Abstract
Background & Aims
Chronic stress exacerbates or causes relapse of symptoms such as abdominal pain and cramping in patients with irritable bowel syndrome (IBS). We investigated whether chronic stress increases plasma norepinephrine and sensitizes colon-specific dorsal root ganglion (DRG) neurons by increasing the expression of nerve growth factor (NGF) in the colon wall.
Methods
Heterotypic chronic stress (HeCS) was induced in male Wistar rats and neurologic and molecular responses were analyzed. Tissues were analyzed for NGF expression.
Results
HeCS significantly increased the visceromoter response to colorectal distension; expression of NGF increased in colonic muscularis externa and mucosa/submucosa. Rheobase decreased, resting membrane potential was depolarized, and electrogenesis of action potentials increased in colon-specific thoracolumbar DRG neurons. Luminal administration of resiniferatoxin in distal colon, systemic administration of anti-NGF antibody, or inhibition of the NGF receptor TrkA by k252A or antisense oligonucleotides in thoracolumbar DRG blocked the chronic stress-induced visceral hypersensitivity to colorectal distension. Blockade of α1/α2- and β1/β2-adrenergic receptors prevented the stress-induced visceral hypersensitivity and increased expression of NGF in the colon wall. HeCS did not induce any inflammatory response in the colon wall.
Conclusion
The peripheral stress mediator norepinephrine induces visceral hypersensitivity to colorectal distension in response to HeCS by increasing the expression of NGF in the colon wall, which sensitizes primary afferents in the absence of an inflammatory response.
Keywords: irritable bowel syndrome, nerve growth factor, dorsal root ganglia, norepinephrine, corticotrophin releasing hormone
Abdominal discomfort/cramping and altered bowel habits are the defining symptoms of irritable bowel syndrome (IBS)1. The etiologies of both symptoms are not fully understood. However, clinical studies show that chronic stress exacerbates or precipitate both symptoms of IBS2, 3. This suggests that chronic stress may impair the cellular functions of some of the same cells that cause motility dysfunction or visceral hypersensitivity in IBS patients in the first place. However, the mechanisms by which chronic stress causes cellular dysfunction in these cells may differ from those that cause the underlying dysfunction in IBS. Animal studies support this hypothesis. A recent study found that alterations in the transcription rates of genes encoding key cell-signaling proteins of the excitation-contraction coupling in colonic circular smooth muscle cells underlie motility dysfunction of faster colonic transit and increase in defecation rate in a model of post-infective IBS4. Another study found that chronic stress enhances the transcription rate of some of the same genes encoding key cell-signaling proteins that accelerate colonic transit and defecation rate, but by different mechanisms5.
Clinical studies show that the primary spinal afferents mediate visceral hypersensitivity to colorectal distension (CRD) in IBS patients6, 7. However, all psychological stress responses begin in the CNS. The release of corticotrophin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus is an early and essential step in the initiation of all psychological stress responses8. The central release of CRH and other mediators, such as arginine vasopressin, stimulate the neuroendocrine system comprised of autonomic neurons and the hypothalamus-pituitary-adrenal (HPA)-axis, which modulates the adaptive and maladaptive responses of peripheral organs in a stress- and cell-type specific manner. Both acute and chronic stressors induce visceral hypersensitivity to colorectal distension in rats by releasing CRH in the hypothalamus9, 10. However, we do not know which pathways or which stress mediators of the neuroendocrine system transmit the central signal to colon-specific primary afferent neurons in the dorsal root ganglia (DRG) to modulate their sensitivity to CRD.
In a recent study5, we found that nine-day heterotypic chronic stress (HeCS) significantly increases the plasma concentration of norepinephrine. In the present study, we tested the hypothesis that norepinephrine released by the central component of the stress response acts as a messenger to induce the expression of neurotrophin, nerve growth factor (NGF), in the distal colon, which sensitizes the colon-specific neurons in the thoracolumbar DRG to induce visceral hypersensitivity to CRD. We chose chronic stress because clinical findings indicate that chronic stress, rather than short-term acute stress2, 3 exacerbates the symptoms of IBS. In addition, variable stressors are less likely to show adaptation compared to repeated applications of the same stressor.
Methods
Animals
We used six to ten week-old male Wistar rats housed at 22°C with a 12-hour light/dark cycle. The Institutional Animal Care and Use Committee at UTMB approved all procedures performed on animals.
Heterotypic chronic stress protocol
The rats were subjected to nine consecutive days of a heterotypic stress protocol comprised of three randomly arranged stressors, 60-minutes of water avoidance stress, 45-minutes of cold restraint stress at 4°C or 20-minutes of forced swimming stress, as described previously5. The control rats were brought to the lab and handled identically without subjecting them to the stress protocol.
Measurement of visceromoter response to graded CRD
Electromyographic activity from the external oblique muscle was recorded in response to colorectal distention (CRD). CRD was performed by rapidly inflating the balloon to constant pressures: 20, 30, 40, 50, 60 & 80 mm Hg, for 20 seconds followed by 2-minute rest. The net value for each distension period was calculated by subtracting the baseline value derived from the average AUC for 20 seconds before and 20 seconds after the distention period. Additional details are provided in the Supplement.
Tissue isolation
About six cm length of the distal colon was opened along the mesentery. Mucosa/submucosa was separated from the muscularis externa. Tissues were either snap frozen in liquid nitrogen or were divided into 8 strips and washed in Hanks balanced salt solution and incubated in Dulbecco’s modified eagle medium + 10% fetal calf serum + penicillin/streptomycin containing norepinephrine for 24-hours.
Electrophysiology
Under general 2% isoflurane anesthesia, the lipid soluble fluorescent dye, DiI-I (1,1′dioleyl-3,3,3′,3′-tetramethylindocarbocyanine methanesulfonate, Invitrogen), 25 mg in 0.5 ml methanol was injected in 2 μl volumes at eight to ten sites in the distal colon wall starting at the pelvic girdle and moving toward the cecum. These sites were within the colon segment used for colorectal distension. Thoracolumbar DRG neurons were isolated from DRG T13, L1 and L2, 16 days later. The methods used for isolation of DRG and current clamp recordings have been described previously11 (see supplement for additional details).
Intrathecal Catheter/osmotic pumps
K252a, a non-specific antagonist of tyrosine kinase receptors including the high affinity trkA receptors12, or previously validated antisense and mismatched oligonucleotides13 were administered by osmotic pump attached to an intrathecal catheter. Please see Supplement for details.
NGF neutralizing antibody (16 μg/kg i.p. in 0.5 ml in phosphate buffer saline (PBS)) was purchased from R&D systems (Minneapolis, MN). Control rats received PBS containing an equal concentration of goat non-immune serum.
Please see supplement for details on mast cell counts, myeloperoxidase and tryptase measurements and pharmacological reagents
Statistics
Data are expressed as mean ± SE. One-way ANOVA followed by Fisher post-hoc analysis and t-test were used for comparison of means. The effect of HeCS and treatments were analyzed using two-way repeated measures ANOVA.
Results
Chronic Stress-induced Visceral Hypersensitivity is Associated with Increase in Excitability of Colon-specific TL DRG Neurons
We found that 9-day HeCS significantly increases the visceromoter response to graded CRD at pressures of 40, 50, 60 and 80 mm Hg, compared to pre-stressed baseline response (Figure 1A) (n=14 rats). The increase in visceromoter response persists for at least 8-hours after the last stressor, but it returns to basal levels 24-hours post-HeCS. By contrast, 9-day sham stress in age-matched control rats had no significant effect on the visceromoter response to CRD (Figure 1B) at 8 and 24 hours (n=10 rats). However, there was a significant decline in the VMR to CRD in sham stressed rats at 7 days compared to baseline at 40, 50, 60, and 80 mm Hg.
Figure 1.
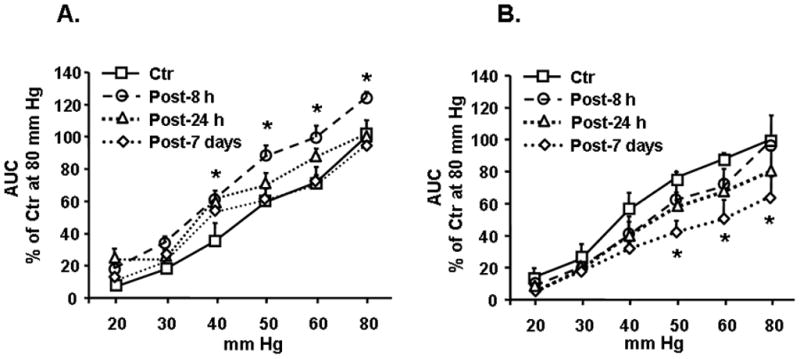
(A) HeCS significantly increased visceromoter to graded CRD, n=14 rats, *p<0.05 compared to pre-stress baseline responses 8-hours following the last stressor. No significant increase was observed at 24-hours and 7-days after HeCS. (B) Sham stressed control rats showed no change in their visceromoter response at 8 and 24 hours but, significant differences were found between baseline and 7 days at 40, 50, 60, 80 mmHg p<0.05, n=10 rats.
Acutely dissociated thoracolumbar colon-specific afferent neurons, identified by the presence of retrograde label DiI injected into the colon wall, showed a significant decrease in Rheobase (Figures 2A and 2B), depolarization of resting membrane potential (Figure 2C), and a significant increase in the number of action potentials evoked at 2X rheobase (Figures 2D and 2E), compared to neurons from the sham-stressed control rats (n=22 and 31 neurons respectively). HeCS did not alter the other electrophysiological characteristics, such as cell diameter, capacitance, input resistance and action potential amplitude, duration, threshold and overshoot, of DRG neurons (data not shown).
Figure 2.

Effects of HeCS and colonic resiniferatoxin (RTX) treatment on electrophysiological properties of DiI labeled colon-specific thoracolumbar DRG neurons. Changes in electrophysiological properties of colon specific DRG neurons from control (Ctr, n=31 neurons), HeCS (n=22 neurons), HeCS rats pretreated with RTX (n=23 neurons) and control rats treated with RTX (n=20 neurons). (A) Current clamp traces showing action potentials induced by current injection at rheobase. (B) Graph showing changes in rheobase. (C) Graph showing changes in resting membrane potential. (D) Current clamp traces showing the number of action potentials at 2X rheobase. (E) Number of action potentials (AP) induced by current injection at 2X rheobase. *p<0.05, control vs HeCS, #p<0.05 HeCS vs HeCS + RTX.
The role of afferent nerve endings in the above remodeling of colon-specific DRG neurons was investigated by desensitizing them with intraluminal infusion of 200 μg/kg resiniferatoxin14 in the distal colon, one-day prior to the beginning of HeCS protocol. The treatment of sham-stressed rats with resiniferatoxin had no significant effect on rheobase and resting membrane potential of colon-specific DRG neurons (Figures 2B and 2C) (n=20 neurons). However, in rats subjected to HeCS, resiniferatoxin-treatment significantly blocked the reduction in rheobase and increase in the number of action potentials at 2X (Figures 2A, 2B, 2D and 2E) (n=23 neurons).
HeCS Induces NGF Expression in the Distal Colon
We found that HeCS significantly increases the expression of NGF in the muscularis externa and in mucosa/submucosa of the distal colon, when compared with those in sham-stressed rats (Figure 3A and Supplement Figure 1). Immunohistochemical staining of cross-sections detected NGF immunoreactivity in smooth muscle cells and in the myenteric plexus in muscularis externa and in epithelial cells in mucosa/submucosa. NGF immunoreactivity increased in both tissue-types of the chronically stressed rats, compared with and sham-stressed rats (Figure 3B). By contrast, HeCS had no significant effect on the expression of trkA receptors in the muscularis externa (100 ± 11% vs 108 ± 27%) or in mucosa/submucosa (100 ± 17% vs. 92 ± 5%).
Figure 3.

(A) HeCS significantly increased NGF in muscularis externa and mucosa/submucosa, which was blocked by inhibition of α1/α2 and β1/β2-adrenergic receptors prior to each daily stress session. n=8 rats in each group, *p<0.01, control (Ctr) vs. HeCS and HeCS vs. HeCS + adrenergic receptor antagonists. (B) Immunohistochemical staining for NGF (brown) in distal colon cross-sections from control and HeCS rats. Sections were counterstained with hematoxilyn. (C) NGF antagonism by systemic administration of neutralizing antibody significantly reduced HeCS-induced increase in visceromoter response. HeCS, n=4 rats, HeCS + NGF Ab, n=5 rats, *p<0.05 baseline vs HeCS; +p<0.05 HeCS vs. HeCS + NGF Ab. (D) Intrathecal administration of trkA antagonist k252A (n=5) or antisense oligonucleotide (n=3) suppressed HeCS-induced increase in visceromoter response. *p<0.05 baseline vs. HeCS, +p<0.05, HeCS + mismatch oligonucleotide (MM) vs. HeCS + NGF Ab. (E) Western blots showing the effects of intrathecal treatment with either trkA antisense (AS) or mismatch (MM) oligonucleotide on trkA receptor expression in thoracolumbar (TL), thoracic (Th) or lumbosacral (LS) DRG. n=4, *p<0.05 mismatch vs antisense oligonucleotides.
Effect of Antagonism of Peripheral NGF on HeCS-induced Increase in visceromoter response
The rats treated with 16 μg/kg i.p. NGF antibody15, 30 minutes before each daily stress session, showed no significant increase in visceromoter response to CRD following HeCS, compared to their pre-stress baseline responses (Figure 3C). Rats receiving non-immune serum showed significant increase in visceromoter response following HeCS (Figure 3C).
Effects of Antagonism of trkA Receptors in thoracolumbar DRG on HeCS-induced Increase in Excitability of Colon-specific Afferent Neurons
Next, we investigated whether NGF high affinity receptor trkA in DRG mediates the HeCS-induced increase in visceromoter response to CRD. We administered either trkA pharmacological inhibitor k252a, or trkA anti-sense oligonucleotide intrathecally by a surgically implanted osmotic pump for the entire nine-day period of HeCS. The intrathecal infusions started 24-hours prior to the first session of HeCS. The control rats received infusion of mismatch oligonucleotides. The rats treated with intrathecal k252a showed no significant difference between their post-HeCS and baseline responses to CRD (Figure 3D). The control rats that received mismatch oligonucleotide during stress showed significant increase in visceromoter response to CRD following HeCS, compared to baseline responses (Figure 3D). There was no significant difference between the post-HeCS and baseline responses of rats treated intrathecally with the trkA antisense oligonucleotides (Figure 3D).
The expression of trkA in the thoracolumbar DRG of rats treated with antisense oligonucleotides decreased significantly to about 50% of that in rats treated with mismatch oligonucleotides (Figure 3F). No significant difference in trkA levels were observed in the thoracic DRG, several segments rostral to the catheter tip, or in lumbosacral DRG, several segments caudal to the catheter tip, indicating that the knockdown effect was localized to the DRG near the catheter tip (Figure 3F).
Intrathecal application of k252a significantly attenuated the HeCS-induced decrease in rheobase (Figure 4A), depolarization of resting membrane potential (Figure 4B), and increase in the number of action potentials at 2X rheobase (Figure 4C). Taken together, these findings support an essential role of NGF receptor trkA and NGF in colon-specific thoracolumbar DRG neurons in the induction of visceral hypersensitivity by HeCS.
Figure 4.

Intrathecal treatment with trkA antagonist k252a blocked the HeCS-induced increase in excitability of colon-specific thoracolumbar DRG neurons (Ctr= control, n=13 neurons from 5 rats; HeCS n=22 neurons from 5 rats; HeCS pre-treated with resiniferatoxin, n=23 neurons from 5 rats. (A) Rheobase. (B) Resting membrane potential. (C) Number of action potentials (AP) at 2X rheobase. *p<0.05, control vs. HeCS, #p<0.05 HeCS vs. HeCS + k252a.
Effect of NGF on colon-specific thoracolumbar DRG neuronal excitability in vitro
Next, we investigated whether exposure to NGF in vitro alters the excitability of colon-specific thoracolumbar DRG neurons. We incubated acutely dissociated thoracolumbar DRG neurons from naïve rats with either high NGF (250 ng/ml) or low NGF (2.5 ng/ml) for 24 hours and measured passive and active electrophysiological properties of DiI labeled colonic sympathetic afferents. The resting membrane potential (Figure 5A) and rheobase (Figure 5B) significantly decreased in neurons treated with 250 ng/ml NGF, when compared with those treated with 2.5 ng/ml NGF. The number of action potentials generated at 2X rheobase was greater in neurons treated with high NGF than that with low NGF controls, but it did not reach statistical significance (p= 0.11, data not shown). These findings demonstrate that exposure to higher concentrations of NGF in vitro produces changes in electrophysiological properties of colon-specific thoracolumbar DRG neurons that are similar to those produced by HeCS.
Figure 5.
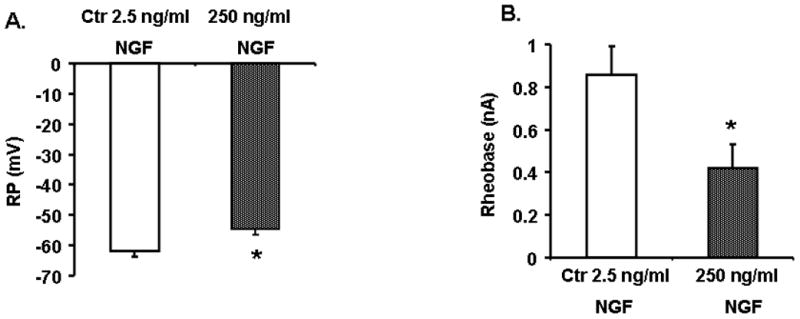
Electrophysiological properties of colon-specific thoracolumbar DRG neurons that were incubated for 24 hours with either high NGF (250 ng/mL or low NGF (2.5 ng/mL) in vitro. Neurons incubated with high NGF showed a significant decline in resting membrane potential (A) and rheobase (B), *p<0.05, low NGF vs high NGF.
The Role of Norepinephrine in Inducing Visceral Hypersensitivity and NGF Expression in Distal Colon
We reported recently that nine-day HeCS significantly elevates plasma concentration of norepinephrine5. To determine whether norepinephrine contributes to the induction of visceral hypersensitivity, rats subjected to HeCS were treated once daily before each stress session with phentolamine (2 mg/kg i.p.) + propranolol (2 mg/kg i.p.). Sham-treated rats served as controls. Visceromoter responses to CRD were compared with their respective pre-stress baselines (Figure 6A). Phentolamine plus propranolol blocked the HeCS-induced increase in visceromoter response to CRD and elevation of NGF in the muscularis externa and mucosa/submucosa (Figure 3A).
Figure 6.

(A) In vivo intraperitoneal administration of α1/α2- and β1/β2-adrenergic receptor antagonists blocked the HeCS-induced increase in the visceromoter response to graded CRD (n=3). Rats subjected to HeCS were treated once daily before each stress session with a combination of phentolamine (2 mg/kg i.p.) +and propranolol (2 mg/kg i.p.). In vitro incubation of muscularis externa/serosa (B) and mucusa/submucosa (C) for 24-hours with norepinephrine concentration-dependently increased the expression of NGF, *p<0.05, n=6 strips (Thirty strips of each tissue type were prepared from distal colon of 4 rats (about 8 strips/rat) and evenly distributed among the experimental groups.
We incubated strips of muscularis externa or mucosa/submucosa with norepinephrine for 24 hours in vitro. Norepinephrine (Figures. 6B and 6C) concentration-dependently increased the expression of NGF in muscularis externa and in mucosal/submucosa.
The Roles of Mast cells, Inflammatory Mediators and Peripheral CRH1/CRH2 Receptors in the Induction of Visceral Hypersensitivity by HeCS
We observed no differences in mast cell numbers in either muscularis externa or in mucosa/submucosa between HeCS-treated and sham-stressed controls (Figure 7A). HeCS reduced the spontaneous release of tryptase from muscularis externa, but not that induced by ionomycin (Figure 7B). HeCS had no effect on the spontaneous or ionomycin-induced release of tryptase in mucosal/submucosal tissues (Figure 7C). Nine-day HeCS had no significant effect on MPO in the muscularis externa or the mucosa/submucosa (Figure 7D). It also did not alter the concentrations of TNFα and IL-1β in these tissues (data not shown). Finally, the inhibition of CRH1/CRH2 receptors by astressin had no significant effect on the generation of NGF by norepinephrine in muscularis externa (Figure 7E), but astressin blocked this effect in the mucosa/submucosa (Figure 7F).
Figure 7.

(A). HeCS did not increase the number of mast cells in the muscularis externa or mucosa/submucosa (n=4 rats). (B) and (C). HeCS did not increase the spontaneous or ionomycin-induced release of tryptase from the muscularis externa/or the mucosa/submucosa. Instead, it decreased the spontaneous release of tryptase in the muscularis externa. n=4 rats, *p< 0.05, (D) HeCS did not increase MPO protein in the muscularis externa or mucosa/submucosa. n=4 rats. (E) and (F). CRH1/CRH2 receptor antagonist astressin (AST) had no effect on the norepinephrine-induced expression of NGF in the muscularis externa/serosa, but it blocked this increase in mucosa/submucosa, *p<0.05, n=4 strips. Strips were incubated with 10 μM astressin for 30 min prior to the addition of norepinephrine (NE).
Discussion
Our findings show that HeCS induces visceral hypersensitivity to CRD that lasts for at least 8 hours, but it returns to baseline by 24 hours. Choudhury et al.5 found that HeCS induces colonic circular smooth muscle hyperreactivity to ACh that also lasts for about 8 hours and returns to baseline by 24 hours. We found that adrenergic stimulation mediates the induction of visceral hypersensitivity by HeCS. The blockade of α1/α2- and β1/β2-adrenergic receptors before the daily application of stress prevented the induction of visceral hypersensitivity by HeCS. By contrast, Bradesi et al.10 found that 10-day homotypic water avoidance stress induces visceral hypersensitivity that lasts for about 40 days. However, they did not investigate which peripheral stress hormone/neurotransmitter mediates the prolonged induction of hypersensitivity in their model. Note that plasma/urine levels of norepinephrine are elevated in IBS patients16, 17.
The mechanoreceptors in the gut wall are distributed throughout its thickness18–21. Accumulating evidence from human7 and animal studies19, 20 shows that splanchnic neurons with mechanoreceptors in the muscularis externa/serosa mediate afferent responses to rapid step distensions that mimic the physiological rapid compression of the gut wall by giant migrating contractions22. In vitro, the mucosal afferents respond primarily to stimulation by von Frey hairs that mimics the flow of digesta19, 20; the mucosal afferent nerves do not respond to circumferential stretch19. In vivo, the mucosal afferents respond to slow distension that mimics slow lumen filling by fecal material, but not to rapid step balloon distension6. In addition, desensitization of the mucosal afferent mechanoreceptors by lidocane does not affect pain-perception to rapid balloon distension in IBS patients6. However, lidocane blocks the sensory perception to slow distension. It seems that the mucosal and muscularis externa afferent neurons may respond differentially to slow and rapid distensions of the gut wall. Therefore, we chose to investigate the effects of HeCS on the expression of various potential mediators of DRG-sensitization separately in the muscularis externa and in mucosa/submucosa.
Western blotting and immunohistochemical staining with NGF antibody and ELISA measurements showed that HeCS enhances the expression of NGF in the muscularis externa as well as in mucosa/submucosa. The inhibition of α1/α2- β1/β2 adrenergic receptors blocks the increase in the expression of NGF in both tissues, suggesting that they mediate the expression of NGF in the colon wall in response to HeCS. In addition, in vitro incubation of both tissue-types with norepinephrine enhances the expression of NGF. Prior reports show that numerous cell-types, including smooth muscle cells23, glia24, immune cells25 epithelial cells26 and neurons27 are capable of generating NGF. In our study, the smooth muscle cells and mucosa seemed to show the largest increase in NGF immunoreactivity in the colon wall, but we did not quantitate it. We found that neutralization of peripheral NGF by its antibody blocks the increase of visceromoter response to CRD. Together, the above data suggest that the up regulation of NGF throughout the thickness of the distal colon wall by HeCS-induced release of norepinephrine is an intermediate step in the induction of visceral hypersensitivity to CRD.
NGF in the periphery complexes with trkA receptors and migrates retrograde to the DRG neurons28, 29. The inhibition of retrograde migration of this complex by desensitization of afferent nerve endings with resiniferatoxin blocked the induction of visceral hypersensitivity to CRD. The pharmacological blockade of trkA receptors or their suppression by antisense oligonucleotide in the thoracolumbar DRG also blocked the induction of visceral hypersensitivity to CRD. Taken together, NGF expression in the colon wall is critical for the induction of visceral hypersensitivity to CRD by HeCS.
Patch-clamp recordings from colon-specific thoracolumbar DRG neurons showed that HeCS decreases rheobase, depolarizes resting membrane potential and increases the electrogenesis of action potentials, when compared with those in age-matched sham-stressed controls. Systemic administration of NGF antibody that does not cross the blood-brain barrier blocked these effects. This suggests that the alterations in the electrophysiological characteristics of colon-specific thoracolumbar DRG neurons may primarily be due to increase of NGF in the colon wall, rather than due to a direct effect of plasma norepinephrine on the DRG neurons30 or due to descending inhibition from the CNS31.
Voltage-gated sodium channels (Nav) play a critical role in regulating the sensitivity of DRG neurons to peripheral insult. The C-type DRG neurons express predominantly the slowly inactivating TTX-R Nav1.8 and TTX-R Nav1.9 channels32, 33. The Nav1.8 channels make major contribution to the electrogenesis of action potentials32, 34, whereas the Nav1.9 channels regulate the resting membrane potential of these neurons35. The ionic mechanisms underlying visceral hypersensitivity are specific to the pathophysiology of pain and its targeted organ36. Visceral hypersensitivity in rat colonic inflammation results mainly from alterations in the electrogenesis of action potentials due to remodeling of the Nav1.8 channels37. Our data show that visceral hypersensitivity induced by HeCS in rats is due to alterations in the electrogenesis of action potentials and changes in the resting membrane potentials of the colon-specific DRG neurons. According to the above-established roles of Nav1.8 and Nav1.9 in regulating the electrophysiological characteristics of the DRG neurons, HeCS may remodel both types of Nav channels. The persistence of hypersensitivity in isolated DRG neurons for at least 8-hours after the last stressor suggests altered gene expression of the Nav channels38, 39.
NGF is a key signaling protein in the sensitization of visceral afferent neurons during inflammation40–43.The administration of exogenous NGF mimics inflammatory hyperalgesia43, while systemic administration of NGF antibody blocks the induction of inflammatory hyperalgesia15. During inflammation, NGF interacts with mast cells in a reciprocal synergistic reaction25, 44. NGF acts on trkA receptors on mast cells to degranulate them, while the degranulation of mast cells releases NGF. It is noteworthy that while both NGF and trkA receptors are up regulated during inflammation43, only NGF expression is enhanced in the muscularis externa by non-inflammatory stress mediator norepinephrine.
Other studies found increase in mast cell numbers or their activation and increase in MPO activity and proinflammatory cytokines in mucosa/submucosa in response to chronic stress9, 10 or maternal deprivation45. However, the consensus seems to be that the overall intensity of inflammatory responses in these models is several-fold less than that in models of TNBS-induced inflammation. By contrast, we did not find any evidence of increase in MPO, proinflammatory cytokines or mast cell activation/hyperplasia in the muscularis externa or mucosa/submucosa in response to HeCS, which induces robust visceral hypersensitivity to CRD. In addition, NGF increases in both tissue-types in response to HeCS, which was blocked by inhibiting α1/α2- β1/β2-adrenergic receptors. Norepinephrine increases the expression of NGF in vitro in muscularis externa mucosa/submucosa, and this increase is blocked by the inhibition of adrenergic receptors. Taken together, norepinephrine induces the expression of NGF in the colon wall in the absence of any detectable inflammatory response.
CRH receptors have been identified in the mucosa as well as in the myenteric and submucosal plexi46, 47. Our findings show that these receptors in the muscularis externa that includes the myenteric and submucosal plexi do not mediate the generation of NGF by norepinephrine. However, the CRH receptors in the mucosa mediate the expression of NGF in mucosal/submucosal tissues.
In conclusion, 9-day heterotypic chronic stress induces visceral hypersensitivity to CRD that lasts for at least 8-hours after the last stressor. We reported previously that HeCS elevates the plasma level of norepinephrine that alters gene expression of key cell signaling proteins of the excitation-contraction coupling in colonic circular smooth muscle cells to induce hyperreactivity to acetylcholine5. Our findings in this study in relation to the well-established elements of the stress response are summarized in a schematic diagram in Figure 8: 1) Chronic stress releases CRH and angiotensin vasopressin from the paraventricular nucleus in the hypothalamus. 2) CRH and arginine vasopressin stimulate the locus ceruleus/norepinephrine system. In parallel, CRH releases adrenocorticotropic hormone from the pituitary, which releases corticosterone from the adrenal cortex. 3) The activation of the greater splanchnic sympathetic preganglionic neurons releases norepinephrine from the chromaffin cells in the adrenal medulla into the blood stream48, 49. The activation of the sympathetic neurons also releases norepinephrine locally in peripheral organs. 4) Norepinephrine enhances the expression of NGF in the colon wall. 5) NGF complexes with trkA receptors and the complex transports retrograde to the thoracolumbar DRG29. 6) NGF/trkA complex in the DRG sensitizes the ion channels. 7) The hypersensitization of these ion channels amplifies the afferent signals in response to colonic distension to increase in its perception. This sensitization occurs in the absence of a detectable inflammatory response in the muscularis externa or in mucosa/submucosa. Based on the topology and phenotypes of afferent nerve endings in the colon wall7, 18–21, increase of NGF in muscularis externa may mediate the induction of visceral hypersensitivity by HeCS, whereas, increase of NGF in mucosa/submucosa may mediate altered physiological responses to digesta in the lumen.
Figure 8.

A schematic illustrating the proposed mechanism of HeCS-induced visceral hypersensitivity to CRD in relation to the well-established elements of the stress response. Step 1: Stress releases of CRH and angiotensin vasopressin from the paraventricular nucleus in the hypothalamus. CRH releases adrenocorticotropic hormone and other mediators from the pituitary. Step 2. CRH and arginine vasopressin stimulate the locus ceruleus/norepinephrine system. Step 3. The activation of the sympathetic preganglionic neurons releases norepinephrine/epinephrine in blood stream from the adrenal medulla. Step 4: Norepinephrine elevates the expression of NGF in colonic muscularis externa and mucosa/submucosa. Step 5: NGF complexes with trkA receptors and the complex transports retrograde to the thoracolumbar DRG. Step 6: NGF/trkA complex sensitizes colon-specific neurons by modulating the expression and characteristics of ion channels Step 7: The amplification of afferent signal in response to CRD is perceived as abdominal pain/discomfort.
Supplementary Material
Acknowledgments
Supported by NIDDK Grants DK 032346 and DK 072414 (SKS)
Abbreviations
- IBS
Irritable bowel syndrome
- (HPA)-axis
Hypothalamus-pituitary-adrenal
- CRH
Corticotrophin-releasing hormone
- DRG
Dorsal root ganglia
- HeCS
Heterotypic Chronic Stress
- NGF
Nerve Growth Factor
- (trkA) [NGF high affinity receptor]
Tropomyosin related kinase A
- CRD
Colorectal Distention
- DiI
1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocynine perchlorate
Footnotes
There are no conflicts of interest to disclose for all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions by the authors:
| Topic | Winston | Xu | Sarna |
| Study concept And design | X | ||
| Analysis of data | X | X | |
| Acquisition of data | X | X | |
| Interpretation of data | X | ||
| Drafting manuscript | X | ||
| Revision | X | ||
| Statistical analysis | X | X | |
| Funding | X | ||
| Study supervision | X |
References
- 1.Drossman DA, Camilleri M, Mayer, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 2.Bennett EJ, Tennant CC, Piesse C, et al. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–61. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead WE, Crowell MD, Robinson JC, et al. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–30. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol. 2009;296:G632–42. doi: 10.1152/ajpgi.90673.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1238–47. doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lembo T, Munakata J, Mertz H, et al. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–96. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 7.Lembo T, Munakata J, Naliboff B, et al. Sigmoid afferent mechanisms in patients with irritable bowel syndrome. Dig Dis Sci. 1997;42:1112–20. doi: 10.1023/a:1018817132213. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 9.Gue M, Del Rio-Lacheze C, Eutamene H, et al. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil. 1997;9:271–9. doi: 10.1046/j.1365-2982.1997.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 10.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 11.Xu GY, Winston JH, Shenoy M, et al. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–92. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Berg MM, Sternberg DW, Parada LF, et al. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–6. [PubMed] [Google Scholar]
- 13.Summer GJ, Puntillo KA, Miaskowski C, et al. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain. 2006;7:884–91. doi: 10.1016/j.jpain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–52. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf CJ, Safieh-Garabedian B, Ma QP, et al. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–31. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 16.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–13. [PubMed] [Google Scholar]
- 17.Posserud I, Agerforz P, Ekman R, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leek BF. Abdominal and pelvic visceral receptors. Br Med Bull. 1977;33:163–8. doi: 10.1093/oxfordjournals.bmb.a071417. [DOI] [PubMed] [Google Scholar]
- 19.Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol. 1999;518 (Pt 1):271–82. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brierley SM, Jones RC, 3rd, Gebhart GF, et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–78. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–60. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- 22.Sarna SK. Enteric descending and afferent neural signaling stimulated by giant migrating contractions: essential contributing factors to visceral pain. Am J Physiol Gastrointest Liver Physiol. 2007;292:G572–81. doi: 10.1152/ajpgi.00332.2006. [DOI] [PubMed] [Google Scholar]
- 23.Clemow DB, Steers WD, Tuttle JB. Stretch-activated signaling of nerve growth factor secretion in bladder and vascular smooth muscle cells from hypertensive and hyperactive rats. J Cell Physiol. 2000;183:289–300. doi: 10.1002/(SICI)1097-4652(200006)183:3<289::AID-JCP1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.von Boyen GB, Steinkamp M, Reinshagen M, et al. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol. 2006;18:820–5. doi: 10.1111/j.1365-2826.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- 25.Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–43. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanzel RD, Lourenssen S, Blennerhassett MG. Inflammation causes expression of NGF in epithelial cells of the rat colon. Exp Neurol. 2008;211:203–13. doi: 10.1016/j.expneurol.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Yamataka A, Fujimoto T, et al. Mast cells and gut nerve development: implications for Hirschsprung’s disease and intestinal neuronal dysplasia. J Pediatr Surg. 1999;34:543–8. doi: 10.1016/s0022-3468(99)90069-6. [DOI] [PubMed] [Google Scholar]
- 28.Barker PA, Hussain NK, McPherson PS. Retrograde signaling by the neurotrophins follows a well-worn trk. Trends Neurosci. 2002;25:379–81. doi: 10.1016/s0166-2236(02)02199-9. [DOI] [PubMed] [Google Scholar]
- 29.Delcroix JD, Valletta JS, Wu C, et al. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 30.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–12. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 31.Pyner S, Coote JH. Rostroventrolateral medulla neurons preferentially project to target-specified sympathetic preganglionic neurons. Neuroscience. 1998;83:617–31. doi: 10.1016/s0306-4522(97)00355-2. [DOI] [PubMed] [Google Scholar]
- 32.Sangameswaran L, Delgado SG, Fish LM, et al. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–6. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 33.Dib-Hajj S, Black JA, Cummins TR, et al. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 2002;25:253–9. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- 34.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–40. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 35.Herzog RI, Cummins TR, Waxman SG. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J Neurophysiol. 2001;86:1351–64. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- 36.Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol. 2004;91:2524–31. doi: 10.1152/jn.00866.2003. [DOI] [PubMed] [Google Scholar]
- 37.Beyak MJ, Ramji N, Krol KM, et al. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–55. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- 38.Gould HJ, 3rd, Gould TN, England JD, et al. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000;854:19–29. doi: 10.1016/s0006-8993(99)02216-7. [DOI] [PubMed] [Google Scholar]
- 39.Fjell J, Cummins TR, Davis BM, et al. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999;57:39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 40.Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int. 1992;12:213–6. doi: 10.1007/BF00302155. [DOI] [PubMed] [Google Scholar]
- 41.Chien CC, Fu WM, Huang HI, et al. Expression of neurotrophic factors in neonatal rats after peripheral inflammation. J Pain. 2007;8:161–7. doi: 10.1016/j.jpain.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Winston JH, Toma H, Shenoy M, et al. Acute pancreatitis results in referred mechanical hypersensitivity and neuropeptide up-regulation that can be suppressed by the protein kinase inhibitor k252a. J Pain. 2003;4:329–37. doi: 10.1016/s1526-5900(03)00636-9. [DOI] [PubMed] [Google Scholar]
- 43.Delafoy L, Raymond F, Doherty AM, et al. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105:489–97. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- 44.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Brain Res Mol Brain Res. 2001;97:177–85. doi: 10.1016/s0169-328x(01)00314-x. [DOI] [PubMed] [Google Scholar]
- 45.Barreau F, Cartier C, Ferrier L, et al. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–34. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Chatzaki E, Crowe PD, Wang L, et al. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–16. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Gao N, Hu HZ, et al. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi-Shima N, Okada S, Shimizu T, et al. Adrenal adrenaline- and noradrenaline-containing cells and celiac sympathetic ganglia are differentially controlled by centrally administered corticotropin-releasing factor and arginine-vasopressin in rats. Eur J Pharmacol. 2007;564:94–102. doi: 10.1016/j.ejphar.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 49.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1763–75. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


