquire intravenous self-administration of cocaine, methamphetamine and nicotine faster than males. Female rats also self-administer more cocaine with longer duration of “binges” and greater loss of circadian control over drug intake in an escalation model, and exhibit greater extinction responding on the drug associated lever after drug removal and greater reinstatement after drug priming than males (see Carroll et al., 2004 and references therein). Thus, research that incorporates male and female subjects can identify sex-specific mechanisms as well as general mechanisms underlying aspects of addiction.
Steroid hormones exert genomic effects via binding to their receptors and altering the transcription of genes, but rapid membrane effects of steroids provide another level of diversity in the mechanisms by which steroids can influence brain function and behavior (e.g., Brann et al., 1995; Lösel & Wehling, 2003; McEwen, 1991; Rupprecht & Holsboer, 1999). For instance, a vast variety of brain functions are affected by sex steroids (e.g., testosterone, Hajszan et al., 2008; estrogen, Tokuyama et al., 2008; progesterone, Quesada & Micevych, 2008) and adrenal steroids (e.g., corticosterone, Uchoa et al., 2009; aldosterone, Shen et al., 2009). Genomic or rapid membrane effects have been observed via steroid interaction at androgen receptors (e.g., Claessens et al., 2008), glucocorticoid and mineralocorticoid receptors (e.g., Kawata et al., 2008), liver X receptors (e.g., Matsumoto et al., 2009), estrogen receptors (e.g., Kelly & Rønnekleiv, 2008) progesterone receptors (e.g., Brinton et al., 2008) and vitamin D receptors (e.g., Garcion et al., 2002). Besides membrane effects of steroid hormones at their “classic” receptor, some steroids and their derivatives have rapid membrane actions via an interaction with ligand-gated ion channels (e.g., Belelli & Lambert, 2005; Finn et al., 2004a; Paul & Purdy, 1992; Rupprecht & Holsboer, 1999). This evidence for rapid non-genomic effects of steroids gave rise to the term “neuroactive steroids.”
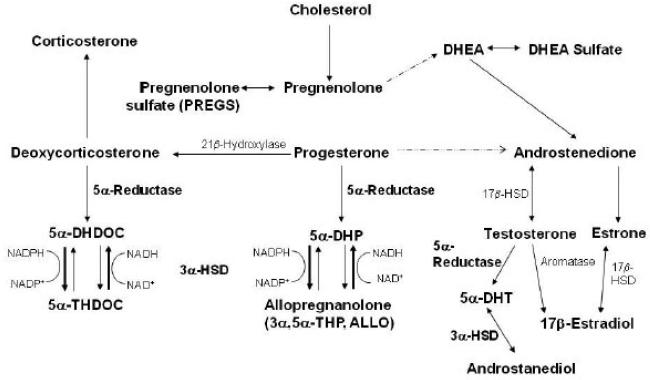
The term “neurosteroid” was introduced by Baulieu (1981) to designate a steroid hormone derivative found in brain at concentrations that were independent of its plasma concentration, and the story of their discovery and function has been reviewed recently (Baulieu et al., 2001). Given that the enzymes identified in classic steroidogenic tissues are found in the brain (Mellon & Vaudry, 2001), brain “neuroactive steroid” levels most likely reflect a combination of neuroactive compounds produced de novo as well as peripherally derived steroids that are metabolized to neuroactive compounds in the brain. Thus, it has been suggested that the term “neurosteroid” be broadened to include both sources of “neuroactive steroids” (Mellon & Griffin, 2002). Consistent with this idea, we will use the term neurosteroids to refer to both sources of neuroactive steroids in this review and will focus on the effects of neurosteroids that potentiate the action of γ-aminobutyric acid (GABA) at GABAA receptors (Figure 1).
Figure 1. The biosynthesis of select steroids with genomic and non-genomic effects.
Depicted is the biosynthetic pathway for ALLO, 5α-THDOC, and androstanediol, which are potent positive modulators of GABAA receptors and are formed from the two step reduction of the parent steroids, progesterone, deoxycorticosterone, and testosterone, respectively. The rate-limiting enzyme in GABAergic steroid biosynthesis is 5a-reductase. Also depicted are the sulfated derivatives of pregnenolone and DHEA, which are negative modulators of GABAA receptors, and corticosterone, which has been shown to have excitatory effects. The broken lines indicate that 17-OH pregnenolone and 17-OH progesterone are omitted from the figure in the formation of DHEA and androstenedione, respectively.
Abbreviations: DHEA, dehydroepiandrosterone; DHDOC, dihydrodeoxycorticosterone; THDOC, tetrahydrodeoxycorticosterone; DHP, dihydroprogesterone; DHT, dihydrotestosterone; 3α-HSD, 3α-hydroxysteroid dehydrogenase. (Adapted from Finn et al., 2006a)
The progesterone derivative allopregnanolone (ALLO) is the most potent endogenous modulator of GABAA receptors identified (reviewed in Belelli & Lambert, 2005; Belelli et al., 1990; Finn et al., 2004a; Purdy et al., 1990; Rupprecht & Holsboer, 1999; Veleiro & Burton, 2009). Endogenous ALLO levels fluctuate within a range of concentrations previously shown to potentiate the action of GABA at GABAA receptors. Exposure to various stressors (including injection or consumption of alcohol) increased brain ALLO levels to the equivalent of 10 – 30 nM in male rats (e.g., Barbaccia et al., 2001; Finn et al., 2004b; Paul & Purdy, 1992; VanDoren et al., 2000). Similar concentrations are achieved in female rats at estrus, and increase to approximately 100 nM during pregnancy (Paul & Purdy, 1992). These findings suggest that fluctuations in endogenous ALLO levels could modify the functioning of central GABAA receptors in vivo – an idea that is supported by the finding that increasing endogenous ALLO levels in the dentate gyrus revealed a physiologically relevant neurosteroid tone that was sufficient to modulate GABAA receptors (Belelli & Herd, 2003).
Alcohol (ethanol) has many pharmacodynamic properties (Spanagel, 2009; Vengeliene et al., 2008;), but its ability to potentiate the action of GABA at GABAA receptors appears to be integral for its reinforcing and discriminative stimulus effects (Chester & Cunningham, 2002; Grant, 1999) as well as its effects on withdrawal-related hyperexcitability (Devaud et al., 2006; Grobin et al., 1998). Given that ethanol injection and consumption can increase cortical ALLO to pharmacologically active levels in male rodents (Barbaccia et al., 1999; Finn et al., 2004b; VanDoren et al., 2000), the ability of ethanol to increase endogenous ALLO levels may potentiate or prolong ethanol's effect via dual (i.e., ethanol + ALLO) actions at GABAA receptors. Likewise, a reduction in ethanol's steroidogenic effect (e.g., use of finasteride (FIN), a 5α-reductase inhibitor that blocks the metabolism of progesterone to ALLO) may reduce the action of ethanol at GABAA receptors. Consistent with this idea, ethanol was found to have a direct and indirect effect on GABAA receptor function, with the indirect effect being due to steroidogenesis, in that it was blocked by FIN (Sanna et al., 2004). Thus, the underlying hypothesis of the studies summarized here is that fluctuations in neurosteroid levels (and the resultant change in GABAergic inhibitory tone) alter sensitivity to ethanol, due to parallel interactions of GABAergic neurosteroids and ethanol at GABAA receptors, thereby leading to changes in the motivational- or withdrawal-related effects of ethanol.
The present review discusses sex differences in two preclinical models of ethanol reward and withdrawal following manipulation of GABAergic neurosteroid levels. Voluntary consumption of ethanol by laboratory animals may be relevant to human consumption of ethanol, and in any case is a tool that can be used to study learned associations between intake and subjective effects of the drug. Seizure susceptibility during ethanol withdrawal is an indication of neuroadaptation that occurs in response to ethanol, so studying the contribution of neurosteroids in convulsive behavior during ethanol withdrawal can increase our understanding about neural responses to ethanol (Finn et al., 2004a).
Neurosteroid Manipulation of Ethanol-Drinking Behavior
Investigations of sex differences in drug abuse and self-administration behavior have gained momentum in the past decade (Becker & Hu, 2008; Carroll et al., 2004; Lynch et al., 2002; Wiren et al., 2006). The literature indicates that marked sex differences in self-administration patterns are observed at every stage throughout the time course of drug exposure history, from acquisition to maintenance to relapse. This phenomenon also appears salient across multiple classes of abused drugs, as sex differences in self-administration have been noted for psychostimulants, opiates, nicotine, and ethanol. While the potential role of organizational steroid effects in controlling sex differences in response to ethanol cannot be ruled out, the leading hypothesis in our laboratory to explain these disparities in self-administration behavior involves sex-dependent expression of the steroids testosterone, estrogen and progesterone, and their neuroactive metabolites. The importance of arriving at a more complete understanding of the neuroendocrine mechanisms underlying these sex differences is clear, as treatment strategies and their effectiveness are likely to hinge upon sex differences in the endogenous neurosteroid environment. In addition, variations in neurosteroid physiology may also help to explain individual differences in susceptibility to drug dependence as well as vulnerability to relapse and negative health consequences of drug intake.
This section reviews research conducted over the past several years that queried whether manipulations in endogenous GABAergic neurosteroids impact patterns of ethanol self-administration in intact male and female C57BL/6 mice. The 3α,5α-reduced pregnane neurosteroid ALLO was the focus of this work. Females demonstrate greater endogenous levels of ALLO when compared to males, and they exhibit marked fluctuations of ALLO in an estrous/menstrual cycle-dependent manner (Finn et al., 2004b; Genazzani et al., 1998; Paul & Purdy, 1992). An earlier finding from our laboratory was that either an ethanol injection (2.0 g/kg) or ethanol self-administered (1.8 – 2.2 g/kg) over a 2-hr period elicited approximately a 1.7-fold elevation in brain ALLO concentrations in male, but not in female mice (Finn et al., 2004b). Given the known sex differences in physiological ALLO levels and the observed disparities in response to acute ethanol exposure, it was hypothesized that sex differences in the ability of this neurosteroid to alter ethanol intake would be observed.
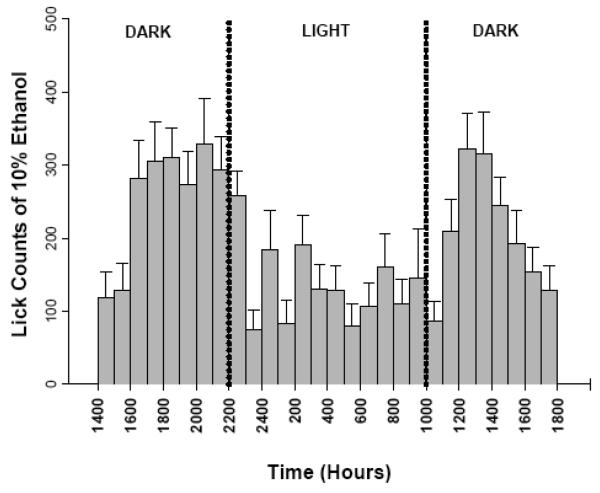
In an initial 2-bottle choice study in which a 10% v/v ethanol (10E) solution and water were available, ALLO (3.2 and 10 mg/kg, administered IP) significantly increased ethanol intake in male, but not in female mice, during the first hour of a 2-hr limited access session (Sinnott et al., 2002). In subsequent experiments this apparent sex difference in ALLO modulation was further examined by optimizing several experimental factors. First, the drinking session started at 2-hr post dark phase onset when mice are the most active and demonstrate the highest level of ethanol intake (Figure 2). Second, we have used a lickometer apparatus that records individual licks from a sipper tube, allowing us to analyze drinking behavior with high temporal resolution. Then, drinking patterns were examined following a more extensive range of acute exogenous ALLO doses. In male mice, a biphasic response to ALLO was observed in which the lowest dose tested (3.2 mg/kg) significantly increased total 10E intake but the highest dose tested (24 mg/kg) suppressed consumption (Figure 3A; Ford et al., 2005b). For comparative purposes, systemic administration of 3.2 mg/kg ALLO to male mice was previously shown to generate steroid plasma concentrations in the physiological range (i.e., 34 ng/ml), whereas doses ≥ 17 mg/kg produced supra-physiological levels of this neurosteroid (Finn et al., 1997). This biphasic effect of ALLO on ethanol self-administration in males has been replicated by alternate routes of ALLO administration (i.e., intracerebroventricular) with an operant self-administration procedure (Ford et al., 2007), and in male rats (Janak et al., 1998; Janak & Gill, 2003).
Figure 2. Lickometer assessment of continuous access ethanol drinking patterns.
Consumption of 10% v/v ethanol (10E) versus water was examined in male C57BL/6 mice with the use of lickometers, an experimental method that allows for the analysis of self-administration behavior at a fine or microstructure levels by recording individual licks. Based on the hourly distribution, studies examining neurosteroid effects on 10E intake focused on 2 hr limited access sessions, beginning 2-3 hrs after lights out. Values are the mean ± SEM for 24 mice.
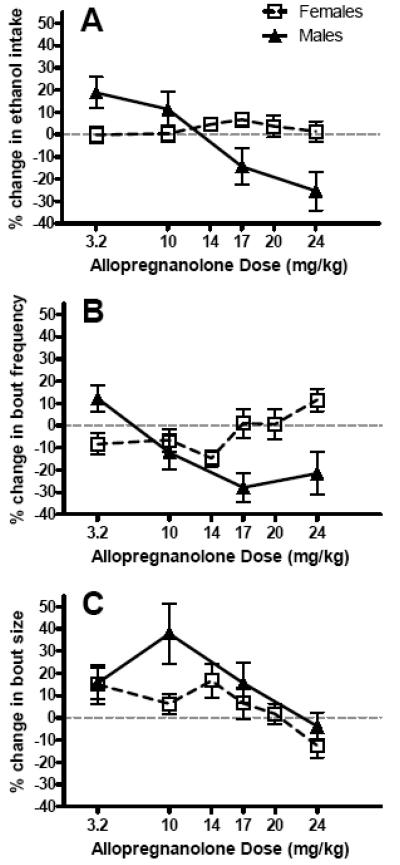
Figure 3. Sex differences in ALLO modulation of (A) ethanol intake, (B) bout frequency, and (C) bout size.
All data are presented as % change from baseline values derived from vehicle treatment throughout the dose-response assessment, and are reported as the mean ± SEM of 18 male and 24 female mice. The dashed line depicts the within-subject baseline values for each experimental variable. Graphs depict transformations of previously published data (Ford et al., 2005b, 2008).
Lickometer analysis of the drinking pattern microarchitecture revealed that ALLO altered levels of 10E consumption primarily via an increase (following the 3.2 mg/kg dose) and significant decrease (following the 17 and 24 mg/ kg doses) in bout frequency (Figure 3B & 3C; Ford et al., 2005b). Although not mirroring the general trend in overall 10E intake consumed, the 10-mg/kg ALLO dose elicited a notable enlargement of the average bout size in male mice. Interestingly, when the 3β-epimer of ALLO, epipregnanolone, was tested under the same conditions in male mice, no effects on g/kg intake or drinking patterns were observed (Ford et el., 2005b), demonstrating the stereospecificity of ALLO's behavioral influence.
In contrast to ALLO modulation of ethanol drinking behavior in males, a subsequent evaluation in females revealed a relative insensitivity to ALLO pretreatment on 10E intake, as well as the drinking patterns expressed (Ford et al., 2008). A sex comparison on response magnitude with regard to 10E dose consumed, bout frequency, and bout size is illustrated in Figure 3 as percent change from baseline values. When challenging the female mice with exogenous ALLO, additional doses were selected to ensure that a potentially narrower effective dose range of ALLO was not overlooked. Multiple interpretations can be furnished to explain the sex-dependent fashion in which exogenous ALLO differentially modulated 10E consumption. First, the higher endogenous levels of ALLO expressed in females may coincide with GABAA receptor subunit compositions that confer relative insensitivity to modulation of this ethanol-related behavior (Follesa et al., 2004; Lambert et al., 2003). A second explanation could involve a sex-dependent difference in the phosphorylation state of GABAA receptors within the neural substrates mediating ethanol intake regulation, as sensitivity to neurosteroid is contingent upon the balance between net phosphatase versus kinase activities (Brussaard and Koksma, 2003).
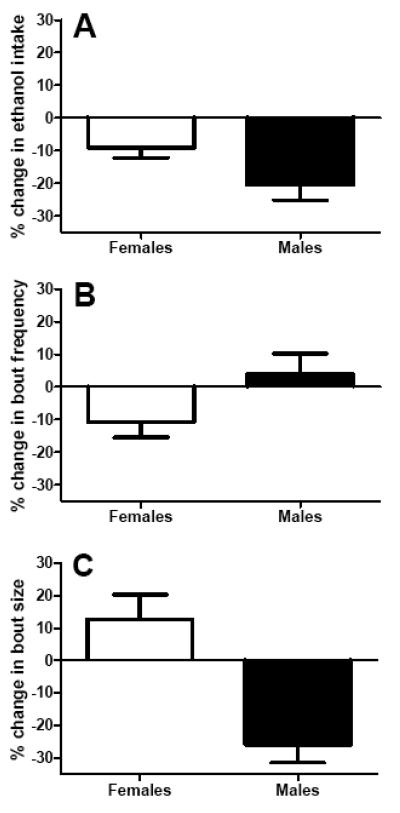
Since a marked sex difference was observed following exogenous challenge with ALLO, subsequent experiments then sought to determine the role of endogenous neurosteroid tone in supporting the ongoing maintenance of established limited access 10E drinking in male and female mice. As depicted in Figure 1, the 5α-reductase enzyme represents a rate-limiting step in the biosynthesis of 5α-reduced metabolites of progesterone (i.e., ALLO), deoxycorticosterone (i.e., tetrahydrodeoxycorticosterone; THDOC) and testosterone (i.e., androstanediol). The activity of the 5α-reductase enzyme can be blocked with the use of the non-competitive inhibitor, FIN (reviewed in Finn et al. 2006a). In male mice, acute pretreatment (i.e., days 1-3) with 50 mg/kg FIN resulted in a 33% decrease in 10E intake throughout the 2-hr limited access session (Ford et al., 2005a). While exogenous ALLO administration primarily affected bout frequency in males, the FIN-induced suppression of consumption maintenance was attributable mainly to attenuation in bout size. In comparison, female mice were relatively insensitive to the ability of 50 mg/kg FIN to suppress 10E intake. Neither the 10E dose consumed nor the drinking patterns in females exhibited significant changes from vehicle treatment (Ford et al., 2008). A sex comparison of the effects of 50 mg/kg FIN on 10E dose consumed, bout frequency, and bout size is illustrated in Figure 4 as percent change from baseline values during days 1-3 of treatment. Since these studies were conducted in intact animals, we should note that preliminary evidence suggested that 50 mg/kg FIN was more efficacious in reducing endogenous ALLO levels in males when compared to females. Specifically, our earlier work in male mice suggested that systemic administration of 50 mg/kg FIN suppressed ALLO levels by greater than 50%, whereas only a 30% decline in this steroid was observed in females following the same pretreatment (Ford et al., 2008).
Figure 4. Sex differences in FIN modulation of (A) ethanol intake, (B) bout frequency, and (C) bout size.
All data are presented as % change from baseline values derived from vehicle treatment prior to acute FIN pretreatment (3-days), and are reported as the mean ± SEM of 16 male and 14 female mice. The solid line depicts within-subject baseline values for each experimental variable. Graphs depict transformations of previously published data (Ford et al., 2005a, 2008).
Because females exhibit higher basal levels of ALLO and the suppression in levels with the 50-mg/kg dose of FIN was less than that in males, it was rationalized that a higher dose of FIN pretreatment would be necessary to elicit significant alterations in the drinking patterns of female mice. In fact, acute pretreatment with 100 mg/kg FIN resulted in a pronounced 35% decrease in the 10E dose consumed, a decrease that was primarily attributable to a significant drop in bout frequency (Ford et al. 2008). Although inhibition of 5α-reductase decreased 10E drinking in both males and females with differential efficacy, it is noteworthy that this modulation was achieved in a sex-dependent fashion. While FIN suppressed ethanol intake in males via a decrease in bout size, this enzyme inhibitor achieved a similar result in female mice by significantly reducing frequency. The differential modulation of drinking patterns between male and female mice may point to sex-dependent divergence in the mechanism of action (details in Conclusions section). The variation in mechanism of ethanol intake modulation may also reflect the influence of FIN treatment on 5α-reduced neurosteroids other than ALLO, such as THDOC and androstanediol. Unfortunately, these neuroactive steroids and their potential role in modulating ethanol-related behaviors remain under-studied, and will need to be examined in future research efforts.
Neurosteroid Manipulation of Ethanol Withdrawal
Neuroadaptations occur with a history of dependence to offset the acute depressant effects of ethanol and include a recruitment of corticotropin releasing factor systems (Heilig & Koob, 2007) as well as a shift in the central excitation-inhibition balance toward excitation (i.e., decrease in GABAergic inhibitory transmission and increase in glutamatergic excitatory transmission; Devaud et al., 2006; Morrow, 1995; Tabakoff & Hoffman, 1996). In many cases, dependence is inferred from the expression of a withdrawal syndrome that transpires upon removal of ethanol, and which is characterized by a rebound hyperexcitability of the nervous system. In humans, the withdrawal syndrome peaks about 72 hours after the cessation of ethanol intake and consists of a wide range of symptoms including: agitation, anxiety, auditory, tactile and/or visual disturbances, disorientation, headache, nausea, vomiting, sweats, and tremors or convulsions (e.g., Ballenger & Post, 1978; Sellers, 1988). While men experience more severe withdrawal from chronic ethanol exposure than women (Deshmukh et al., 2003; Wojnar et al., 1997), such as an increased risk in experiencing withdrawal-induced convulsions (Brathen et al., 1999; Wojnar et al., 1997), women are more vulnerable than men to some of the medical consequences of ethanol abuse (see Devaud et al., 2006; Hashimoto & Wiren, 2008). These observations led to investigations on the potential contribution of the hormonal milieu to sex differences in ethanol withdrawal.
This section reviews information related to the impact of GABAergic steroids on ethanol withdrawal severity. Available data in humans are suggestive of an inverse relationship between endogenous GABAergic neurosteroid levels and behavioral changes in excitability during ethanol withdrawal. Specifically, increased subjective ratings of anxiety and depression during the early withdrawal phase (i.e., 4 – 5 days of withdrawal) corresponded to a significant decrease in ALLO and THDOC levels in a small cohort of alcoholic patients, when compared with control subjects (Romeo et al., 1996). Similar to what has been reported for patients suffering from depression (Romeo et al., 1998; Ströhle et al., 2000; Uzunova et al., 1998), treatments that normalized plasma ALLO levels in the alcoholic patients reduced the reports of anxiety and depression during early withdrawal (Hill et al., 2005; Romeo et al., 2000).
The preclinical models described below focused on measuring changes in seizure susceptibility during ethanol withdrawal, since chronic ethanol exposure and withdrawal elicited symptoms in rodents that were similar to the human condition (e.g., Goldstein & Pal, 1971). In parallel to the human condition, seizure susceptibility during ethanol withdrawal was increased in male versus female rodents (e.g., Veatch et al., 2007) and remained elevated for a longer duration in the male rodents (Alele & Devaud, 2007; Devaud & Chadda, 2001), which was indicative of a faster recovery from ethanol withdrawal in the female rodents. Additional studies determined that male rats displayed motor incoordination during ethanol withdrawal, while female rats were not impaired (Koirala et al., 2008), suggesting that sex differences in susceptibility and duration of convulsive behavior during withdrawal extended to other withdrawal-related behaviors.
Studies in our laboratory examined seizure susceptibility during ethanol withdrawal after pretreatments aimed at manipulating endogenous GABAergic neurosteroid levels in male and female mice from two different genetic animal models of ethanol withdrawal severity: the Withdrawal Seizure-Prone (WSP) and Withdrawal Seizure-Resistant (WSR) selected lines and the C57BL/6 and DBA/2 inbred strains. Selective breeding involves the systematic mating of individuals with extremes in the phenotype of interest [in this case, an increase (WSP) or no change (WSR) in handling-induced convulsions (HIC) during withdrawal]. This selection pressure alters the allele frequencies for genes important for the selected behavior (i.e., withdrawal-induced HICs) so that all trait-relevant loci become homozygously fixed. Following equivalent exposure to 72 hr of ethanol vapor (i.e., the method to induce physical dependence), withdrawal-induced HICs were more than 10-fold greater in two independent replicates of the WSP versus WSR selected lines, with negligible withdrawal in the WSR lines (Crabbe et al., 1985). WSP mice also exhibit significant elevations in withdrawal-induced HICs several hours after a single injection of a sedative dose of ethanol (i.e., acute withdrawal; see Metten & Crabbe, 1996). A major advantage of selected lines is that they can be used to study correlated responses to selection. That is, if lines selected for alcohol withdrawal severity differ on other traits, it can be inferred that those traits are influenced by some of the same genes affecting withdrawal (discussed in Crabbe et al., 1990). With regard to inbred strain differences, by conducting more than 20 generations of brother-sister matings, virtually all genetic variability has been eliminated (i.e., all genes are homozygously fixed). Each member of an inbred strain is essentially a clone of all others (although males and females differ on XX and XY chromosomes), so differences in behavior or neurobiology between inbred strains are attributed to genetic differences. Relevant to the present review, acute (Roberts et al., 1992) and chronic (Crabbe, 1998; Crabbe et al., 1983) ethanol withdrawal-induced HICs are significantly greater in the DBA/2 versus the C57BL/6 inbred strains. A detailed discussion of the effects of GABAergic neurosteroids on ethanol withdrawal in male mice from both genetic animal models as well as a comparison of the withdrawal HIC profile has recently been reviewed (Finn et al., 2004a).
The underlying hypothesis for the withdrawal-related studies in our laboratory is that the modulatory effects of GABAergic neurosteroids on ethanol withdrawal severity are due, in part, to alterations in sensitivity of GABAA receptors to the neurosteroids or alterations in their biosynthesis/metabolism (see Figure 1). Although sex differences in hormone levels during ethanol withdrawal have yet to be examined, data in the WSP and WSR selected lines indicate that plasma ALLO is significantly decreased whereas plasma corticosterone (CORT) is significantly increased during ethanol withdrawal in male WSP versus WSR mice (Tanchuck et al., in press). These results in WSP mice are consistent with data indicating that CORT exhibits proconvulsant effects and that ALLO exhibits anticonvulsant effects in some preclinical models (discussed in Gililland & Finn, 2007; Gorin-Meyer et al., 2007). That is, a significant decrease in an anticonvulsant steroid and significant increase in a proconvulsant steroid could contribute to the high ethanol withdrawal in the WSP selected line. However, it is not known whether sex differences in steroid levels during ethanol withdrawal contribute to the observed disparities in the severity and duration of ethanol withdrawal in male and female rats (reviewed in Devaud et al., 2006). This possibility is worthy of future investigation.
As mentioned above, ethanol dependence reduces GABAergic neurotransmission (e.g., Grobin et al., 1998; Morrow, 1995), so sex differences in the effects of GABAergic steroids at GABAA receptors during withdrawal may represent another potential mechanism contributing to the discrepancy in the withdrawal profile of male versus female rodents. While ethanol withdrawal produces cross-tolerance to the anticonvulsant effect of benzodiazepines (Devaud et al., 1996), male and female rodents exhibited enhanced sensitivity to the anticonvulsant effects of ALLO and pregnanolone (PREG, 5β isomer with a similar pharmacological profile to ALLO; Alele & Devaud, 2007; Devaud et al., 1996). Sensitization to the anticonvulsant effect of ALLO and PREG was greater in female versus male rats (Alele & Devaud, 2007; Devaud et al., 1996) when tested at peak withdrawal (i.e., 8 hrs), and this enhanced behavioral sensitivity corresponded to an increase in functional sensitivity of GABAA receptors to ALLO (males; Devaud et al., 1996) and to THDOC (males and females; Devaud et al., 1998). An interesting finding was that sensitization to the anticonvulsant effect of PREG persisted at 3 days of ethanol withdrawal only in the male rats (Alele & Devaud, 2007), consistent with the longer duration of withdrawal in male rodents and with the idea that sensitization to GABAergic steroids during ethanol withdrawal may represent a compensatory neuroadaptive mechanism.
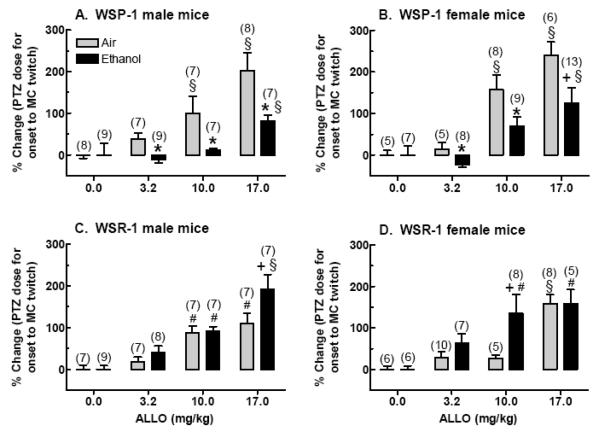
In the WSP and WSR selected lines, female and male WSR mice from the replicate-1 line exhibited sensitization to ALLO's anticonvulsant effect during ethanol withdrawal (Figure 5C & 5D; Beckley et al., 2008). However, when data were collapsed across both replicates of the WSR line, sensitivity to the anticonvulsant effect of ALLO was unchanged in male WSR mice (Finn et al., 2006b). Although not identical, this finding is consistent with the enhanced sensitivity to ALLO's anticonvulsant effect in female versus male rats (Devaud et al., 1996). In contrast, female WSP mice exhibited cross-tolerance to the anticonvulsant effect of ALLO during ethanol withdrawal (Figure 5B; Beckley et al., 2008). Male WSP mice also exhibited cross-tolerance to the anticonvulsant effect of ALLO during ethanol withdrawal, and this decrease in behavioral sensitivity corresponded to a right-ward shift in the functional sensitivity of GABAA receptors to ALLO during withdrawal (Figure 5A; Finn et al., 2006b). These results indicate that cross-tolerance to ALLO is a correlated response to selection in the male and female WSP and WSR mice. In other words, the results suggest that some of the genes that confer reduced sensitivity of GABAA receptors to ALLO during ethanol withdrawal also impart increased severity of ethanol withdrawal.
FIGURE 5. Genotype (but no sex) difference in the change in sensitivity to the anticonvulsant effect of ALLO, measured by the percent change in pentylenetetrazol (PTZ) threshold dose for onset to MC twitch, in (A) WSP male, (B) WSP female, (C) WSR male, and (D) WSR female mice.
PTZ was administered at 20 min post-injection of ALLO or vehicle. Since ethanol withdrawal significantly decreased the PTZ dose, these data were transformed to % change in PTZ threshold dose (i.e., for each line, sex, treatment and steroid group, the % change was calculated for each animal as the change from the mean for the respective vehicle-injected group – air or ethanol). An increase in PTZ threshold dose following ALLO injection indicated that the dose of ALLO was anticonvulsant. Values represent the mean (± SEM) for the number of animals in parentheses. Data (from replicate-1 animals) were adapted from previously published data (Beckley et al., 2008; Finn et al., 2006b).
+P < 0.10, *P < 0.05 vs. respective air-exposed mice
#P < 0.05, §P < 0.01 vs. respective vehicle-injected mice
To further characterize the influence of GABAergic neurosteroids on ethanol withdrawal, the 5α-reductase inhibitor FIN was used as a tool to decrease levels of ALLO and other GABAergic steroids (Figure 1; reviewed in Finn et al., 2006a). We hypothesized that a FIN-induced reduction in GABAergic neurosteroid levels would significantly increase ethanol withdrawal severity, and that there would be sex and genotype differences in FIN's effect. In contrast to our prediction, systemic administration of FIN (50 mg/kg) to male and female C57BL/6 and DBA/2 mice during the development of physical dependence significantly decreased ethanol withdrawal severity in female DBA/2 mice, produced a non-selective suppressive effect on HICs in male DBA/2 and C57BL/6 mice, and did not significantly alter HICs in female C57BL/6 mice (Table 1; Finn et al., 2004c). In male WSP and WSR mice, systemic FIN pretreatment significantly reduced chronic withdrawal severity, measured by HICs and anxiety-related behavior, but only in the WSP line (Gorin et al., 2005). However, in both of these studies, FIN pretreatment also significantly decreased blood ethanol concentration (BEC) upon the initiation of withdrawal (Finn et al., 2004c; Gorin et al., 2005), suggesting that the suppression in chronic ethanol withdrawal severity was due to an indirect effect on ethanol pharmacokinetics.
TABLE 1.
Neurosteroid Manipulations Alter the Severity of Acute and Chronic Ethanol Withdrawal in C57BL/6 and DBA/2 Inbred Strains: Sex Differences
| Strain | Sex | Change in Withdrawal (versus Control Group) | ||
|---|---|---|---|---|
| Systemic FINa Chronic Withdrawal |
Systemic FINb Acute Withdrawal |
ADX + GDXc Acute Withdrawal |
||
| DBA/2J | male | Non-selective ↓ | Significant ↓ | Significant ↑ |
| female | Significant ↓ | Significant ↑ | Significant ↑ | |
| C57BL/6J | male | Non-selective ↓ | Significant ↓ | Significant ↑ |
| female | No change | Significant ↑ | No change | |
Data are summarized for the effects of experimental manipulations designed to deplete GABAergic neurosteroids (50 mg/kg dose of finasteride, FIN) or to deplete peripherally-derived steroids (adrenalectomy and gonadectomy, ADX + GDX) versus the respective control group (i.e., vehicle injection or SHAM surgery). Withdrawal was indexed by measuring handling-induced convulsions (HICs) and calculating the area under the withdrawal curve.
Mice were pretreated with FIN (4 daily injections) during the development of physical dependence. The non-selective decrease refers to a decrease in HICs in air- and ethanol-exposed mice. Adapted from Finn et al. (2004c).
Mice were pretreated with FIN (1 injection) prior to a single high dose of ethanol. Adapted from Gorin-Meyer et al. (2007).
Mice received a single high dose of ethanol at least 1 week following SHAM or ADX +GDX surgery. Adapted from Gililland and Finn (2007).
In an effort to minimize the effect of FIN on ethanol metabolism, a subsequent study examined the effect of FIN on acute ethanol withdrawal severity (i.e., a model of acute hyperexcitability or “hangover” – Prediger et al., 2006). Depending on the sex or genotype of the mouse, total clearance time for a 4-g/kg dose of ethanol ranges from 4.2 – 8.5 hrs, with the appearance of increased anxiety or HICs occurring after ethanol elimination (Gililland & Finn, 2007; Gorin-Meyer et al., 2007; Prediger et al., 2006). For this study, male and female C57BL/6 and DBA/2 mice were pretreated with FIN (50 mg/kg) or vehicle prior to a single injection of ethanol (4 g/kg) or saline, with HIC scores measured over 24 hrs. In both strains of mice, FIN pre-treatment increased acute ethanol withdrawal severity in females, while decreasing it in males (Table 1; Gorin-Meyer et al., 2007). FIN did not alter BECs, ethanol clearance, estradiol or CORT levels in a manner that appeared to contribute to the sex difference in FIN's effect on acute ethanol withdrawal severity. These findings suggest that male and female C57BL/6 and DBA/2 mice differ in their sensitivity to changes in ALLO or other endogenous GABAergic steroid levels during acute ethanol withdrawal.
As mentioned in the Introduction, both central and peripheral sources of steroids can contribute to neurosteroid concentrations in the brain, but it is not known whether the contributions of these sources differ between the sexes. Previous work found that removal of the peripheral sources of neurosteroids in male rats eliminated the ability of an acute ethanol injection to increase cortical ALLO levels (O'Dell et al., 2004) and altered several behavioral measures of ethanol intoxication (Hirani et al., 2002, 2005; Khisti et al., 2003), suggesting that peripheral sources of GABAergic steroids are important for the ability of ALLO to modulate ethanol sensitivity. To determine the relevance of these findings toward withdrawal-related behavior, male and female C57BL/6 and DBA/2 mice were tested for acute ethanol withdrawal following removal of the adrenals (ADX), gonads (GDX), both organs (ADX+GDX), or a sham surgery (SHAM). We hypothesized that peripheral sources of steroids (i.e., adrenal and gonads) were important during withdrawal from a single high dose of ethanol (4 g/kg) and that the direction of change in acute ethanol withdrawal severity would provide insight into the relative importance of pro- versus anti-convulsant steroids to the acute withdrawal profile in intact animals. In other words, an increase in withdrawal following organ removal would suggest that endogenous anticonvulsant steroids were protective against HICs during withdrawal in intact animals, whereas a decrease in withdrawal following organ removal would suggest that endogenous proconvulsant steroids were important for the withdrawal profile in intact animals.
The results indicated that the magnitude of acute ethanol withdrawal was significantly increased by ADX and ADX+GDX surgeries in male mice from both genotypes (Table 1; Gililland & Finn, 2007). In female DBA/2 mice, the duration of acute ethanol withdrawal was increased by ADX+GDX surgery, whereas surgical status did not alter acute ethanol withdrawal severity in female C57BL/6 mice (Gililland & Finn, 2007). Surgical status had negligible effects on ethanol pharmacokinetics, indicating that the increases in acute ethanol withdrawal severity were not due to a change in ethanol metabolism. Overall, the results indicate the removal of peripherally-derived steroids with anticonvulsant properties contributed to the increase in acute ethanol withdrawal severity in male and female DBA/2 mice and in male C57BL/6 mice. Removal of the adrenals was sufficient for this effect in male C57BL/6 and DBA/2 mice (i.e., similar effect with ADX versus ADX+GDX), whereas removal of both the adrenals and gonads was necessary for this effect in female DBA/2 mice. These findings suggest that testosterone and its GABAergic metabolites may exert a minor role in the acute withdrawal profile in intact animals (i.e., since GDX alone was ineffective). Consistent with this idea, preliminary results from steroid replacement studies indicate that pretreatment with progesterone or deoxycorticosterone to ADX+GDX male and female DBA/2 mice significantly restored the acute ethanol withdrawal profile to that seen in intact animals, and that this effect was reversed when FIN was co-administered with progesterone or deoxycorticosterone to block metabolism to GABAergic neurosteroids (Kaufman et al., 2009). Thus, endogenous GABAergic derivatives of progesterone and deoxycorticosterone may be more important than derivatives of testosterone in contributing to acute ethanol withdrawal severity in intact animals.
Discussion and Conclusions
The present review focused on animal models relevant to two aspects of addiction: alcohol self-administration, which is dominated in the early stage by positive reinforcement, and alcohol withdrawal, which is dominated by negative reinforcement (e.g., Le Moal and Koob, 2007; Spanagel, 2009). Given that alcohol addiction develops across time, one should consider that the addictive behavior is the result of cumulative responses to alcohol exposure (e.g., sensitivity) and a complex interaction of genetic and environmental variables (Bevilacqua and Goldman, 2009; Spanagel, 2009). Additionally, sex differences exist in all phases of drug abuse, with females generally more sensitive to the rewarding effects of drugs than males (see Carroll et al., 2004). As described earlier, female rodents exhibit increased ethanol consumption and decreased withdrawal severity versus male rodents. The fact that these differences are eliminated by gonadectomy (Alele and Devaud, 2007; Almeida et al., 1998; Cailhol and Mormède, 2001) argues that the hormone milieu contributes to these sex differences.
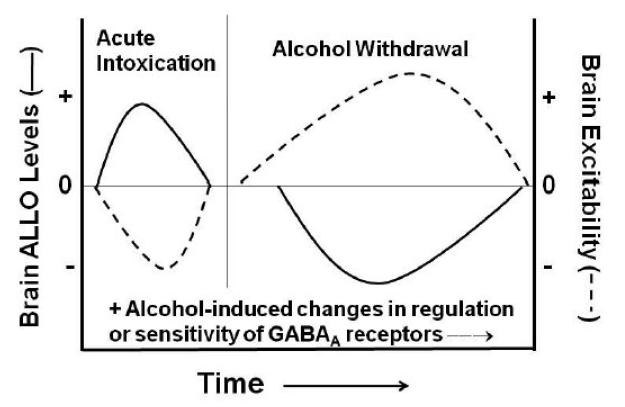
Sex differences exist in ethanol-related behaviors as well as in endogenous levels of GABAergic neurosteroids. When one considers that ethanol injection and consumption can increase GABAergic steroid levels in rodent models (Barbaccia et al., 1999; Eva et al., 2008; Finn et al., 2004b; VanDoren et al., 2000), that there can be sex differences in the steroidogenic effect of ethanol (Finn et al., 2004b), and that the steroidogenic effect of alcohol consumption is controversial in humans (Holdstock et al., 2006; Pierucci-Lagha et al., 2006; Torres & Ortega, 2003, 2004), it is likely that the interaction of ethanol and GABAergic steroids at GABAA receptors and the concomitant behavioral outcome is multi-faceted. An additional layer of complexity is provided by recent observations regarding the correspondence between estrous cycle- and pregnancy/post-partum- related changes in the plasticity of GABAA receptors and behavioral measures of seizure susceptibility, anxiety, or depression in mice (Maguire et al., 2005; Maguire & Mody, 2008), although differences in this relationship have been reported (Sanna et al., 2009). Finally, it is conceivable that voluntary ethanol consumption (as with ethanol drinking studies) and forced chronic ethanol administration (to induce physical dependence) may differentially alter GABAergic neurosteroid levels in males and females. If this is the case, sex differences in endogenous neurosteroid tone may interact with the ethanol-induced plasticity of GABAA receptors and contribute to the discrepancies between sexes in the effects of neurosteroid manipulations on ethanol drinking- versus withdrawal-related behaviors (Figure 6).
Figure 6. Hypothetical model for interaction of alcohol intoxication- versus alcohol withdrawal-related effects on endogenous ALLO levels and brain excitability.
The effect of acute intoxication or alcohol withdrawal on endogenous ALLO levels is depicted by the solid line, while the subsequent change in brain excitability is depicted by the dashed line. In general, the impact of endogenous neurosteroid tone on GABAA receptor-mediated inhibition exhibits an inverse relationship on brain excitability (i.e., ↑ ALLO = ↓ excitation; ↓ ALLO = ↑ excitation). Disparate influences of alcohol intoxication and withdrawal on ALLO levels, in conjunction with differential alterations in GABAA receptor plasticity within discrete brain regions, likely underly sex differences in the effects of GABAergic neurosteroid manipulations on measures of alcohol self-administration and withdrawal.
With regard to ethanol drinking behavior, established limited access 10E intake in male C57BL/6 mice was sensitive to manipulations in endogenous GABAergic neurosteroid levels. In contrast, female C57BL/6 mice were insensitive to the effects of exogenous ALLO challenge and required a higher FIN dose to suppress 10E intake than male mice. Taken in conjunction with the finding that ethanol consumption increased cortical ALLO levels only in male mice (Eva et al., 2008; Finn et al., 2004b), the sex differences in the ability of exogenous ALLO challenge to manipulate ethanol intake likely is due to the sex and estrous cycled-related differences in the plasticity of GABAA receptors to ALLO in combination with the concomitant effects of ethanol drinking on ALLO levels. Another possible explanation is that the insensitivity of the female mice to ALLO challenge was due to a more rapid metabolism of ALLO within neurocircuitry fundamental to the regulatory processes underlying ethanol intake than in males. This suggestion is based on the finding that regional differences in the potency of ALLO to enhance GABAergic transmission in the dentate versus CA1 of the hippocampus was due to alterations in ALLO metabolism, since preventing the metabolism of ALLO unmasked an endogenous neurosteroid tone (Belelli & Herd, 2003). In this circumstance, use of a synthetic neurosteroid analog such as ganaxolone (Beekman et al., 1998; Carter et al.., 1997; Gasior et al., 1997; Reddy & Rogawski, 2000), which has a similar pharmacological profile to ALLO but an additional 3β-methyl group to protect the steroid from metabolic attack at the 3α position and prolong availability, might avoid the potential confound of ALLO metabolism. Consistent with this idea, preliminary data found that a 10 mg/kg dose of ganaxolone significantly decreased 24 hr ethanol intake in male mice (Finn & Ford, unpublished).
Another consideration is that full dose effect curves are not conducted in ethanol self-administration studies, so one does not know whether a drug manipulation is producing a leftward (i.e., ethanol more reinforcing) or rightward (i.e., ethanol less reinforcing) shift in the dose effect curve. Depending on the point of manipulation in the inverted U dose effect curve, both an agonist and antagonist can decrease ethanol self-administration. Since data to date are consistent with ALLO producing a leftward shift in the ethanol dose effect curve, the predicted outcome of a high dose ALLO challenge would be to decrease ethanol intake (which is what we observe in male mice). Likewise, a FIN-induced decrease in ethanol reinforcement could produce a decrease in ethanol intake (also what we observe in male and female mice). Even though both high dose ALLO and FIN decreased ethanol intake, the suppression occurred via distinct effects on the regulatory processes influencing ethanol consumption (i.e., ↓ bout frequency versus ↓ bout size). Importantly, the examination of bout parameters in the mouse is analogous to the quantity and frequency determinations that are considered “gold standards” for the assessment of drinking in humans (Feunekes et al., 1999). Furthermore, efficacy of treatment (e.g., naltrexone) can be contingent upon the demonstration of a drinking pattern that is susceptible to manipulation (Anton et al., 2004). Although additional studies are necessary, it is possible that either high dose ganaxolone or FIN may be a useful pharmacotherapeutic strategy to decrease ethanol consumption.
With regard to ethanol dependence and withdrawal, female mice were sensitive to several GABAergic steroid manipulations, suggesting that the insensitivity of female mice to effects of exogenous ALLO challenge on ethanol drinking behavior did not extend to all ethanol-related phenotypes. The results summarized above (section on ethanol withdrawal) clearly indicate that there are sex differences in the severity and duration of ethanol withdrawal, yet it is not known whether these differences are due to sex differences in the expression of specific GABAA receptor subunits in dependent animals (Devaud et al., 1998), sex differences in expression or activity of biosynthetic enzymes and the concomitant alteration in endogenous levels of steroid hormones that can be altered during dependence and withdrawal, sex differences in the sensitivity of GABAA or other receptors to steroids during withdrawal, or other factors.
Sex differences may also interact with genetic background, as genotypes with mild versus severe withdrawal exhibit marked differences in sensitivity to ALLO during ethanol withdrawal (sensitization with mild withdrawal, cross-tolerance with severe withdrawal). Taken in conjunction with the finding that other seizure-prone genotypes, such as the DBA/2 inbred strain (Finn et al., 2000) also exhibit cross-tolerance to ALLO during ethanol withdrawal, an understanding of the GABAergic neural differences that underlie ethanol withdrawal severity may lead to improved therapies for the treatment of ethanol dependence and withdrawal. However, it also should be noted that expression profiling of male and female WSP and WSR mice indicated that the transcriptional response following dependence and withdrawal correlated with sex rather than the selected withdrawal phenotype at a time point corresponding to peak withdrawal (Hashimoto & Wiren, 2008), but correlated with selected withdrawal phenotype at a time point corresponding to 3 weeks of withdrawal (Hashimoto et al., 2008). These results suggest that sex differences in distinct neuroadaptive responses are more important than withdrawal severity, at least in the early phase of withdrawal, and may guide sex specific strategies for the treatment of early versus late phases of withdrawal.
In conclusion, male and female mice differ in their sensitivity to changes in ALLO or other GABAergic neurosteroid levels with regard to the effects on alcohol's motivational- and withdrawal-related properties, suggesting interesting sex differences in the sensitivity of GABAA receptors to GABAergic steroids within circuits relevant to alcohol reward versus withdrawal. Thus, sex differences in the modulation of GABAergic neurosteroids may be an important consideration in understanding and developing therapeutic interventions in alcoholics. This unique approach will advance our understanding of risk for alcohol abuse as well as probe the feasibility of targeting neurosteroid biosynthesis or developing neurosteroid analogs for therapeutic intervention in the treatment of alcoholism.
Acknowledgements
The work presented was supported by grants from the Department of Veterans Affairs (VA Merit to DAF) and the NIAAA (AA12439 to DAF and AA10760, Portland Alcohol Research Center component to DAF). MMF is supported by a KO1 award from NIAAA (AA016849). EHB is supported by a predoctoral NRSA from NIMH (F31 MH081560). KRK is supported by a predoctoral NRSA from NIAAA (F31 AA017019). We thank Michelle Tanchuck, Andrea Fretwell, Naomi Yoneyama, Moriah Strong, Chris Snelling, Sarah Eddy, Jeffrey Nickel, and Rebecca Gorin-Meyer for technical assistance on the described studies. We also thank Dr. John Crabbe for supplying the WSP and WSR mice and the Dependence Core of the Portland Alcohol Research Center for their assistance in the induction of physical dependence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J. Pharmacol. Exp. Ther. 2007;320:427–436. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Almeida OFX, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats: The role of the gonadal steroid environment. J. Clin. Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br. J. Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur. J. Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int. Rev. Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Steroid hormones in the brain: Several mechanisms? In: Fuxe K, Gustafsson JA, Wetterberg L, editors. Steroid Hormone Regulation of the Brain. Pergamon; Oxford: 1981. pp. 3–14. [Google Scholar]
- Baulieu EE, Robel P, Schumacher M. Neurosteroids: Beginning of the story. Int. Rev. Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckley EH, Fretwell AM, Tanchuck MA, Gililland KR, Crabbe JC, Finn DA. Decreased anticonvulsant efficacy of allopregnanolone during ethanol withdrawal in female Withdrawal Seizure-Prone vs. Withdrawal Seizure-Resistant mice. Neuropharmacology. 2008;54:365–374. doi: 10.1016/j.neuropharm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Ungard JT, Gasior M, Carter RB, Dijkstra D, Goldberg SR, Witkin JM. Reversal of behavioral effects of pentylenetetrazol by the neuroactive steroid ganaxolone. J. Pharmacol. Exp. Ther. 1998;284:868–877. [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: Evidence for endogenous neurosteroids? J. Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci. Biobehav. Rev. 1990;14:315–322. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genes and addictions. Clin. Pharmacol. Ther. 2009;85:359–361. doi: 10.1038/clpt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Hendry LB, Mahesh VB. Emerging diversities in the mechanism of action of steroid hormones. J. Steroid Biochem. Mol. Biol. 1995;52:113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- Brathen G, Brodtkorb E, Helde G, Sand T, Bovim G. The diversity of seizures related to alcohol use. A study of consecutive patients. Eur. J. Neurol. 1999;6:697–703. doi: 10.1046/j.1468-1331.1999.660697.x. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard AB, Koksma JJ. Conditional regulation of neurosteroid sensitivity of GABAA receptors. Ann. N.Y. Acad. Sci. 2003;1007:29–36. doi: 10.1196/annals.1286.003. [DOI] [PubMed] [Google Scholar]
- Caihol S, Mormède P. Sex and strain differences in ethanol drinking: Effects of gonadectomy. Alcohol. Clin. Exp. Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J. Pharmacol. Exp. Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol. Clin. Exp. Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl. Recept. Signal. 2008;6:e008. doi: 10.1621/nrs.06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD Recombinant Inbred mice. J. Pharmacol. Exp. Ther. 1998;286:263–271. [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav. Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: Interpretation of experiments using selectively bred and inbred animals. Alcohol. Clin. Exp. Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr., Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol. Biochem. Behav. 1983;18(Suppl 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Sassoon S, O'Reilly A, Pfefferbaum A, Sullivan EV. Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. J. Stud. Alcohol. 2003;64:375–379. doi: 10.15288/jsa.2003.64.375. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol. Clin. Exp. Res. 2001;25:1689–1696. [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Morrow AL. Influence of gender on chronic ethanol-induced alterations in GABAA receptors in rats. Brain Res. 1998;796:222–230. doi: 10.1016/s0006-8993(98)00357-6. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J. Pharmacol. Exp. Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Devaud LL, Risinger FO, Selvage D. Impact of the hormonal milieu on the neurobiology of alcohol dependence and withdrawal. J. Gen. Psychol. 2006;133:337–356. doi: 10.3200/GENP.133.4.337-356. [DOI] [PubMed] [Google Scholar]
- Eva C, Mele P, Collura D, Nai A, Pisu MG, Serra M, Biggio G. Modulation of neuropeptide Y and Y1 receptor expression in the amygdala by fluctuations in the brain content of neuroactive steroids during ethanol drinking discontinuation in Y1R/LacZ transgenic mice. J. Neurochem. 2008;104:1043–1054. doi: 10.1111/j.1471-4159.2007.05077.x. [DOI] [PubMed] [Google Scholar]
- Feunekes GI, van 't Veer P, Van Staveren WA, Kok FJ. Alcohol intake assessment: The sober facts. Am. J. Epidemiol. 1999;150:105–112. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug. Rev. 2006a;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Beadles-Bohling AS, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes Brain Behav. 2006b;5:53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: Behavioral genetic approaches. Pharmacol. Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J. Pharmacol. Exp. Ther. 2000;292:394–405. [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: Sex and strain differences. Pharmacol. Biochem. Behav. 2004c;78:435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Lotrich F, Gallaher EJ. Genetic differences in behavioral sensitivity to a neuroactive steroid. J. Pharmacol. Exp. Ther. 1997;280:820–828. [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004b;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur. J. Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alters ethanol intake patterns in male C57BL/6J mice: Potential role of endogenous neurosteroids? Alcohol. 2005a;37:23–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol. Clin. Exp. Res. 2005b;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav. Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA. Ethanol intake patterns in female mice: Influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend. 2008;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Goldberg SR, Witkin JM. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. J. Pharmacol. Exp. Ther. 1997;282:543–553. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J. Clin. Endocrinol. Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Gililland KR, Finn DA. The impact of gonadectomy and adrenalectomy on acute withdrawal severity in male and female C57BL/6J and DBA/2J mice following a single high dose of ethanol. Alcohol. Clin. Exp. Res. 2007;31:1846–1857. doi: 10.1111/j.1530-0277.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: Grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Gorin RE, Crabbe JC, Tanchuck MA, Long SL, Finn DA. Effects of finasteride on chronic and acute ethanol withdrawal severity in the WSP and WSR selected lines. Alcohol. Clin. Exp. Res. 2005;29:939–948. doi: 10.1097/01.alc.0000167742.11566.01. [DOI] [PubMed] [Google Scholar]
- Gorin-Meyer RE, Wiren KM, Tanchuck MA, Long SL, Yoneyama N, Finn DA. Sex differences in the effect of finasteride on acute ethanol withdrawal severity in C57BL/6J and DBA/2J mice. Neuroscience. 2007;146:1302–1315. doi: 10.1016/j.neuroscience.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol. Biochem. Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm. Behav. 2008;53:638–646. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Anacker AMJ, Wiren KM. Selected line differences in the ethanol response: Gene expression differences following abstinence in male and female WSP and WSR mice. Alcohol. Clin. Exp. Res. 2008;32(s1):149A. [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: Expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology. 2008;33:1084–1096. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlíková H, Lyudmila K, Vrbíková J, Kancheva R, Pouzar V, Černý I, Stárka L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids. 2005;70:515–524. doi: 10.1016/j.steroids.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt's forced swim test: modulation by 3alpha-hydroxy-5alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Holdstock L, Penland SN, Morrow AL, de Wit H. Moderate doses of ethanol fail to increase plasma levels of neurosteroid 3α-hydroxy-5α-pregnan-20-one-like immunoreactivity in healthy men and women. Psychopharmacology. 2006;186:442–450. doi: 10.1007/s00213-005-0187-0. [DOI] [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol. Clin. Exp. Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Kaufman KR, Tanchuck MA, Strong MN, Finn DA. Replacement with GABAergic steroids or their precursors alters the acute ethanol withdrawal profile. Alcohol. Clin. Exp. Res. 2009;33(s1):156A. [Google Scholar]
- Kawata M, Nishi M, Matsuda K, Sakamoto H, Kaku N, Masugi-Tokita M, Fujikawa K, Hirahara-Wada Y, Takanami K, Mori H. Steroid receptor signaling in the brain—Lessons learned from molecular imaging. J. Neuroendocrinol. 2008;20:673–676. doi: 10.1111/j.1365-2826.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Rønnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol. Cell. Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, Matthews DB, Morrow AL. Ethanol-induced elevation in 3α-hydroxy-5α-pregnan-20-one does not modulate motor incoordination in rats. Alcohol. Clin. Exp. Ther. 2003;28:1249–1256. doi: 10.1097/01.alc.0000134232.44210.06. [DOI] [PubMed] [Google Scholar]
- Koirala B, Alele PE, Devaud LL. Influence of hormonal status on behavioral responses to an acute ethanol challenge during ethanol withdrawal in male and female rats. Pharmacol. Biochem. Behav. 2008;90:691–700. doi: 10.1016/j.pbb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog. Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol. Clin. Exp. Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Koob GF. Drug addiction: Pathways to the disease and pathophysiological perspectives. Eur. Neuropsychopharmacology. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell. Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hashimoto K, Yamada M, Satoh T, Hirato J, Mori M. Liver X receptor-α regulates proopiomelanocortin (POMC) gene transcription in the pituitary. Mol. Endocrinol. 2009;23:47–60. doi: 10.1210/me.2007-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol. Sci. 1991;12:141–147. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Vaudry H. Biosynthesis of neurosteroids and regulation of their synthesis. Int. Rev. Neurobiol. 2001;46:33–78. doi: 10.1016/s0074-7742(01)46058-2. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Dependence and withdrawal. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; New York: 1996. pp. 269–290. [Google Scholar]
- Morrow AL. Regulation of GABAA receptor function and gene expression in the central nervous system. Int. Rev. Neurobiol. 1995;38:1–41. doi: 10.1016/s0074-7742(08)60523-1. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur. J. Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Khisti RT, Morrow AL, Marx CE, Shampine LJ, Kranzler HR. Subjective effects and changes in steroid hormone concentrations in humans following acute consumption of alcohol. Psychopharmacology. 2006;186:451–461. doi: 10.1007/s00213-005-0231-0. [DOI] [PubMed] [Google Scholar]
- Prediger RD, daSilva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;10:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J. Med. Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych P. Estrogen and progesterone modulate [35S]GTPγS binding to nociceptin receptors. Neuroendocrinology. 2008;88:35–42. doi: 10.1159/000XXXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J. Pharmacol. Exp. Ther. 2000;295:1241–1248. [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;596:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin. Neuropharmacology. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, Pasinib A. Effects of fluoxetine, indomethacine and placebo on 3alpha, 5alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J. Biol. Psychiatry. 2000;1:101–104. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatments on neuroactive steroids in major depression. Am. J. Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J. Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J. Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM. Alcohol, barbiturate and benzodiazepine withdrawal syndromes: Clinical management. Can. Med. Assoc. J. 1988;139:113–20. [PMC free article] [PubMed] [Google Scholar]
- Shen J-W, Geerling JC, Loewy AD. Vagal innervations of the aldosterone-sensitive HSD2 neurons in the NTS. Brain Res. 2009;1249:135–147. doi: 10.1016/j.brainres.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α,5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. J. Psychiatry Res. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Alcohol and glutamate receptors. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. CRC Press; New York: 1996. pp. 73–93. [Google Scholar]
- Tanchuck MA, Long SL, Ford MM, Hashimoto J, Crabbe JC, Roselli CE, Wiren KM, Finn DA. Selected line differences in the effects of ethanol dependence and withdrawal on allopregnanolone levels and 5α-reductase enzyme activity and expression. Alcohol. Clin. Exp. Res. 2009 doi: 10.1111/j.1530-0277.2009.01047.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Reddy AP, Bethea CL. Neuroprotective actions of ovarian hormones without insult in the raphe regions of rhesus macaques. Neuroscience. 2008;154:720–731. doi: 10.1016/j.neuroscience.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:1207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology. 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- Uchoa ET, Sabino HAC, Ruginsk SG, Antunes-Rodrigues J, Elias LLK. Hypophagia induced by glucocorticoid deficiency is associated with increased activation of satiety-related responses. J. Appl. Physiol. 2009;106:596–604. doi: 10.1152/japplphysiol.90865.2008. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthew DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J. Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol. Clin. Exp. Res. 2007;31:477–485. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr. Med. Chem. 2009;16:455–472. doi: 10.2174/092986709787315522. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br. J. Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, Grant KA, Finn DA. Impact of sex: Determination of alcohol neuroadaptation and reinforcement. Alcohol. Clin. Exp. Res. 2006;30:233–242. doi: 10.1111/j.1530-0277.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- Wojnar M, Wasilewski D, Matsumoto H, Cedro A. Differences in the course of alcohol withdrawal in women and men: a Polish sample. Alcohol. Clin. Exp. Res. 1997;21:1351–1355. [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Becker JB. Sex matters: Gonadal steroids and the brain. Neuropsychopharmacology. 2009;34:537–538. doi: 10.1038/npp.2008.221. [DOI] [PubMed] [Google Scholar]