Abstract
Objective
Medial vascular fibrosis contributes to arterial stiffening and reduced compliance, increasing the risk of cardiovascular events. We undertook the first comprehensive histopathologic study of medium-to-large caliber blood vessels (carotid, coronary, dorsalis pedis, internal mammary, iliac, mesenteric, pulmonary, and renal arteries) in 100 autopsy subjects to characterize medial fibrosis in relation to cardiovascular risk factors.
Methods and Results
Masson Trichrome staining of vascular tissue microarrays (TMAs) was digitally analyzed to determine the percent fibrosis (% collagen) of over 700 vascular segments. The percent fibrosis of the tunica media was strongly correlated within subjects across all systemic blood vessels (average r = 0.53), suggesting that fibrosis is a global process independent of the predilection of the vessel towards the development of atherosclerosis. Hypertension, diabetes, age and poor renal function were significantly associated with increased systemic vascular fibrosis (p≤0.03). By multivariable analysis, only poor renal function (p=0.003) was an independent predictor of higher levels of fibrosis. Finally, in a subset of 13 individuals we observed a significant correlation between pre-mortem pulse pressure and systemic vascular fibrosis (p<0.001).
Conclusions
This study demonstrates that vascular fibrosis is a global process associated with diseases of aging and elevated pulse pressures.
Keywords: Arteriosclerosis, fibrosis, image analysis, tissue microarrays
1. Introduction
Blood vessels are dynamic conduits, undergoing significant changes in response to the aging process and the development of vascular diseases. These pathologic changes affect the intima, media, and adventitia. While the term “arteriosclerosis” can be taken as an all-encompassing definition of vascular disease, including atherosclerosis, herein we make a distinction between the two. We use the term “arteriosclerosis” to refer to vascular changes in the tunica media. “Atherosclerosis,” the term we use for focal intimal lesions, causes luminal narrowing and is the major cause of acute vascular events (e.g., myocardial infarctions, strokes, and aortic dissections). Risk factors for atherosclerosis have been extensively reported and include age, sex, hypertension, diabetes, smoking, dyslipidemia, obesity, psychosocial factors, diet, and alcohol consumption [1,2]. Arteriosclerosis does not markedly change the caliber of a vessel per se, but as a physiologic phenomenon, it increases arterial stiffness with a resultant decrease in compliance and widening of the pulse pressure [3]. Arterial fibrosis contributes to the development of left ventricular hypertrophy, dementia and renal fibrosis [4]. Pulse wave velocity (PWV) and pulse pressure (PP) are non-invasive measures of central and conduit arterial stiffness that have been used extensively in human studies. In healthy subjects, PWV is an independent predictor of hypertension, stroke and coronary heart disease [5,6]. It is also an independent predictor of mortality in general populations, hypertensive populations, older populations and in subjects with end-stage renal disease [7–10]. PP is similarly an independent marker of coronary heart disease and all-cause mortality [11].
Histologically, arteriosclerotic changes are recognized by alterations in the media. Injured or aged blood vessels have increased collagen formation and develop elastic fiber fragmentation/degeneration, laminar medial necrosis, and increased advanced glycation end products deposition [3,12–14].
There have been surprisingly few thorough examinations of histologic medial fibrosis in arteriosclerosis across multiple patient samples. Studies of vascular fibrosis have generally investigated a few select blood vessels at a time, with most data being generated in the aorta. Only rarely have these studies distinguished between vessel layers [15–22]. These previous studies have demonstrated that older age and hypertension are associated with increased collagen formation.
We sought to rigorously characterize the association between clinical characteristics and the degree of vascular fibrosis in a variety of atherosclerosis-prone and atherosclerosis-resistant vessels from throughout the body. We utilized tissue microarray (TMA) technology to quantify the degree of fibrosis in 8 different blood vessels taken from 100 adult subjects undergoing autopsy.
2. Materials and Methods
A detailed description of the methods is presented in an online supplement.
2.1 Population
Tissues from 100 adult autopsies were harvested and processed as described [23]. Eight medium-to-large caliber vessels (carotid, coronary, dorsalis pedis, iliac, internal mammary, mesenteric, pulmonary, and renal arteries), were used for this study. Demographic and clinical information was abstracted from patient medical records as described [23]. Eight subjects were previously enrolled in the Baltimore Longitudinal Study of Aging (BLSA) [24]. This study was approved by the Johns Hopkins Medicine institutional review board (IRB).
2.2 Tissue microarray creation
Seventeen 99-core tissue microarrays (TMAs) were created from 1,683 vascular tissues as described in detail elsewhere [23].
2.3 Digital analysis of staining
Stained slides were digitized using a ScanScope CS digital slide scanner (Aperio, San Diego, CA) and processed/analyzed using the program FrIDA [25].
2.4 Statistical analysis
Statistical analysis was performed using Pearson’s correlations, t-tests for univariate analysis and multiple regression for multivariate analysis.
3. Results
3.1 Population characteristics
Subjects ranged in age from 20 to 101 years (mean 64±16 years). Seventy-six subjects were Caucasian, 20 subjects were African-American, 2 subjects were Asian and 2 subjects were Hispanic. Thirty subjects had a previous diagnosis of diabetes and an overlapping 62 subjects were hypertensive. The average post-mortem interval (PMI) was 18 hours (range, 4 to 28 hours). Nine subjects were characterized as having poor renal function (estimated GFR<30mL/min/1.73 m2). Ten subjects were missing information on kidney function.
Multiple BP measurements were available on 13 subjects. The 8 BLSA subjects had a median of 11.5 BP measures over a median of 20 years of clinic visits. The 5 non-BLSA subjects had a median of 20 BP measures over a median of 5 years of clinic visits.
3.2 Characterization of Masson Trichrome staining
The percent fibrosis of the tunica media (N=725) and tunica intima (N=505) were measured. Average medial fibrosis varied between vessels from a low of 27±13% in the pulmonary artery to a high of 62±21% in the dorsalis pedis (“dorsalis”) artery (Supplemental Table I). Fibrosis of the media and intima were strongly correlated (r = 0.47 to 0.72, p<0.0001) for all arteries except the renal artery (r=0.29, p=0.10, Supplemental Table II). The percent fibrosis measures of duplicate coronary artery segments within the same TMA and across TMAs (intra- and inter-slide comparisons) demonstrated strong reproducibility of our measurements. Both the intra-slide (r = 0.78, p<0.0001, N=83) and interslide (r = 0.74, p=0.001, N=16) measurements were strongly correlated in a linear fashion (Supplemental Fig. I).
3.3 Correlation of percent medial fibrosis across blood vessels from the same individual and its relationship with medial thickness
The percent medial fibrosis was strongly correlated between all blood vessels in the same individual (average r = 0.53, range 0.33 to 0.72) with the exception of the pulmonary artery. The correlations between the pulmonary artery and other blood vessels were significant, but less robust (average r = 0.34, range 0.25 to 0.40) (Table 1). For all blood vessels except the iliac artery, increasing medial thickness was modestly associated with greater fibrosis (Supplemental Table III).
Table 1.
Pearson’s correlation (r) of medial fibrosis across all 8 blood vessels
| Blood vessel |
Carotid | Coronary | Dorsalis | Iliac | Internal Mammary |
Mesenteric | Pulmonary | Renal |
|---|---|---|---|---|---|---|---|---|
| Carotid | -- | |||||||
| Coronary | 0.54 | -- | ||||||
| Dorsalis | 0.47 | 0.46 | -- | |||||
| Iliac | 0.51 | 0.59 | 0.63 | -- | ||||
| Internal Mammary |
0.56 | 0.58 | 0.35 | 0.45 | -- | |||
| Mesenteric | 0.50 | 0.57 | 0.56 | 0.66 | 0.55 | -- | ||
| Pulmonary | 0.39 | 0.28 | 0.40 | 0.36 | 0.28 | 0.25 | -- | |
| Renal | 0.52 | 0.46 | 0.58 | 0.72 | 0.33 | 0.53 | 0.39 | -- |
All correlations are significant at p<0.01 except the mesenteric-to-pulmonary correlation, where p=0.03.
3.4 Association of subject characteristics with vascular fibrosis
Increasing age was correlated with greater fibrosis in the dorsalis (r = 0.27), iliac (r = 0.29), mesenteric (r = 0.27), and renal arteries (r = 0.31) (Fig. 2). Increasing age also significantly correlated with systemic vascular average (r = 0.22, p = 0.03). A history of hypertension was associated with higher levels of medial fibrosis in all blood vessels except the pulmonary artery (Table 2). The presence of diabetes was associated with higher levels of medial fibrosis in the coronary, carotid, and internal mammary arteries (Table 2). Poor renal function (estimated GFR<30mL/min/1.73 m2) was associated with significantly higher levels of medial fibrosis in the carotid, coronary, internal mammary, and mesenteric arteries (Table 2). The degree of medial fibrosis was not correlated with body mass index (BMI), smoking history, post-mortem interval, or days of fixation of tissue in formalin for any blood vessel (data not shown).
Fig. 2.
Scatter plots for percent medial fibrosis from each blood vessel and patient age. *p≤0.05. X axes are age (years). Open circle (○) is a 27 year old poorly controlled type 1 diabetic male and the open triangle (Δ) is a 101 year old female without hypertension or diabetes. (Note that data points for the subject Δ are missing for 2 blood vessels.)
Table 2.
Association between hypertension, diabetes, poor renal function and percent medial fibrosis
| Hypertension | Diabetes | Poor Renal Function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Vessel | Yes | No | p | N | Yes | No | p | N | Yes | No | p | N |
| Carotid | 43 ± 17 | 35 ± 15 | 0.02 | 92 | 46 ± 18 | 37 ± 15 | 0.009 | 92 | 52 ± 16 | 38 ± 16 | 0.04 | 82 |
| Coronary | 32 ± 21 | 19 ± 16 | 0.003 | 97 | 36 ± 21 | 23 ± 19 | 0.005 | 97 | 46 ± 24 | 24 ± 19 | 0.002 | 88 |
| Dorsalis | 67 ± 20 | 55 ± 22 | 0.01 | 85 | 65 ± 23 | 61 ± 20 | 0.36 | 85 | 72 ± 15 | 60 ± 22 | 0.17 | 76 |
| Iliac | 53 ± 22 | 44 ± 20 | 0.05 | 80 | 55 ± 23 | 47 ± 20 | 0.11 | 80 | 59 ± 21 | 47 ± 21 | 0.17 | 73 |
| Internal Mammary | 24 ± 16 | 16 ± 12 | 0.02 | 95 | 26 ± 18 | 18 ± 13 | 0.02 | 95 | 41 ± 23 | 18 ± 12 | <0.0001 | 85 |
| Mesenteric | 40 ± 21 | 26 ± 19 | 0.001 | 96 | 37 ± 24 | 34 ± 20 | 0.48 | 96 | 54 ± 28 | 31 ± 19 | 0.002 | 86 |
| Pulmonary | 28 ± 13 | 26 ± 13 | 0.34 | 87 | 29 ± 11 | 27 ± 14 | 0.57 | 87 | 32 ± 11 | 27 ± 14 | 0.31 | 78 |
| Renal | 59 ± 17 | 47 ± 17 | 0.0009 | 93 | 60 ± 17 | 52 ± 18 | 0.07 | 93 | 64 ± 08 | 53 ± 18 | 0.09 | 84 |
| Systemic Vasc. Avg. | 45 ± 14 | 34 ± 13 | 0.0003 | 100 | 47 ± 17 | 38 ± 13 | 0.008 | 100 | 56 ± 17 | 39 ± 14 | 0.0008 | 100 |
Poor renal function = GFR<30mL/min/1.73 m2
Values for Yes/No are mean % medial fibrosis ± SD
N= number of samples for each vessel.
3.5 Multivariable analysis of medial fibrosis
A multivariable regression analysis was performed using the Z-transformed average of medial fibrosis in the 7 systemic blood vessels. In a model with age, diabetes, hypertension and renal function, only poor renal function was an independent predictor of higher levels of medial fibrosis (Table 3). Additional multivariable analyses performed separately on the muscular arteries (renal, mesenteric, iliac, coronary, and dorsalis) and elastic arteries (internal mammary and carotid) again identified only poor renal function as an independent predictor of higher levels of fibrosis (data not shown).
Table 3.
Multivariable linear regression model
| Model (n=100) | |||
|---|---|---|---|
| Variable | β | SE | p |
| Age (per 1 year) | 0.0005 | 0.001 | 0.68 |
| Diabetes (yes vs no) | 0.04 | 0.03 | 0.26 |
| Hypertension (yes vs no) | 0.07 | 0.04 | 0.07 |
| Renal Function | |||
| Estimated GFR (>60 mL/min/1.73 m2) | 1.0 (ref.) | 1.0 (ref.) | -- |
| Estimated GFR (30–60 mL/min/1.73 m2) | 0.04 | 0.03 | 0.29 |
| Estimated GFR (<30mL/min/1.73 m2) | 0.15 | 0.05 | 0.003 |
Model of overall systemic vascular fibrosis (Z-transformed) and clinical variables age, diabetes, hypertension, and renal function.
SE, standard error
GFR, glomerular filtration rate.
3.6 Pulse pressure is associated with systemic fibrosis
For the 13 subjects in whom adequate blood pressure (BP) measurements were available (8 subjects from the BLSA study and 5 non-BLSA subjects), we compared calculated PP at time of death with level of systemic vascular fibrosis. There was a strong correlation between PP, a surrogate marker of arterial stiffness, and overall systemic fibrosis in these 13 subjects (r=0.79, p=0.001). We also observed robust correlations (r=0.46 to 0.74) between PP and medial fibrosis levels in each individual artery (with the exception of the pulmonary artery) but these were not statistically significant, probably owing to the small sample size (Supplemental Table IV). The correlation between PP and overall systemic fibrosis (average across all vessels) was also significant among the subsample of BLSA subjects, who had more standardized measures of BP over time (r = 0.75, p = 0.03, n = 8).
4. Discussion
This is the first comprehensive histopathologic study of arteriosclerosis performed by digital analysis of medial fibrosis. These data suggest that the development of arteriosclerosis, as represented by degree of fibrosis, is a global process that occurs across the major arteries of the systemic circulation. Higher levels of fibrosis were associated with only a modest increase in medial thickness. Age, hypertension, diabetes, and poor renal function were the primary clinical correlates of medial fibrosis. In a subsample of subjects for whom adequate measurements were available, elevated pulse pressure values were also significantly associated with higher levels of systemic fibrosis.
A novel component of this study is the use of histopathologic methods with tissue microarray technology and quantitative digital scoring to characterize arteriosclerosis. These methods allow for robust, cost-effective, and high throughput analysis of hundreds of tissues, enabling us to consider a comprehensive collection of eight major vessels, rather than only one or two as had been the previous standard. The pulmonary artery, which is not subjected to systemic circulatory pressures, served as a useful control in this project, and was the only vessel studied not correlated with hypertension. It was fortuitous that 8 subjects in our study had blood pressure measurements obtained using rigorous and standardized protocols in annual or biannual visits as part of the BLSA [24].
An important limitation of this study is the diversity of disease and death in this population. Nonetheless, only in deceased subjects can one collect the wide range of blood vessels used in this study, and such a collection is determined by the availability of autopsy subjects. To harvest 100 subjects who met inclusion criteria, this collection took 3 years at two large university hospitals and was not as selective as had been anticipated. There was a wide range in heterogeneity in disease length and severity which certainly negatively impacted on the strength of associations with disease variables that were measured categorically. For example, the duration of diabetes in this cohort ranged from 2 weeks to 37 years and was unknown for some subjects. One outlier in particular was a 27-year-old type 1 diabetic subject with a history of renal transplantation and who was found to have severe fibrosis throughout his vasculature. This outlier was influential in the relationship of arteriosclerosis and aging as seen in Fig. 2. Another limitation was the lack of a healthy control group since all hospital deaths involved an underlying disease process. Finally, this study is too small to weigh the impact of genetic variation on vascular fibrosis, and thus this parameter was not analyzed.
A second limitation of this study was the lack of traditional biochemical measures of fibrosis. Thus, our study is unable to determine an exact amount of collagen or differentiate between collagen isoforms [22]. We determined the level of fibrosis histochemically based on the proportion of “blue” stain to total stained tissue (Fig. 1), thus the degree of fibrosis was dependent on the exact range of hue, saturation, and intensity values chosen for “blue.” This is a limitation of all quantitative methods based on color space segmentation [26]. Therefore this value is more realistically a relative value between vessels, rather than an absolute value of collagen. Also, we were not able to evaluate elastic fiber fragmentation, cystic medial degeneration or other acquired vascular pathologies.
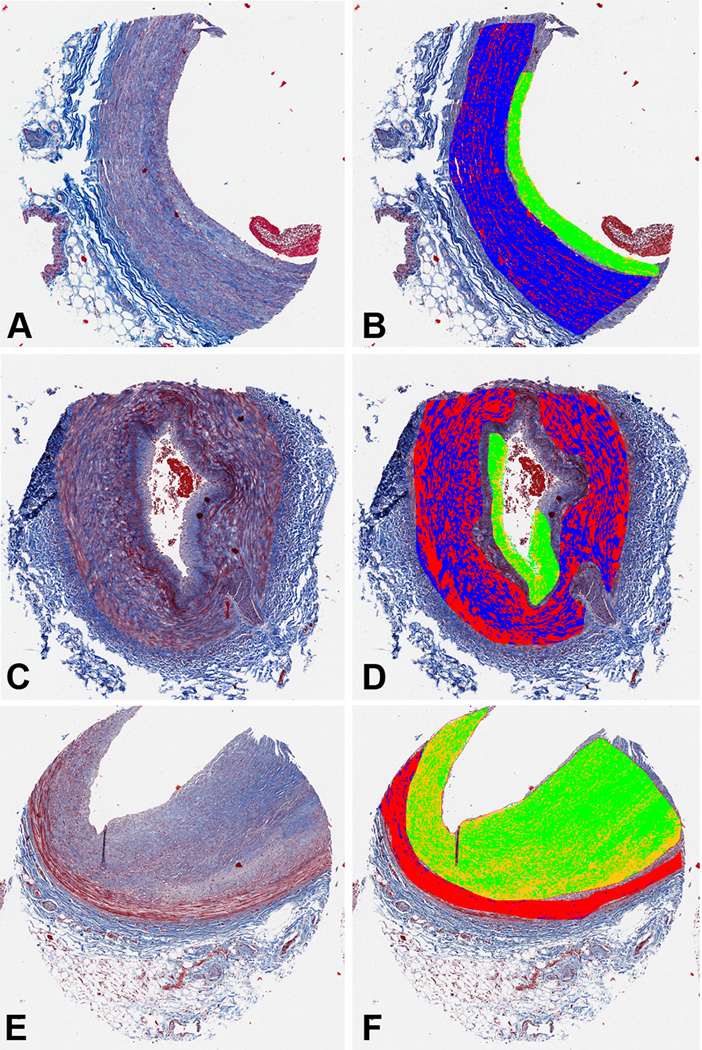
Fig. 1.
Determination of vascular fibrosis by digital analysis of TMA core images. The left panels (A, C, E) show 3 examples of blood vessels stained with Masson Trichrome. The right panels (B, D, F) show these images segmented into four areas: medial fibrosis (blue), medial non-fibrosis (red), intimal fibrosis (green), and intimal non-fibrosis (yellow). (A, B) Internal mammary artery from a 65 year old diabetic, hypertensive male. (C, D) Dorsalis pedis from a 43 year old non-hypertensive, non-diabetic male. (E, F) Coronary artery from a 59 year old non-hypertensive, non-diabetic male.
Clinical studies have identified age as one of the most potent determinants of arterial stiffness [27]. In our study, we found that age was associated with fibrosis in some, but not all, arterial segments. However, age was no longer significantly associated with fibrosis in multivariable analyses, even when the analyses were repeated in a subset of the population free of hypertension and diabetes (data not shown), albeit the sample size was small. These findings suggest that the robust association of age with arterial stiffness may be mediated through processes other than vascular fibrosis, such as elastic fiber fraying/fragmentation, medial elastocalcinosis, inflammation, and covalent cross-linking of advanced glycation end-products on adjacent collagen molecules [28].
Hypertension is also an important determinant of arterial stiffness. In this study, we found that hypertension was associated with fibrosis in all systemic vascular segments, and was borderline significant (p=0.07) in multivariable analysis. This indicates that fibrosis of the medial layer, most likely through the deposition of collagen, is one of the main adaptive responses to the increased blood pressure. It should be noted that in addition to the effects of fibrosis, the effective stiffness of the arterial wall segment is also modulated by additional adaptive mechanisms that are used to compensate for wall stress, such as alterations in vessel diameter and in wall hypertrophy, which vary among the various arterial beds [29]. We did not have sufficient clinical data to determine duration of hypertension or the effectiveness of blood pressure control. These data would have likely enhanced our finding [23].
Poor renal function (estimated GFR <30mL/min/1.73 m2) was associated with higher levels of fibrosis in several vessels (Table 2), and was the only variable independently associated with fibrosis after multivariable adjustment (Table 3). Not surprisingly, subjects with poor renal function were frequently hypertensive (89%) or diabetic (67%). This variable may be a surrogate to identify subjects in our cohort with the most severe hypertension or diabetes as renal failure is a complication of both diseases. The cross-sectional nature of this study limits our ability to draw conclusions regarding the temporality of the observed associations. Thus it is not clear if poor renal function is a cause of, or result of medial fibrosis in our population. It should be noted that PWV has been shown to be an independent predictor of mortality in patients with ESRD [10]. Furthermore, insensitivity of PWV to blood pressure lowering has also been shown to be an independent predictor of mortality in these patients [30]. Our findings suggest that one possible mechanism for the insensitivity of arterial stiffness to blood pressure in patients with ESRD may be accelerated vascular fibrosis.
We took advantage of the limited clinical blood pressure information that was available, to examine the relationship between pulse pressure, a surrogate marker of arterial stiffness, and vascular fibrosis. We found that the correlation coefficients were high in each of the systemic vessels, even though the p-values were not statistically significant, likely due to the small sample size. Interestingly, the highest correlation coefficient was for the systemic average fibrosis (r=0.79, p=0.001). Methodologically, this may be due to normalization of sampling variability in individual blood vessels. Physiologically, this could be due to the fact that PP is determined from SBP and DBP, which, in turn, are dependent on cardiac contractility, heart rate, peripheral resistance, arterial stiffness and reflected waves, and thus are influenced by the collective properties (including fibrosis) of several arterial beds. A potential limitation of our approach is that the values of pulse pressure used in this analysis were derived from models that predicted the longitudinal changes in blood pressure based on repeated measurements previously collected. We felt this measurement would be superior to a single BP measurement taken shortly before death, which would be highly confounded by super-imposed effects of disease and medications. However, this measurement maybe inferior in that it may not take into account potential BP lowering measures initiated by the patient towards the end of the patient’s life.
5. Conclusion
This study provides further evidence that global increases in medial fibrosis are caused by many of the same risk factors that cause atherosclerosis. Local factors (distance to heart, atherosclerosis-prone vs. atherosclerosis-resistant, muscular vs. elastic) probably affect the baseline amount of fibrosis in a given vessel, but global processes appear to augment fibrosis (Supplemental Table I). In any given individual, increased arteriosclerosis in one vessel was associated with increased arteriosclerosis in all other vessels (Table 1). This systemic nature of fibrosis suggests that interventions aimed at lowering arterial stiffness will need to target systemic processes, not just regional hemodynamics. Additional studies should focus on the cellular and molecular signaling cascades that underlie the process of global fibrosis described herein.
Supplementary Material
Acknowledgments
This study was supported by a junior faculty award from the American Diabetes Association (1-05-JF-20 to MKH), by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK076595 to ES) and supported in part by the Intramural Research Program of the National Institutes of Health/National Institute on Aging.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
References
- 1.Grundy SM, Pasternak R, Greenland P, Smith S, Jr., Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481–1492. doi: 10.1161/01.cir.100.13.1481. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 4.O'Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 5.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 6.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 8.Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 11.Najjar SS, Lakatta EG. 29 Vascular Aging. In: Runge MS, Patterson C, editors. Contemporary Cardiology: Principles of Molecular Cardiology. Totowa, NJ: Humana Press, Inc.; 2005. pp. 517–547. [Google Scholar]
- 12.Homme JL, Aubry MC, Edwards WD, et al. Surgical pathology of the ascending aorta: a clinicopathologic study of 513 cases. Am J Surg Pathol. 2006;30:1159–1168. doi: 10.1097/01.pas.0000213270.38091.69. [DOI] [PubMed] [Google Scholar]
- 13.Halushka MK, Selvin E, Lu J, Macgregor AM, Cornish TC. The use of human vascular tissue microarrays for measurement of advanced glycation end products. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.953273. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 15.Spina M, Garbisa S, Hinnie J, Hunter JC, Serafini-Fracassini A. Age-related changes in composition and mechanical properties of the tunica media of the upper thoracic human aorta. Arteriosclerosis. 1983;3:64–76. doi: 10.1161/01.atv.3.1.64. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 17.Clausen B. Influence of age on connective tissue. Hexosamine and hydroxyproline in human aorta, myocardium, and skin. Lab Invest. 1962;11:229–234. [PubMed] [Google Scholar]
- 18.Toda T, Tsuda N, Nishimori I, Leszczynski DE, Kummerow FA. Morphometrical analysis of the aging process in human arteries and aorta. Acta Anat (Basel) 1980;106:35–44. doi: 10.1159/000145167. [DOI] [PubMed] [Google Scholar]
- 19.Ruengsakulrach P, Sinclair R, Komeda M, et al. Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis. Circulation. 1999;100:II139–II144. doi: 10.1161/01.cir.100.suppl_2.ii-139. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JR, Schwartz CJ. Relationship between arterial disease in different sites. A study of the aorta and coronary, carotid, and iliac arteries. Br Med J. 1962;1:1293–1301. doi: 10.1136/bmj.1.5288.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 22.Cattell MA, Anderson JC, Hasleton PS. Age-related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245:73–84. doi: 10.1016/0009-8981(95)06174-6. [DOI] [PubMed] [Google Scholar]
- 23.Halushka MK, Cornish TC, Lu J, Selvin L, Selvin E. Creation, validation, and quantitative analysis of protein expression in vascular tissue microarrays. Cardiovasc Pathol. 2009 doi: 10.1016/j.carpath.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shock NW, Gruelich RC, Andres RA, et al. Normal Human Aging. Washington, DC: U.S. Government Printing Office; The Baltimore Longitudinal Study of Aging. 1984
- 25.Cornish TC, De Marzo AM, Gurel B, Morgan J. FRIDA: An open source framework for image dataset analysis. Arch Pathol Lab Med. 2008;132:856. [Google Scholar]
- 26.Ruifrok AC, Katz RL, Johnston DA. Comparison of quantification of histochemical staining by hue-saturation-intensity (HSI) transformation and color-deconvolution. Appl Immunohistochem Mol Morphol. 2003;11:85–91. doi: 10.1097/00129039-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 28.Lakatta EG. Central arterial aging and the epidemic of systolic hypertension and atherosclerosis. JASH. 2007;1:302–340. doi: 10.1016/j.jash.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Safar ME, Blacher J, Mourad JJ, London GM. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke. 2000;31:782–790. doi: 10.1161/01.str.31.3.782. [DOI] [PubMed] [Google Scholar]
- 30.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.