Abstract
The primary function of O6-alkylguanine-DNA alkyltransferase (AGT) is to maintain genomic integrity in the face of damage by both endogenous and exogenous alkylating agents. However, paradoxically, bacterial and mammalian AGTs have been shown to increase cytotoxicity and mutagenicity of dihaloalkanes and other bis-electrophiles when expressed in bacterial cells. We have extended these studies to mammalian cells using CHO cells that lack AGT expression and CHO cells stably transfected with a plasmid that expresses human AGT. The cytotoxicity of 1,2-dibromoethane, dibromomethane and epibromohydrin was significantly increased by the presence of AGT but cytotoxicity of butadiene diepoxide was not affected. Mutations caused by these agents were assessed using hypoxanthine-guanine phosphoribosyltransferase (HPRT) as a reporter gene. There was a small (c. 2–3-fold) but statistically significant AGT-mediated increase in mutations caused by 1,2-dibromoethane, dibromomethane and epibromohydrin. Analysis of the mutation spectrum induced by 1,2-dibromoethane showed that the presence of AGT also altered the types of mutations with an increase in total base substitution mutants due to a rise in transversions at both G:C and A:T sites. AGT expression also led to mutations arising from the transcribed strand, which were not seen in cells lacking AGT. Although the frequency of deletion mutations was decreased by AGT expression, the formation of large deletions (≥3 exons) was increased. This work demonstrates that interaction of AGT with some bis-electrophiles can cause mutagenicity and diminished cell survival in mammalian cells. It is consistent with the hypothesis that DNA-AGT cross-links, which have been characterized in experiments with purified AGT protein and such bis-electrophiles, can be formed in mammalian cells.
Keywords: dihaloalkanes, epihalohydrins, butadiene diepoxide, alkyltransferase, DNA-protein cross-links
1. Introduction
Dibromomethane (DBM) and 1,2-dibromoethane (DBE) are halogenated aliphatic hydrocarbons that were used globally as gasoline additives, chemical intermediates for the manufacture of dyes and pharmaceuticals and as industrial solvents [1,2]. However, the ability of these bifunctional alkylating agents to induce mutations, tumorigenesis and toxicity in rat and mouse models in vivo led to significant curtailing of their usage after the 1970s [2–4].
Although both DBE and DBM were shown to be directly mutagenic in vitro [5–8], the presence of a metabolizing system significantly enhanced the mutagenic and carcinogenic potential of these compounds. Cytochrome P450 monooxygenases and glutathione-S-transferases are two separate pathways that serve to biotransform the dibromoalkanes yielding metabolites capable of reacting with cellular nucleophiles resulting in a broad spectrum of genotoxic outcomes [5–15]. These pathways may lead to the mutagenicity and carcinogenicity of such dihaloalkanes.
However, more recently, an additional pathway has been demonstrated in which DNA damage leading to cytotoxicity and mutagenicity is paradoxically mediated via the DNA repair protein, O6-alkylguanine-DNA alkyltransferase (AGT). It is well established that AGT protects cells from alkylating agents that form genotoxic O6-alkylguanine adducts in DNA. This protection occurs via a direct repair mechanism in which the alkyl group is transferred onto a cysteine acceptor residue located within the AGT generating S-alkylcysteine in the protein and restoring the DNA in a single step [16–18]. In contrast, studies in which AGTs from various species were expressed in Escherichia coli or Salmonella typhimurium have shown that their presence can greatly increase mutations and cytotoxicity of dihaloalkanes [3,19–25]. Detailed studies of this interaction using purified proteins as well as expression of human AGT (hAGT) in bacterial cells exposed to dihaloalkanes have provided a plausible mechanism for this enhancement of DNA damage. An initial reaction occurs between the dibromoalkane and hAGT to produce a reactive intermediate at Cys145 in the active site that can subsequently attack DNA yielding covalent AGT-DNA adducts [3,23]. The formation of hAGT-DNA conjugates in the presence of DBE in vitro was shown to occur with a preference for guanine but A:T pairs were also targeted. Mutation spectrum analysis revealed that AGT-mediated base-pair changes produced by DBE consisted of 76% G:C to A:T transitions and 20% G:C to T:A transversions [23]. Since mass-spectrometry (MS) analysis of the tryptic digest of the reaction products indicated adducts at the N7 position of guanine, apurinic sites generated from the N7-guanine adducts were hypothesized to be responsible for the G:C to T:A transversions [23]. However, the presence of adducts at positions other than N7-guanine and the predominance of transition mutations supports the existence of additional mutagenic pathway(s) for this mechanism of toxicity. Further experiments with DBM and other dihaloalkanes revealed that hAGT exhibited a similar mechanism of genotoxicity upon reaction with these compounds with increasing activities in the order I > Br ≫ Cl [24,25].
Exposure to other bis-electrophiles including butadiene diepoxide (BDO) [25–27], epibromohydrin (EBH) [28] and nitrogen mustards [29] was subsequently shown to lead to hAGT-mediated toxicity and mutagenesis due to the formation of hAGT-DNA cross-links in a similar bacterial test system. Epoxides such as EBH are widely used in the chemical industry [30]. They have been shown to be direct acting alkylating agents inducing DNA damage [31,32]. Similarly, BDO, which is a metabolite of 1,3-butadiene, an important industrial compound and environmental pollutant, is a known carcinogen, and bifunctional alkylating agent that has been shown to cause numerous genotoxic effects [33,34].
Except for a brief report that Chinese hamster lung fibroblasts transfected to express the E. coli AGTs, Ogt or Ada, were sensitized to the growth inhibitory effects of DBE and DBM [35], AGT-mediated toxicity of bis-electrophiles has not been studied in mammalian cells. Therefore, we have examined the effects of hAGT expression on the toxicity and mutagenicity of DBE, DBM, BDO and EBH in Chinese hamster ovary (CHO) cells. The results show that an AGT-mediated pathway leading to cell death and to an increase in mutations does occur in these cells for all of these agents except BDO. However, the results differ from those seen in bacterial cells with a relatively lower increase in mutations and higher increase in toxicity.
2. Materials & Methods
2.1 Chemicals and reagents
DBE (99% purity), DBM (99% purity), EBH (98% purity) and BDO (97%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Cell culture reagents were from Gibco-Invitrogen (Carlsbad, CA). Geneticin was purchased from CellGro (Herndon, VA).
2.2 Cell culture
Experiments were carried out with CHO cells, stably transfected with either the empty pCMV expression vector or pCMV-hAGT [36]. The cells were seeded at 3 × 105 cells/75 cm2 flask and passaged weekly. Cells were grown in α-MEM media that did not contain ribonucleosides or deoxyribonucleosides (Gibco-Invitrogen, Carlsbad, CA) containing 26 mM NaHCO3, 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, CA), 100 U/ml penicillin and 100 μg/ml streptomycin. In order to maintain plasmid expression, 1 mg/ml Geneticin (CellGro, Herndon, VA) was added to the culture medium. Prior to all experiments, Western blot analysis was used to confirm appropriate hAGT protein expression in the CHO cells [36].
2.3 Cytotoxicity and hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene mutation frequency assay
Prior to treatment with the mutagens, the CHO cells were pretreated with α-MEM media containing 100 μM sodium hypoxanthine, 0.4 μM aminopterin and 16 μM thymidine (HAT) (Carlsbad, CA) for 7–10 days in order to eliminate any spontaneous HPRT mutants. Aminopterin inhibits de novo synthesis of nucleosides in cells, while HPRT and thymidine kinase supply them from hypoxanthine and thymidine. Only cells expressing wild-type HPRT and thymidine kinase enzymes can survive in the HAT selection media. Following HAT selection, aliquots of the treated cells were frozen down in freezing media [α-MEM media prepared as mentioned above but with 20% fetal bovine serum and 10% dimethyl sulfoxide (DMSO)] and stored in liquid nitrogen. These HAT-treated CHO cell aliquots were used for subsequent experiments.
For the cytotoxicity and mutation frequency assays, the HAT-treated CHO cells were plated at a density of 2 × 106 cells/75 cm2 flask and grown for 24 h. Cells were then exposed to different doses of freshly prepared DBE, DBM, BDO or EBH or up to 1% DMSO for 2 h. The mutagens were first dissolved in DMSO and then diluted to their final concentration in serum-free α-MEM media. Following mutagen exposure, the cells were washed twice with 10 ml of phosphate-buffered saline and harvested with trypsin. A portion of the treated cells were used for cytotoxicity measurements while the rest were used to estimate HPRT mutation frequency.
Cytotoxicity was determined using a colony forming assay essentially as described [36,37]. The mutagen-treated cells were plated at a density of 100–1000/25 cm2 flasks and grown for 7–8 days until discrete colonies could be observed. These colonies were then washed with a 0.9% saline solution, stained with 0.5% crystal violet in ethanol and counted. Survival rates of the mutagen-treated cells were expressed as a percentage of survival rates with the DMSO-treated cells.
For the HPRT mutation frequency assay, the mutagen-treated CHO cells were maintained in exponential growth for 7 days in order to allow cells that had incurred mutations in the HPRT gene to express this mutated protein. Next, a total of 2 × 106 cells (2 × 105cells/10 cm2 dish) were plated with fresh culture media containing 40 μM 6-thioguanine (6-TG) and incubated for 10 days to allow for the formation of HPRT mutant colonies. Plating efficiency of the mutagen-treated cells was also determined by simultaneously plating 200 cells/25 cm2 flasks in normal α-MEM media without 6-TG and maintaining them for 10 days. The cells were then stained with 0.5% crystal violet solution in ethanol and the colonies counted. The HPRT mutation frequency was calculated by counting the total number of 6-TG resistant colonies and adjusting it with the plating efficiency of the cells in non-selective media. The value was expressed as the number of HPRT mutants per 106 surviving cells.
2.4. HPRT Mutation Spectra
In order to analyze the types of mutations induced at the HPRT locus by DBE, the HAT-treated CHO cells were plated at a density of 2 × 106 cells/75 cm2 flask and grown for 24 h prior to treatment with 1.5 mM DBE for 2 h as described above. The cells were harvested and seeded into twenty 25 cm2 tissue culture flasks. In subsequent steps, each flask was treated separately. After a week of propagation in normal media, each flask of cells was harvested and 1 × 106 cells from each flask were plated (2.5 × 105 cells/10 cm2 dish) using fresh culture media containing 40 μM 6-TG. Following 10 days in the selection media, one to two HPRT mutant clones were picked from each dish and expanded in α-MEM media containing 40 μM 6-TG.
Total cytoplasmic RNA was isolated from 3 × 106 cells from each HPRT mutant clone using the RNAqueous® kit from Ambion (Austin, TX) according to manufacturer instructions. cDNA was then synthesized from 1–2 μg of this RNA using oligo d(T) as the primer with the SuperScript™ II First-Strand Synthesis System for RT-PCR kit (Invitrogen, Carlsbad, CA). The cDNA was amplified using gene-specific primers [38]. A high-fidelity PCR kit (Roche, Indianapolis, IN) was used in order to minimize secondary mutations introduced by polymerase. The forward HPRT primers (primers 1 and 2) used for this experiment are located 100-50 bps before the beginning of the open reading frame. The reverse HPRT primers (primers 3 and 4) are located 50–100 bps after the end of the open reading frame. For the first round of PCR, a 789 bp product was generated using oligonucleotide primers HPRT-1 [5′-d(CTCGGCGCCTCCTCTG-CGGG)-3′] and HPRT-4 [5′-d(GGTAATTTTACTGGGAAC)-3′] [38]. The PCR cycling conditions were as follows: initial melting (92°C, 4 min), 30 cycles of denaturation (92°C, 30 s), annealing (52°C, 30 s), extension (72°C, 1 min) followed by a last extension step at 72°C for 5 min. The size of the cDNA fragment was verified by electrophoresis in a 0.8% agarose gel in Tris-Acetate-EDTA buffer. If no product was observed, 0.1 μl of the PCR reaction was used as template in a second round of PCR with oligonucleotide primers HPRT-2 [5′-d(CTCCTCACAC-CGCTCTTCGC)-3′] and HPRT-3 [5′-d(CTCCTCGTGTTTGCAGATTC)-3′] [38]. The PCR cycling conditions differed from those described above in that the annealing temperature was 60°C and 25 cycles were used. The 657 bp product generated from this PCR reaction was confirmed with a 0.8% agarose gel. The PCR products were purified using the QiaQuick PCR purification kit from Qiagen (Valencia, CA) and eluted into water. When multiple RT-PCR products were generated, the fragments were excised from a 0.8% agarose gel, purified using the QiaQuick Gel Extraction Kit (Qiagen, Valencia, CA) and eluted into water. All purified PCR products were then sequenced at the Macromolecular Core Facility of the Milton Hershey Medical Center, Pennsylvania State University using the appropriate forward and reverse HPRT primers 1, 2, 3 and 4.
3. Results
3.1. Effect of AGT expression on cytotoxicity induced by bis-electrophiles in CHO cells
Substantial hAGT-mediated increases in both lethality and mutagenesis were observed in bacterial cells after treatment with DBE, DBM, BDO and EBH [3,19–21,23–25,27,39]. Therefore, we tested these compounds in mammalian CHO cells, which had been stably transected with a construct expressing hAGT or an empty vector control. CHO cells do not express an endogenous hAGT and the cell line transfected with an empty vector has been confirmed to lack AGT activity [37].
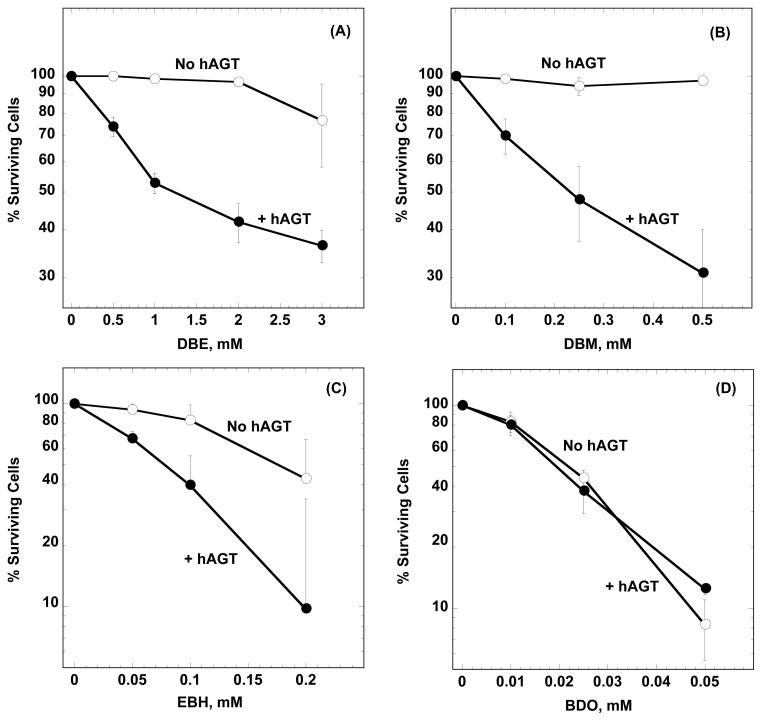
The cytotoxic effects were determined by assaying the ability of CHO cells to form colonies following exposure to the bis-electrophiles or DMSO vehicle. As indicated in Fig. 1A–C, CHO cells expressing hAGT are much more susceptible to the cytotoxic effects of DBE, DBM or EBH compared to cells with the empty pCMV vector. Thus, following a 2-hour exposure to 3 mM DBE, the survival rate of the CHO-hAGT cells decreased to ~35% while the survival of -CHO-pCMV cells was about ~75% compared to the cells that had only been exposed to DMSO. Similar values were ~30% and ~97% following exposure to 0.5 mM DBM. The concentration at which these bis-electrophiles caused 50% lethality (LC50) in CHO-hAGT cells were as follows: DBE ~ 1.3mM; DBM ~ 0.23mM; EBH ~0.08mM. In all cases, cytoxicity was dose dependent and hAGT expression increased toxicity at every dose tested. In contrast, despite the dose-dependent increase in cytotoxicity following BDO exposure at concentrations of 10–50 μM, hAGT expression had no impact on cell-killing (Fig. 1D). It should be noted that BDO was much more cytotoxic towards CHO cells than the other bis-electrophiles tested (LC50 for CHO-hAGT cells exposed to BDO ~ 0.02mM).
Figure 1. Effect of hAGT expression on toxicity of DBE, DBM, EBH and BDO in CHO cells.
CHO cells stably transfected with a pCMV expression vector or with hAGT were treated with the compounds shown for 2 h. Cell survival rates were determined as described in Materials and Methods and expressed as the percentage of survival rates of DMSO treated cells. Results are shown in Panel (A) for DBE, Panel (B) for DBM, Panel (C) for EBH and Panel (D) for BDO. Two-way ANOVA was used to analyze the effect of hAGT expression: Panel (A), pCMV versus hAGT: P <0.01; Panel (B), pCMV versus hAGT: P < 0.01; Panel (C), pCMV versus hAGT: P < 0.01; Panel (D) pCMV versus hAGT: P = 0.6.
3.2. Effect of AGT expression on mutations induced by bis-electrophiles in CHO cells
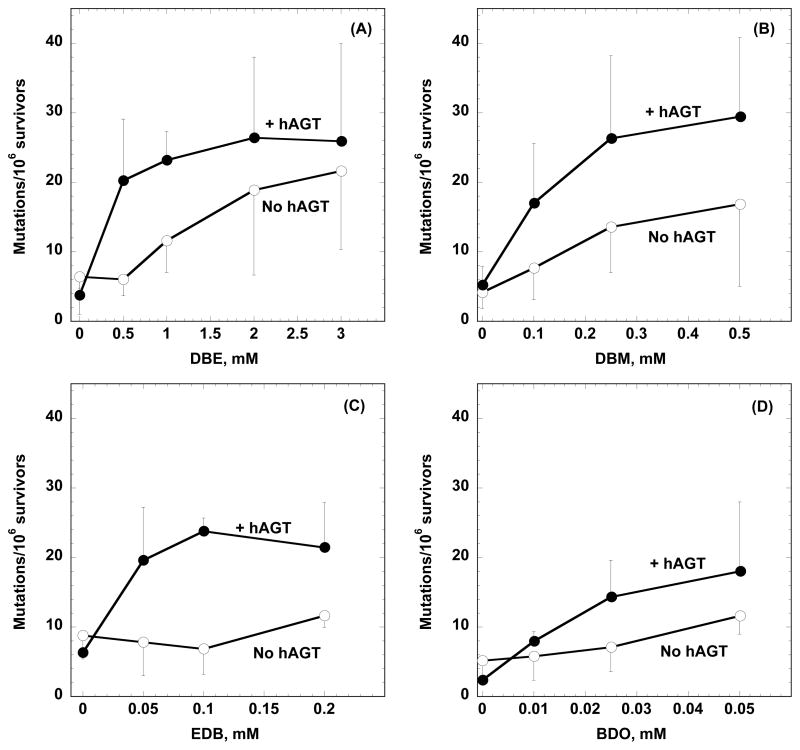
The ability of the bis-electrophiles to produce mutations was assessed using the HPRT gene as a test locus. Mutants lacking HPRT activity were isolated by growing in medium containing 6-TG. The mutation frequency was higher in cells expressing hAGT after treatment with DBE, DBM or EBH (Fig. 2A–C). The increase was small (~1.2–3.4 fold for DBE, ~1.3–2.2 fold for DBM and ~1.8–3.5 fold for EBH) but statistically significant as analyzed by a two-way ANOVA statistical test. BDO induced a slight increase in mutations in cells expressing hAGT but this difference was not statistically significant (Fig. 2D). It can be seen from Fig. 2 that the difference in mutagenesis between control and hAGT-expressing CHO cell lines exposed to DBE, DBM and EDB is much more prominent at lower doses with the mutation frequency curves converging at higher doses. This is reflected in statistical analysis comparing the hAGT effect for each dose where only the values of 0.5 mM and 1 mM for DBE, 0.1 and 0.25 mM DBM and 0.05 and 0.1 mM for EDB were significantly different.
Figure 2. Effect of hAGT expression on the productions of mutations in the HPRT gene by DBE, DBM, EBH and BDO in CHO cells.
CHO cells stably transfected with a pCMV expression vector or with hAGT were treated with the compounds shown for 2 h. Mutations were determined as described in Materials and Methods and the mutation rate expressed per 106 surviving cells grown in the absence of 6-TG. Results are shown in Panel (A) for DBE, Panel (B) for DBM, Panel (C) for EBH and Panel (D) for BDO. Two-way ANOVA was used to analyze the effect of hAGT expression: Panel (A), pCMV versus hAGT, P < 0.01; Panel (B), pCMV versus hAGT: P = 0.02; Panel (C), pCMV versus hAGT: P < 0.01; Panel (D), pCMV versus hAGT: P = 0.1. Comparison of the individual points plus and minus hAGT for each dose showed that the number of mutations for DBE doses 0.5 and 1 mM, DBM doses 0.1 and 0.25 mM and all of the doses of EDB were significantly different (P < 0.05).
3.3. Effect of AGT expression on the mutation spectrum induced by DBE in CHO cells
In order to analyze the types of mutations caused by DBE in the presence and absence of hAGT expression, 40–45 HPRT mutants were collected from each cell line following treatment with 1.5 mM DBE. RT-PCR was used for molecular characterization of these HPRT mutations. The HPRT mutation frequency at 1.5 mM DBE was 15 mutants/106 viable cells for CHO-pCMV cells and increased to 26 mutants/106 survivors with the CHO-hAGT cells which is in good agreement with that extrapolated from Figure 2A.
In the cells lacking hAGT expression, ~55% were base-pair substitution mutations the majority of which were located at G:C base pairs (Table 1). The predominant mutations by far were G:C to A:T transitions (~41% of total) followed by equal proportions of A:T to G:C transitions and G:C to T:A transversions (~5% of each). Approximately 38% of all the mutations observed were deletion mutations, which consisted mostly of one and two exon deletions (~25% of total). When hAGT was expressed there was a significant change in the mutation spectrum. There was a greater proportion (~78% of total) of base substitution mutations (*p = 0.04; Fisher’s exact test). There was a clear decrease in transition mutations (~29% of total) and proportional increases in all classes of transversion mutations (~49% of total) at both G:C (*p = 0.02; Fisher’s exact test) and A:T loci (*p = 0.002; Fisher’s exact test). The frequency of deletion mutations was decreased although within the pool of deletion mutants (~13% of total), the majority were missing entire exons with a preference for ≥ 3 exon deletions.
Table 1.
Comparison of DBE-induced mutations in CHO-pCMV and CHO-hAGT cells
| CHO-pCMV | CHO-hAGT | |||||
|---|---|---|---|---|---|---|
| Mutations | Total | % | Frequency (mutants/106 survivors) | Total | Percentage | Frequency (mutants/106 survivors) |
| Base Substitutions | 23 | 54.8 | 8.2 | 35 | 77.8 | 20.5 |
| Transitions | 19 | 45.2 | 6.8 | 13 | 28.9 | 7.6 |
| G:C → A:T | 17 | 40.5 | 6.1 | 13 | 28.9 | 7.6 |
| A:T → G:C | 2 | 4.8 | 0.7 | - | - | - |
| Transversions | 4 | 9.6 | 1.4 | 22 | 48.9 | 12.9 |
| G:C → T:A | 2 | 4.8 | 0.7 | 6 | 13.3 | 3.5 |
| G:C → C:G | 1 | 2.4 | 0.4 | 6 | 13.3 | 3.5 |
| A:T → T:A | - | - | - | 5 | 11.1 | 2.9 |
| A:T → C:G | 1 | 2.4 | 0.4 | 5 | 11.1 | 2.9 |
| Deletions | 16 | 38.1 | 5.7 | 6 | 13.3 | 3.5 |
| One exon | 9 | 21.4 | 3.2 | 1 | 2.2 | 0.6 |
| Two exon | 2 | 4.8 | 0.7 | - | - | - |
| ≥ 3 exons | - | - | - | 4 | 8.9 | 2.3 |
| Other deletions | 5 | 11.9 | 1.8 | 1 | 2.2 | 0.6 |
| Other mutations | 3 | 7.2 | 1.1 | 4 | 8.8 | 2.4 |
| Duplication | - | - | - | 1 | 2.2 | 0.6 |
| Duplication/Inversion | 2 | 4.8 | 0.7 | - | - | - |
| Complex | - | - | - | 1 | 2.2 | 0.6 |
| Unknown | 1 | 2.4 | 0.4 | 2 | 4.4 | 1.2 |
| Mutants collected | 42 | 45 | ||||
| Estimated DBE -induced mutation frequency | 15 | 26.4 | ||||
Characteristics of the base-pair mutations are shown in Table 2. There was no obvious hAGT-related difference in the specific sites of genomic alterations. With the assumption that all base-pair substitutions observed in this study are due to miscoding with a premutagenic lesion on a purine, which would be in accordance with previous studies that this is the predominant site of DBE induced DNA damage in either the presence or absence of AGT [3,10,23,40–44], it appears that the control CHO cells demonstrated a preference for mutations at the second purine of 5′-PuPu sequences, many of which were at 5′-TGG or 5′-CAG sites. This was consistent with previously reported experiments [45] and this pattern was also seen in the CHO-hAGT cells. However, expression of hAGT increased the mutations arising from primary damage on the transcribed strand, which were not seen in the CHO-pCMV cells.
Table 2.
Types and locations of base-pair substitution mutations in the coding region of the hprt gene in mutants derived from DBE-treatment
| Positiona (Exon) | Mutationb | Amino Acid Change | Target sequencec | Number | Strand with mutagenic lesiond |
|---|---|---|---|---|---|
| CHO-pCMV cells. | |||||
| 119 (2) | GC → AT | Gly → Glu | CAT GGA GTG | 1 | NTS |
| 134 (2) | GC → AT | Arg → Lys | GAC AGG ACT | 4 | NTS |
| 197 (3) | GC → AT | Cys → Tyr | CTC TGT GTG | 1 | NTS |
| 208 (3) | GC → AT | Gly → Arg | AAG GGG GGC | 6 | NTS |
| 539 (8) | GC → AT | Gly → Glu | GTT GGA TTT | 1 | NTS |
| 569 (8) | GC → AT | Gly → Glu | GTT GGA TAT | 2 | NTS |
| 580 (8) | GC → AT | Asp → Asn | CTT GAC TAT | 1 | NTS |
| 601 (8) | GC → AT | Asp → Asn | AAG GAT TTG | 1 | NTS |
| 212 (3) | GC → TA | Gly → Val | GGG GGC TAT | 2 | NTS |
| 580 (8) | GC → CG | Asp → His | CTT GAC TAT | 1 | NTS |
| 136 (3) | AT → CG | Thr → Pro | AGG ACT GAA | 1 | NTS |
| 404 (6) | AT → GC | Asp → Gly | GAG GAC ATA | 2 | NTS |
| CHO-hAGT cells | |||||
| 119 (2) | GC → AT | Gly → Glu | CAT GGA GTG | 1 | NTS |
| 134 (2) | GC → AT | Arg → Lys | GAC AGG ACT | 2 | NTS |
| 149 (3) | GC → AT | Ala → Val | CTT GCC CGA | 1 | TS |
| 212 (3) | GC → AT | Gly → Asp | GGG GGC TAT | 3 | NTS |
| 419 (6) | GC → AT | Gly → Asp | ACT GGT AAA | 2 | NTS |
| 569 (8) | GC → AT | Gly → Glu | GTT GGA TAT | 1 | NTS |
| 577 (8) | GC → AT | Leu → Phe | GCC CTT GAC | 1 | TS |
| 589 (8) | GC → AT | Glu → Lys | AAG GAG TAC | 2 | NTS |
| 47 (2) | GC → TA | Gly → Val | CCA GGC TAT | 2 | NTS |
| 119 (2) | GC → TA | Gly → Val | CAT GGA GTG | 1 | NTS |
| 143 (2) | GC → TA | Arg → Ile | GAA AGA CTT | 1 | NTS |
| 191 (3) | GC → TA | Ala → Asp | GTG GCC CTC | 2 | TS |
| 130 (2) | GC → CG | Asp → His | ATG GAC AGG | 3 | NTS |
| 403 (6) | GC → CG | Asp → His | GAG GAC ATA | 1 | NTS |
| 606 (8) | GC → CG | Leu → Phe | GAT TTG AAT | 2 | NTS |
| 299 (3) | AT → CG | Ile → Ser | TTT ATC AGA | 2 | TS |
| 605 (8) | AT → CG | Leu → Trp | GAT TTG AAT | 3 | TS |
| 64 (2) | AT → TA | Phe → Ile | TTA TTT TGT | 1 | TS |
| 133 (2) | AT → TA | Arg → Trp | GAC AGG ACT | 3 | NTS |
| 217 (3) | AT → TA | Lys → Stop | TAT AAA TTC | 1 | NTS |
Positions in the coding region are numbered in such a manner that A of the ATG initiation codon is designated as the first nucleotide.
Mutations were characterized at the cDNA level
The altered bases are underlined.
NTS = non-transcribed strand; TS = transcribed strand. This classification assumes that the pre-mutagenic lesion occurs on a purine.
4. Discussion
These results demonstrate that the presence of hAGT can increase both mammalian cell killing and mutagenesis after exposure to dihaloalkanes or EBH. Activation mediated via an hAGT-linked pathway is therefore an additional way in which the genotoxicity of these compounds may occur. Although we did not test this directly, it is very likely that the underlying mechanism of this enhanced toxicity is the formation of DNA-hAGT cross-links as indicated in studies with bacterial cells and in vitro experiments [3,23,24,28]. Attempts to isolate the hAGT-DNA crosslink directly from the mammalian cells were not successful but this experiment is technically very challenging since the number of adducts is likely to be very low and the presence of other proteins interferes with immunoprecipitation techniques.
A significant difference in the current results of mammalian cells and those previously published using E. coli system is the relative degree of response between cytotoxicity and mutagenesis. TRG8 E. coli cells expressing hAGT demonstrated a very large increase (2–3 orders of magnitude) in the mutation rate with a lesser decrease in cell survival in response to these bifunctional agents [3,23,24,28]. In contrast, the sensitization of the CHO-hAGT cells to the cytotoxic effects of DBE, DBM and EBH was much more drastic than the increase in mutagenic potential in these cells.
Although the pCMV vector is a very strong promoter, the level of AGT protein expression on a per cell basis in the CHO cells is c. 20 % of that in the bacteria used in previous studies. It is therefore possible, but unlikely, that the differences in genotoxicity between mammalian and bacterial cells are due to different expression levels of hAGT in the systems. However, if that were indeed the case, then one would expect the degree of reduction in cell survival to be similar to the increase in mutation frequency. Instead, our CHO cell results demonstrated that the cell-killing mediated by hAGT was far greater than the increase in mutation frequency. Thus, a more plausible explanation may be found in differences in pathways for processing DNA damage. Studies have shown that mammalian processing of DNA adducts may occur differently in bacteria leading to the differential degrees of response to cytotoxicity and mutagenesis observed between such model systems [46,47].
Protein-DNA cross-links may be more toxic to mammalian cells. Furthermore, the modification of such lesions allowing DNA polymerases to copy past them yielding point mutations may also occur more readily in the bacterial system. The bacterial experiments specifically assayed for single base-pair mutations affecting gene function. Although such mutations were the majority of those seen in the current investigation with mammalian cells using the HPRT gene as reporter, the spectrum observed was more comprehensive ranging from base-pair substitutions to large deletions or insertions at the hprt locus [48]. It is likely that protein-DNA cross-links pose a significant ‘road-block’ to replicative polymerases and transcription complexes [3,23,25]. Subsequent inefficient processing causing DNA strand breakage, errors in DNA replication and/or mis-repair may yield large-scale deletions [49,50], which can significantly reduce cell survival while simultaneously decreasing the number of viable mutants [51]. The difference in mutation frequency between control and hAGT-expressing CHO cell lines treated with DBM, DBE and EBH was more obvious at lower doses. In contrast, the cytotoxicity results continue to diverge even at higher doses (Fig. 1). This suggests that the DNA-protein adducts, in the context of these studies, are more efficient at inducing lethality rather than mutagenesis.
Although the hAGT-mediated increase in mutation frequency was small, it was statistically significant. Another argument supporting the hypothesis that hAGT does influence mutations is provided in the analysis of the mutation spectra after DBE treatment since there was a marked difference in the types of genomic alterations observed. The HPRT mutation spectrum demonstrated by our CHO-pCMV cells was similar to that obtained by Ballering et al. who analyzed HPRT mutants for untransfected CHO-9 cells exposed to 1.0 mM DBE [45]. They showed that ~58% of the HPRT mutants sequenced were base-pair substitutions, while we observed ~55% base-pair substitutions. The majority of the base-pair substitution mutations detected in both studies were G:C to A:T transition mutations (~45% of all the sequenced mutants), which could be attributed to S-[2-(N7-guanyl)ethyl]glutathione, a major DBE adduct which is formed by conjugation to glutathione, and has been shown in vitro to miscode for these mutations with different polymerases [44,45,52]. Assuming that the point mutations that were found occurred from miscoding with a premutagenic lesion on a purine [3,10,23,40–44], all the base-pair substitutions seen with the CHO-pCMV cells in our study were located on the non-transcribed strand. This can be accounted for by the relatively efficient repair of lesions in the coding strand by transcription-coupled repair. As previously reported [45], there were a number of single exon deletions, which may be due to DNA rearrangements or point mutations of splice acceptor sites at intron-exon boundaries [45,53–55].
When hAGT was expressed, there was a significant increase in base-pair substitutions and a change in the type of mutations with a significant increase in transversions. One plausible explanation for the increase in G:C to T:A transversions mutations is that they are due to depurination of adducts formed by DBE-activated hAGT at the guanine-N7 position. Such an adduct has been characterized from the reaction between DBE, hAGT and DNA [23]. The resulting apurinic site and similar non-informational or non-coding lesions are known to lead to the incorporation of A resulting in the G:C to T:A change [56–58]. Although such an adduct has not yet been characterized, attachment of the reactive hAGT-S(CH2)2 to either the N7 or N3 position of adenine may lead to the increased A:T to T:A transversions.
Other explanations for the increased base-pair mutations are also possible. The extremely bulky nature of the covalent DNA-protein cross-links induced by the DBE and hAGT interaction pose major obstacles to canonical DNA replicative polymerases [59]. Such lesions probably require error-prone trans-lesion polymerases to restart the replication fork. It is possible that the adduct can rotate the damaged base about its N-glycosyl bond altering it from an anti- to syn- conformation [60,61]. This conformational rearrangement may facilitate mis-incorporation of incorrect nucleotides by error-prone translesion polymerases [60,61] generating the other base substitutions (G:C to A:T, G:C to C:G and A:T to C:G) observed in our mutation analysis of CHO-hAGT cells. Proteolytic degradation of the DBE-induced hAGT-DNA adduct into a smaller lesion [59,62] may be required for the DNA damaged to be transcribed by bypass polymerases.
The presence of hAGT enhanced the number of point mutations at G:C sites in the transcribed strand, from 0/20 in CHO-pCMV cells to 4/25 in the CHO-hAGT cells (Table 2). This suggests that the bulky adducts formed by the hAGT-DNA cross-links are poorly or inaccurately repaired irrespective of the strand to which the adduct is attached. There is also the possibility that hAGT expression facilitated mutations in the transcribed strand at A:T sites, which increased from 0/3 to 6/10 (Table 2). However, it is not certain that all of these mutations arise from damage on A since hAGT can be cross-linked by DBE to T residues in in vitro experiments [3,23].
Interestingly, there was a significant decrease in the frequency of deletion mutations caused by DBE when hAGT was expressed. This suggests that the bulk of the of the hAGT-mediated mutations are not due to inaccurate homologous recombination repair resulting stalled replication forks or a double stranded DNA breaks resulting from the replicative stress caused by the DNA-protein crosslinks. Such repair would be likely to lead to increased inversions, duplications, and deletions.
Both DBM and EBH gave similar results to DBE with a strong enhancement of cytotoxicity and a small increase in mutations. In the presence of hAGT, both were more toxic than DBE with 50% killing at about 1 mM for DBE, 0.2 mM for DBM and 0.08 mM for EBH. At these doses, there was little effect on viability in the control CHO cells that lack AGT. As with DBE, the increase in mutations by DBM and EBH was only 2–3-fold in contrast to the 1000-fold increase seen in bacterial systems. Although the enhanced mutation frequency was relatively small, it should be noted that over the dose range studied it accounted for a significant percentage of the total mutations suggesting that hAGT-mediated DNA damage is one of the factors contributing to the mutagenicity of these agents. Loeber et al. [29] have shown that nitrogen mustards form DNA-hAGT cross-links in mammalian cells, suggesting that these agents also cause mutagenic and cytotoxic damage in this manner.
Previous studies of the mechanism of enhancement of DNA damage by these compounds have shown that DBE and DBM exert their effects solely by reacting first with hAGT and that interaction occurs only at the Cys145 acceptor site [23,24]. The intermediate formed is highly unstable in an aqueous environment but can react with cellular DNA. EBH may act predominantly in the same way but can also form adducts at Cys150 of hAGT and can react first directly with DNA with the resulting adduct then forming a covalent bond with hAGT [28]. The improved stability of the intermediates formed in the these reactions may account for the huge increase (about 1400-fold) in mutations when EBH was used in bacterial test systems [28]. However, this was not seen in the mammalian system where the increase in mutations was small and similar to that seen with DBM and DBE.
The presence of hAGT did not produce a significant effect on cell killing or mutations induced by BDO. This contrasts strongly with the effects seen in bacteria and in in vitro studies that have demonstrated its ability to form covalent hAGT-DNA adducts at both Cys145 and Cys150 residues [25–27]. However, BDO was very toxic to the control CHO cells with a 50% reduction in viability at c. 20 μM and it is possible that it may rapidly initiate cytotoxic cellular damage triggering substantial reductions in cell viability before any hAGT-DNA cross-linking can develop. BDO is known to cause numerous genotoxic effects both in vitro and in vivo, including double strand breaks, DNA cross-links, sister chromatin exchanges, and chromosomal aberrations [63,64], all of which likely contribute to its potency at diminishing cell survival.
Overall, our experiments have demonstrated the potentiation of mammalian cell killing and mutagenesis by bis-electrophiles in the presence of hAGT. This genetic damage is most likely due to the DNA-protein adducts detected in vitro [3,23,26,29]. Based on the HPRT mutation spectra analyses, we can conclude that there are at least two different mutagenic mechanisms for the induction of genome alterations by AGT-DNA cross-links in CHO cells. The presence of G:C to T:A transversion mutations observed in CHO cells expressing hAGT when they are exposed to either dibromoalkanes or EBH are suggestive of toxic abasic site processing likely resulting from an N7-guanine adduct that spontaneously depurinates [23]. Currently, the mechanism(s) underlying the other types of mutations and the cytotoxicity observed in bacterial and mammalian cell model systems have not been fully defined. Although the frequency of AGT-mediated mutations was much less than seen in bacteria, such mutations may represent a significant fraction of the genetic alterations caused by these bifunctional agents in cells such as liver that express high levels of AGT [65]. The relatively greater ratio of cell killing to mutations seen in CHO-hAGT cells compared to bacterial cells may be due to the tendency of mammalian cells to undergo apoptosis in response to significant and persistent DNA damage such as DNA-protein cross-links. The translation of the hAGT-DNA cross-link into lethality or mutations will depend on the chemical stability and structure of the lesion in question and the surrounding DNA sequence context, which in turn will affect its ability to either be repaired or trigger error-prone or error-free DNA damage tolerance pathways.
Acknowledgments
This work was supported by grants CA-018137 and CA-071976. We thank Dr. Natalia A. Loktionova for help with the manuscript preparation.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Komsta E, Chu I, Secours VE, Valli VE, Villeneuve DC. Results of a short-term toxicity study for three organic chemicals found in Niagara River drinking water. Bull Environ Contam Toxicol. 1988;41:515–522. doi: 10.1007/BF02020995. [DOI] [PubMed] [Google Scholar]
- 2.Rannug U. Genotoxic effects of 1,2-dibromoethane and 1,2-dichloroethane. Mutat Res. 1980;76:269–295. doi: 10.1016/0165-1110(80)90020-2. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Pegg AE, Williams KM, Guengerich FP. Paradoxical enhancement of the toxicity of 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase. J Biol Chem. 2002;277:37920–37928. doi: 10.1074/jbc.M205548200. [DOI] [PubMed] [Google Scholar]
- 4.Poelarends GJ, van Hylckama Vlieg JE, Marchesi JR, Freitas Dos Santos LM, Janssen DB. Degradation of 1,2-dibromoethane by Mycobacterium sp. strain GP1. J Bacteriol. 1999;181:2050–2058. doi: 10.1128/jb.181.7.2050-2058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osterman-Golkar S, Hussain S, Walles S, Anderstam B, Sigvardsson K. Chemical reactivity and mutagenicity of some dihalomethanes. Chem Biol Interact. 1983;46:121–130. doi: 10.1016/0009-2797(83)90011-x. [DOI] [PubMed] [Google Scholar]
- 6.Rannug U, Sundvall A, Ramel C. The mutagenic effect of 1,2-dichloroethane on Salmonella typhimurium I. Activation through conjugation with glutathione in vitro. Chem Biol Interact. 1978;20:1–16. doi: 10.1016/0009-2797(78)90076-5. [DOI] [PubMed] [Google Scholar]
- 7.Roldan-Arjona T, Pueyo C. Mutagenic and lethal effects of halogenated methanes in the Ara test of Salmonella typhimurium: quantitative relationship with chemical reactivity. Mutagenesis. 1993;8:127–131. doi: 10.1093/mutage/8.2.127. [DOI] [PubMed] [Google Scholar]
- 8.van Bladeren PJ, Breimer DD, Rotteveel-Smijs GM, de Jong RA, Buijs W, van der Gen A, Mohn GR. The role of glutathione conjugation in the mutagenicity of 1,2-dibromoethane. Biochem Pharmacol. 1980;29:2975–2982. doi: 10.1016/0006-2952(80)90047-7. [DOI] [PubMed] [Google Scholar]
- 9.Guengerich FP, Kim MS, Muller M, Lowe LG. Chemical mechanisms of formation of DNA-carcinogen adducts, elucidation of potential of adducts for mutagenicity, and mechanisms of polymerase fidelity and mutation in the presence of adducts. Recent Results Cancer Res. 1997;143:49–63. doi: 10.1007/978-3-642-60393-8_4. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa N, Guengerich FP. Evidence for formation of an S-[2-(N7-guanyl)ethyl]glutathione adduct in glutathione-mediated binding of the carcinogen 1,2-dibromoethane to DNA. Proc Natl Acad Sci U S A. 1983;80:5266–5270. doi: 10.1073/pnas.80.17.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thier R, Pemble SE, Kramer H, Taylor JB, Guengerich FP, Ketterer B. Human glutathione S-transferase T1-1 enhances mutagenicity of 1,2-dibromoethane, dibromomethane and 1,2,3,4-diepoxybutane in Salmonella typhimurium. Carcinogenesis. 1996;17:163–166. doi: 10.1093/carcin/17.1.163. [DOI] [PubMed] [Google Scholar]
- 12.Thier R, Taylor JB, Pemble SE, Humphreys WG, Persmark M, Ketterer B, Guengerich FP. Expression of mammalian glutathione S-transferase 5-5 in Salmonella typhimurium TA1535 leads to base-pair mutations upon exposure to dihalomethanes. Proc Natl Acad Sci USA. 1993;90:8576–8580. doi: 10.1073/pnas.90.18.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bladeren PJ, Breimer DD, Rotteveel-Smijs GM, Hoogeterp JJ, Mohn GR, de Groot A, van Zeeland AA, van der Gen A. The activating role of glutathione in the mutagenicity of 1,2-dibromoethane. Adv Exp Med Biol. 1981;136(Pt A):809–820. doi: 10.1007/978-1-4757-0674-1_63. [DOI] [PubMed] [Google Scholar]
- 14.van Bladeren PJ, Breimer DD, Rotteveel-Smijs GM, Mohn GR. Mutagenic activation of dibromomethane and diiodomethane by mammalian microsomes and glutathione S-transferases. Mutat Res. 1980;74:341–346. doi: 10.1016/0165-1161(80)90192-2. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler JB, Stourman NV, Thier R, Dommermuth A, Vuilleumier S, Rose JA, Armstrong RN, Guengerich FP. Conjugation of haloalkanes by bacterial and mammalian glutathione transferases: mono- and dihalomethanes. Chem Res Toxicol. 2001;14:1118–1127. doi: 10.1021/tx010019v. [DOI] [PubMed] [Google Scholar]
- 16.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 17.Margison GP, Santibáñez-Koref MF. O6-Alkylguanine-DNA alkyltransferase: role in carcinogenesis and chemotherapy. BioEssays. 2002;24:255–266. doi: 10.1002/bies.10063. [DOI] [PubMed] [Google Scholar]
- 18.Tubbs JL, Pegg AE, Tainer JA. DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy. DNA Repair (Amst) 2007;6:1100–1115. doi: 10.1016/j.dnarep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abril N, Luque-Romero FL, Christians FC, Encell LP, Loeb LA, Pueyo C. Human O6-alkylguanine-DNA alkyltransferase: protection against alkylating agents and sensitization to dibromoalkanes. Carcinogenesis. 1999;20:2089–2094. doi: 10.1093/carcin/20.11.2089. [DOI] [PubMed] [Google Scholar]
- 20.Abril N, Luque-Romero FL, Prieto-Alamo MJ, Margison GP, Pueyo C. Ogt alkyltransferase enhances dibromoalkane mutagenicity in excision repair-deficient Escherichia coli K-12. Mol Carcinog. 1995;12:110–117. doi: 10.1002/mc.2940120208. [DOI] [PubMed] [Google Scholar]
- 21.Abril N, Luque-Romero FL, Prieto-Alamo MJ, Rafferty JA, Margison GP, Pueyo C. Bacterial and mammalian DNA alkyltransferases sensitize Escherichia coli to the lethal and mutagenic effects of dibromoalkanes. Carcinogenesis. 1997;18:1883–1888. doi: 10.1093/carcin/18.10.1883. [DOI] [PubMed] [Google Scholar]
- 22.Abril N, Luque-Romero FL, Yamada M, Nohmi T, Pueyo C. The effectiveness of the O6-alkylguanine-DNA alkyltransferase encoded by the ogt(ST) gene from S. typhimurium in protection against alkylating drugs, resistance to O6-benzylguanine and sensitisation to dibromoalkane genotoxicity. Mutat Res. 2001;497:111–121. doi: 10.1016/s1383-5718(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Hachey DL, Valadez G, Williams KM, Guengerich FP, Loktionova NA, Kanugula S, Pegg AE. Characterization of a mutagenic DNA adduct formed from 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase. J Biol Chem. 2004;279:4250–4259. doi: 10.1074/jbc.M311105200. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Williams KM, Guengerich FP, Pegg AE. O6-Alkylguanine-DNA alkyltransferase has opposing effects in modulating the genotoxicity of dibromomethane and bromomethyl acetate. Chem Res Toxicol. 2004;17:742–752. doi: 10.1021/tx049958o. [DOI] [PubMed] [Google Scholar]
- 25.Valadez JG, Liu L, Loktionova NA, Pegg AE, Guengerich FP. Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase. Chem Res Toxicol. 2004;17:972–982. doi: 10.1021/tx049897u. [DOI] [PubMed] [Google Scholar]
- 26.Loeber R, Rajesh M, Fang Q, Pegg AE, Tretyakova N. Cross-linking of the human DNA repair protein O6-alkylguanine-DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem Res Toxicol. 2006;19:645–654. doi: 10.1021/tx0600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalapila AG, Loktionova NA, Pegg AE. Alkyltransferase-mediated toxicity of 1,3-butadiene diepoxide. Chem Res Toxicol. 2008;21:1851–1861. doi: 10.1021/tx800178t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalapila AG, Loktionova NA, Pegg AE. Effect of O6-alkylguanine-DNA alkyltransferase on genotoxicity of epihalohydrins. Environ Mol Mutagen. 2009;50:502–514. doi: 10.1002/em.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeber R, Michaelson E, Fang Q, Campbell C, Pegg AE, Tretyakova N. Cross-linking of the DNA repair protein O6-alkylguanine-DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards. Chem Res Toxicol. 2008;21:787–795. doi: 10.1021/tx7004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolman A, Chovanec M, Osterman-Golkar S. Genotoxic effects of ethylene oxide, propylene oxide and epichlorohydrin in humans: update review (1990–2001) Mutat Res. 2002;512:173–194. doi: 10.1016/s1383-5742(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Ehrenberg L, Hussain S. Genetic toxicity of some important epoxides. Mutat Res. 1981;86:1–113. doi: 10.1016/0165-1110(81)90034-8. [DOI] [PubMed] [Google Scholar]
- 32.Mlejnek P, Kolman A. Effects of three epoxides -ethylene oxide, propylene oxide and epichlorohydrin- on cell cycle progression and cell death in human diploid fibroblasts. Chem Biol Interact. 1999;117:219–239. doi: 10.1016/s0009-2797(98)00109-4. [DOI] [PubMed] [Google Scholar]
- 33.Adler ID, Cochrane J, Osterman-Golkar S, Skopek TR, Sorsa M, Vogel E. 1,3-Butadiene working group report. Mutat Res. 1995;330:101–114. doi: 10.1016/0027-5107(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 34.Van Duuren BL, Langseth L, Goldschmidt BM, Orris L. Carcinogenicity of epoxides, lactones, and peroxy compounds. VI. Structure and carcinogenic activity. J Natl Cancer Inst. 1967;39:1217–1228. [PubMed] [Google Scholar]
- 35.Abril N, Margison GP. Mammalian cells expressing Escherichia coli O6-alkylguanine-DNA alkyltransferases are hypersensitive to dibromoalkanes. Chem Res Toxicol. 1999;12:544–551. doi: 10.1021/tx980250h. [DOI] [PubMed] [Google Scholar]
- 36.Loktionova NA, Xu-Welliver M, Crone TM, Kanugula S, Pegg AE. Protection of CHO cells by mutant forms of O6-alkylguanine-DNA alkyltransferase from killing by 1,3-bis-(2-chloroethyl)-1-nitrosourea (BCNU) plus O6-benzylguanine or O6-benzyl-8-oxoguanine. Biochem Pharmacol. 1999;58:237–244. doi: 10.1016/s0006-2952(99)00095-7. [DOI] [PubMed] [Google Scholar]
- 37.Loktionova NA, Pegg AE. Point mutations in human O6-alkylguanine-DNA alkyltransferase prevent the sensitization by O6-benzylguanine to killing by N,N′-bis (2-chloroethyl)-N-nitrosourea. Cancer Res. 1996;56:1578–1583. [PubMed] [Google Scholar]
- 38.Lee DH, Kim TH, Lee SY, Kim HJ, Rhee SK, Yoon B, Pfeifer GP, Lee CS. Mutations induced by 1,3-butadiene metabolites, butadiene diol epoxide, and 1,2,3,4-diepoxybutane at the Hprt locus in CHO-K1 cells. Mol Cells. 2002;14:411–419. [PubMed] [Google Scholar]
- 39.Liu H, Xu-Welliver M, Pegg AE. The role of human O6-alkylguanine-DNA alkyltransferase in promoting 1,2-dibromoethane-induced genotoxicity in Escherichia coli. Mutat Res. 2000;452:1–10. doi: 10.1016/s0027-5107(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 40.Koga N, Inskeep PB, Harris TM, Guengerich FP. S-[2-(N7-guanyl)ethyl]glutathione, the major DNA adduct formed from 1,2-dibromoethane. Biochemistry. 1986;25:2192–2198. doi: 10.1021/bi00356a051. [DOI] [PubMed] [Google Scholar]
- 41.Kim DH, Guengerich FP. Formation of the DNA adduct S-[2-(N7-guanyl)ethyl]glutathione from ethylene dibromide: effects of modulation of glutathione and glutathione S-transferase levels and lack of a role for sulfation. Carcinogenesis. 1990;11:419–424. doi: 10.1093/carcin/11.3.419. [DOI] [PubMed] [Google Scholar]
- 42.Kim DH, Humphreys WG, Guengerich FP. Characterization of S-[2-(N1-adenyl)ethyl]glutathione as an adduct formed in RNA and DNA from 1,2-dibromoethane. Chem Res Toxicol. 1990;3:587–594. doi: 10.1021/tx00018a015. [DOI] [PubMed] [Google Scholar]
- 43.Cmarik JL, Inskeep PB, Meredith MJ, Meyer DJ, Ketterer B, Guengerich FP. Selectivity of rat and human glutathione S-transferases in activation of ethylene dibromide by glutathione conjugation and DNA binding and induction of unscheduled DNA synthesis in human hepatocytes. Cancer Res. 1990;50:2747–2752. [PubMed] [Google Scholar]
- 44.Inskeep PB, Guengerich FP. Glutathione-mediated binding of dibromoalkanes to DNA: specificity of rat glutathione-S-transferases and dibromoalkane structure. Carcinogenesis. 1984;5:805–808. doi: 10.1093/carcin/5.6.805. [DOI] [PubMed] [Google Scholar]
- 45.Ballering LA, Vogel EW, Vrieling H, Nivard MJ. Strand-specific mutation induction by 1,2-dibromomethane at the hypoxanthine-guanine phosphoribosyltransferase locus of Chinese hamster ovary cells. Mutagenesis. 1998;13:61–65. doi: 10.1093/mutage/13.1.61. [DOI] [PubMed] [Google Scholar]
- 46.Kanuri M, Minko IG, Nechev LV, Harris TM, Harris CM, Lloyd RS. Error prone translesion synthesis past gamma-hydroxypropanodeoxyguanosine, the primary acrolein-derived adduct in mammalian cells. J Biol Chem. 2002;277:18257–18265. doi: 10.1074/jbc.M112419200. [DOI] [PubMed] [Google Scholar]
- 47.Moriya M, Pandya GA, Johnson F, Grollman AP. Cellular response to exocyclic DNA adducts. IARC Sci Publ. 1999:263–270. [PubMed] [Google Scholar]
- 48.Dogliotti E, Vitelli A, Terlizzese M, Di Muccio A, Calcagnile A, Saffiotti U, Bignami M. Induction kinetics of mutations at two genetic loci, DNA damage and repair in CHO cells after different exposure times to N-ethyl-N-nitrosourea. Carcinogenesis. 1987;8:25–31. doi: 10.1093/carcin/8.1.25. [DOI] [PubMed] [Google Scholar]
- 49.Seigneur M, Bidnenko V, Ehrlich SD, Michel B. RuvAB acts at arrested replication forks. Cell. 1998;95:419–430. doi: 10.1016/s0092-8674(00)81772-9. [DOI] [PubMed] [Google Scholar]
- 50.Wu XC, Marcinkowski K, Turner PM, Ferguson LR. Mutations induced by some DNA minor groove binding alkylators in AS52 Chinese hamster cells. Mutat Res. 2000;448:35–45. doi: 10.1016/s0027-5107(99)00229-8. [DOI] [PubMed] [Google Scholar]
- 51.Koberle B, Roscheisen C, Helbig R, Speit G. Molecular characterization of methyl methanesulphonate (MMS)-induced HPRT mutations in V79 cells. Mutat Res. 1993;301:65–71. doi: 10.1016/0165-7992(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 52.Kim MS, Guengerich FP. Polymerase blockage and misincorporation of dNTPs opposite the ethylene dibromide-derived DNA adducts S-[2-(N7-guanyl)ethyl]glutathione, S-[2-(N2-guanyl)ethyl]glutathione, and S-[2-(O6-guanyl)ethyl]glutathione. Chem Res Toxicol. 1998;11:311–316. doi: 10.1021/tx970206m. [DOI] [PubMed] [Google Scholar]
- 53.Wei SJ, Chang RL, Bhachech N, Cui XX, Merkler KA, Wong CQ, Hennig E, Yagi H, Jerina DM, Conney AH. Dose-dependent differences in the profile of mutations induced by (+)-7R,8S-dihydroxy-9S,10R-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in the coding region of the hypoxanthine (guanine) phosphoribosyltransferase gene in Chinese hamster V-79 cells. Cancer Res. 1993;53:3294–3301. [PubMed] [Google Scholar]
- 54.Xu Z, Yu Y, Schwartz JL, Meltz ML, Hsie AW. Molecular nature of spontaneous mutations at the hypoxanthine-guanine phosphoribosyltransferase (HPRT) locus in Chinese hamster ovary cells. Environ Mol Mutagen. 1995;26:127–138. doi: 10.1002/em.2850260206. [DOI] [PubMed] [Google Scholar]
- 55.Yang JL, Hsieh YC, Wu CW, Lee TC. Mutational specificity of chromium(VI) compounds in the hprt locus of Chinese hamster ovary-K1 cells. Carcinogenesis. 1992;13:2053–2057. doi: 10.1093/carcin/13.11.2053. [DOI] [PubMed] [Google Scholar]
- 56.Kunkel TA. Mutational specificity of depurination. Proc Natl Acad Sci USA. 1984;81:1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eckert KA, Ingle CA, Klinedinst DK, Drinkwater NR. Molecular analysis of mutations induced in human cells by N-ethyl-N-nitrosourea. Mol Carcinogenesis. 1988;1:50–56. doi: 10.1002/mc.2940010111. [DOI] [PubMed] [Google Scholar]
- 58.Grevatt PC, Donahue JM, Bhanot OS. The role of N3-ethyldeoxythymidine in mutagenesis and cytotoxicity by ethylating agents. J Biol Chem. 1991;266:1269–1275. [PubMed] [Google Scholar]
- 59.Reardon JT, Cheng Y, Sancar A. Repair of DNA-protein cross-links in mammalian cells. Cell Cycle. 2006;5:1366–1370. doi: 10.4161/cc.5.13.2892. [DOI] [PubMed] [Google Scholar]
- 60.Scholdberg TA, Merritt WK, Dean SM, Kowalcyzk A, Harris CM, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Structure of an oligodeoxynucleotide containing a butadiene oxide-derived N1 beta-hydroxyalkyl deoxyinosine adduct in the human N-ras codon 61 sequence. Biochemistry. 2005;44:3327–3337. doi: 10.1021/bi0482452. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Shapiro R, Broyde S. Molecular dynamics of a food carcinogen-DNA adduct in a replicative DNA polymerase suggest hindered nucleotide incorporation and extension. Chem Res Toxicol. 2005;18:1347–1363. doi: 10.1021/tx050132b. [DOI] [PubMed] [Google Scholar]
- 62.Minko IG, Kurtz AJ, Croteau DL, Van Houten B, Harris TM, Lloyd RS. Initiation of repair of DNA-polypeptide cross-links by the UvrABC nuclease. Biochemistry. 2005;44:3000–3009. doi: 10.1021/bi0478805. [DOI] [PubMed] [Google Scholar]
- 63.Antsypovich S, Quirk-Dorr D, Pitts C, Tretyakova N. Site specific N6-(2-hydroxy-3,4-epoxybut-1-yl)adenine oligodeoxynucleotide adducts of 1,2,3,4-diepoxybutane: synthesis and stability at physiological pH. Chem Res Toxicol. 2007;20:641–649. doi: 10.1021/tx060178k. [DOI] [PubMed] [Google Scholar]
- 64.Goggin M, Swenberg JA, Walker VE, Tretyakova N. Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res. 2009;69:2479–2486. doi: 10.1158/0008-5472.CAN-08-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pegg AE, Roberfroid M, von Bahr C, Foote RS, Mitra S, Bresil H, Likachev A, Montesano R. Removal of O6-methylguanine from DNA by human liver fractions. Proc Natl Acad Sci USA. 1982;79:5162–5165. doi: 10.1073/pnas.79.17.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]