Abstract
We have previously reported protective effects of atrial natriuretic peptide (ANP) against endothelial cell (EC) permeability induced by thrombin via suppression of Rho GTPase pathway of barrier dysfunction by protein kinase A and Epac-Rap1-Tiam1-Rac signaling cascades. This study tested effects of ANP on EC barrier dysfunction induced by inflammatory mediators lipopolysaccharide (LPS) and TNFα and linked them with activation of mitogen-activated protein kinase (MAPK) and NFκB signaling cascades known to promote EC hyperpermeability in the models of lung inflammation and sepsis. LPS and TNFα increased permeability in human pulmonary EC monitored by measurements of transendothelial electrical resistance, and caused disruption of EC monolayer integrity monitored by immunofluorescence staining for adherens junction marker protein VE-cadherin. Both disruptive effects were markedly attenuated by ANP. Both LPS and TNFα caused sustained activation of p38 and ERK1/2 MAP kinases, increased phosphorylation and degradation of negative regulator of NFκB signaling IkBα, and increased Rho-kinase mediated phosphorylation of myosin phosphatase MYPT1 leading to accumulation of phosphorylated myosin light chains. Consistent with protective effects on EC permeability and monolayer integrity, ANP dramatically attenuated activation of inflammatory signaling by LPS and TNFα in pulmonary EC. These results strongly suggest inhibitory effects of ANP on the LPS and TNFα induced inflammatory signaling as additional mechanism of EC barrier preservation in the models of acute lung injury and sepsis.
Keywords: natriuretic peptide, cytoskeleton, pulmonary endothelium, vascular leak
INTRODUCTION
The lung endothelium forms a semi-selective barrier between circulating blood and interstitial fluid, which is dynamically regulated by a counterbalance of barrier protective and barrier disruptive mediators present in the circulation. Prevalence of barrier disruptive stimuli results in increased permeability and movement of fluid, macromolecules and leukocytes into the interstitium. Because of its enormous surface area, the lung vasculature is particularly sensitive to barrier dysregulation with increases in vascular permeability leading to alveolar flooding (pulmonary edema) during inflammatory processes such as sepsis and acute respiratory distress syndrome (ARDS). Mechanisms which govern increased vascular permeability are under intense investigation, and various models of agonist-induced endothelial cell (EC) permeability have been developed (Dudek and Garcia, 2001; Lum and Malik, 1996; Mehta and Malik, 2006). However, little is known about intracellular processes, which determine EC barrier preservation in acute lung injury (ALI), and effective barrier-protective substances for ALI treatment remain to be identified.
Natriuretic peptides (atrial, brain, and C-type) belong to a family of mediators that regulate a variety of physiological functions including vascular tone, plasma volume, and renal function. The investigations of the action of atrial natriuretic peptide (ANP) in the cardiovascular system have concentrated mainly on the cGMP-dependent diuretic, natriuretic and vasodilating ANP effects (see (Baxter, 2004) for review). However, ANP exhibits much broader range of biological activities including effects on endothelial function and inflammation, which suggest its role in the regulation of the lung function in the settings of acute lung injury, sepsis, lung inflammation, and ventilator-induced lung injury (Eison et al., 1988; Mitaka et al., 1992). In vivo and in vitro models of lung injury show that ANP can protect endothelial barrier function apart from its vasodilatory and natriuretic action (Furst et al., 2005; Imamura et al., 1988; Irwin et al., 2005; Mitaka et al., 1992).
Lipopolysaccharide (LPS) is an important structural component of the outer membrane of Gram-negative bacteria which can induce systemic inflammation and sepsis in mammals. Genetic mapping and animal models have identified Toll-like receptor 4 (TLR4) as an essential receptor for LPS signaling (Lu et al., 2008). TLR play an important role in activating signal transduction pathways leading to the induction of the inflammatory response. After binding to a ligand, TLR sequentially recruits the adaptor molecules MyD88, IL-1R-associated kinase (IRAK), and TNF receptor-associated factor 6 (TRAF6). In turn, these adaptor molecules activate MAP kinases JNK, p38, ERK1/2 and IκB, a cytoplasmic inhibitor of NFκB (Lu et al., 2008). The MAPK cascade activation contributes to the production of proinflammatory cytokines. Activation of IκB kinase complex results in phosphorylation of its components IκBα and IκBβ, ubiquitination and degradation of IκB, and release of the active NFκB complex which then translocates to the nucleus and binds to the promoters of various genes, activating transcription (see (Lu et al., 2008) for review). Therefore, phosphorylation of the MAPKs and IκB degradation is a necessary step in the signaling cascade leading to transcription of inflammatory target genes. It is important to note that in addition to regulation of gene expression, MAPK signaling is also involved in remodeling of EC cytoskeleton and permeability changes by edemagenic and inflammatory mediators (Bogatcheva et al., 2003).
In this work we studied effects of ANP on the pulmonary EC permeability and cytoskeletal remodeling induced by pro-inflammatory agonists LPS and TNFα and linked them with activation of stress MAPK and NFκB cascades.
MATERIALS AND METHODS
Cell culture and reagents
Human pulmonary artery endothelial cells (HPAEC) and cell culture basal medium with growth supplements were obtained from Lonza Inc (Allendale, NJ). Cells were cultured according to the manufacturer's protocol, and used at passages 5-9. ANP was purchased from AnaSpec Inc (San Jose, CA). TNFα was obtained from R&D Systems (Minneapolis, MN). Di-phospho-MLC, phospho-p38, phospho-Erk1/2, phospho-IκBα, and IκBα antibodies were obtained from Cell Signaling (Beverly, MA); phospho-MYPT1 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY); VE-cadherin antibodies were obtained from BD Transduction Laboratories (San Diego, CA). All reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Immunofluorescence staining
Endothelial monolayers plated on glass cover slips were treated with the agonist of interest, fixed in 3.7% formaldehyde solution in PBS for 10 min at 4° C, washed three times with PBS, permeabilized with 0.1% Triton X-100 in PBS-Tween (PBST) for 30 min at room temperature, and blocked with 2% BSA in PBST for 30 min. Incubation with VE-cadherin antibodies were performed in blocking solution (2% BSA in PBST) for 1 hr at room temperature followed by staining with Alexa 488-conjugated secondary antibodies. Actin filaments were stained with Texas Red-conjugated phalloidin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech, Tokyo, Japan) as described elsewhere (Birukov et al., 2004; Birukova et al., 2006; Birukova et al., 2008a).
Western immunoblotting
After stimulation, cells were washed with PBS and lysed with cell lysis buffer containing 10 mM Tris (pH 7.4), 1% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 0.2 mM EGTA, 0.2 mM vanadate, 0.2 mM PMSF, and 0.5% phosphatase inhibitor cocktail. Total cell lysates were cleared by centrifugation and boiled with the same amount of 3 × SDS sample buffer for 5 min. Protein extracts were separated by SDS-PAGE on 10% gels for detection of Erk1/2, p38, and IκBα; 7.5% gels for detection of MYPT and β-tubulin; and 15% gels for detection of MLC. The separated proteins were transferred to nitrocellulose membranes by electrotransfer (30 V for 18 h, or 90 V for 2 h). The blots were subsequently blocked with 5% non-fat dry milk in PBST at room temperature for 1 h, and then incubated at 4°C overnight with primary specific antibodies of interest. After washing three times for 10 min with PBST, the membrane was incubated with HRP-linked IgG secondary antibodies at room temperature for 1 h, followed by washing three times for 10 min with PBST. Immunoreactive proteins were detected using an enhanced chemiluminescence detection system according to the manufacturer's protocol (Amersham, Little Chalfont, UK).
Measurement of transendothelial electrical resistance
The endothelial monolayer barrier properties were evaluated by the highly sensitive biophysical assay with an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) that allows measurements of transendothelial electrical resistance in real time, which reflects agonist-induced EC permeability changes (Birukov et al., 2004; Birukova et al., 2008a).
Statistical Analysis
Results are expressed as means ± SD of three to eight independent experiments. Stimulated samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way variance analysis (ANOVA) and post hoc multiple comparisons tests were used. P<0.05 was considered statistically significant.
RESULTS
Effects of ANP on pulmonary EC hyper-permeability induced by inflammatory mediators
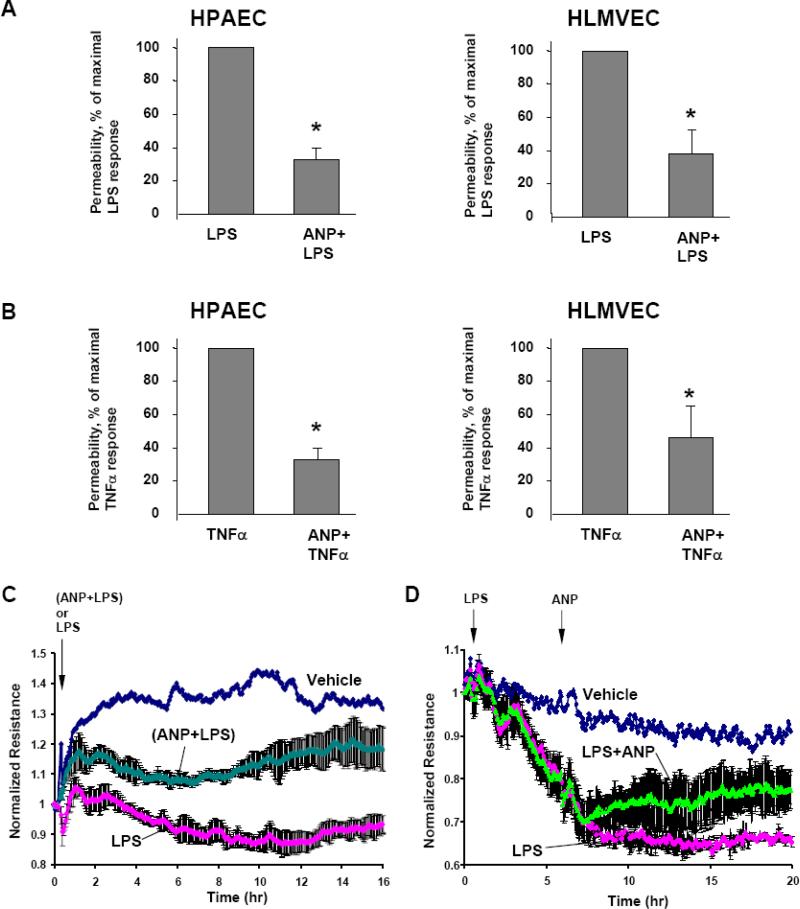
Effects of ANP on endothelial barrier dysfunction induced by inflammatory mediators LPS and TNFα were evaluated by measurements of permeability across the human pulmonary EC monolayers grown on gold microelectrodes. Our previous report showed that both, LPS and TNFα significantly decreased transendothelial electrical resistance (TER) in human pulmonary EC, reflecting endothelial barrier compromise, with maximal response at 6 hrs of agonist stimulation (Birukova et al., 2007). In the current experiments cells were pretreated with ANP immediately followed by LPS or TNFα challenge, and permeability changes were analyzed after 6 hr of agonist stimulation. In both, macro- and micro-vascular EC, ANP exhibited strong protective effects against EC permeability caused by LPS (Figure 1A) or TNFα (Figure 1B). Moreover, in experiments when LPS and ANP were added simultaneously (Figure 1C), or when cells were stimulated with LPS 5 hours prior to ANP treatment (Figure 1D), ANP also exhibited strong protective effect against LPS-induced EC permeability.
Figure 1. Effect of ANP on agonist-induced EC permeability.
A and B: HPAEC or HLMVEC plated on microelectrodes were pretreated with ANP (100 nM, 20 min) followed by stimulation with LPS (200 ng/ml) (A) or TNFα (20 ng/ml) (B). Transendothelial electrical resistance was measured over the time. Summary of permeability measurements illustrates ANP protective effects against maximal agonist-induced permeability increase, observed at 6 hours after agonist stimulation. C: HPAEC were stimulated with LPS (200 ng/ml) or with combination of ANP (100 nM) and LPS followed by permeability measurements. D: HPAEC were pretreated with LPS (200 ng/ml) for 5 hrs followed by stimulation with ANP (100 nM); transendothelial electrical resistance was measured over the time. Permeability data are expressed as mean ± SD of three to eight independent experiments; *p<0.05.
Effects of ANP on LPS- or TNFα-induced EC monolayer disruption
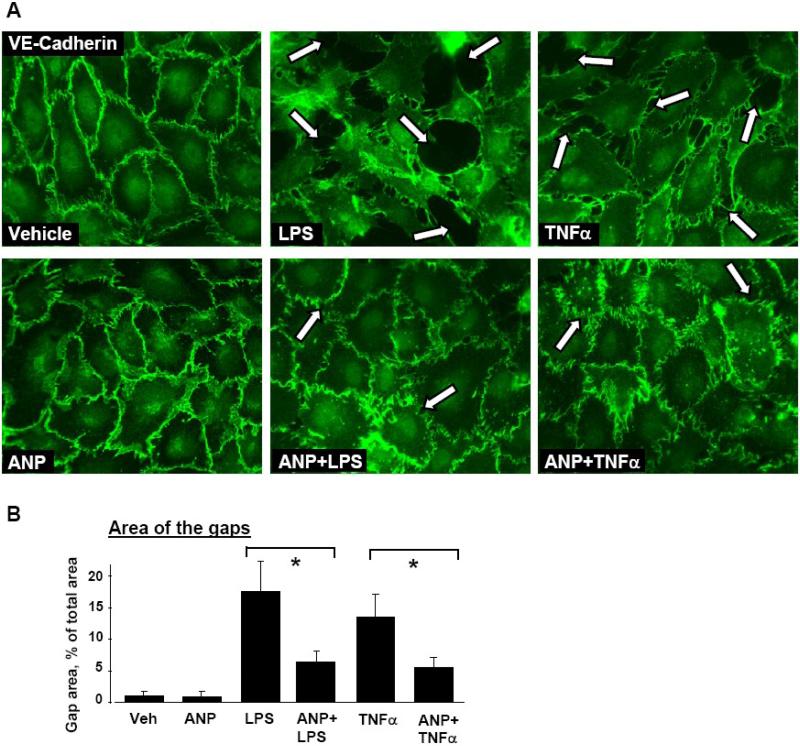
In these experiments, EC pretreated with ANP or vehicle were challenged with LPS or TNFα for 6 hours, and effects of ANP on agonist-induced adherens junction disruption were examined by immunofluorescence staining with VE-cadherin antibodies. HPAEC stimulation with either LPS or TNFα caused dramatic disruption of cell-cell contacts, reflecting EC barrier compromise (Figure 2, upper panels). ANP pretreatment preserved continuous adherens junction pattern in the LPS- or TNFα–challenged EC monolayers and thus prevented agonist-induced paracellular gap formation and disruption of EC monolayer integrity (Figure 2A, lower panels, and Figure 2B).
Figure 2. Effect of ANP on agonist-induced adherens junction remodeling.
Endothelial monolayers were pretreated with vehicle or ANP (100 nM, 20 min) and stimulated with LPS (200 ng/ml) or TNFα (20 ng/ml) for 6 hours. A: Immunofluorescence staining for VE-cadherin was performed to visualize adherens junctions. LPS- or TNFα-induced disruption of adherens junctions is shown by arrows. B: Quantitative analysis of agonist-induced paracellular gap formation. Data are expressed as mean ± SD of three independent experiments; *p<0.05.
Effects of ANP on LPS-induced activation of inflammatory signaling
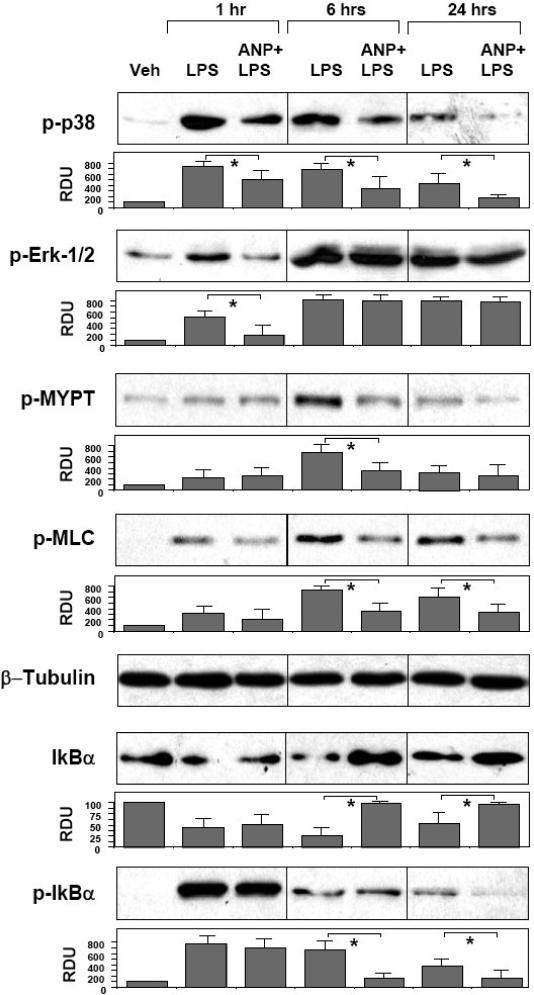
A number of signaling cascades, such as p38 and Erk1/2 MAPK, Rho pathway, and NFκB cascade become activated by LPS and have been shown to be involved in control of EC permeability and inflammation (Bogatcheva et al., 2003; Lu et al., 2008). Recent reports, including our work have suggested a potential role of Rho-dependent signaling in the development of LPS-induced acute lung injury (Bogatcheva et al., 2003; Essler et al., 2000; Fu et al., 2009; Lu et al., 2008; Tasaka et al., 2005). In the following experiments we investigated effects of ANP on the regulation of MAPK-, Rho-, and NFκB-dependent signaling cascades activated by LPS. Confluent lung EC were pretreated with ANP and stimulated with LPS for various periods of time. Control stimulations were performed with LPS alone. Protein phosphorylation profiles were analyzed by western blotting with phospho-specific antibodies. LPS challenge induced rapid phosphorylation/activation of stress MAP kinase p38 and Erk1/2. Maximal p38 MAPK phosphorylation was observed at 1 hr of LPS treatment and remained elevated at all time points. Initial Erk1/2 phosphorylation was detected as early as 1 hr of LPS treatment, reached maximum at 6 hr and remained elevated up to 24 hr of LPS stimulation. ANP pretreatment significantly attenuated p38 MAPK phosphorylation at all time points. Interestingly, ANP reduced initial (1 hr) Erk1/2 phosphorylation but was without effect on Erk1/2 activation observed at later time points 6-24 hrs, which corresponds to developed EC barrier dysfunction (Figure 3, upper panels). Activation of Rho pathway was evaluated by analysis of site-specific phosphorylation of Rho-kinase substrate myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT1) and myosin light chain (MLC). LPS caused significant MYPT1 phosphorylation at 6 hours of treatment and MLC phosphorylation at all examined time points. ANP pretreatment markedly suppressed LPS-induced increases in MYPT1 and MLC phosphorylation (Figure 3, middle panels). Next, we analyzed phosphorylation and degradation status of IκBα, the events critical for activation of NFκB-dependent transcription. LPS induced rapid degradation IκBα with partial recovery at 24 hr post LPS treatment. IκBα degradation was accompanied by pronounced protein phosphorylation with maximum at 1 hr. ANP pretreatment did not reduce IκBα degradation at 1 hr, but promoted protein recovery and restored IκBα content by 6 hrs post LPS treatment. These ANP effects were accompanied by significant attenuation IκBα phosphorylation detected at 6 and 24 hrs of LPS challenge (Figure 3, lower panels).
Figure 3. Effects of ANP on LPS-induced inflammatory signaling.
HPAEC were pretreated with vehicle or ANP (100 nM, 20 min) followed by stimulation with LPS (200 ng/ml) for 1, 6, or 24 hours. Phosphorylation of p38, Erk1/2, MYPT1, MLC, and IκBα was detected by western blot with corresponding phospho-specific antibodies. Degradation of IκBα was detected using antibodies against non-phosphorylated protein. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. P-IκBα was normalized to the total IκBα content. Results are representative of three to six independent experiments. Result of densitometry shown as mean ± SD, * p<0.05.
Effects of ANP on inflammatory cascades activated by TNF α
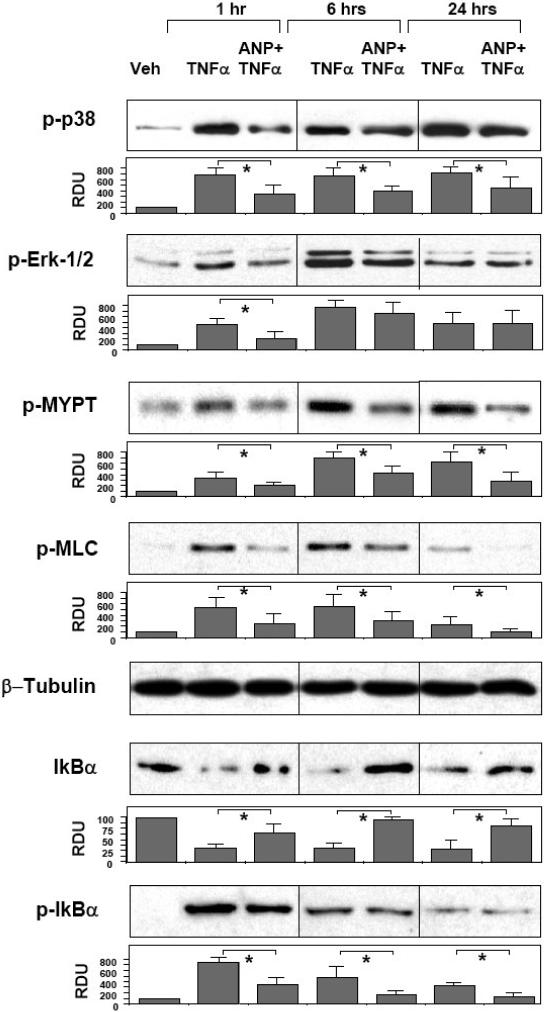
Similarly to LPS, inflammatory signaling activated in pulmonary EC in response to TNFα included p38 and Erk1/2 MAP kinases, Rho pathway, and NFκB cascade. To examine ANP protective effects in the TNFα model of EC barrier dysfunction, human lung EC were pretreated with vehicle or ANP followed by TNFα challenge for 1, 6, or 24 hours. TNFα-induced inflammatory response was detected by activation of MAPK-, Rho-, and NFκB-dependent signaling. Western blot analysis showed that TNFα induced robust and sustained activation/phosphorylation of p38 and Erk1/2 MAPK. Similarly to the data observed in the LPS model, ANP suppressed p38 MAPK phosphorylation at all time points, but showed inhibitory effect on Erk1/2 activation only during initial phase (1 hr) of TNFα treatment, and was without effect at the later time points (6-24 hrs) (Figure 4, upper panels). Evaluation of Rho-dependent signaling revealed marked MYPT1 and MLC phosphorylation in response to TNFα. These effects were significantly attenuated by ANP pretreatment (Figure 4, middle panels). Analysis of NFκB-dependent signaling revealed rapid and sustained degradation of IκBα subunit at all time points of TNFα treatment, which was accompanied by increased protein phosphorylation with maximum at 1 hr. At the later time points, IκBα phosphorylation gradually decreased but remained elevated for up to 24 hrs of TNFα stimulation. Pretreatment of HPAEC with ANP blunted both IκBα degradation and phosphorylation at all times of TNFα challenge (Figure 4, lower panels).
Figure 4. Effect of ANP on TNFα-induced inflammatory signaling.
HPAEC were pretreated with vehicle or ANP (100 nM, 20 min) followed by stimulation with TNFα (20 ng/ml) for 1, 6, or 24 hours. Phosphorylation of p38, Erk1/2, MYPT1, MLC, and IκBα was determined by western blot with corresponding phospho-specific antibodies. Degradation of IκBα was detected using pan IκBα antibodies. Equal protein loading was confirmed by determination of β-tubulin content in total cell lysates. P-IκBα was normalized to the total IκBα content. Results are representative of three to six independent experiments. Result of densitometry shown as mean ± SD, * p<0.05.
DISCUSSION
This study shows that ANP exhibits potent protective effects on the pulmonary vascular EC exposed to inflammatory mediators LPS and TNFα. Observed protective effects on the EC barrier may explain in part dramatic increases in survival rates in the ANP-treated mice with induced septic shock (Ladetzki-Baehs et al., 2007). These protective effects of ANP may be mediated by several mechanisms discussed below.
Attenuation of Rho pathway as vascular barrier protective mechanism triggered by ANP has been previously tested in the aseptic model of thrombin-induced EC hyper-permeability (Birukova et al., 2008b; Klinger et al., 2006). ANP also inhibited VEGF-induced signaling and attenuated EC permeability in part by preserving the endothelial cell tight junction functional morphology (Pedram et al., 2002). Mechanistic analysis revealed that ANP stimulated Rac GTPase via PKA- and Epac-Rap1/Tiam1/Vav2-dependent signaling cascades (Birukova et al., 2008b). In turn, activated Rac inhibited thrombin-induced activation of Rho and Rho-kinase signaling and abrogated Rho-kinase induced inactivation of myosin phosphatase (MYPT1), accumulation of phospho-MLC, increased EC contractility and eventually lung endothelial barrier disruption (Birukova et al., 2008b). LPS and TNFα also activate Rho signaling (Petrache et al., 2003) and cause vascular endothelial leak (Birukova et al., 2007; Essler et al., 2000; Tasaka et al., 2005). Although precise mechanisms of Rho activation by inflammatory mediators remain to be explored, pharmacological inhibition of Rho associated kinase by fasudil and Y-27632 attenuated endotoxin-induced acute lung injury and leukocyte accumulation in the inflamed blood vessels (Slotta et al., 2006; Tasaka et al., 2005). Our results show that Rho-kinase mediated phosphorylation of MYPT1 and accumulation of phospho-MLC in LPS- and TNFα-stimulated pulmonary EC was dramatically attenuated by ANP, and strongly suggest Rho inhibition as a barrier protective mechanism of ANP in the models of sepsis and inflammation.
Activation of stress-induced MAP kinases p38 and JNK observed in this study may increase permeability via weakening of adherens junctions and remodeling of actin cytoskeleton (Becker et al., 2001; Garcia et al., 2002; Kiemer et al., 2002). Essential role of p38 MAPK in development of endotoxin-induced inflammation or ALI induced by an agent of complement origin was further demonstrated in the animal studies (Nash and Heuertz, 2005). Inhibition of p38 MAPK by specific inhibitor SB203580 attenuated the pulmonary inflammatory responses, neutrophil recruitment, total protein content in bronchoalveolar lavage fluid, activation of NFκB, inflammatory cytokine release, and reduced the mortality rate of LPS-induced acute lung injury (Kim et al., 2006; Liu et al., 2008). Our data show that that both LPS and TNFα induced phosphorylation of p38 and Erk1/2 MAP kinases. Pretreatment with ANP attenuated effects of LPS and TNFα on p38 MAPK, but not Erk1/2 MAPK phosphorylation. Taken together with the data showing effects of ANP on the LPS- and TNFα-induced permeability increase and EC monolayer disruption, these results strongly suggest that p38 MAPK-mediated mechanism of EC permeability may be abrogated by ANP, whereas Erk1/2 activation is neither involved in the LPS-induced EC barrier disruption, nor affected by ANP. Studies by Kiemer et al. propose a potential mechanism explaining attenuation of LPS- and TNFα-induced p38 MAPK activity by ANP observed in this study, which may be due to ANP-induced activation of the MAPK phosphatase MKP-1 protein expression in ANP-treated cells (Kiemer et al., 2002).
Activation of NFκB upon LPS challenge results in a rapid release of TNFα into the circulation leading to additional inflammatory insult and vascular barrier compromise (Reinhart and Karzai, 2001). In turn, by inhibiting NFκB signaling cascade, ANP suppresses TNFα and IL-1β synthesis upon LPS stimulation (Kiemer et al., 2000). Consistent with these observations, our data show ANP-induced inhibition of IkB phosphorylation caused by LPS and TNFα, which indicates abrogation of NFκB signaling, and link these effects with preservation of endothelial barrier properties by ANP. General application of chemical NFκB inhibitors may cause problems related to their efficacy and side effects. In contrast, ANP is an endogenous peptide that has been approved for therapeutic treatment of acute myocardial failure, and the dose tested in this study is well within the range used in clinical trials (Suwa et al., 2005).
In summary, this study shows that ANP attenuates three pathways of endothelial barrier dysfunction induced by inflammatory agents LPS and TNFα: p38 MAPK, Rho-, and NFκB signaling. Our results strongly suggest a potential therapeutic role of ANP in the treatment of ALI and other lung inflammatory syndromes.
Acknowledgments
Supported by HL89257 from the National Heart, Lung, and Blood Institute, the American Heart Association Midwest Affiliate Grant-in-Aid, and the American Lung Association Biomedical Research Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99:71–5. doi: 10.1007/s00395-004-0457-8. [DOI] [PubMed] [Google Scholar]
- Becker PM, Verin AD, Booth MA, Liu F, Birukova A, Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1500–11. doi: 10.1152/ajplung.2001.281.6.L1500. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168:1749–61. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is Involved in the Differential Regulation of Endothelial Barrier by HGF and VEGF. Am J Respir Cell Mol Biol. 2008a doi: 10.1165/rcmb.2008-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L924–35. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008b;215:715–24. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Dudek SM, Garcia JG, Verin AD. Mitogen-activated protein kinases in endothelial pathophysiology. J Investig Med. 2003;51:341–52. doi: 10.1136/jim-51-06-30. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Eison HB, Rosen MJ, Phillips RA, Krakoff LR. Determinants of atrial natriuretic factor in the adult respiratory distress syndrome. Chest. 1988;94:1040–5. doi: 10.1378/chest.94.5.1040. [DOI] [PubMed] [Google Scholar]
- Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling [In Process Citation]. J Immunol. 2000;164:6543–9. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33:612–24. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Wang P, Schaphorst KL, Becker PM, Borbiev T, Liu F, Birukova A, Jacobs K, Bogatcheva N, Verin AD. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganization and lung permeability. Faseb J. 2002;16:1064–76. doi: 10.1096/fj.01-0895com. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ohnuma N, Iwasa F, Furuya M, Hayashi Y, Inomata N, Ishihara T, Noguchi T. Protective effect of alpha-human atrial natriuretic polypeptide (alpha-hANP) on chemical-induced pulmonary edema. Life Sci. 1988;42:403–14. doi: 10.1016/0024-3205(88)90078-1. [DOI] [PubMed] [Google Scholar]
- Irwin DC, Tissot van Patot MC, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol. 2005;288:L849–59. doi: 10.1152/ajplung.00294.2004. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Hartung T, Vollmar AM. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J Immunol. 2000;165:175–81. doi: 10.4049/jimmunol.165.1.175. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Weber NC, Furst R, Bildner N, Kulhanek-Heinze S, Vollmar AM. Inhibition of p38 MAPK activation via induction of MKP-1: atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ Res. 2002;90:874–81. doi: 10.1161/01.res.0000017068.58856.f3. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology. 2006;225:36–47. doi: 10.1016/j.tox.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Klinger JR, Warburton R, Carino GP, Murray J, Murphy C, Napier M, Harrington EO. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp Cell Res. 2006;312:401–10. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ladetzki-Baehs K, Keller M, Kiemer AK, Koch E, Zahler S, Wendel A, Vollmar AM. Atrial natriuretic peptide, a regulator of nuclear factor-kappaB activation in vivo. Endocrinology. 2007;148:332–6. doi: 10.1210/en.2006-0935. [DOI] [PubMed] [Google Scholar]
- Liu S, Feng G, Wang GL, Liu GJ. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584:159–65. doi: 10.1016/j.ejphar.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Hirata Y, Nagura T, Sakanishi N, Tsunoda Y, Amaha K. Plasma alpha-human atrial natriuretic peptide concentration in patients with acute lung injury. Am Rev Respir Dis. 1992;146:43–6. doi: 10.1164/ajrccm/146.1.43. [DOI] [PubMed] [Google Scholar]
- Nash SP, Heuertz RM. Blockade of p38 map kinase inhibits complement-induced acute lung injury in a murine model. Int Immunopharmacol. 2005;5:1870–80. doi: 10.1016/j.intimp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Deciphering vascular endothelial cell growth factor/vascular permeability factor signaling to vascular permeability. Inhibition by atrial natriuretic peptide. J Biol Chem. 2002;277:44385–98. doi: 10.1074/jbc.M202391200. [DOI] [PubMed] [Google Scholar]
- Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–9. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:S121–5. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- Slotta JE, Braun OO, Menger MD, Thorlacius H. Fasudil, a Rho-kinase inhibitor, inhibits leukocyte adhesion in inflamed large blood vessels in vivo. Inflamm Res. 2006;55:364–7. doi: 10.1007/s00011-006-6013-2. [DOI] [PubMed] [Google Scholar]
- Suwa M, Seino Y, Nomachi Y, Matsuki S, Funahashi K. Multicenter prospective investigation on efficacy and safety of carperitide for acute heart failure in the ‘real world’ of therapy. Circ J. 2005;69:283–90. doi: 10.1253/circj.69.283. [DOI] [PubMed] [Google Scholar]
- Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32:504–10. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]