Abstract
Collagen fiber assembly affects many physiological processes and is tightly controlled by collagen binding proteins. However to what extent membrane bound vs. cell secreted collagen binding proteins affect collagen fibrillogenesis is not well-understood. In our previous studies we had demonstrated that the membrane anchored extracellular domain (ECD) of the collagen receptor Discoidin Domain Receptor2 (DDR2) inhibits fibrillogenesis of collagen endogenously secreted by the cells. These results led to a novel functional role of the DDR2 ECD. However since soluble forms of DDR1 and DDR2 containing its extracellular domain (ECD) are known to naturally exist in the extracellular matrix, in this work we investigated if these soluble DDR ECDs may have a functional role in modulating collagen fibrillogenesis. For this purpose we created mouse osteoblast cell lines stably secreting DDR1 or DDR2 ECD as soluble proteins. Transmission electron microscopy, fluorescence microscopy, and hydroxyproline assays were used to demonstrate that DDR ECD expression reduced the rate and quantity of collagen deposition and induced significant changes in fiber morphology and matrix mineralization. Collectively, our studies advance our understanding of DDR receptors as powerful regulators of collagen deposition in the ECM and elucidate their multifaceted role in ECM remodeling.
Introduction
Collagen fibrillogenesis, the assembly of collagen fibers, is a critical process in the development, maturation and repair of mammalian tissue. Alterations in the structure and amount of deposited collagen fibers can greatly alter the integrity of the whole tissue. Even a single point mutation in collagen type I can severely compromise the strength of cortical bone tissue leading to osteogenesis imperfecta1. Further, the interaction between collagen-binding proteins and collagen molecules during fibrillogenesis can promote significant alterations in the resulting collagen fiber structure and subsequent extracellular matrix (ECM) remodeling2; 3. For example, soluble collagen binding-proteins such as decorin, biglycan, fibronectin and vitronectin are thought to play a significant role in the process of collagen fibrillogenesis and bone mineralization due to their interaction with collagen molecules4.
The collagen-binding membrane proteins discoidin domain receptors (DDR1 and DDR2) are transmembrane receptors belonging to the family of receptor tyrosine kinase and have been studied for ECM remodeling in atherosclerosis5; 6; 7 , osteoarthritis8; 9; 10 and several malignancies7. It is well established that activation of the DDR1 and DDR2 kinase domain up-regulates the expression of various matrix metalloproteinases (MMPs) 5; 11 and alters the biosynthesis of collagen5. The extracellular domain (ECD) of DDRs is known to be necessary and sufficient for its interaction with collagen11. Besides the full-length receptor, the DDR1 ECD is also found in five distinct isoforms12 and as a shedded protein in the ECM13; 14. Several protein5 and mRNA15; 16; 17 species consisting of the DDR2 ECD have also been observed in vivo. However the functional role(s) of these ECDs of DDRs, lacking their kinase domain is not well understood. We had previously elucidated that DDR1 ECD18 and DDR2 ECD19 inhibit collagen fibrillogenesis in-vitro when used as purified proteins. Further we have recently demonstrated that the DDR2 ECD when anchored on the cell surface preserves the capacity to inhibit collagen fibrillogenesis independent of its kinase activity2. It is therefore likely that the expression of soluble ECD of DDRs by cells such as is found in the shedding of DDR1 ECD12 may play an important role in matrix remodeling.
The fibrillogenesis process of collagen is understood to initiate in the extracellular space near the plasma membrane where secretory vesicles form regions of deep invagination20. However, it is not clear how and when collagen-binding proteins interact with collagen molecules during fibrillogenesis or to what extent membrane-bound versus soluble collagen-binding proteins affect the collagen fibrillogenesis process by cells. In this study we seek to elucidate the alterations in collagen fibrillogenesis arising due to soluble DDR1 and DDR2 ECDs secreted by the cells and compare the results with our previous findings utilizing the membrane-bound DDR2 ECD (DDR2-/KD). Similar to our previous study, we created stably transfected mouse osteoblast cell lines to express DDR1/ECD or DDR2/ECD as a soluble protein. We utilized a number of ultrastructural and biochemical analyses to elucidate how alterations in the collagen matrix, due to DDR ECDs, affects collagen fibrillogenesis and matrix mineralization.
Results
Characterization of Stable Cell Lines
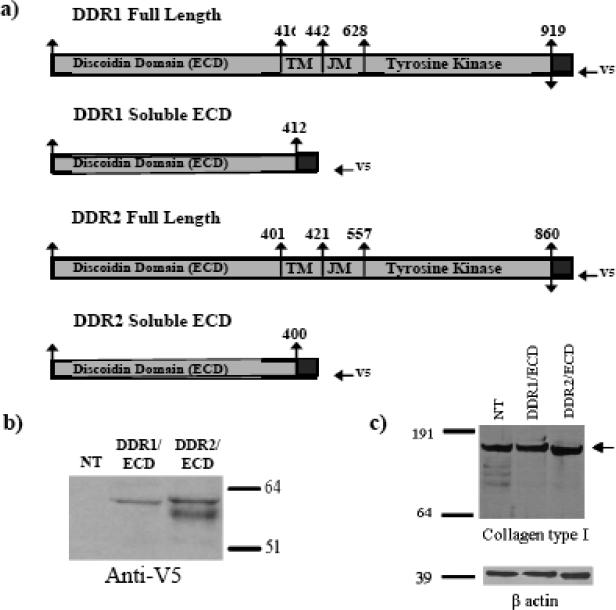
We used mouse pre-osteoblast cell line MC3T3-E1 to ascertain the effects of cell-secretion of DDR1/ECD and DDR2/ECD on collagen fibrillogenesis by the cells. Based on previous studies by us2 and others21 these cells secrete and form well-defined collagen fibers in the ECM over a period of one to several weeks. These cells were stably transfected with V5-epitope tagged DDR1/ECD and DDR2/ECD expression constructs (Figure 1 a) to ensure long term secretion of these proteins in the ECM. The expression of DDR1/ECD and DDR2/ECD in our transiently transfected (data not shown) and stable cell lines was confirmed using western blotting (Figure 1b) of the conditioned media obtained from these samples. DDR2/ECD was expressed as a doublet in western blots, consistent with it being a glycosylated protein as observed by other investigators5; 22; 23; 24. Cells stably transfected with DDR1/ECD or DDR2/ECD did not show alterations in collagen protein expression as shown by western blotting of whole cell lysates of these samples (Figure 1c).
Fig. 1.
Creation of stable cell lines expressing the recombinant protein DDR1/ECD and DDR2/ECD. (a) A schematic representation of the V5 His-tagged full-length mouse DDR1 and DDR2 and the V5 His-tagged soluble ECD proteins, DDR1/ECD and DDR2/ECD. (b) Verification of DDR1/ECD and DDR2/ECD in conditioned media of stable cell lines. Western blot analysis using anti-V5 antibodies was performed on conditioned media from each cell line as indicated. Presence of DDR1/ECD and DDR2/ECD was observed at their expected molecular weights, slightly under 64 kDa as a single band (for DDR1/ECD) and as a doublet for DDR2/ECD. (c) Western blotting of whole cell lysates from nontransfected or stably transfected cells (as indicated) shows no significant difference(s) in collagen expression in the various samples.
DDR ECDs Disrupt the Structure of Collagen Fibers
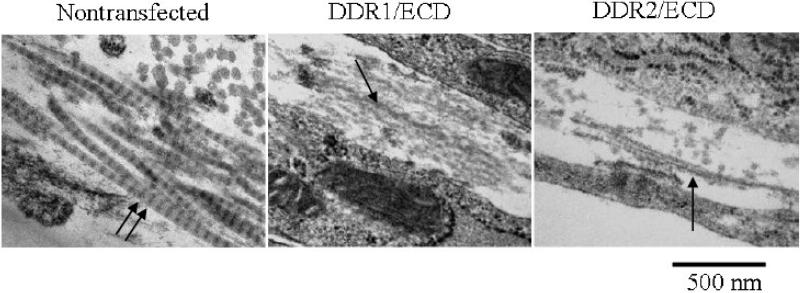
To determine if secretion of DDR1/ECD and DDR2/ECD by the cells affects the structure of collagen fibers assembled by the cells in the ECM, we employed ultrastructural morphological analysis using transmission electron microscopy (TEM) of cell cultures. Figure 2 shows the collagen fiber morphology of nontrasfected, DDR1/ECD and DDR2/ECD stable cell lines at 3 weeks of culture (similar fiber morphology was observed for all time points of culture duration). Collagen fibers formed in the ECM of nontransfected cells have well defined banded structures exhibiting D-periodicity. Nontransfected samples exhibited a D-periodicity average of 61 ± 5 nm (n=100) across weeks 1 through 3. In contrast, the collagen fibers in the ECM of both DDR1/ECD and DDR2 ECD stable cell lines exhibited poorly formed fiber morphology lacking the characteristic D-periodicity at all time points.
Fig. 2.
TEM micrographs showing ultrastructure of collagen fibers assembled in the ECM of 3T3 cells after three weeks of culture. Nontransfected samples show well-formed collagen fibers with defined D-periodic structure (double arrow). This banded structure of collagen fibers is hindered in DDR1/ECD and DDR2/ECD stable cell lines (indicated by single arrows) for all time points (week three is shown). Magnification of micrographs is 50,000x.
DDR ECDs Affect Collagen Fiber Diameter
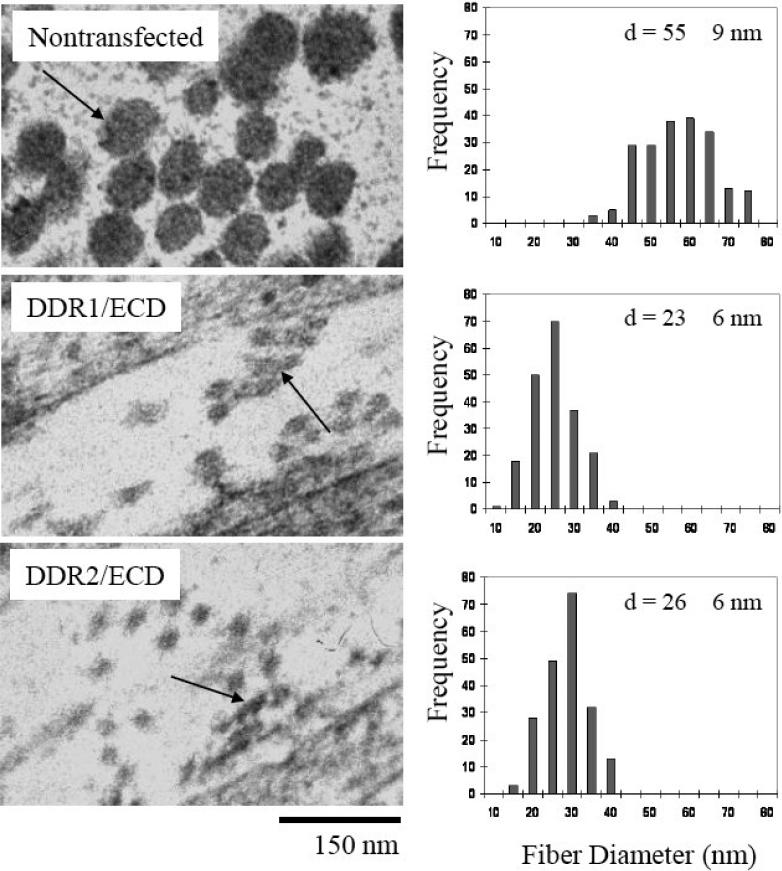
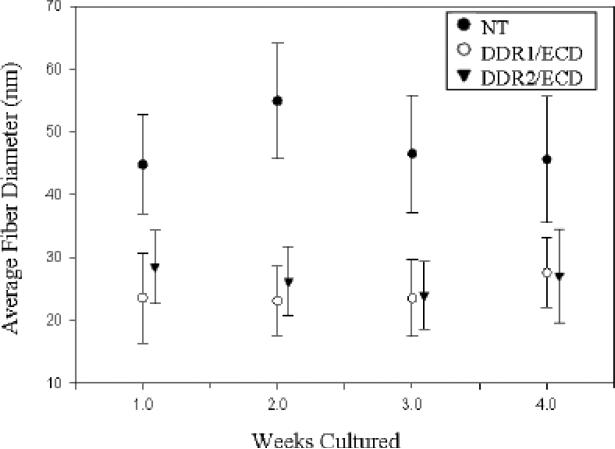
Fiber diameter analysis was conducted on the collagen fibers present in the ECM of nontransfected, DDR1/ECD and DDR2/ECD cell lines (Figure 3). The TEM micrographs clearly show larger fiber diameters for the native cells as compared to cell lines over expressing DDR1/ECD or DDR2/ECD. While nontransfected cells exhibited an average fiber diameter of 55 nm, the average fiber diameter for DDR1/ECD and DDR2/ECD samples was 23 nm and 26 nm respectively. This disparity in collagen fiber diameter between native cells and DDR1/ECD and DDR2/ECD cell lines was consistent with other time intervals as well (Figure 4). DDR1/ECD samples had an average fiber diameter in the range of 23.1 nm to 27.6 nm across the four week time intervals, while DDR2/ECD samples exhibited fiber diameters ranging from 23.8 nm to 28.4 nm. The nontransfected cell cultures had consistently larger fiber diameters, producing average fiber diameters of 45 to 55 nm across the four week time intervals. These observations confirm that DDR1/ECD and DDR2/ECD expression in the ECM reduces collagen fiber diameter. Standard deviation of the average fiber diameter was observed to be slightly higher in the nontransfected cell samples (9-10 nm) as compared to those of DDR1/ECD or DDR2/ECD samples (5-7 nm). Further, the cross-section of collagen fibers present in DDR1/ECD and DDR2/ECD samples often show fused fibers with non-circular cross-sections while the nontransfected samples exhibited well-separated fibers with distinct circular cross-sections. SS
Fig. 3.
Collagen fiber diameter is affected by over expression of soluble DDR1/ECD and DDR2/ECD in the ECM of cultured cell lines. The average fiber diameters for both DDR1/ECD and DDR2/ECD samples are notably smaller than the nontransfected samples (NT). ‘d’ is the average diameter (nm) obtained by measuring 200 fibers from each sample type after two weeks of culture. The fiber cross-sections of DDR1/ECD and DDR2/ECD display fused fibers (arrow) while the nontransfected samples show characteristic circular cross-sections (arrow). Magnification of micrographs is 50,000x.
Fig. 4.
Over-expression of soluble DDR1/ECD and DDR2/ECD in the ECM reduces the lateral growth of collagen fibers. All cell lines had little to no increase in fiber diameter throughout the four week period. The nontransfected cell line maintained an average fiber diameter 40% to 60% higher than DDR1/ECD and DDR2/ECD cell lines at all time intervals. Analysis was conducted for 200 fibers for each sample type at every time interval.
DDR ECDs Inhibit the Kinetics of Collagen Fibrillogenesis
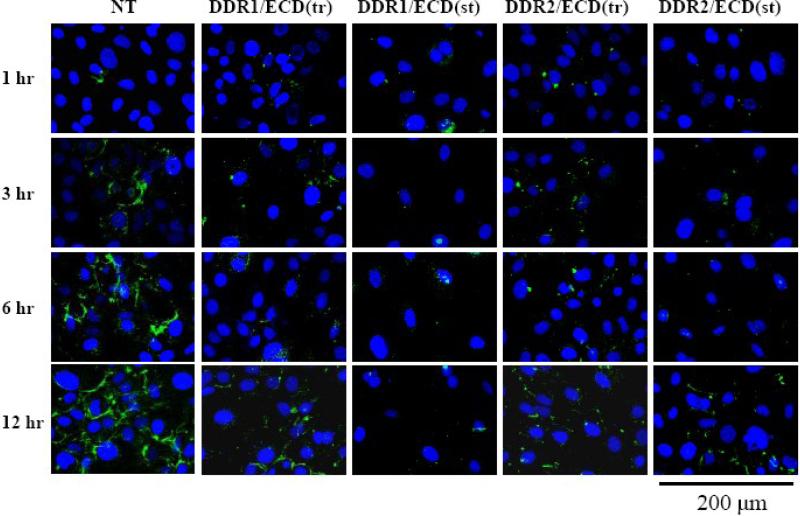
To ensure that the inhibition of collagen fibrillogenesis observed in the DDR ECD stable cell lines was due to the expression of DDR1/ECD or DDR2/ECD and not due differences in the amount of collagen being secreted by these cells, fluorescent microscopy was used to observe how DDR/ECD altered fibrillogenesis of exogenously added collagen (Figure 5). Nontransfected cells and cells transiently or stably transfected with DDR1/ECD and DDR2/ECD, were cultured in the absence of ascorbic acid and monomeric collagen I labeled with fluorescein isothiocyanate (FITC) was exogenously added for various time intervals. The nontransfected cells show evidence of fiber formation as early as 1 hour after addition of collagen with continued growth in collagen fibrillogenesis throughout the 12 hour period. During this same period, there was negligible fiber formation in the cell samples stably or transiently transfected with DDR/ECDs.
Fig. 5.
DDR1/ECD and DDR2/ECD impedes exogenous collagen fibrillogenesis. FITC-labeled collagen was added to native and DDR ECD transiently and stably transfected cell cultures and incubated for 1 hr, 3 hr, 6 hr, and 12 hr intervals. Growth of polymeric collagen fiber formation is observed from 1 hour through the 12 hour period for the native cells (NT). Some fiber formation occurs at the 6 and 12 hour time intervals for the transiently transfected samples, DDR1/ECD(tr) and DDR2/ECD(tr) cell cultures with little to no collagen fiber formation occurring in the stably transfected samples, DDR1/ECD(st) and DDR2/ECD(st). Images were collected using fluorescent microscopy at 63x magnification.
DDR ECDs Decrease Collagen Deposition in ECM
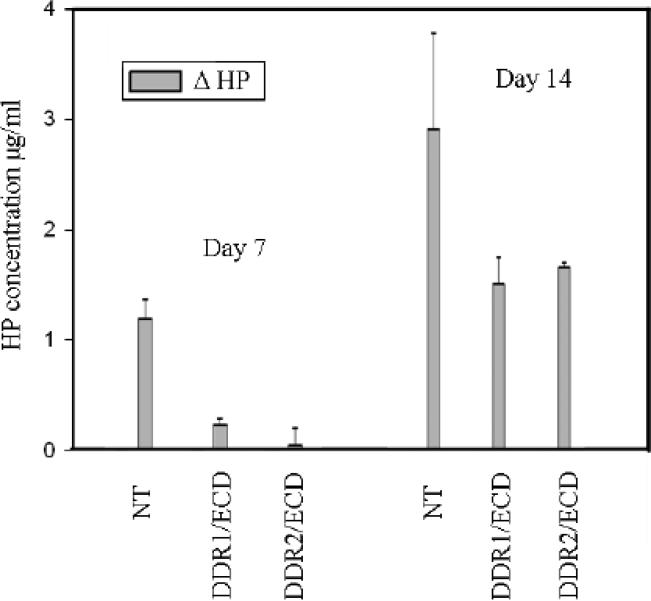
Our TEM analysis (Figures 2 and 3) and fluorescence microscopy data (Figure 5) indicate suppression of fibrillar collagen content in cells over-expressing DDR1/ECD and DDR2/ECD. To ascertain and quantify the total collagen content in the adherent ECM, we carried out the hydroxyproline (HP) assay, a biochemical technique used for routine measurement of collagen content in tissue specimens2; 25. Nontransfected cells, DDR1/ECD and DDR2/ECD stable cell lines were cultured in the presence and absence of ascorbic acid for 1 and 2 weeks and the adhered cell layer was subjected to HP analysis (Figure 6). Little difference was observed in the HP content for all the samples prepared without ascorbic acid, consistent with our western blot analysis which showed equal levels of collagen type I in all samples (Figure 1c). However in the presence of ascorbate the differences in HP content measured between nontransfected and stably transfected cells were significant. The HP concentration reported, ΔHP is the difference in HP between samples grown in the presence and absence of ascorbic acid and is estimated to be the HP present in the ECM. After 1 week, samples over-expressing DDR1/ECD (or DDR2/ECD) exhibited only 18% (or 3%) of the HP content of nontransfected cells. After 2 weeks only 52% (or 57%) of the HP content present in nontransfected cell samples was observed for our stably transfected cells. Since hydroxyproline concentration is indicative of collagen abundance25 our results show that collagen deposition in the ECM was significantly reduced in DDR/ECD cell samples.
Fig. 6.
The abundance of hydroxyproline present in the ECM of nontransfected (NT) cells is greater than that of DDR ECD transfected cells indicative of collagen content. Δ HP is the difference in hydroxyproline (HP) content between samples grown with and without ascorbic acid. In the absence of ascorbic acid, there is negligible difference in the quantity of HP present across all samples (data not shown). Differences in Δ HP demonstrate collagen content is reduced in cell lines over-expressing DDR1/ECD and DDR2/ECD. Nontransfected cells clearly displayed higher hydroxyproline content after both 7 and 14 days of culture.
DDR ECDs Promote Matrix Mineralization
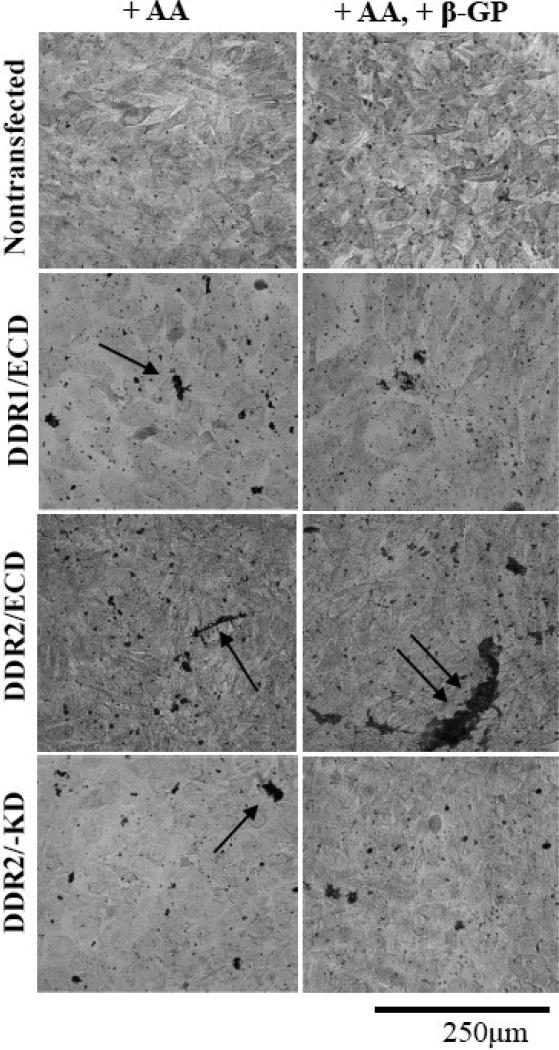
The osteoblastic cell line MC3T3-E1 has previously been utilized to study bone matrix formation including mineralized plaques21; 26. In order to ascertain how DDR ECDs alter the formation of mineralized calcium deposits in vitro a modified von Kossa staining protocol was employed and visualized with DIC microscopy. Samples of nontransfected, stably transfected DDR1/ECD, DDR2/ECD and DDR2/-KD2 cells were cultured for two weeks in the presence of ascorbic acid, and in the presence of both 25μg/ml ascorbic acid and the osteogenic supplement 2mM β-glycerolphosphate. As seen in figure 7, all nontransfected cells showed heterogeneity in crystal size and distribution of crystal formation throughout the sample. The nontransfected cells in the presence of β-glycerophosphate exhibited increased crystal sizes compared to those without the osteogenic supplement. All stably transfected cell lines exhibited a striking increase and a wider distribution of crystal sizes as compared to non transfected cells even in the absence of β-glycerolphosphate. In the presence of β-glycerolphosphate, the DDR2/ECD cells displayed especially pronounced crystal sizes compared to all other samples.
Fig. 7.
DDR ECD alters the formation of mineralized plaque in cells. Nontransfected MC3T3-E1 cells display small crystalline calcium deposits (black) throughout the sample; the size of the crystals in these samples increases slightly upon addition of the osteogenic supplement β-glycerophosphate (β-GP) to the culture media. Stable cell lines DDR1/ECD, DDR2/ECD and DDR2/-KD show increased crystal size as compared to nontransfected cells when cultured in the presence of Ascorbic acid (AA), indicated by arrows. Addition of β-glycerophosphate (β-GP) further increases the size of calcium deposits in stable cell lines DDR2/ECD and DDR2/-KD as compared to nontransfected cells. The DDR2/ECD cell line shows the most pronounced calcified crystals, indicated by double arrow. All samples were cultured for two weeks.
Discussion
In this study we elucidate how cell-secreted, soluble ECD of DDR1 and DDR2 inhibit collagen fibrillogenesis and enhance matrix mineralization for ECM endogenously generated by the cells. Using similar experimental approaches, in our earlier work2 we had demonstrated that kinase-deficient and membrane-anchored DDR2 ECD (DDR2/-KD) also inhibits collagen fibrillogenesis. Such an inhibition of collagen fibrillogenesis by DDR118 and DDR219 ECD was originally observed by us using purified proteins in-vitro. Thus our current investigations along with our previous studies2; 18; 19 enable us to compare the role of membrane bound vs. soluble proteins (DDR-ECD) in regulating collagen fibrillogenesis.
Multiple protein species are known to naturally exist for the transmembrane receptors DDR1 and DDR2. Five splice variants have been characterized for DDR1 (‘a’ through ‘e’) 12. The d and e isoforms lack the intracellular kinase domain of DDR1. The splicing of DDR1 to various extents has been reported in human ovarian cancer27, breast cancer15 and fetal brain15 and has thus far been best characterized in colon cancer cell lines12. In normal and diseased arteries of non-human primates, three isoforms (a, b and d) have been detected; these isoforms are differently expressed in advanced atherosclerotic lesions5 and have been detected in normal human lung tissue and cultured human smooth muscle cells (SMCs) 5. Although splice variants for DDR2 have not yet been characterized there is ample evidence to support they exist. In one report Northern blot analysis using a cDNA probe corresponding to the ECD of DDR2 has revealed multiple mRNA species in melanoma carcinoma and virus-transformed normal embryonic lung cell lines15. In a separate study, a major 10kb transcript and a minor 4.5 kb transcript for DDR2 were detected in human and rat heart and other tissues using Northern analysis16 along with additional weak bands at 0.8, 3.6, 2.4, and 1.7 kb. In mouse and rat heart tissue, a DDR2 probe hybridized to multiple RNAs of varying lengths (~4kb and 7kb)17. At least two transcripts for DDR2 (9.5 and 4.5kb) and several protein species (130, 90, 50 and 45 kD) have been found in cultured human SMCs using antibodies against DDR2 ECD5. Besides alternate splicing of DDRs, shedding of the DDR1 ECD as a soluble protein in the ECM is another naturally occurring phenomenon reported for several mammalian cells13; 14. While no direct evidence for DDR2 ECD shedding exists, the western blots with DDR2 antibodies on SMCs transiently transfected with full-length DDR2 show several smaller molecular species (90, 50 and 45 kDa) besides the full-length 130kDa receptor suggesting the likelihood of shedding of DDR2 ECD. Our current investigations highlight the relevance of further characterizing DDR2 isoforms and understanding the different functional roles of DDR1 and DDR2 protein variants.
Several reports by us and others have highlighted that dimerization or oligermization of DDR1 ECD and DDR2 ECD enhances their binding to triple helical collagen18; 19; 22; 28; 29; 30; 31. The ECD of DDRs consists of a discoidin domain and a stalk region. It has been reported that independent deletion of the cytoplasmic domain of DDR1 did not inhibit receptor dimerization28. More recently, Abdulhussein et al found that the cysteine residues, 303 and 348 present in stalk region of DDR1 ECD are essential for receptor dimerization and deletion of the stalk region prevented receptor dimerization30. Further, Abdulhussein et al demonstrated that GST tagged DDR1 ECD and DDR2 ECD when expressed as a soluble protein bound to collagen29. The molecular weight of the DDR1 ECD-GST and DDR2 ECD-GST proteins was found to be around 62kDa29, similar to the molecular weight of the DDR1/ECD and DDR2/ECD proteins utilized in this study. We have also recently shown that DDR1 exists as a dimer on the cell surface independent of the presence of collagen and undergoes further oligomarization upon ligand stimulation31. Since the DDR1/ECD and DDR2/ECD proteins in our stable cell lines preserve the capacity to interact with collagen, they are likely expressed as dimers and may undergo further oligomerization upon interaction with collagen.
Our results signify that both membrane anchored and soluble isoforms of DDR ECD proteins may be important in ECM remodeling. In this regard several soluble collagen-binding proteins secreted in the ECM: decorin3; 32; 33; 34, lumincan3; 34; 35, biglycan34; 36, fibromodulin34; 35; 36, periostin37, aggrecan34 and versican34 are known to regulate collagen fibrillogenesis. Decorin and lumican are known to regulate collagen fibril diameter3; 38 and the absence of biglycan and fibromodulin inhibits the maturation of collagen fibrils36. Animal studies have begun to reveal the importance of soluble collagen binding proteins in the regulation of collagen maturation and fiber diameter. Studies on knockout mice for decorin39, lumican35, fibromodulin35; 36 and periostin37 have demonstrated that these proteins are critical to generate a uniform collagen fiber diameter in tissues. For example, ultrastructural analyses of the cornea, skin and tendon from lumican knockout mice shows collagen fibers with increased fiber diameters while the tendon from fibromodulin knockout mice contains higher frequency of smaller fiber diameters35. Limited studies exist on effects of membrane anchored proteins on fibrillogenesis of type 1 collagen. Integrins α5β1 and α2β1 are understood to modulate collagen fibrillogenesis predominantly with fibronectin polymerization as a prerequisite40. Although the integrin α1(I) and α2 (I) domains have been shown to affect collagen fibrillogenesis as soluble proteins in-vitro41, no changes in collagen fiber density or organization were observed in the integrin α1β142 or α2 subunit-deficient mice43. Interestingly, the knockout mice for the orphan receptor Gpr4844 and the transmembrane collagen XIII45 showed disrupted collagen fibrils although the mechanism(s) of their interaction with collagen type 1 are not well understood. Our current results, along with our previous findings indicate that DDR ECDs serve as a robust model system to compare and contrast how membrane anchored vs. soluble proteins may regulate collagen fiber structure and deposition.
We had earlier reported that collagen fibers with intact native banded structure were occasionally observed in the kinase-deficient, membrane-anchored DDR2 ECD (DDR2/-KD) samples; however in our DDR1/ECD and DDR2/ECD samples, observation of native banded structure of collagen was far more infrequent. D-periodicity of collagen fibers from native cultures were measured at 61 ± 5 nm which is in agreement with previous studies by us and others46. Previously we found that the membrane-anchored DDR2/-KD inhibited lateral fiber growth, compared to native cultures. While fiber diameter measurements for the first week of culture for DDR1/ECD and DDR2/ECD gave similar results to that of DDR2/-KD samples (25.0 to 28.9 nm), fibers in DDR2/-KD cultures exhibited lateral growth of around 10 nm over the three weeks of culture observed. In contrast a sustained inhibition of lateral growth of collagen fibers was observed by DDR ECD proteins resulting in average collagen fiber diameters between 20 and 30 nm throughout a four week period. Taken together our results show that soluble DDR2 ECD inhibits collagen fibrillogenesis in the ECM consistent with membrane-anchored DDR2 albeit with a slightly higher potential. We speculate that this stronger inhibition of collagen fiber structure and lateral diameter is due to the soluble DDR ECD being distributed throughout the ECM and thus having more ability to affect collagen fiber formation even in ECM regions away from the pericellular regions.
We found that both DDR1 and DDR2 ECD increased matrix mineralization as compared to native cells, with the effect of DDR2 ECD being more prominent. Both soluble (DDR2/ECD) and membrane-bound DDR2 ECD (DDR2/-KD), when compared to wild-type cells induced larger mineral deposits. In this regard, a recent study has reported abnormal calcification arising due to mutations in the DDR2 gene in spondylo-meta-epiphyseal dysplasia (SMED) in humans47. It is interesting to note that all the mutations reported were found in the DDR2 intracellular domain and not in its ECD. Although the expression levels of DDR2 were not reported in this study47, it is likely that expression of DDR2 ECD present in the full-length mutated receptor in SMED cases along with impaired signaling of the mutated receptor may lead to increased calcification. Matrix mineralization in both DDR1 and DDR2 knockout mice have not been reported in detail, however in DDR1-knock mice, a reduced bone calcification was described in the fibula bone48. Our observations suggest the importance of evaluating matrix mineralization with respect to expression of both the full length DDR receptors and their isoforms containing the ECDs. Since the collagen type I binding site for decorin is in close proximity to that of DDR249, further investigations are needed to understand if binding of DDR2 ECD to collagen type I promotes crystal formation by interfering with decorin binding. It is interesting to compare the effect of DDRs on collagen fibrillogenesis and matrix mineralization to those of decorin. Both DDR ECDs and decorin inhibit collagen fibrillogenesis and result in reduction of collagen fiber diameters33. In contrast while DDR ECDs enhance matrix mineralization, decorin is found to be an inhibitor of collagen calcification4. No reports elucidating the ultrastructure of native ECM in DDR1 or DDR2 knock out mice have yet been made.
We conclude that expression of both membrane-bound and soluble DDR1 and DDR2 ECDs can alter the morphology of endogenous collagen fibers, thus perturbing the overall ECM structure. We speculate that such perturbations, if observed in vivo, may significantly alter the integrity and biomechanical properties of resulting tissues. Further studies need to be addressed to elucidate which DDR1 and DDR2 isoforms are modulated in pathological states in vivo and how their expression alters ECM morphology and tissue biomechanics.
Materials and Methods
Creation of Expression Constructs For Soluble DDR ECD
Expression plasmids encoding the ECD of DDR1 and DDR2 were generated using the full-length mouse DDR1-myc and DDR2-myc constructs obtained from Regeneron Pharmaceuticals, Tarrytown, NY 50. The coding regions of the DDR1/ECD (amino acid Met-1 to Ser-412) and DDR2/ECD (amino acids Met-1 through Ile-400) were amplified by polymerase chain reaction utilizing the Pfu TURBO polymerase (Stratagene, La Jolla, CA) and the following primers:
- DDR1/ECD:
- Forward: 5’-GAAGGATGGGGACAGGGACCCTC-3’
- Reverse: 5’-GCTCCCCTCCGCCTTGCCCAC-3’
- DDR2/ECD:
- Forward: 5’-AGGATGATCCCGATTCCCAGA-3’
- Reverse: 5’-GATCCGAGTGTTGCTATCATCAAC-3’
The resulting PCR products (DDR1/ECD: 1241 bp and DDR2/ECD: 1203 bp) were subjected to Taq polymerase to include 3’ A-overhangs in the PCR product to enable ligation immediately into the pcDNA3.1/V5-His-TOPO vector using the Top10 chemically competent cells from Invitrogen. Recombinant clones were identified by restriction analysis using double digestion with Age1 and Kpn1 (for DDR1/ECD) and Kpn1 and EcoRV (for DDR2/ECD). The authenticity (i.e. correct orientation and in frame with the V5 coding region) of the resulting clones was verified by dideoxynucleotide sequencing. Creation of expression plasmid encoding for the DDR2/-KD construct was reported earlier 2.
Creation of Stable Cell Lines
The pre-osteoblast mouse cell line MC3T3-E1 (ATCC) has been used extensively to elucidate functional roles of osteoblastic cells and collagen fibrillogenesis21; 26. Cells were seeded (60 –80% confluent) on two 100 mm dishes in MEM-α with 10% (v/v) fetal bovine serum and 1% Penicillin Streptomyosin (Gibco). Cells were transfected with DDR1/ECD and DDR2/ECD expression construct using FuGene 6 (Roche). Thirty hours after transfection, the cells were incubated with selection media containing 475 μg/ml of geneticin. After 12 to 14 days of culture in selection media, surviving cells from DDR1/ECD and DDR2/ECD colonies were transferred to 35 mm dishes. Of the many stable cell lines generated, two were selected. Selection was done on the basis of healthy cell morphology and expression level of the DDR ECD proteins. Western blotting was performed on whole cell lystates or conditioned media from serum starved cultures for verification of protein expression. One DDR1/ECD and one DDR2/ECD stable colony was selected based on protein expression and healthy cell morphology.
Transmission Electron Microscopy
Nontransfected MC3T3-E1 and stably transfected DDR1/ECD and DDR2/ECD samples were prepared for transmission electron microscopy (TEM) as previously reported 2. Briefly, cells were cultured in the presence of ascorbic acid (25 μg/ml) for one to four weeks. Ascorbic acid is critical for prolyl hydroxylation and for lysine hydroxylase acitvity in the biosynthesis and assembly of collagen 51. Samples were fixed in 4% (v/v) gluteraldehyde, stained with 1% osmium tetroxide and uranyl acetate. Dehydration was carried out by a graded ethanol series (30%–100%) and samples were embedded in an epoxy resin. 80 nm thick sections were obtained by use of a Leica Ultracut UCT ultramicrotome (Leica-Micro-systems Wien, Austria). Sections were examined with a Zeiss EM 900 TEM (Carl-Zeiss SMT, Peabody, New York) operating at 80 kV. An Olympus SIS Megaview III camera (Lakewood, Colorado), was used to obtain digital micrographs at magnifications ranging from 7,000 to 85,000x.
TEM Image Analysis
Image J software (NIH) was used on TEM images to measure the diameter of collagen fibers present in the ECM. Fiber diameters were measured on cross-sectional or longitudinal images of collagen fibers. TEM micrographs with magnifications of 50,000 and 85,000x were utilized. For each specimen type, at least three independent cell cultures were made and processed. At least three TEM grids were made from each sample, and numerous regions on each grid were imaged. 200 fiber diameters were measured for each specimen type, and a statistical analysis provided average diameter, standard deviation and frequency distribution data. The morphology of collagen fibers was qualitatively analyzed in TEM micrographs to identify presence or absence of D-periodicity in collagen fibers.
Hydroxyproline (HP) Assay
Hydroxyproline assays were performed on nontransfected MC3T3-E1, DDR1/ECD and DDR2/ECD stably transfected cell cultures to determine the collagen content in the adherent cell layer as previously described 2; 25. Briefly, cell cultures were prepared in triplicate, with and without ascorbic acid, and cultured for 7 and 14 days. A cell proliferation assay was used to normalize the cell population of each sample using Calcein-Am (BioChemika 17783) in order to reduce discrepancies due to variation in growth rate of the samples. The adherent cell layers were scraped and pipetted into individual eppendorf tubes and brought to a volume of 50 μl with a final concentration of 4N sodium hydroxide and autoclaved for 20 minutes at 120°C. 450 μl of chloromine T reagent was added to each sample and incubated at room temperature for 25 minutes. Lastly, 500 μl of Ehrlich's reagent was added to each sample and incubated at 65°C for 20 minutes. Absorbance was measured at 560 nm using a Beckman DU730 spectrophotometer. The HP content was obtained using calibration against a standard curve ranging from 0.5 μg/ml to10 μg/ml. For each cell line the difference in hydroxyproline content, ΔHP , between cells grown in the presence and absence of ascorbic acid was calculated. ΔHP is thus indicative of the amount of collagen present in the ECM.
Fluorescence Microscopy
Fluorescence microscopy was used to ascertain the rate of collagen fibrillogenesis by nontransfected MC3T3-E1 cells and cells stably and transiently transfected with DDR1/ECD and DDR2/ECD for exogenously added fluorescently labeled collagen. Cells were cultured on 1% (w/v) poly-L-lysine coated glass coverslips and grown in the absence of ascorbic acid in order to ensure that no endogenous collagen fibrillogenesis by the cells occurs in the assay. Cells were incubated with Fluorescein Isothiocyanate (FITC) conjugated collagen type 1 (Sigma C4361) at a final concentration of 1 μg/ml for the appropriate time intervals: 30 minutes, 1,3,6 and 12 hours. Cells were washed and fixed with 2% formalin (Fisher Scientific, Kalamazoo, Michigan) for 30 minutes. Thereafter, cells were washed and incubated for 20 minutes with 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining. Glass coverslips were mounted onto microscope slides using ProLong Gold antifade reagent (Invitrogen Molecular Probes P36934). Slides were examined with a 63x objective on a Zeiss Axiovert 200 microscope. An EXFO mercury lamp was used to excite the sample and appropriate filter cubes were used for fluorochrome observation: YFP-2427A (Semrock) was used to observe FITC and filter set # 49 (Zeiss) was used for observance of DAPI nuclear stain.
Mineralization Assay
Nontransfected MC3T3-E1 cells and stably transfected DDR1/ECD, DDR2/ECD and DDR2/-KD (previously published 2) cells were seeded onto 25mm glass coverslips. For each cell type samples were grown in duplicate either in the presence of 25μg/ml ascorbic acid or in the presence of both 25μg/ml ascorbic acid and 2mM β-glycerophosphate (an osteogenic supplement). After 14 days of culture cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature and washed three times in ultra pure water. To visualize calcium deposits a modified von Kossa staining procedure was employed 26. 2.5% (w/v) AgNO3 was placed on the cells and let to incubate for 30 minutes in the dark after which cells were exposed to UV light for 50 minutes. Cultures were washed three times in ultra pure water, counterstained with Toluidine Blue for 3 minutes, washed repeatedly and finally mounted onto microscope slides. Micrographs were imaged using differential interference contrast (DIC) with a 20x objective lens on a Zeiss Axiovert 200 microscope. The experiment was repeated three independent times.
Acknowledgements
This work was supported by the NIH award K25 HL81442-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: DDR2, DDR1, ECD, RTK, MMP, TEM, HP, KD, SMC, DIC
References
- 1.Jepsen KJ, Schaffler MB, Kuhn JL, Goulet RW, Bonadio J, Goldstein SA. Type I collagen mutation alters the strength and fatigue behavior of Mov13 cortical tissue. J Biomech. 1997;30:1141–7. doi: 10.1016/s0021-9290(97)00088-2. [DOI] [PubMed] [Google Scholar]
- 2.Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. J Mol Biol. 2009;385:902–11. doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–63. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesmann HP, Meyer U, Plate U, Hohling HJ. Aspects of collagen mineralization in hard tissue formation. Int Rev Cytol. 2005;242:121–56. doi: 10.1016/S0074-7696(04)42003-8. [DOI] [PubMed] [Google Scholar]
- 5.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164:1575–85. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–35. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel WF. Collagen-receptor signaling in health and disease. Eur J Dermatol. 2001;11:506–14. [PubMed] [Google Scholar]
- 8.Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, Xu L, Li Y. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56:3685–92. doi: 10.1002/art.22970. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–73. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- 10.Lam NP, Li Y, Waldman AB, Brussiau J, Lee PL, Olsen BR, Xu L. Age-dependent increase of discoidin domain receptor 2 and matrix metalloproteinase 13 expression in temporomandibular joint cartilage of type IX and type XI collagen-deficient mice. Arch Oral Biol. 2007;52:579–84. doi: 10.1016/j.archoralbio.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 12.Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. FASEB J. 2001;15:1321–3. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- 13.Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514:175–80. doi: 10.1016/s0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- 14.Slack BE, Siniaia MS, Blusztajn JK. Collagen type I selectively activates ectodomain shedding of the discoidin domain receptor 1: involvement of Src tyrosine kinase. J Cell Biochem. 2006;98:672–84. doi: 10.1002/jcb.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–18. [PubMed] [Google Scholar]
- 16.Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, Strebhardt K. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993;8:3433–40. [PubMed] [Google Scholar]
- 17.Lai C, Lemke G. Structure and expression of the Tyro 10 receptor tyrosine kinase. Oncogene. 1994;9:877–83. [PubMed] [Google Scholar]
- 18.Agarwal G, Mihai C, Iscru DF. Interaction of discoidin domain receptor 1 with collagen type 1. J Mol Biol. 2007;367:443–55. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 19.Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006;361:864–76. doi: 10.1016/j.jmb.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Alberts B. Molecular biology of the cell. Reference edition. 5th edit Garland Science; New York: 2008. [Google Scholar]
- 21.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J Bone Miner Res. 2005;20:81–7. doi: 10.1359/JBMR.041026. [DOI] [PubMed] [Google Scholar]
- 22.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–9. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 23.Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277:3606–13. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, Friedman SL, Lin HC. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 2002;277:19206–12. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- 25.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 26.Coetzee M, Haag M, Kruger MC. Effects of arachidonic acid and docosahexaenoic acid on differentiation and mineralization of MC3T3-E1 osteoblast-like cells. Cell Biochem Funct. 2009;27:3–11. doi: 10.1002/cbf.1526. [DOI] [PubMed] [Google Scholar]
- 27.Laval S, Butler R, Shelling AN, Hanby AM, Poulsom R, Ganesan TS. Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell Growth Differ. 1994;5:1173–83. [PubMed] [Google Scholar]
- 28.Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281:22744–51. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- 29.Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–70. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- 30.Abdulhussein R, Koo DH, Vogel WF. Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1. J Biol Chem. 2008;283:12026–33. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihai C, Chotani M, Elton TS, Agarwal G. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol. 2009;385:432–45. doi: 10.1016/j.jmb.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velleman SG. The role of the extracellular matrix in skeletal muscle development. Poult Sci. 1999;78:778–84. doi: 10.1093/ps/78.5.778. [DOI] [PubMed] [Google Scholar]
- 33.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–55. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 34.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 35.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–93. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 36.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–80. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 37.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7:105–14. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- 39.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jokinen J, Dadu E, Nykvist P, Kapyla J, White DJ, Ivaska J, Vehvilainen P, Reunanen H, Larjava H, Hakkinen L, Heino J. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956–63. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 42.Louis H, Kakou A, Regnault V, Labat C, Bressenot A, Gao-Li J, Gardner H, Thornton SN, Challande P, Li Z, Lacolley P. Role of alpha1beta1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2597–604. doi: 10.1152/ajpheart.00299.2007. [DOI] [PubMed] [Google Scholar]
- 43.Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277:10789–94. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 44.Weng J, Luo J, Cheng X, Jin C, Zhou X, Qu J, Tu L, Ai D, Li D, Wang J, Martin JF, Amendt BA, Liu M. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc Natl Acad Sci U S A. 2008;105:6081–6. doi: 10.1073/pnas.0708257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kvist AP, Latvanlehto A, Sund M, Eklund L, Vaisanen T, Hagg P, Sormunen R, Komulainen J, Fassler R, Pihlajaniemi T. Lack of cytosolic and transmembrane domains of type XIII collagen results in progressive myopathy. Am J Pathol. 2001;159:1581–92. doi: 10.1016/S0002-9440(10)62542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen DH, Smithson SF, Borochowitz Z, Belostotsky R, Raas-Rothschild A. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet. 2009;84:80–4. doi: 10.1016/j.ajhg.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–17. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–31. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 50.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 51.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78:2879–82. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]