Abstract
Examination of anterior cruciate ligament (ACL) anatomy is of great interest both in studying injury mechanisms and surgical reconstruction. However, after a typical acute ACL rupture it is not possible to measure the dimensions of the ACL itself due to concomitant or subsequent degeneration of the remaining ligamentous tissue. The contralateral ACL may be an appropriate surrogate for measuring anatomical dimensions, but it remains unknown whether side-to-side differences preclude using the contralateral as a valid surrogate for the ruptured ACL. This study examined whether the ACL volume is significantly different between the left and right knees of uninjured subjects. ACL volumes were calculated for the left and right sides of 28 individuals using a previously-validated MRI-based method. The mean ACL volume was not significantly different (p=0.2331) between the two sides in this population. Side-to-side ACL volume was also well correlated (correlation=0.91, p<0.0001). The results of this study show that the volume of the contralateral ACL is a valid surrogate measure for a missing ACL on the injured side. This non-invasive, in vivo technique for measuring ACL volume may prove useful in future large-scale comprehensive studies of potential risk factors for ACL rupture, in quantification potential loading effects on ACL size as a prophylactic measure against ACL rupture, and in the use of ACL volume as a screening tool for assessing risk of injury.
Keywords: anterior cruciate ligament, anatomy, injury, MRI
Introduction
Various intrinsic anatomical and structural factors have been investigated as potential risk factors in non-contact anterior cruciate ligament (ACL) injuries. Since female athletes have been shown to have up to eleven times the relative risk of sustaining an ACL rupture than their male counterparts (Arendt and Dick, 1995; Gwinn et al., 2000; Lindenfeld et al., 1994), many studies have sought to understand the relevance of ACL morphology for injury by performing gender-based comparisons of ACL properties. Studies using in vivo magnetic resonance imaging have shown that female ACLs have significantly smaller cross sectional areas, even when controlling for weight (Anderson et al., 2001) and height (Dienst et al., 2007) than males, and that female ACLs are also narrower than male ACLs (Davis et al., 1999). Cadaveric studies have found that female ACLs have a smaller cross sectional area, are shorter, and have a smaller volume than male ACLs (Chandrashekar et al., 2005); they also have lower mechanical properties, including modulus of elasticity, than male ACLs (Chandrashekar et al., 2006). While these studies have observed gender differences that might explain the increased risk of ACL rupture in females, direct comparison of injured and non-injured individuals would be more appropriate to establish causality between ACL properties and rupture risk.
One recent study did compare ACL volume between injured subjects and gender, height, age, and weight matched controls, thereby directly drawing an association between ACL volume and injury events (Chaudhari et al., 2009). In that study, the contralateral ACL volume was used as a surrogate measure for the volume of the ruptured ACL. The contralateral ACL volumes of injured subjects were significantly smaller than the ACL volumes of matched control subjects. Ideally, if it were possible to measure the volume or other properties of a torn ACL then those could be compared to uninjured controls to directly infer the importance of these properties to the injury event. However, because the volume of a ruptured ACL cannot be measured in vivo, it is desirable to use the contralateral ACL volume as a surrogate measure. Unfortunately, to date no study has been performed examining the side-to-side differences in ACL volume in healthy, uninjured knees to determine whether contralateral ACL volume is a valid surrogate measure for the volume of a ruptured ACL. The purpose of this study was to test the hypothesis that the volume of the ACL is significantly different between the left and right knees of uninjured subjects.
Methods
Subjects and Imaging
Twenty-eight subjects (17 males, 11 females; body mass 72.9±13.9kg; height 1.75±0.08m) participated in the study after providing IRB-approved written informed consent. All subjects were recruited through community advertising, and the only exclusion criteria were a history of prior lower extremity soft-tissue injury requiring surgical repair/reconstruction; meniscectomy of more than 25% of the meniscus; or fracture requiring internal fixation. MR images (GE Signa 1.5T, sagittal 3D-SPGR, voxel size 0.55mm × 0.55mm × 1.5mm) were taken of both knees using a lower extremity coil. Given a nominal midsubstance ACL width of approximately 5 mm (Anderson et al., 2001), these scan parameters result in approximately 3–4 sagittal slices where the midsubstance of the ACL is visible, with additional slices showing the full extent of the insertions. Both scans were performed during the same session. Each knee was placed in full extension and padding was placed around the knee so that it remained relaxed and in full extension during imaging.
ACL Segmentation
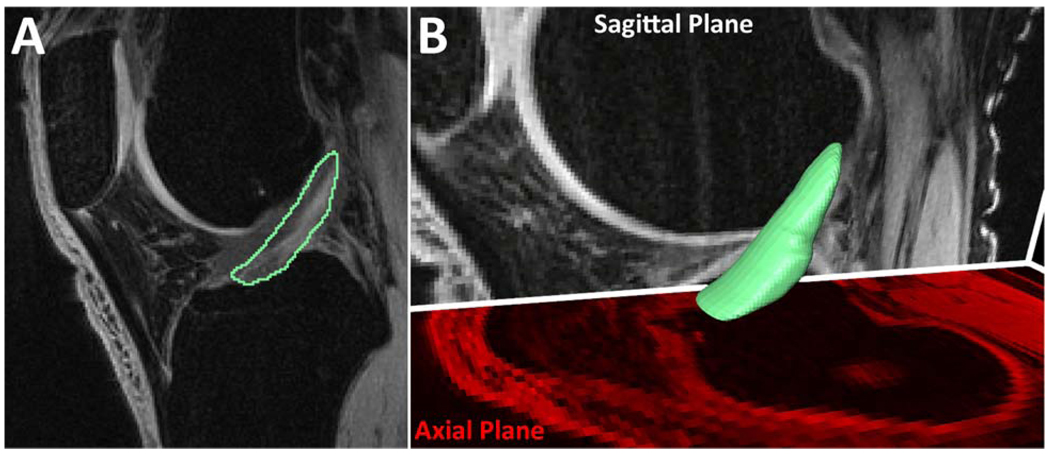
The entire ACL of each knee, including femoral and tibial insertions, was manually segmented from the MR images using standard software (Amira 5.0.0, Visage Imaging, Carlsbad, CA) under the guidance of an experienced orthopedic surgeon (Figure 1a). The volume of each ACL was then determined from these segmented slices using Amira (Figure 1b). Each ACL was segmented on two independent occasions, and the two measurements were averaged to get the final ACL volume. This technique has been previously validated using porcine ACLs (Chaudhari et al., 2009). The validation study showed a 0.98 correlation between MRI-based ACL volume estimates using a similar imaging sequence and true ACL volume measurements after dissection. The range of porcine ACL volumes (1850–2636 mm3) encompasses the mean of our ACL volumes (1895 mm3), giving support to the use of porcine ACL volumes in the validation of this technique for human ACL volume measurements.
Figure 1.
Manual ACL segmentation process using Amira. A) Manual segmentations of an ACL in a sagittal plane slice. While this is initially done in the sagittal plane, the outlines can be seen and modified in the coronal and axial planes. B) Smoothed, 3-D surface generation from manual segmentations. The surface was smoothed for visualization purposes only and was not used for volume calculations.
Statistical Analyses
We used a multi-factor ANOVA model with ACL volume as response, and gender, age, height, side, and trial number as fixed effect predictors. Subjects were used as random effects nested within gender. Association between the left and right side average ACL volumes were studied using correlation coefficient. Differences in the right and left average ACL volumes were examined for association with height. Summary statistics are reported as mean ± standard deviation (Table 1). SAS JMP Version 8 (SAS Institute, Cary NC) software was used for analyses. Level of significance was set at 0.05.
Table 1.
Summary Statistics (N = 28)
| Variable | Mean±SD | Range |
|---|---|---|
| Gender | 17 Male, 11 Female | |
| Age | 35.0±12.2 yrs | 20 to 66 yrs |
| Height | 1.75±0.08 m | 1.60 to 1.91 m |
| Right average ACL volume | 1907.7±432.7 mm3 | 1343.6 to 2986.0 mm3 |
| Left average ACL volume | 1881.6±367.7 mm3 | 1248.7 to 2990.2 mm3 |
| Difference in Mean ACL Volumes (R-L) | 26.2±181.0 mm3 | −485.9 to +425.0 mm3 |
| 95% CI for Difference in Means | (−44.0 to + 96.4 mm3) | |
| Interquartile range | (−75.1 to 137.3 mm3) | |
| Absolute Difference in Mean ACL Volumes | 132.8±123.3 mm3 | 4.3 to 485.9 mm3 |
Results
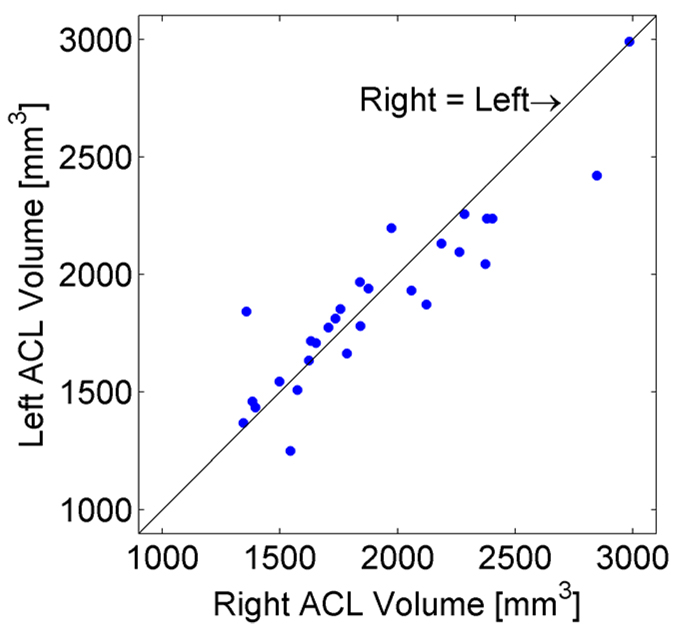
Height was the only significant predictor of ACL volume in the multi-factor model (p=0.0275). In an ANOVA model with only height and side as fixed effects, height was highly significant (p=0.0002). Upon adjusting for height, the mean ACL volume was not significantly different (p=0.2331) between the right and left sides. Least squares estimates (±Standard Error) of the mean ACL volumes at the average height of 68.8” were R=1907.74 (±58.66) mm3 and L=1881.55 (±58.66) mm3. Average right and left ACL volumes were also well correlated (correlation=0.91, p<0.0001) (Figure 2). There was no association between their differences and height (p=0.1149).
Figure 2.
Left vs. Right ACL volumes for healthy subjects. The two measurements were well correlated (correlation = 0.91, p <0.0001).
There appears to be no training effect with this technique, since the difference between the 1st and 2nd measurements was not significant (p=0.3510). The coefficient of variation (CV) between the repeated measures ranged from 0–6%, with a mean of 2.8%.
Discussion
With the right and left ACL volumes having a significant correlation (correlation=0.91, p<0.0001) and having no association between their differences and height (p=0.1149), the results of this study show that the volume of the contralateral ACL is a valid surrogate measure for a missing ACL on the injured side. Though only based on two repeated measures, with a mean CV of 2.8% and a range of 0–6%, this technique also shows good precision, making it an acceptable technique for measuring ACL volume in vivo and enabling future studies on the importance of ACL morphology to injury risk. These results are consistent with the findings of Dargel et al. (2009), who reported that neither total length nor cross-sectional area of the ACL were different between right and left knees of cadaveric specimens.
While it was well within the error of the measurement, a slightly higher average volume was measured on the right side than the left side (26.2 mm3). However, this difference does not appear to be clinically significant as it only amounts to about 1% of the average volume, whereas the previously reported average difference between injured and matched control subjects was 231 mm3 (Chaudhari et al., 2009).
The primary limitation of this technique is that it requires manual segmentation of the ACL from the MRI. Depending on the field strength, chosen excitation sequence, and individual subject variation, it can sometimes be very difficult to distinguish the ACL from surrounding tissues. The 3D-SPGR sequence was chosen as the best sequence for cartilage contrast rather than ACL contrast, so it is possible that the precision and accuracy of this technique might be improved by choosing a different sequence and/or by intra-articular injection of a contrast agent.
To compare our results to previously published studies, average ACL volumes were calculated for both males (2040±396 mm3) and females (1671±269 mm3). The ACL volumes calculated for this study fall within the range of previously reported values. An earlier cadaveric study using 3-D scanning software found larger ACL volumes (2722±706 mm3 in males; 1996±530 mm3 in females) than were found during this study (Chandrashekar et al., 2006). Their larger ACL volumes might be due in part to over-estimation by the 3-D scanning method, because it cannot detect surface concavities (Hashemi et al., 2005). ACL volumes found by Charlton et al. (2002) using an MRI-based method similar to ours were smaller (839mm3 in males, 652mm3 in females) than those found in our study. For their study, however, a 2.5mm slice thickness and a low-field MRI scanner (0.2T) were used. It was our experience that ACL boundaries were not always clear, especially at the femoral and tibial insertions, which could have led to an under-estimation of actual ACL volume. The clarity of the ACL in the MRI would only be degraded with a lower-field MRI scanner, and this degraded image quality could propagate to a greater under-estimation of ACL volume. Moreover, the segmented ACL is often truncated if an insertion lies between image slices. The larger the slice thickness that is chosen, the greater this truncation effect is.
Nevertheless, Chaudhari et al. (2009) showed, using porcine ACLs, that MRI-based measurements of ACL volume correlate very well with actual ACL volumes. That data combined with the data from this study showing the side-to-side similarity in ACL volume in this population suggests that the contralateral volume can serve as an appropriate surrogate measure for ruptured ACL volume. Future larger-scale studies can be used to confirm that this relationship holds true for the general population, to evaluate the potential of ACL volume as a predictor of ACL rupture and screening tool for assessing risk of injury, and to investigate the potential for the ACL to respond to its loading environment as a prophylactic measure to avoid injury.
Acknowledgements
The authors gratefully acknowledge Tom Andriacchi, Sean Scanlan, and Chris Dyrby of Stanford University for providing the MR images and subject demographic data analyzed in this study. Support for H.N. Nagaraja came from Clinical and Translational Science Award (CTSA) Number UL1RR025755 from the National Institutes of Health, National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of Anthropometric Measurements, Strength, Anterior Cruciate Ligament Size, and Intercondylar Notch Characteristics to Sex Differences in Anterior Cruciate Ligament Tear Rates. American Journal of Sports Medicine. 2001;29(1):58–66. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- Arendt E, Dick R. Knee Injury Patterns Among Men and Women in Collegiate Basketball and Soccer : NCAA Data and Review of Literature. American Journal of Sports Medicine. 1995;23(6):694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. Journal of Biomechanics. 2006;39(16):2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N, Slauterbeck J, Hashemi J. Sex-based differences in the anthropometric characteristics of the anterior cruciate ligament and its relation to intercondylar notch geometry: a cadaveric study. American Journal of Sports Medicine. 2005;33(10):1492–1498. doi: 10.1177/0363546504274149. [DOI] [PubMed] [Google Scholar]
- Charlton WPH, St. John TA, Ciccotti MG, Harrison N, Schweitzer M. Differences in Femoral Notch Anatomy between Men and Women : A Magnetic Resonance Imaging Study. American Journal of Sports Medicine. 2002;30(3):329–333. doi: 10.1177/03635465020300030501. [DOI] [PubMed] [Google Scholar]
- Chaudhari AMW, E.A Z, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior Cruciate Ligament-Injured Subjects Have Smaller Anterior Cruciate Ligaments Than Matched Controls: A Magnetic Resonance Imaging Study. American Journal of Sports Medicine. 2009;37(7):1282–1287. doi: 10.1177/0363546509332256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargel J, Feiser J, Gotter M, Pennig D, Koebke J. Side differences in the anatomy of human knee joints. Knee Surg Sports Traumatol Arthrosc. 2009 doi: 10.1007/s00167-009-0870-5. [DOI] [PubMed] [Google Scholar]
- Davis TJ, Shelbourne KD, Klootwyk TE. Correlation of the intercondylar notch width of the femur to the width of the anterior and posterior cruciate ligaments. Knee surgery, sports traumatology, arthroscopy. 1999;7(4):209–214. doi: 10.1007/s001670050150. [DOI] [PubMed] [Google Scholar]
- Dienst M, Schneider G, Altmeyer K, Voelkering K, Georg T, Kramann B, Kohn D. Correlation of intercondylar notch cross sections to the ACL size: a high resolution MR tomographic in vivo analysis. Archives of orthopaedic and trauma surgery. 2007;127(4):253–260. doi: 10.1007/s00402-006-0177-7. [DOI] [PubMed] [Google Scholar]
- Gwinn DE, Wilckens JH, Mcdevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. American Journal of Sports Medicine. 2000;28(1):98–102. doi: 10.1177/03635465000280012901. [DOI] [PubMed] [Google Scholar]
- Hashemi J, Chandrashekar N, Cowden C, Slauterbeck J. An alternative method of anthropometry of anterior cruciate ligament through 3-D digital image reconstruction. Journal of Biomechanics. 2005;38(3):551–555. doi: 10.1016/j.jbiomech.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Lindenfeld TN, Schmitt DJ, Hendy MP, Mangine RE, Noyes FR. Incidence of injury in indoor soccer. American Journal of Sports Medicine. 1994;22(3):364–371. doi: 10.1177/036354659402200312. [DOI] [PubMed] [Google Scholar]