Abstract
Background & Aims
Patients with acute hepatitis C virus (HCV) infection that receive treatment achieve high rates of sustained virological response (SVR), but few studies have examined outcomes among injecting drug users (IDUs). We evaluated the efficacy of treatment of recent HCV infection in IDUs with acute and early chronic HCV.
Methods
We analyzed data from the Australian Trial in Acute Hepatitis C (ATAHC)—a prospective study of the natural history and treatment outcomes of patients with recent HCV infection. Participants eligible for the study had their first anti-HCV antibody positive test result within the past 6 months and either acute clinical HCV within the past 12 months or documented anti-HCV seroconversion within 24 months. Participants with HCV received pegylated interferon (PEG-IFN)α-2a (180 μg/week, n=74); those with HCV/HIV co-infection received PEG-IFNα-2a (180 μg/week) with ribavirin (n=35) for 24 weeks.
Results
From June 2004 to February 2008, 167 participants were enrolled in the ATAHC; 79% had injected drugs in the previous 6 months. Among 74 with only HCV, the SVRs were 55% and 72% by intention-to-treat and per protocol analysis, respectively. In multivariate analyses, baseline factors independently associated with lower SVR included decreased social functioning and current opiate pharmacotherapy. Adherent participants had higher SVR rates (63% vs 29%, P=0.025). Of the 35 participants with HCV/HIV co-infection, the SVRs were 74% and 75% by intention-to-treat and per protocol analysis, respectively.
Conclusion
Treatment of recent HCV infection among IDUs, including those with HIV co-infection, is effective. Strategies to engage socially marginalized individuals and increase adherence should improve treatment outcomes in this population.
Keywords: hepatitis C, HCV, acute hepatitis C, pegylated interferon, injection drug users
Introduction
An estimated 75% of people with acute hepatitis C virus (HCV) infection progress to chronic infection (1), and experience an increased risk of impaired quality of life (2) and progressive liver disease (3). Several studies have demonstrated that treatment based on interferon-α in acute HCV infection can yield much higher levels of sustained virological response (SVR) than the treatment of chronic HCV infection (4-10).
While these findings are encouraging, questions remain about the most effective treatment strategies in recent HCV infection. For example, data are very limited on the feasibility and outcome of treatment for acute HCV in injecting drug users (IDU), even though they represent the population group at greatest risk for infection in many countries. Most acute HCV treatment studies have been performed in settings where injecting drug use is uncommon (4, 8, 10), or have chosen to predominantly recruit participants whose infection was acquired through other modes of percutaneous exposure (7, 9).
Another important issue is timing: since the majority of people who spontaneously clear virus following acute HCV infection do so within 16 weeks of symptomatic presentation (20 – 24 weeks following infection) (4, 8, 11), it appears reasonable to delay therapeutic intervention for this time period to avoid unnecessary treatment (12, 13). On the other hand, treatment strategies for individuals with asymptomatic presentation but evidence of recent infection through anti-HCV antibody seroconversion are less certain. Repeat HCV screening is common among IDUs, but the variability of testing intervals means that many of those with diagnosed recent HCV infection will have early chronic HCV infection.
The Australian Trial in Acute Hepatitis C (ATAHC) study was specifically designed to investigate HCV treatment in people whose infection was recently acquired through injecting drug use. Here we report on the treatment outcomes, and the role and predictors of treatment adherence in determining these outcomes.
Methods
Study design
ATAHC was a multicenter, prospective cohort study of the natural history and treatment of recent HCV infection. Study recruitment commenced in June 2004 through an Australian network of tertiary hospitals (n=13) and general practice/primary care clinics (n=3). Recent infection included participants with either acute or early chronic HCV infection with the following eligibility criteria:
First positive anti-HCV antibody within 6 months of enrolment; and either
Acute clinical hepatitis C infection, defined as symptomatic seroconversion illness or alanine aminotransferase (ALT) level greater than 10 times the upper limit of normal (>400 IU/mL) with exclusion of other causes of acute hepatitis, at most 12 months before the initial positive anti-HCV antibody; or
Asymptomatic hepatitis C infection with seroconversion, defined by a negative anti-HCV antibody in the two years prior to the initial positive anti-HCV antibody.
Other eligibility criteria included being age 16 years or above, having a negative pregnancy test, and ability to provide written, informed consent. All participants with detectable HCV RNA at screening or baseline were assessed for HCV treatment eligibility. HCV treatment was not offered to people who had positive serology for anti-hepatitis A virus IgM, hepatitis B surface antigen or anti-hepatitis B core IgM; concurrent additional causes of liver disease; or other standard laboratory-based exclusion criteria for interferon therapy. Having received investigational drugs within the previous 6 weeks was also an exclusion criterion for treatment. Heavy alcohol intake and active illicit drug use were not exclusion criteria, however a drug and alcohol assessment was performed for treatment suitability.
People diagnosed with recent HCV infection at one of the participating sites who satisfied these inclusion and exclusion study were invited to participate in the study, regardless of their or their doctors’ intentions regarding treatment. Participants were followed from baseline at 4 weekly intervals to week 12, then at 12 weekly for up to 144 weeks.
All study participants provided written informed consent prior to study procedures. The study protocol was approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee (primary study committee) as well as through local ethics committees at all study sites, and was conducted according to the Declaration of Helsinki and ICH/GCP guidelines. The study was registered with clinicaltrials.gov registry (NCT00192569).
HCV treatment and virological assessment
Participants who began HCV treatment received pegylated interferon-α2a (PEG-IFN) 180 micrograms weekly for 24 weeks. Due to non-response at week 12 in the initial two participants with HCV/HIV coinfection, the study protocol was amended to provide PEG-IFN and ribavirin combination therapy for 24 weeks in this group. Ribavirin was prescribed at a dose of 1000-1200 mg for those with genotype 1 infection and 800 mg in those with genotype 2/3. Medical supervision of PEG-IFN injections was not mandatory but was available for use on a case-by-case basis.
The presence of HCV RNA was assessed at all scheduled study visits (including screening, baseline, week 4, 8, 12, and 24 on-treatment), with a qualitative HCV-RNA assay (TMA assay, Versant, Bayer, Australia, lower limit of detection 10 IU/ml) and if positive a quantitative HCV RNA assay (Versant HCV RNA 3.0 Bayer, Australia lower limit of detection 615 IU/ml). HCV genotype (Versant LiPa2, Bayer, Australia) was assessed on participants found to be viremic at screening. A questionnaire was administered at screening and every 12 weeks through follow up, to obtain information on injection of illicit drugs, social functioning (Opiate Treatment Index Social Functioning Scale) (14) and psychological parameters [Mini-International Neuropsychiatric Interview (M.I.N.I.) (15) and the Depression Anxiety Stress Scale (DASS-21) (16)]. Adverse events were collected on all treated participants from the commencement of treatment to week 48.
Study definitions
The presentation of recent HCV at the time of diagnosis was classified as either acute clinical or asymptomatic infection. Acute clinical infection included those with either a documented clinical history of symptomatic seroconversion illness and those without clinical symptoms but with a documented peak ALT above 400 IU/ml at or prior to the time of diagnosis. Participants with asymptomatic infection included participants with anti-HCV antibody seroconversion but no acute clinical symptoms or documented peak ALT above 400 IU/ml. The estimated date of infection for acute clinical infection was calculated as six weeks prior to onset of seroconversion illness if present or six weeks prior to the first ALT reading above 400 IU/ml. The estimated date of infection for asymptomatic infection was calculated as the mid-point between the last negative anti-HCV antibody and the first positive anti-HCV antibody test result. For participants who were anti-HCV antibody negative and HCV RNA positive at screening, the estimated date of infection was designated to be six weeks prior to screening.
Adherence was defined as the receipt of at least 80% of scheduled PEG-IFN alfa-2a doses and therapy for 80% of the scheduled treatment period. For participants in whom therapy was terminated at 12 weeks due to virological non-response, the scheduled treatment period was defined as 12 weeks. HCV relapse and breakthrough were distinguished from reinfection by the detection of HCV viremia with a viral sequence that differed from that of the initial infection, as confirmed by viral sequence analysis (17).
Study outcomes
Evaluation of HCV treatment response was based on intention-to-treat (ITT) analyses that included all participants who received at least one injection of PEG-IFN therapy. Additional per-protocol analyses included all adherent individuals with follow-up virological data (≥week 48). Primary endpoints for treatment were the proportion of participants with undetectable qualitative HCV RNA rates at weeks 4 (rapid virological response, RVR), 12, 24 (end-of-treatment response, ETR) and 48 (sustained virological response, SVR). If HCV RNA had not been assessed at week 48, the result of next available HCV RNA assessment was used to calculate SVR. HCV treatment outcomes were separately assessed in participants with and without HIV infection.
Statistical analyses
Logistic regression analyses were used to identify predictors of HCV treatment response. Potential predictors were determined a priori and included sex, age, weight, education, employment, accommodation, social functioning, methadone or buprenorphine treatment, mental health status (depression and suicidality, based on the MINI), ethnicity, injecting drug use characteristics, alcohol consumption, estimated duration of HCV infection, presentation (acute clinical, asymptomatic), peak and baseline ALT level, baseline HCV RNA levels and HCV genotype. Social functioning was calculated using a validated scale from the Opiate Treatment Index (14) that addresses employment, residential stability, and inter-personal conflict. The scale also addresses social support, and the role of drug use in the participant’s social networks, and a higher number means poorer functioning. This scale has been validated among opiate users in Australia (range, 0-48) (14). Current depression and suicide risk were evaluated using the Mini-International Neuropsychiatric Interview (M.I.N.I.) (15).
Additional analyses were performed to evaluate time to clearance among treated and untreated groups and the impact of treatment on clearance of HCV infection. Among untreated subjects, spontaneous HCV clearance was defined as two consecutive negative qualitative tests for HCV RNA over an interval of ≥4 weeks. The estimated date of spontaneous clearance was determined by calculating the midpoint between the date of the last HCV RNA qualitative positive test and first qualitative HCV RNA negative test. Among treated subjects, the estimated date of initial HCV clearance (in those with subsequent SVR) was determined by calculating the midpoint between the date of the last HCV RNA qualitative positive test and first qualitative HCV RNA negative test. Kaplan-Meier analyses were used to estimate the time to spontaneous HCV clearance and initial HCV clearance (in those with subsequent SVR). The impact of treatment on HCV clearance was evaluated using Cox Proportional Hazards Analyses, while adjusting for factors associated with spontaneous HCV clearance and SVR. These factors included sex, age, history of injecting, estimated duration of HCV infection, presentation (acute clinical, asymptomatic), peak ALT level, baseline HCV RNA levels, HCV genotype and HIV infection.
The multivariate model for predictors of treatment response and HCV clearance were determined using a forward stepwise approach, considering factors that were significant at the 0.10 level in univariate analysis. The final models included only factors that remained significant at the 0.05 level. All analyses were performed using the statistical packages SAS and STATA.
Results
Over the period June 2004 through February 2008, 200 people with recent HCV infection were screened for potential inclusion in the study (Figure 1). Ultimately, 167 participants were enrolled, through tertiary hospitals (n=150) or through general practice or primary care clinics (n=17). Of those who consented to enrol four did not return for a subsequent baseline visit and were excluded from further analysis, leaving a total participant population of 163.
Figure 1.
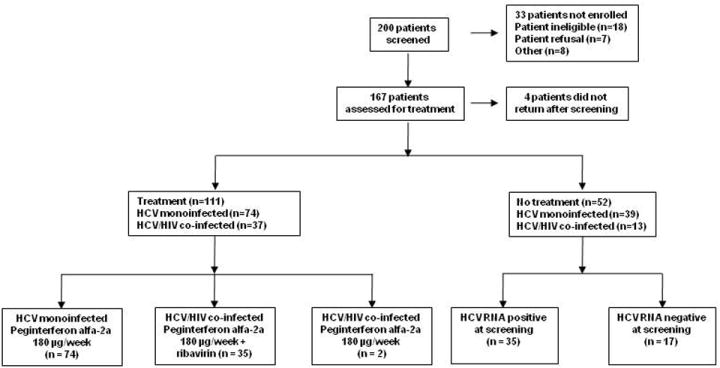
Overview of study population. At least 1 dose of PEG-IFN was administered to 111 participants. Two HCV/HIV co-infected participants received PEG-IFN monotherapy (both non-responders) prior to a protocol amendment in which HCV/HIV participants then received PEG-IFN and ribavirin combination therapy.
Diagnosis of recent HCV infection was on the basis of acute clinical hepatitis in 61% (99 of 163), that included symptomatic seroconversion illness in 41% (67 of 163, including 36 with jaundice) and ALT >400 IU/mL in 20% (32 of 163), respectively. Diagnosis of recent HCV infection was on the basis of anti-HCV antibody seroconversion in the absence of an acute clinical presentation in 39% (64 of 163). Overall, anti-HCV antibody seroconversion was documented in 86% (n=140). The enrolment characteristics of treated (n=111) and untreated (n=52) participants, with the latter group stratified by HCV RNA status (35 positive, 17 negative) at screening, are shown in Table 1.
Table 1.
Enrolment characteristics of participants (n=163)
| Total study population | Treated | Untreated |
||
|---|---|---|---|---|
| HCV RNA positive at screening | HCV RNA negative at screening | |||
| Total participants, (n) | 163 | 111 | 35 | 17 |
| Male, n (%) | 116 (71%) | 83 (75%) | 23 (66%) | 10 (59%) |
| Age (yrs), mean/SD | 34.3 ± 9.9 | 34.5 ± 10.4 | 34.9 ± 8.9 | 31.9 ± 8.5 |
| Weight (kg), mean/SD | 73.2 ± 14.1 | 72.6 ± 11.7 | 70.6 ± 12.8 | 82.7 ± 24.5 |
| BMI (kg/m2), mean/SD | 24.0 ± 4.3 | 23.7 ± 3.3 | 23.3 ± 4.1 | 27.7 ± 8.1 |
| Caucasian ethnicity, n (%) | 149 (91%) | 99 (89%) | 34 (97%) | 16 (94%) |
| Tertiary education or greater, n (%) | 35 (22%) | 32 (29%) | 3 (9%) | 0 (0%) |
| Full-time or part-time employment, n (%) | 63 (39%) | 52 (47%) | 9 (26%) | 2 (12%) |
| Methadone or buprenorphine treatment | ||||
| Ever (not current) | 17 (10%) | 12 (11%) | 4 (11%) | 1 (6%) |
| Current | 22 (14%) | 12 (11%) | 6 (17%) | 4 (24%) |
| Social functioning score, median (IQR) | 13 (8-18) | 11 (6-17) | 15 (10-19) | 18 (13-20) |
| Current major depression, n (%) | 19 (12%) | 8 (7%) | 9 (26%) | 2 (12%) |
| Injecting drug use ever, n (%) | 125 (77%) | 84 (76%) | 30 (86%) | 11 (65%) |
| Last time injected, n (%)¥ | ||||
| Within the last month | 53 (42%) | 31 (37%) | 15 (50%) | 7 (64%) |
| 1 and 6 months ago | 46 (37%) | 35 (42%) | 9 (30%) | 2 (18%) |
| >6 months ago | 25 (20%) | 18 (21%) | 5 (17%) | 2 (18%) |
| Estimated duration of infection at screening (wks), median (range) | 25 (6-74) | 25 (6-74) | 19 (7-62) | 26 (15-66) |
| Presentation of recent HCV, n (%) | ||||
| Acute clinical (symptomatic) | 67 (41%) | 46 (41%) | 12 (34%) | 9 (53%) |
| Acute clinical (ALT >400 IU/mL) | 32 (20%) | 24 (22%) | 6 (17%) | 2 (12%) |
| Asymptomatic seroconversion | 64 (39%) | 41 (37%) | 17 (49%) | 6 (35%) |
| Symptoms and signs in acute clinical (symptomatic) cases, n (%)* | ||||
| Jaundice | 36 (54%) | 24 (52%) | 5 (42%) | 7 (78%) |
| Nausea | 45 (67%) | 29 (63%) | 8 (67%) | 8 (89%) |
| Abdominal pain | 42 (63%) | 30 (65%) | 8 (67%) | 4 (44%) |
| Hepatomegaly | 16 (24%) | 11 (24%) | 4 (33%) | 1 (11%) |
| HIV infection, n (%) | 50 (31%) | 37 (33%) | 12 (34%) | 1 (6%) |
| ALT (IU/L) | ||||
| Peak ALT prior to enrolment, median (IQR) | 468 (175-1206) | 479 (207-1179) | 393 (114-1174) | 382 (44-2206) |
| ALT at screening, median (IQR) | 118 (52-312) | 185 (70-403) | 104 (39-155) | 27 (20-49) |
| HCV RNA (IU/L) | ||||
| Log10 HCV RNA - screening, median | 4.5 | 5.0 | 3.3 | <1.0 |
| <400,000 IU/mL, n (%) | 119 (73%) | 73 (66%) | 29 (83%) | 17 (100%) |
| HCV genotype, n (%) | ||||
| Genotype 1 | 76 (47%) | 63 (57%) | 13 (37%) | 0 (0%) |
| Genotype 2 | 6 (4%) | 4 (4%) | 2 (6%) | 0 (0%) |
| Genotype 3 | 56 (34%) | 40 (36%) | 16 (46%) | 0 (0%) |
| Genotype 4 | 1 (1%) | 0 (0%) | 1 (3%) | 0 (0%) |
| Genotype missing | 24 (15%) | 4 (4%) | 3 (9%) | 17 (100%) |
includes 4 participants with missing data,
at time of screening,
denominator is in total number of people reporting documented illness,
among those having reported injecting ever,
IQR, interquartile range.
For the majority of participants, injecting drug use was recorded as the most likely mode of HCV acquisition (n=119, 73%). Other modes of reported HCV acquisition included male to male sexual contact (n=24, 15%), heterosexual contact (n=5, 3%), body piercing (n=1, 1%), medical procedure (n=1, 1%), occupational needle stick (n=1, 1%), tattoos (n=1, 1%) and other forms of percutaneous exposure (n=1, 2%). In 6% of participants (n=10), no risk factor could be identified.
The study population had a low proportion of participants who were in full- or part-time employment (39%) or who had completed tertiary education (22%). Social functioning was low, with a median score of 13 (interquartile range, IQR: 8-18; possible range 0 to 48). Overall, 125 (77%) participants had ever injected illicit drugs and 39 (24%) reported having ever received methadone or buprenorphine treatment. Among participants who reported injection drug use ever, recent injecting was common, with 42% (53 of 125) injecting in the previous month and an additional 37% (46 of 125) in the period one to six months prior to screening. Among those having reported injecting drug use in the past 6 months, the drugs most often injected were methamphetamine (48%) and heroin (39%).
HCV treatment uptake
As the 17 participants with undetectable HCV RNA at screening were ineligible for treatment, the uptake of HCV treatment was 76% (111 of 146) among those who were eligible on the basis of positive HCV RNA. Uptake was 76% (74 of 97) in participants without HIV and 76% (37 of 49) among those with HIV infection.
Among those who were HCV RNA positive at screening or baseline and therefore potentially eligible for treatment (n=146), untreated participants had slightly poorer social functioning (i.e higher scores, 15 vs. 11), lower tertiary education (9% vs. 29%) and were less frequently in full- or part-time employment (26% vs. 47%) when compared to treated participants. A greater proportion of untreated participants also had current major depression (26% vs. 7%) and reported injecting drug use in the last month (50% vs. 37%). Untreated participants had a shorter estimated duration of infection at screening (19 vs. 25 weeks), lower peak ALT (393 vs. 479 IU/L) or screening ALT (104 vs. 185 IU/L) and lower median HCV RNA at screening (3.3 vs. 5.0 log10 HCV RNA).
HCV treatment outcomes
Due to the different treatment regimens employed, treatment outcomes were assessed separately for HCV mono-infected receiving PEG-IFN monotherapy (n=74) and the HCV/HIV co-infected participants receiving PEG-IFN and ribavirin combination therapy (n=35). The initial two participants with HCV/HIV co-infection treated with PEG-IFN monotherapy were excluded from outcome analyses (both were non-responders at week 12).
Among treated participants, those with HCV/HIV co-infection were older, more likely to be male (100% vs. 62%), and more likely to have acquired HCV through sexual contact (63% vs. 5%), and had better social functioning [i.e lower scores, 8 vs. 14 (Table 2)].
Table 2.
Baseline characteristics among treated HCV and HCV/HIV infected participants with recently acquired HCV infection (n=109)€
| HCV infected | HCV/HIV infected | |
|---|---|---|
| Total participants, (n) | 74 | 35 |
| Male, n (%) | 46 (62%) | 35 (100%) |
| Age (yrs), mean/SD | 31.0 ± 9.0 | 42.0 ± 9.5 |
| Age category (yrs) | ||
| ≤ 25 | 18 (24%) | 1 (3%) |
| 26 – 30 | 21 (28%) | 4 (11%) |
| 31 – 40 | 24 (32%) | 9 (26%) |
| > 40 | 11 (15%) | 21 (60%) |
| Weight (kg), mean/SD | 69.5 ± 11.8 | 78.1 ± 9.2 |
| BMI (kg/m2), mean/SD | 23.0 ± 3.6 | 24.7 ± 2.3 |
| Caucasian ethnicity, n (%) | 63 (85%) | 34 (97%) |
| Tertiary education or greater, n (%) | 13 (18%) | 17 (49%) |
| Full-time or part-time employment, n (%) | 26 (35%) | 24 (69%) |
| Methadone or buprenorphine treatment | ||
| Ever (not current) | 12 (16%) | 0 (0%) |
| Current | 12 (16%) | 0 (0%) |
| Social functioning score, median (IQR) | 14 (9-19) | 8 (4-13) |
| Current major depression, n (%) | 7 (10%) | 1 (3%) |
| Mode of infection, n (%) | ||
| Injecting drug use | 62 (84%) | 13 (37%) |
| Sexual exposure with person(s) of same sex | 1 (1%) | 22 (63%) |
| Sexual exposure with person(s) of opposite sex | 3 (4%) | 0 (0%) |
| Other | 13 (18%) | 1 (3%) |
| Injecting drug use ever, n (%) | 63 (85%) | 19 (54%) |
| Age at first injection drug use, mean/SD¥ | 23.0 ± 8.5 | 33.8 ± 10.3 |
| Last time injected, n (%)†, ¥ | ||
| Within the last month | 27 (43%) | 4 (21%) |
| 1 and 6 months ago | 25 (40%) | 9 (47%) |
| >6 months ago | 11 (18%) | 6 (32%) |
| Drug(s) most frequently injected, (%)†, ₤ | Opiates (50%) | Methamphetamine (87%) |
| Number of days drinking in last month, mean/SD† | 5.6 ± 6.7 | 7.4 ± 8.9 |
| Estimated duration of infection (wks), median (range) | ||
| Screening | 28 (7-74) | 17 (6-64) |
| Baseline | 34 (18-84) | 30 (10-93) |
| Presentation of recent HCV, n (%)† | ||
| Acute clinical (symptomatic) | 30 (41%) | 15 (43%) |
| Acute clinical (ALT >400 IU/mL) | 13 (18%) | 11 (31%) |
| Asymptomatic seroconversion | 31 (42%) | 9 (26%) |
| Symptoms in acute clinical (symptomatic) cases, n (%)†, * | ||
| Jaundice | 18 (60%) | 6 (40%) |
| Nausea | 20 (67%) | 9 (60%) |
| Abdominal pain | 19 (63%) | 11 (73%) |
| Hepatomegaly | 8 (27%) | 3 (20%) |
| ALT (IU/L) | ||
| Peak prior to screening, median (IQR) | 427 (182-1161) | 557 (286-1151) |
| At screening, median (IQR) | 132 (64-312) | 254 (131-630) |
| At baseline, median (IQR) | 120 (52-215) | 130 (99-422) |
| HCV RNA (IU/L) | ||
| Log10 HCV RNA - baseline, median | 5.0 | 5.8 |
| <400,000 IU/mL, n (%) | 52 (70%) | 16 (46%) |
| HCV genotype, n (%) | ||
| Genotype 1 | 41 (54%) | 19 (56%) |
| Genotype 2 | 1 (1%) | 3 (8%) |
| Genotype 3 | 29 (39%) | 12 (34%) |
| Missing | 3 (4%) | 1 (3%) |
two HCV/HIV co-infected participants receiving PEG-IFN monotherapy are excluded from this analysis,
at time of screening,
denominator is in total number of people reporting documented illness,
among those having reported injecting ever
among those having injected in the last 6 months
As shown in Figure 2, 77% of HCV mono-infected participants (57 of 74) received at least 80% of PEG-IFN alfa-2a doses and therapy for 80% of the scheduled treatment period. Adherence to therapy was not achieved in 23% (17 of 74). These subjects discontinued treatment prematurely, either due to side effects (n=4); death (n=1); lost to follow-up or unwillingness to continue in the study (n=8); late discontinuation at 15 weeks with virological non-response (n=1); and testing HCV RNA positive at screening but negative at treatment commencement (2 participants following week 2 injection; and 1 participant following week 6 injection).
Figure 2.
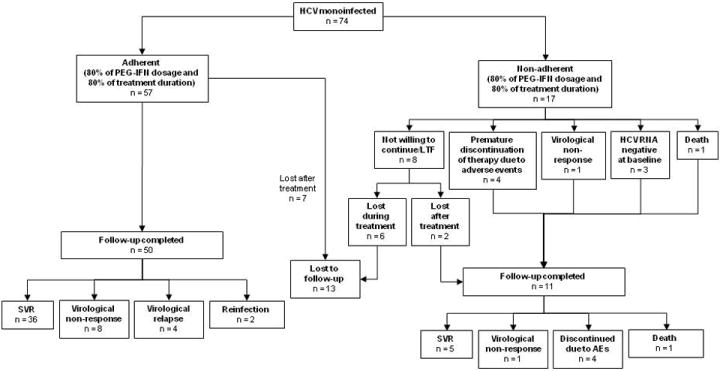
Overview of HCV mono-infected participant population.
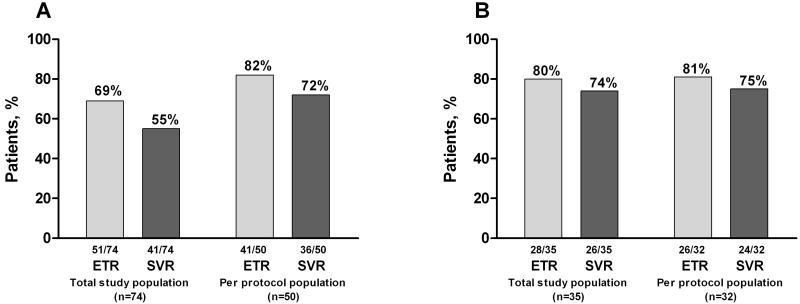
In HCV mono-infected participants, 46% (34 of 74) and 66% (49 of 74) had undetectable HCV RNA (<10 IU/mL) at weeks 4 and 12, respectively, and an ETR was achieved in 69% (51 of 74). Among adherent participants with follow-up virological data (per protocol analysis group) (n=50), 41 (82%) achieved an ETR (Figure 3). SVR was 55% by intention-to-treat and 72% by per protocol analysis.
Figure 3.
Response rates in A) HCV mono-infected participants and B) HCV/HIV co-infected participants in the total study population (intent-to-treat) and per-protocol population.
In univariate analysis, SVR occurred more frequently in people with better social function (i.e. lower scores); not currently receiving methadone or buprenorphine treatment; and not having used injecting drugs ever (Table 3). Although participants reporting ever having injected drugs had a lower frequency of SVR than those who never injected (48% vs. 91%, P=0.030), SVR was not associated with either the duration of abstinence from injecting drug use or the frequency of injecting drug use at baseline among those who injected (Table 3).
Table 3.
Factors associated with sustained virological response (SVR) among HCV mono-infected participants receiving treatment for recently acquired HCV infection (n=74)
| SVR | No SVR | OR | 95% CI | P-value | P-value overall | ||
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 24 | 23 | 1.00 | - | - | - | - |
| Female | 17 | 10 | 1.63 | 0.62 | 4.29 | 0.323 | - |
| Age category (years) | |||||||
| ≤ 25 | 11 | 13 | 1.00 | - | - | - | |
| 26 - 30 | 15 | 12 | 1.48 | 0.49 | 4.46 | 0.489 | 0.107 |
| 31 - 40 | 9 | 7 | 1.52 | 0.43 | 5.43 | 0.519 | - |
| > 40 | 6 | 1 | 7.09 | 0.74 | 68.24 | 0.090 | - |
| Weight (kgs) | |||||||
| ≤ 75 | 26 | 18 | 1.00 | - | - | - | - |
| >75 | 13 | 8 | 1.125 | 0.39 | 3.27 | 0.829 | - |
| Missing | 2 | 7 | - | - | - | - | - |
| Education | |||||||
| Primary/secondary | 26 | 21 | 1.00 | - | - | - | - |
| Other (eg TAFE, tertiary) | 15 | 12 | 1.01 | 0.39 | 2.62 | 0.984 | - |
| Employment | |||||||
| Full-time/part-time | 18 | 8 | 1.00 | - | - | - | - |
| Other | 23 | 25 | 0.41 | 0.15 | 1.12 | 0.082 | - |
| Accommodation | |||||||
| Rental | 25 | 19 | 1.00 | - | - | - | - |
| Privately owned | 13 | 5 | 1.98 | 0.60 | 6.50 | 0.263 | 0.052 |
| Unstable | 3 | 9 | 0.25 | 0.06 | 1.07 | 0.061 | - |
| Social functioning score | |||||||
| ≤14 | 24 | 10 | 1.00 | - | - | - | - |
| >14 | 12 | 19 | 0.26 | 0.09 | 0.74 | 0.011 | 0.011 |
| Missing | 5 | 4 | 0.52 | 0.12 | 2.35 | 0.396 | - |
| Methadone or buprenorphine treatment | |||||||
| Never | 33 | 17 | 1.00 | - | - | - | - |
| Ever (not current) | 5 | 7 | 0.37 | 0.10 | 1.33 | 0.128 | 0.009 |
| Current | 3 | 9 | 0.17 | 0.04 | 0.72 | 0.016 | - |
| Current depression at screening | |||||||
| No | 36 | 29 | 1.00 | - | - | - | - |
| Yes | 5 | 4 | 1.01 | 0.25 | 4.09 | 0.992 | - |
| Suicidality | |||||||
| None/low | 36 | 31 | 1.00 | - | - | - | - |
| Moderate/high | 5 | 2 | 2.15 | 0.39 | 11.89 | 0.379 | - |
| Injecting drug use ever | |||||||
| No | 10 | 1 | 1.00 | - | - | - | - |
| Yes | 31 | 32 | 0.10 | 0.01 | 0.80 | 0.030 | - |
| Last time injected, n (%) | |||||||
| Within the last month | 12 | 15 | 1.00 | - | - | - | - |
| 1 and 6 months ago | 13 | 12 | 1.35 | 0.45 | 4.03 | 0.586 | 0.021 |
| >6 months ago | 6 | 5 | 1.50 | 0.37 | 6.14 | 0.573 | - |
| Never injected | 10 | 1 | 12.50 | 1.40 | 111.83 | 0.024 | - |
| Injecting Frequency | |||||||
| >daily | 8 | 12 | 1.00 | - | - | - | - |
| <daily, >weekly | 6 | 9 | 1.00 | 0.25 | 3.92 | 1.000 | 0.010 |
| <weekly, | 11 | 6 | 2.75 | 0.72 | 10.48 | 0.138 | - |
| Not injected in last 6 months | 4 | 4 | 1.50 | 0.29 | 7.81 | 0.630 | - |
| Never injected | 10 | 1 | 15.00 | 1.59 | 141.16 | 0.018 | - |
| Missing | 2 | 1 | - | - | - | - | |
| Drug injected most often in last 6 months | |||||||
| Heroin/methadone/other opiates | 11 | 13 | 1.00 | - | - | - | - |
| Methamphetamine | 11 | 9 | 1.44 | 0.44 | 4.76 | 0.545 | 0.495 |
| Other | 1 | 5 | 0.24 | 0.02 | 2.34 | 0.217 | - |
| Not injected in last 6 months | 4 | 4 | 1.18 | 0.24 | 5.86 | 0.838 | - |
| Never injected | 10 | 1 | 11.82 | 1.30 | 107.39 | 0.028 | - |
| Missing | 4 | 1 | |||||
| Mean number of alcoholic drinks per day | |||||||
| <4 drinks | 22 | 22 | 1.00 | - | - | - | - |
| ≥4 drinks | 15 | 11 | 1.36 | 0.51 | 3.62 | 0.534 | - |
| Missing | 4 | 0 | - | - | - | - | - |
| Estimated duration of infection at baseline | |||||||
| ≤26 weeks | 6 | 11 | 1.00 | - | - | - | - |
| 27 – 52 weeks | 27 | 10 | 4.95 | 1.45 | 16.96 | 0.011 | 0.911 |
| >52 weeks | 8 | 12 | 1.22 | 0.32 | 4.66 | 0.769 | - |
| Presentation of recent HCV | |||||||
| Acute clinical | 23 | 20 | 1.00 | - | - | - | - |
| Asymptomatic seroconversion | 18 | 13 | 1.20 | 0.47 | 3.06 | 0.814 | - |
| Peak ALT prior to screening (IU/L) | |||||||
| ≤400 | 19 | 14 | 1.00 | - | - | - | - |
| >400 | 21 | 18 | 0.86 | 0.34 | 2.19 | 0.751 | - |
| Missing | 1 | 1 | - | - | - | - | - |
| ALT at screening (IU/L) | |||||||
| ≤100 | 17 | 14 | 1.00 | - | - | - | - |
| >100 | 24 | 19 | 1.04 | 0.41 | 2.63 | 0.934 | - |
| HCV RNA QN at baseline (IU/mL) | |||||||
| >400000 | 21 | 22 | 1.00 | - | - | - | - |
| ≥400000 | 10 | 10 | 1.05 | 0.36 | 3.03 | 0.932 | - |
| Genotype/subtype | |||||||
| Genotype 1 | 21 | 20 | 1.00 | - | - | - | - |
| Genotypes 2 and 3 | 17 | 13 | 1.25 | 0.48 | 3.21 | 0.650 | - |
| Missing genotype | 3 | 0 | - | - | - | - | - |
There was no association between baseline HCV RNA (P=0.93) or HCV genotype (P=0.65) and SVR. However, among the per protocol group, a trend towards lower SVR was seen in those with genotype 1 and high baseline viral load (HCV RNA ≥400,000) (54%) compared to those with genotype 1 with low baseline viral load (HCV RNA <400,000) and genotypes 2/3 with low (<400,000) and high (HCV RNA ≥400,000) baseline viral load (75%, 80%, 75%, respectively) (P=0.61).
In multivariate analysis, the only factors associated with SVR were social functioning (OR=0.21, 95% CI=0.07-0.64, P=0.009) and drug dependency treatment (OR=0.12, 95% CI=0.02-0.54, P=0.004). Participants with higher social functioning scores and no history of drug dependency treatment had higher SVR. In a further analysis, only including participants who had ever injected drugs (n=63), the same two factors were associated with SVR (data not shown).
Further analyses assessed the role of treatment adherence and injection drug use during treatment as predictors of SVR in HCV mono-infected participants. The SVR rates were higher among adherent participants (63% vs. 29%, P=0.025). Among those reporting ever having injected drugs (n=63), the SVR rate was similar for those who said that they had and not injected during treatment (59% vs. 53%, P=0.76) and was not related to frequency of injecting.
Among HIV/HCV co-infected participants treated with PEG-IFN and ribavirin (n=35), 91% (32 of 35) were at least 80% adherent. Two of the other three stopped treatment prematurely as a result of side effects (n=2), and the third stopped treatment after the week 2 injection because the baseline HCV RNA turned out to be negative, despite the screening assessment having been positive. Undetectable HCV RNA (<10 IU/mL) was achieved in 34% and 91% at weeks 4 and 12, respectively. At the end of treatment, HCV RNA was undetectable in 80% (Figure 3). SVR was 74% by intention-to-treat and 75% by per protocol analysis. There was no association between baseline HCV RNA or HCV genotype and SVR.
We also compared the impact of estimated duration of infection at the commencement of treatment on subsequent SVR in HCV mono-infected and HIV/HCV co-infected groups. Among HCV mono-infected participants, SVR was highest in those with an estimated duration of infection of 27 to 52 weeks (73%, 27 of 37), but was reduced both in those with a duration > 52 weeks (40%, 8 of 20) and in those with a duration of ≤ 26 weeks (35%, 6 of 17). The proportion with ≥80% adherence was 65%, 86% and 70% in those with an estimated duration of infection of ≤ 27 weeks, 27 to 52 weeks and >52 weeks respectively. Similarly the proportion receiving all PEG-IFN injections (24 in total) was 35%, 70% and 45% in those same groups respectively. In contrast, among HIV/HCV co-infected participants, SVR was similar within estimated duration of infection groups of ≤ 26 weeks (67%, 10 of 15), 27 to 52 weeks (73%, 11 of 15) and > 52 weeks (100%, 5 of 5).
Among the combined per protocol group (n=82; HCV=50, HCV/HIV=32), we observed 20 participants with ‘virological failure’, including 11 with non-response, 1 with viral breakthrough and 8 with viral relapse.
Safety
Adverse events are shown in Table 4. Three deaths occurred among treated participants in this study. The one death during IFN-based treatment occurred at week 4 of therapy, in a man whose cause of death was reported as methamphetamine toxicity with a contribution of arrhythmogenic right ventricular dysplasia. At baseline, he was assessed as having major depressive symptoms but no suicidality. He had no reported history of injection drug use and the mode of HCV acquisition was recorded as unknown. The other two deaths occurred at 3 and 4 months following treatment completion respectively, with the cause of death given as carbon monoxide toxicity with combined drug effect (amphetamines and three-prescribed medications, including methadone) in one and electrocution in the other. The post-mortem toxicology report in this third case also revealed ongoing polydrug use (ethanol, methamphetamine, methylenedioxymethamphetamine and cannabinoids.) Both cases demonstrated no major depressive symptoms or suicide risk at baseline.
Table 4.
Adverse events among treated HCV and HCV/HIV infected participants with recently acquired HCV infection (n=109)
| HCV infected PEG-IFN α2a (n=74) | HCV/HIV infected PEG-IFN α2a + ribavirin (n=35) | |||
|---|---|---|---|---|
| Adverse event grade* | N | % | N | % |
| Grade 1 | 70 | 94.6 | 35 | 100.0 |
| Grade 2 | 54 | 73.0 | 31 | 88.6 |
| Grade 3 | 28 | 37.8 | 25 | 71.4 |
| Grade 4 | 3 | 4.1 | 1 | 2.9 |
| Most frequent adverse event (>10% of patients)¶ | ||||
| Fatigue | 59 | 79.7 | 32 | 91.4 |
| Headache | 57 | 77.0 | 27 | 77.1 |
| Irritability | 51 | 68.9 | 30 | 85.7 |
| Myalgia | 53 | 71.6 | 25 | 71.4 |
| Insomnia | 48 | 64.9 | 29 | 82.9 |
| Anxiety | 44 | 59.5 | 27 | 77.1 |
| Disturbance in attention | 41 | 55.4 | 27 | 77.1 |
| Arthralgia | 41 | 55.4 | 23 | 65.7 |
| Injection site reaction | 45 | 60.8 | 19 | 54.3 |
| Nausea | 39 | 52.7 | 24 | 68.6 |
| Dry skin | 37 | 50.0 | 25 | 71.4 |
| Abdominal pain | 37 | 50.0 | 22 | 62.9 |
| Cough | 34 | 45.9 | 25 | 71.4 |
| Anorexia | 37 | 50.0 | 21 | 60.0 |
| Pruritus | 37 | 50.0 | 20 | 57.1 |
| Pyrexia | 33 | 44.6 | 22 | 62.9 |
| Diarrhoea | 35 | 47.3 | 19 | 54.3 |
| Dizziness | 32 | 43.2 | 20 | 57.1 |
| Dyspnoea | 28 | 37.8 | 24 | 68.6 |
| Weight decreased | 28 | 37.8 | 24 | 68.6 |
| Pain | 29 | 39.2 | 22 | 62.9 |
| Chills | 27 | 36.5 | 16 | 45.7 |
| Alopecia | 31 | 41.9 | 10 | 28.6 |
| Asthenia | 22 | 29.7 | 19 | 54.3 |
| Dermatitis | 22 | 29.7 | 11 | 31.4 |
| Depression | 17 | 23.0 | 4 | 11.4 |
participants can be counted in ≥1 grade,
Adverse events reported according to MeDRA preferred terms.
Impact of treatment on HCV clearance
Among the 146 participants with detectable HCV RNA at screening or baseline, 35 were not treated for HCV infection and 12 (34%) of these demonstrated spontaneous HCV RNA clearance. As the untreated participants differed from the treated participants, particularly with respect to factors associated with spontaneous HCV RNA clearance (lower screening HCV viral load, shorter estimated duration of infection), adjusted analyses were undertaken to examine the impact of treatment on clearance. In the treated group, only participants who subsequently achieved a SVR were considered to have HCV RNA clearance. The Kaplan Meier analysis of the impact of treatment on HCV RNA clearance among untreated (n=34) and treated (n=106) participants positive for HCV RNA at screening is shown in Figure 4. In Cox Proportional Hazards Analyses, treatment was independently associated with HCV RNA clearance (HR=4.20, 95% CI=1.96-9.00, P<0.0001) after adjusting for sex, age, history of injecting, estimated duration of HCV infection, clinical presentation, peak ALT level, baseline HCV RNA levels, HCV genotype and HIV infection.
Figure 4.
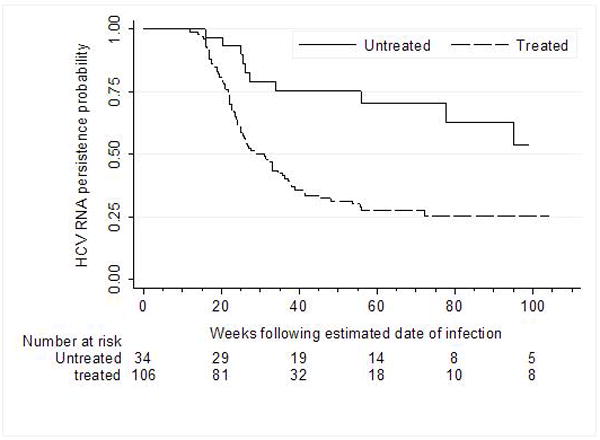
Time to HCV RNA clearance among treated and untreated participants in ATAHC study
Discussion
This study has found that treatment for recent HCV infection is effective in people whose infection was acquired through injecting drug use, even in those with HIV co-infection. Further, it appears that treatment with PEG-IFN alone remains effective when commenced at up to 12 months post-HCV infection. ATAHC is the largest study to examine treatment outcomes for recently acquired HCV among people who inject drugs. It is also the first study to examine outcomes in substantial populations with and without HIV co-infection, and the first to evaluate HCV treatment outcomes across a broad definition of recent HCV infection which encompasses acute and early chronic disease.
An overall intention-to-treat SVR rate of 55-74% with PEG-IFN-based therapy for 24 weeks is very encouraging, given the assumptions that are often made about the feasibility of treatment in this population, and the relatively long estimated duration of HCV infection at treatment initiation. The SVR rate of 74% for HIV/HCV co-infected participants who received PEG-IFN and ribavirin combination therapy was particularly impressive. There was a relatively lower SVR rate of 55% among HCV mono-infected participants, largely related to social factors (poorer social functioning leading to sub-optimal treatment adherence). In addition, PEG-IFN monotherapy may have been sub-optimal for some HCV mono-infected participants including those with genotype 1 and high HCV viral load or duration of HCV infection greater than 12 months.
Previous PEG-IFN (12 – 24 weeks) based studies in acute HCV infection have shown somewhat higher SVR rates of 57 – 88% (4, 6-8, 10, 18, 19). ATAHC differs from most of these investigations in the predominantly injecting drug use related acquisition of the study population and the relatively late enrolment of the participants in the course of their infection. In other studies, mainly made up of cases of acute symptomatic infection, enrolment was within a median of 12 weeks, and many cases may have spontaneously cleared virus, even without being treated. In contrast the median estimated duration of HCV infection at treatment commencement was around 30 weeks in ATAHC, after the time when spontaneous clearance is believed to occur (20).
Adherence clearly plays an important role. In ATAHC, treatment response among HCV mono-infected participants varied considerably by adherence grouping and the overall SVR of 55% was lower than the rate from a recently reported Italian study (n=46, 26 IDU, SVR=72%) (18), in which all injections were medically supervised and adherence was close to 100%. HCV treatment adherence is predictive of chronic HCV treatment outcomes, both in the non-IDU and IDU population (21, 22). In ATAHC poor “adherence” largely related to loss to follow-up rather than either missed doses or dose reductions, therefore strategies to improve engagement for socially marginalized individuals commenced on HCV treatment are required.
Poorer social functioning and current drug dependency treatment were the only factors associated with lower SVR among HCV mono-infected participants in this study. The social functioning scale employed in this study addresses major aspects of social integration such as employment, residential stability, inter-personal conflict, social support and the involvement of the participant in drug using networks. The results from this aspect of the study are novel and suggest this social functioning scale may be a useful tool for future studies of illicit drug users to assess suitability for treatment initiation. Although current opioid maintenance treatment was associated with reduced response rates to therapy, the numbers of participants in this group was small and further investigations of the involvement of the impact of this variable on SVR are required.
ATAHC suggests that a delay in commencement of treatment until such time as spontaneous viral clearance is unlikely, does not seem to adversely influence treatment effectiveness in this population. Although delayed commencement (20 weeks versus 8-12 weeks following acute HCV presentation) produced a lower SVR (76% versus 92-95%) in a prior randomized controlled trial, this was conducted in a largely non-IDU population (4). Estimated duration of HCV infection at commencement of treatment was not a predictor of treatment response in ATAHC in multivariate analysis, however, within the HCV mono-infected population, lower SVR rates were seen among participants with short (≤26 weeks) and longer (>52 weeks) durations of infection. Poorer responses in the most prolonged duration group may reflect sub-optimal therapy with PEG-IFN monotherapy, particularly given the favourable responses in the HIV/HCV co-infected group. On the other hand, poorer responses in the short duration of infection group may be driven by poorer adherence among individuals who more recently acquired HCV infection.
For people with both HIV infection and recently acquired HCV, the SVR rate following PEG-IFN and ribavirin combination therapy (74%) confirms the favourable preliminary data reported on the initial 22 co-infected participants (23). The SVR is higher than other acute HCV studies among co-infected populations with study populations above n=20 (59-61%) (24, 25), and considerably higher than that reported for chronic HCV studies of PEG-IFN and ribavirin therapy in this population (26-40%) (26, 27). It also suggests that 24 weeks is adequate therapy for both acute and early chronic HCV infection, irrespective of HCV genotype and baseline HCV viral load.
Despite the somewhat poorer treatment outcomes in HCV mono-infected participants compared to other acute HCV treatment studies, the ATAHC study demonstrates that in a setting of predominant injecting drug use HCV acquisition participants with acute and early chronic HCV infection can be effectively treated. Although the ATAHC study did not contain a randomized control arm without treatment, the comparison of HCV viral clearance among treated and untreated groups with adjustment for baseline factors associated with clearance provided further evidence of the beneficial impact of early therapeutic intervention. Improved strategies are required to select participants for treatment initiation and to enhance treatment adherence and follow-up, including the potential of supervised therapy, case management, and peer-based support. Ways to improve and support IDUs social functioning prior to commencing treatment should be explored. Drug rehabilitation and harm reduction strategies that reduce rates of injecting drug use and HCV exposure during injecting also need to be the focus of an overall strategy to enhance HCV treatment outcomes among IDUs, both in the acute and chronic HCV infection setting. Finally, a randomized controlled trial of PEG-IFN versus PEG-IFN and ribavirin therapy in this study population would appear justified based on the basis of the data reported here.
Supplementary Material
Acknowledgments
Grant support: This study was funded by the National Institutes of Health grant RO1 DA 15999-01. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, University of New South Wales. Roche Pharmaceuticals supplied financial support for pegylated IFN–alfa-2a/ribavirin. GD, PH and AL were supported by National Health and Medical Research Council Practitioner Research Fellowships. MH was supported by a National Health and Medical Research Council Career Development Award and a VicHealth Senior Research Fellowship. JG was supported by Post Doctoral Fellowships from the Canadian Institutes of Health Research and the National Canadian Research Training Program in Hepatitis C. RF was supported by a National Health and Medical Research Council Industry fellowship. JK was supported by National Health and Medical Research Council Research Fellowship.
ATAHC Study Group
Protocol Steering Committee members
John Kaldor (NCHECR), Gregory Dore (NCHECR), Gail Matthews (NCHECR), Pip Marks (NCHECR), Andrew Lloyd (UNSW), Margaret Hellard (Burnet Institute, VIC), Paul Haber (University of Sydney), Rose Ffrench (Burnet Institute, VIC), Peter White (UNSW), William Rawlinson (UNSW), Carolyn Day (University of Sydney), Ingrid van Beek (Kirketon Road Centre), Geoff McCaughan (Royal Prince Alfred Hospital), Annie Madden (Australian Injecting and Illicit Drug Users League, ACT), Kate Dolan (UNSW), Geoff Farrell (Canberra Hospital, ACT), Nick Crofts (Nossal Institute, VIC), William Sievert (Monash Medical Centre, VIC), David Baker (407 Doctors).
NCHECR ATAHC Research Staff
John Kaldor, Gregory Dore, Gail Matthews, Pip Marks, Barbara Yeung, Jason Grebely, Brian Acraman, Kathy Petoumenos, Janaki Amin, Carolyn Day, Anna Doab, Therese Carroll.
Burnet Institute Research Staff
Margaret Hellard, Oanh Nguyen, Sally von Bibra.
Immunovirology Laboratory Research Staff
UNSW Pathology - Andrew Lloyd, Suzy Teutsch, Hui Li, Alieen Oon, Barbara Cameron.
SEALS – William Rawlinson, Brendan Jacka, Yong Pan.
Burnet Institute Laboratory, VIC – Rose Ffrench, Jacqueline Flynn, Kylie Goy.
Clinical Site Principal Investigators
Gregory Dore, St Vincent’s Hospital, NSW; Margaret Hellard, The Alfred Hospital, Infectious Disease Unit, VIC; David Shaw, Royal Adelaide Hospital, SA; Paul Haber, Royal Prince Alfred Hospital; Joe Sasadeusz, Royal Melbourne Hospital, VIC; Darrell Crawford, Princess Alexandra Hospital, QLD; Ingrid van Beek, Kirketon Road Centre; Nghi Phung, Nepean Hospital; Jacob George, Westmead Hospital; Mark Bloch, Holdsworth House GP Practice; David Baker, 407 Doctors; Brian Hughes, John Hunter Hospital; Lindsay Mollison, Fremantle Hospital; Stuart Roberts, The Alfred Hospital, Gastroenterology Unit, VIC; William Sievert, Monash Medical Centre, VIC; Paul Desmond, St Vincent’s Hospital, VIC.
Footnotes
Manuscript Number: GASTRO-D-09-01046
Disclosures: GD, GM and JK have received research support from Roche Pharmaceuticals. GD is on the speaker’s bureau for Roche Pharmaceuticals. GD and GM are members of advisory board for Roche Pharmaceuticals. GD, PM and BY have received travel grants from Roche Pharmaceuticals. GD is a consultant/advisor for Schering Plough, Tibotec, and Abbott. GM is a consultant/advisor for Schering Plough, Novartis and Astellar.
Author Contributions: Authors GJD, GVM, JMK designed the study and wrote the protocol. All authors assisted with the development of the final protocol. Authors GJD, MH, GVM, JG, KP, BY, PM and JMK assisted with development of data collection instruments. Author JG drafted the primary statistical analysis plan, which was reviewed by KP, GJD, MH, PM, GVM and JMK. The primary statistical analysis was conducted by KP and additional statistical analyses were conducted by JG and GJD. All authors reviewed data analysis. Authors GJD and JG wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Thein HH, Krahn M, Kaldor JM, Dore GJ. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol. 2005;100:643–651. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 4.Kamal SM, Fouly AE, Kamel RR, Hockenjos B, Al Tawil A, Khalifa KE, He Q, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. doi: 10.1053/j.gastro.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 5.De Rosa FG, Bargiacchi O, Audagnotto S, Garazzino S, Cariti G, Raiteri R, Di Perri G. Dose-dependent and genotype-independent sustained virological response of a 12 week pegylated interferon alpha-2b treatment for acute hepatitis C. J Antimicrob Chemother. 2006;57:360–363. doi: 10.1093/jac/dki458. [DOI] [PubMed] [Google Scholar]
- 6.Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, Francavilla R, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005;42:329–333. doi: 10.1016/j.jhep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Wiegand J, Buggisch P, Boecher W, Zeuzem S, Gelbmann CM, Berg T, Kauffmann W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. doi: 10.1002/hep.21043. [DOI] [PubMed] [Google Scholar]
- 8.Kamal SM, Moustafa KN, Chen J, Fehr J, Abdel Moneim A, Khalifa KE, El Gohary LA, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43:923–931. doi: 10.1002/hep.21197. [DOI] [PubMed] [Google Scholar]
- 9.Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, Pastore G, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 10.Kamal SM, Ismail A, Graham CS, He Q, Rasenack JW, Peters T, Tawil AA, et al. Pegylated interferon alpha therapy in acute hepatitis C: relation to hepatitis C virus-specific T cell response kinetics. Hepatology. 2004;39:1721–1731. doi: 10.1002/hep.20266. [DOI] [PubMed] [Google Scholar]
- 11.Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
- 12.Licata A, Di Bona D, Schepis F, Shahied L, Craxi, Camma C. When and how to treat acute hepatitis C? J Hepatol. 2003;39:1056–1062. doi: 10.1016/s0168-8278(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 13.Santantonio T, Wiegand J, Gerlach JT. Acute hepatitis C: current status and remaining challenges. J Hepatol. 2008;49:625–633. doi: 10.1016/j.jhep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multidimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. Br J Addict. 1992;87:733–742. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 16.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 17.White PA, Grebely J, Flynn J, Matthews GV, Renkin M, Pham ST, Bull RA, et al. Hepatitis C virus reinfection following successful treatment of recent infection. Gastroenterology. 2009 Submitted. [Google Scholar]
- 18.Calleri G, Cariti G, Gaiottino F, De Rosa FG, Bargiacchi O, Audagnotto S, Quaglia S, et al. A short course of pegylated interferon-alpha in acute HCV hepatitis. J Viral Hepat. 2007;14:116–121. doi: 10.1111/j.1365-2893.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 19.Broers B, Helbling B, Francois A, Schmid P, Chuard C, Hadengue A, Negro F. Barriers to interferon-alpha therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–328. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 21.McHutchison JG, Fried MW. Current therapy for hepatitis C: Pegylated interferon and ribavirin. Clinics in Liver Disease. 2003;7:149–161. doi: 10.1016/s1089-3261(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 22.Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–747. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- 23.Matthews GV, Hellard M, Haber P, Yeung B, Marks P, Baker D, McCaughan G, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–658. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel M, Nattermann J, Baumgarten A, Klausen G, Bieniek B, Schewe K, Jessen H, et al. Pegylated interferon-alpha for the treatment of sexually transmitted acute hepatitis C in HIV-infected individuals. Antivir Ther. 2006;11:1097–1101. [PubMed] [Google Scholar]
- 25.Gilleece YC, Browne RE, Asboe D, Atkins M, Mandalia S, Bower M, Gazzard BG, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–46. doi: 10.1097/01.qai.0000174930.64145.a9. [DOI] [PubMed] [Google Scholar]
- 26.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, Carosi G, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 27.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, Morand P, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. Jama. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.