Abstract
What are the neural bases of semantic memory? Traditional beliefs that the temporal lobes subserve the retrieval of semantic knowledge, arising from lesion studies, have been recently called into question by functional neuroimaging studies finding correlations between semantic retrieval and activity in left prefrontal cortex. Has neuroimaging taught us something new about the neural bases of cognition that older methods could not reveal or has it merely identified brain activity that is correlated with but not causally related to the process of semantic retrieval? We examined the ability of patients with focal frontal lesions to perform a task commonly used in neuroimaging experiments, the generation of semantically appropriate action words for concrete nouns, and found evidence of the necessity of the left inferior frontal gyrus for certain components of the verb generation task. Notably, these components did not include semantic retrieval per se.
One of the earliest findings in cognitive neuroimaging was that the left inferior frontal gyrus (IFG) is activated when subjects are shown a concrete noun and must generate a semantically appropriate verb (1–5). This finding, which now has been replicated many times, has been interpreted as evidence that the left IFG plays a role in the retrieval of semantic knowledge. This interpretation is supported by other neuroimaging studies finding left IFG activation during different tasks requiring semantic retrieval, such as living/nonliving classification (6–8). In contrast, the literature on cognitive impairments after focal brain lesions reveals no particular association between semantic retrieval and left prefrontal cortex (9). Patients with prefrontal lesions have normal language comprehension; although lesions to either left or right prefrontal cortex do impair the ability to generate semantically related words on a category fluency task, they also impair performance in nonsemantic fluency tasks (10), consistent with an underlying impairment that is not semantic per se (11). Impairments of semantic knowledge are most associated with temporal lobe, not frontal lobe, pathology (12–14).
In this paper, we consider two possible explanations for the discrepancy between the findings from neuroimaging and neuropsychological studies of the role of prefrontal cortex in semantic retrieval. First, differences in the outcomes from these two types of studies may reflect simply the different types of inferences to which neuroimaging studies and lesion studies lend themselves. Neuroimaging studies are limited to inferences about brain regions that are engaged by a cognitive process as revealed by correlated changes in activity related to processing demands. In contrast, a neuropsychological approach allows one to make inferences about brain regions that are necessary for a cognitive process, when that process can be shown to depend on the integrity of a given brain region (15). Thus, one interpretation of the neuroimaging findings that can be tested in patients with frontal lesions is that prefrontal cortex is engaged during the verb generation task but is not necessary.
A second possible explanation for the discrepancy is that the cognitive process that is subserved by left IFG and engaged during the verb generation task is not semantic retrieval per se but some other process, which may be impaired in patients with frontal lesions. The origin for the hypothesis that left IFG is involved in semantic retrieval was the convergence of activity in that region across a variety of tasks that seemed to have in common only demands for semantic retrieval, including not only the verb generation task (1, 2) but also semantic classification tasks (6–8) and other types of generation tasks (3). However, these tasks may have in common other, nonsemantic mechanisms that are in fact responsible for the observed prefrontal activity.
A recent neuroimaging study addressed this possibility by distinguishing between semantic retrieval and the selection of semantic knowledge from among competing alternatives. Evidence for the role of left IFG in the selection of semantic information comes from three functional MRI (fMRI) experiments that found activity in left IFG that was sensitive to increased selection demands during semantic generation, comparison, and classification but not to increased retrieval demands (16). For example, the generation task required subjects to generate a verb in response to a noun that had either high (e.g., scissors) or low (e.g., cat) demands for selection among competing responses. Across all three experiments, selection-related activity was found in left, posterior IFG, around Brodmann’s areas 44/45. This region is consistent with that identified in a review of eight semantic retrieval studies (17), although the interpretation for the activity in that region is fundamentally different than that offered in earlier studies.
The goal of the present study was therefore to test whether left IFG is necessary to perform the verb generation task, and if so whether it is necessary only under conditions that require selection of semantic knowledge among competing alternatives. Patients with focal, unilateral frontal lesions generated verbs related to nouns with high or low demands for selection among competing responses. Based on the considerable number of neuroimaging studies implicating left IFG in verb generation specifically and in semantic retrieval generally, one might predict that patients with lesions including left IFG would be impaired relative to patients with lesions sparing left IFG. Alternatively, if left IFG subserves the selection of semantic knowledge among competing alternatives, the prediction would be that the impairment of patients with left IFG lesions would depend on the demands for selection, resulting in more errors for items with a greater amount of competition among responses. Thus, the results of this study can test whether left IFG is necessary for selection among competing responses and not semantic retrieval per se.
MATERIALS AND METHODS
Participants.
Fourteen patients were selected on the basis of focal frontal cortex lesion evident on computerized tomography or MRI scans. In nine of the patients, lesions were the result of a single infarction of the precentral branch of the middle cerebral artery. In the remaining patients, lesions were the result of the removal of a meningioma, cyst, or arterial-venous malformation. Subjects with a history of psychiatric problems or substance abuse were excluded.
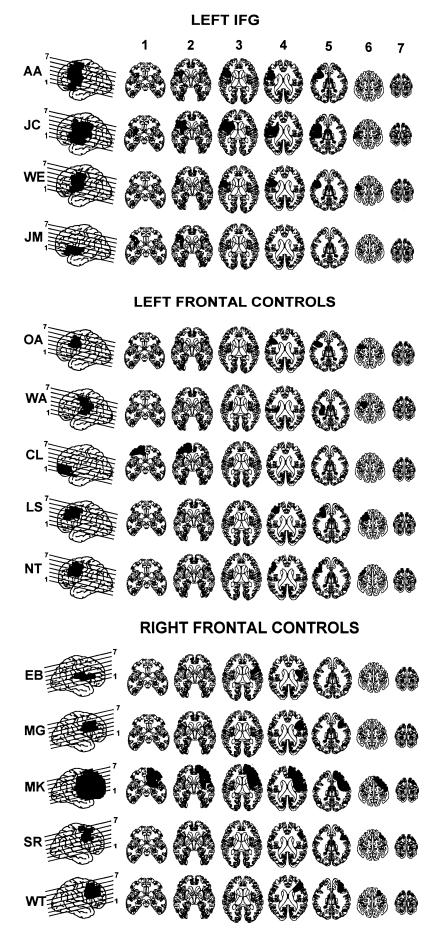
Patients were divided into three groups on the basis of the location of their lesion. In the first group, four patients had lesions that included the posterior region of the left inferior frontal gyrus (pIFG). In the second group (referred to in Tables 1 and 2 as left controls), five patients had lesions of left prefrontal cortex that spared left pIFG. In three of these cases, the lesion was confined to the superior and middle frontal gyri. In one case (WA), the lesion was restricted to the insula, and in the final case (CL), the lesion was of the anterior portion of the left inferior frontal gyrus (aIFG). The final group (referred to in Tables 1 and 2 as right controls) was comprised of five patients with lesions to right prefrontal cortex. Lesions were transcribed onto corresponding axial templates by two independent raters and projected onto a lateral view of the brain (Figs. 1 and 2). The approximate boundaries of relevant Brodmann’s areas were marked on the template by using a landmark-guided method (performed by R.T.K.), and damage within each region could therefore be quantified for each patient.
Table 1.
Demographic and lesion characteristics of patient and control groups
| Patient | Demographic
|
Lesion
|
|||||
|---|---|---|---|---|---|---|---|
| Age | Education | Sex | Hand | Onset | Vol., cc | Etiology | |
| Left IFG | |||||||
| AA | 30 | 10 | F | L | 1993 | 58.5 | Stroke |
| JC | 73 | 16 | M | R | 1987 | 102.6 | Stroke |
| WE | 73 | 14 | M | R | 1995 | 41.1 | Stroke |
| JM | 53 | 11 | M | R | 1997 | 18.8 | Stroke |
| Mean | 56.8 | 12.8 | |||||
| Left controls | |||||||
| OA | 65 | 13 | M | R | 1984 | 17.5 | Stroke |
| WA | 75 | 14 | F | R | 1986 | 26.2 | Stroke |
| CL | 63 | 16 | M | A | 1987 | 40.3 | Meningioma |
| LS | 68 | 16 | F | R | 1981 | 27.9 | Meningioma |
| NT | 56 | 12 | F | R | 1996 | 20.0 | Stroke |
| Mean | 65.4 | 14.2 | |||||
| Right controls | |||||||
| EB | 79 | 12 | F | R | 1983 | 17.3 | Stroke |
| MG | 34 | 12 | M | R | 1984 | 24.5 | AVM |
| MK | 65 | 17 | M | R | 1979 | 200.4 | Aneurysm |
| SR | 77 | 12 | F | R | 1994 | 12.9 | Stroke |
| WT | 53 | 18 | M | R | 1988 | 25.9 | Colloid cyst |
| Mean | 61.6 | 14.2 | |||||
| Elderly controls (n = 16) | |||||||
| Mean | 61.8 | 14.1 | |||||
Table 2.
Summary of errors on generation task
| Patient | Retrieval errors
|
Task errors
|
||||
|---|---|---|---|---|---|---|
| High selection | Low selection | Difference | High selection | Low selection | Difference | |
| Left IFG | ||||||
| AA | 4 | 1 | 3* | 0 | 0 | 0 |
| JC | 12* | 4 | 8* | 14* | 16* | −2 |
| WE | 5* | 1 | 4* | 1 | 3* | −2 |
| JM | 12* | 2 | 10* | 3* | 1 | 2* |
| Mean | 8.3* | 2.0 | 6.3* | 4.5* | 5* | −0.5 |
| Left controls | ||||||
| OA | 4 | 4 | 0 | 0 | 0 | 0 |
| WA | 1 | 0 | 1 | 0 | 1 | −1 |
| CL | 1 | 2 | −1 | 0 | 0 | 0 |
| LS | 2 | 2 | 0 | 0 | 2 | −2 |
| NT | 1 | 1 | 0 | 0 | 0 | 0 |
| Mean | 1.8 | 1.8 | 0 | 0 | 0.6 | −0.6 |
| Right controls | ||||||
| EB | 1 | 1 | 0 | 0 | 0 | 0 |
| MG | 2 | 1 | 1 | 1 | 0 | 1 |
| MK | 2 | 2 | 0 | 0 | 0 | 0 |
| SR | 0 | 0 | 0 | 0 | 0 | 0 |
| WT | 1 | 1 | 0 | 0 | 0 | 0 |
| Mean | 1.2 | 1.0 | 0.2 | 0.2 | 0 | 0.2 |
| Elderly controls (n = 16) | ||||||
| Mean | 1.5 | 1.1 | 0.4 | 0.3 | 0.1 | 0.1 |
| SD | 1.5 | 1.7 | 1.3 | 0.6 | 0.5 | 0.8 |
Number of retrieval errors (i.e., no response, non-verb response, unrelated response) and task errors (i.e., reading noun) for items with high selection demands or low selection demands (max = 48) and the difference between the item types (i.e., effect of competing responses).
Scores that were >2 SDs above the mean of the normal control subjects.
Figure 1.
Reconstructions of lesions based on computerized tomography and MRI scans. For each patient, the lateral view on the far left shows the lesion projected onto the lateral surface of the brain. Lines through the lateral projection show the level of the axial cuts from ventral (1) to dorsal (7). Patients were categorized into three groups based on the locations of the lesions: Patients with left frontal lesions that extended into the IFG (BA 44; Top); patients with left frontal lesions sparing BA 44 (Middle); and patients with right frontal lesions (Bottom).
Figure 2.
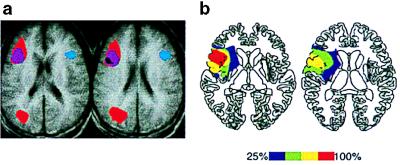
(a) Regions of overlap in selection-related fMRI activity during three semantic retrieval tasks (16) The convergence of activity during all three tasks, including verb generation, is shown in black. (b) Regions of overlap in lesion location for patients who had selection-related deficits on the verb generation task.
Sixteen control subjects (five men, eleven women) were recruited from the Philadelphia area and were screened for prior history of neurological disease, substance abuse, and psychiatric disorders. Control subjects were matched to patients in age and years of education (Table 1). All subjects were paid for their participation and gave their informed consent.
Materials.
Concrete nouns (Kucera–Francis frequency range from 0 to 591, median frequency = 32; word length range from 3 to 8, median = 4) were divided into two groups on the basis of verb generation data from two independent groups of subjects (n = 30 and 50). Subjects were asked to generate a verb from each noun. A ratio of the relative response frequency of the most common completion to the relative response frequency of the second-most common completion was calculated as a measure of response strength. On the basis of these data, two groups of 48 nouns were selected so that nouns in the low selection group (response strength ratio range from 5.0 to 50.0, median = 13.34) differed from nouns in the high selection group (response strength ratio range from 1.0 to 3.0, median = 2.0) in terms of response strength ratio, t (94) = 13.85, P < 0.001, but not word frequency, t (94) = 0.76, P = 0.45 (statistics were performed on the cube-root of ratio and frequency).
Procedure.
On each trial, a noun from either the high selection or low selection group was randomly selected and presented centrally on a computer monitor. Participants were instructed to say a word that described either “what the object does or what you do with the object.” The noun remained on the screen until the subject responded or for a maximum of 30 sec if no response was given. Before the experimental trials, subjects were given a practice set of 16 items. Several subjects required a repetition of the practice set. During the experimental trials, three blocks of 32 nouns (16 high selection and 16 low selection items) were presented; subjects were allowed to rest between blocks and resume testing at their own pace. If a subject made an inappropriate response (e.g., a non-verb response), the error was noted but the instructions for the task were repeated. Responses were recorded on tape for later scoring. Errors were classified as either (i) repetition of the noun, (ii) no response, or (iii) a non-verb response. (Unrelated verb responses, another possible error, were not made by any participants in the study.) This task was administered as part of a larger battery of tests, including working memory and language tasks, that will not be discussed in this report.
RESULTS
Two types of incorrect responses were examined: items for which the subject was unable to retrieve an appropriate verb (i.e., no response or a non-verb response) and items for which the subject read the noun instead of generating a related verb. These two types of responses, retrieval errors and task errors, respectively, were considered separately (see Table 2). For all of the analyses reported here, left frontal controls and right frontal controls were considered together, because there were no differences in performance between these two groups.
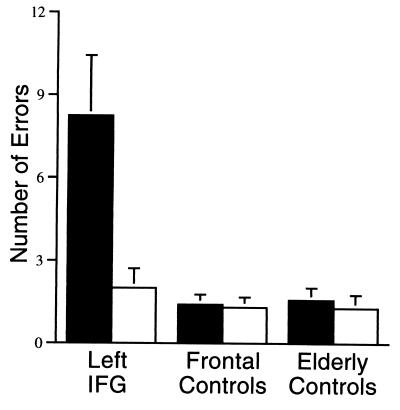
In a group analysis of variance, patients with lesions affecting left IFG made more retrieval errors than control subjects, main effect F(2,27) = 9.70, P < 0.01; however the impairment depended on the amount of competition among responses, interaction F(2,27) = 27.37, P < 0.01. For items with low selection demands, left IFG patients performed comparably to control subjects, F < 1.0. For items with high selection demands, left IFG patients made more retrieval errors than both frontal controls and elderly controls, F(2,27) = 20.52, P < 0.01, as evident in Fig. 3. Tukey’s post-hoc tests indicated no difference between frontal controls and elderly controls in either condition.
Figure 3.
Mean number of errors (maximum = 48) for patients and controls subjects high selection items (filled bars) and low selection items (unfilled bars). Patients with left IFG lesions made more errors (omissions and non-verb responses) generating verbs for high selection items (e.g., cat), but performed comparably to control subjects for low selection items (e.g., scissors), interaction F = 27.37, P < 0.01.
This effect of selection demands can be seen at the individual level as well: The error rate for three of the four left IFG patients (and none of the control patients) was more than two SDs above the elderly control mean error rate for items with high selection demands; yet, all of the patients were in the normal range for items with low selection demands. The effect of selection demands (defined as the number of errors for items with low selection demands subtracted from the number of errors for items with high selection demands) was outside the normal range for all four of the left IFG patients and for none of the control patients.
Because the overall number of retrieval errors made by control subjects was very low, one could argue that the interaction is an artifact of a “floor effect.” That is, if the number of retrieval errors for items with high selection demands is close to zero, it would be difficult to detect an improvement in performance for items with low selection demands. To address this potential artifact, we examined a subset of the control subjects who made a high number of errors (n = 5). For the items with low selection demands, these control subjects made more errors than the left IFG patients (M = 3.6), yet these control subjects did not show an increase in errors for items with high selection demands (M = 3.2) as did the left IFG patients, interaction F(1,7) = 19.14, P < 0.01. The comparable performance of the control subjects on the high and low selection conditions also belies the notion that the high selection condition was simply more difficult and thus more likely to reveal impairments.
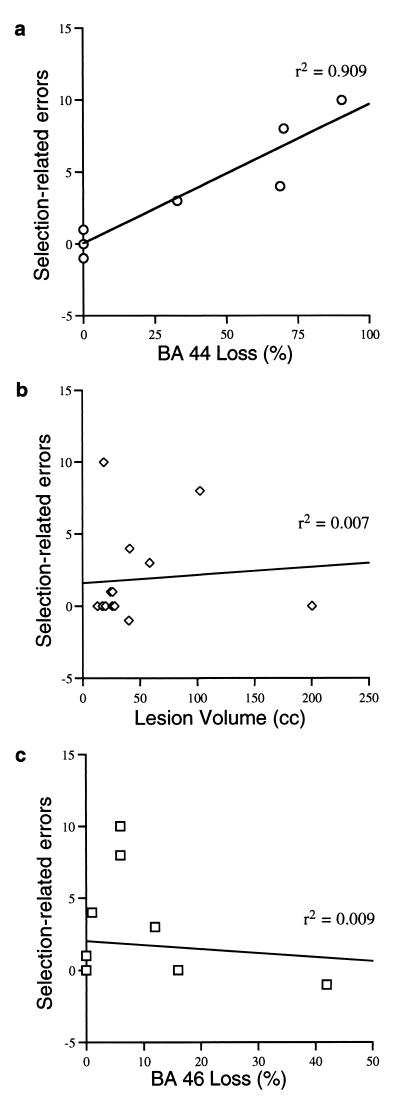
The manner in which the patient groups were defined created the possibility of a confound between lesion size and left IFG damage, because patients were classified as left IFG patients if a lesion in any location included left IFG whereas control patients had lesions that excluded left IFG. In fact, the median lesion size for the left IFG group was 49.8 cc, compared with 25.2 cc in the frontal control group (although this difference was not statistically significant, t < 1.0). Therefore, an alternative explanation of these data could be that the larger the lesion of any location, the more difficulty there would be selecting among competing responses. To address this possibility, the effect of selection demands, as defined above, was regressed on lesion volume, both overall and as a percentage of approximate Brodmann’s areas (BAs) (Fig. 4). Lesion volume, which accounted for <1% of the variance in the data, did not predict patient performance, t < 1.0. The percentage damage to BA 44, which accounted for over 90% of the variance in the data (r = 0.95), was a significant predictor of selection-related errors, t = 10.43, P < 0.01. In contrast, the percentage damage to neighboring Broadmann’s areas (e.g., BA 46) did not account for significant variance in the data. Thus the best anatomical predictor of the number of selection-related errors was the size of the lesion in BA 44.
Figure 4.
The number of selection-related errors (high selection–low selection) was strongly correlated with the percentage loss of BA 44 (a), but was uncorrelated with the overall lesion volume (b), or with percentage loss of neighboring areas (c; e.g., BA 46). Note that some points represent more than one patient.
Patients with lesions affecting left IFG made more task errors than frontal controls or elderly controls, main effect F(2,27) = 6.67, P < 0.01; however, the number of task errors did not depend on the number of competing responses, interaction F < 1.0. Tukey’s post-hoc comparisons indicated that patients with lesions affecting left IFG made more task errors than frontal controls and elderly controls for both high and low selection items. A regression analysis, as described above but using the total number of task errors instead of a difference, found that damage to BA 44 was a better predictor of task errors, t = 2.38, P < 0.05, than was total lesion volume, t = 1.06, although the percentage of variance in the data accounted for by BA 44 damage was only 30%.
DISCUSSION
Patients with lesions that encompassed left IFG showed an impairment in generating semantically appropriate verbs for concrete nouns with high demands for selection among competing responses but not for nouns with low selection demands. Furthermore, nearly all of the variance in the increased error rate could be accounted for by the size of the lesion in BA 44. These findings are consistent with observed increases in blood flow (as measured by fMRI) centered in BA 44 during verb generation for items with high selection demands relative to items with low selection demands (see Fig. 2) (16). Additionally, the impairment observed in patients with damage to left IFG allows one to make conclusions about the necessity of this region for cognitive functioning, namely, that left IFG is necessary for the selection of competing semantic knowledge.
Although previous studies reporting increased activation in the left inferior gyrus during semantic retrieval typically include activations that span the length of the gyrus. There recently has been some speculation that the aIFG at or near BA 47/10 is associated with semantic processing whereas the pIFG at or near BA 44/45 is associated with phonological processing (18). Two observations from the current group of patients are relevant to this conjecture. First, the patients in the left IFG group had lesions that were confined to pIFG. In two of the patients (WE, JC), BA 47 and 10 were completely spared; in the other two patients (AA, JM), BA 10 was spared and damage to BA 47 was limited to the most posterior extent of that region. Despite the integrity of aIFG, all four of these patients showed impairments on this task (when there were high selection demands). This suggests that damage to pIFG may be sufficient to cause this pattern of impairments, but is it necessary? The second relevant observation comes from a single patient in this study (CL) who, after the removal of a meningioma, had focal damage to aIFG that completely obliterated BA 47 and much of BA 10, but that spared pIFG. (Strokes in this region are uncommon because aIFG is an area that is supplied by both the anterior and middle cerebral arteries, which is vulnerable to watershed infarctions but not to the more common middle cerebral infarctions.) The performance of CL was completely normal on this task, suggesting that a lesion of left pIFG is both necessary and sufficient to disrupt the selection of competing semantic information.
Several other patients included in this study help delimit the region that is necessary for selecting among competing knowledge. WA had a lesion to left insular cortex, in the inferior area of left prefrontal cortex but deep to the inferior frontal gyrus. Her performance on this task was normal. MK had a lesion to right IFG (after an aneurysm that destroyed nearly the entire right frontal lobe); his performance was also normal on this task. The only patients that showed impairments on this task (limited to items with many competing responses) were patients with lesions that encompassed left pIFG.
Two previous neuropsychological case studies may be consistent with our assertion that left IFG is necessary for the selection of competing semantic knowledge. Randolph et al. (19) described a patient with bilateral IFG damage who, despite failing to generate exemplars on a standard category fluency task, reached normal levels of performance for more restricted categories such as farm animals. In contrast, patients with Alzheimer’s disease were impaired in both cases. This pattern of this patient’s performance is consistent with an impairment in the ability to select among many competing alternatives; in fact, one would predict a correlation between performance and category “size.” Robinson et al. (20) described a patient with an acute aphasia as a result of a meningioma impinging on left IFG who was impaired on unconstrained verbal tasks (e.g., sentence generation or completion) but who performed normally in highly constrained tasks that required generation of a strong prepotent response. This case study provides another example of the necessity of left IFG in the selection of competing information.
In both the current study and in these previous case studies, the tasks under investigation can be described as word retrieval paradigms. One might wonder whether there is something unique about word retrieval tasks with regard to what we are calling selection demands, that would not be a more general statement about the role of prefrontal cortex across a wider variety of tasks. Indeed, the results of the present study are limited to the verb generation paradigm. However, there is reason to be optimistic that this finding may not be tied specifically to word retrieval. Our initial construal of this selection hypotheses was based on the convergence of selected-related activity in prefrontal cortex across three quite disparate tasks, that required either generation, classification, or comparison of semantic knowledge (16). Future work in patients with focal brain damage will determine whether a similar convergence of selection-related impairments will occur across a wider range of tasks.
One unanticipated result in the current study is worth some speculation. Heretofore, all of the discussion has focused on the retrieval errors (e.g., omissions, non-verb responses). The pattern of results concerning task errors (i.e., reading the noun instead of generating a verb) looks quite different. As with the retrieval errors, only patients with left pIFG lesions made more of these errors than control subjects. However, in contrast to retrieval errors, these errors were made in equal numbers for items with many or few competing responses. That is, the number of competing responses did not affect the likelihood that the stimulus would be erroneously read by the subject. We tentatively offer the following interpretation of this finding. There are two situations during the performance of this task in which the selection among a competing response is required. First, the process of generating an associate is competing with the strongly prepotent response to read the word. Second, once semantic information about the item is retrieved, there is competition among the possible competing alternatives prior to selecting a single response. If the first requirement for selection happens prior to retrieval of semantic knowledge, it would be reasonable to expect that the amount of competition among alternatives in semantic knowledge would have no effect on these errors. In contrast, if the second requirement for selection happens after the retrieval of semantic knowledge, then the amount of competition among alternatives that are retrieved could be expected to impact that process.
This interpretation, although speculative, may have some bearing on a question that is unanswered by both the current study and previous neuroimaging research: Is left pIFG necessary just for the selection of competing semantic knowledge or is it involved in the selection of any information? The notion that there are two types of selection affected in the current study—one that occurs before semantic retrieval and one that occurs during or subsequent to semantic retrieval—is suggestive of a more general selection mechanism. The inability to select a non-dominant response (i.e., generation versus reading) may have something in common with other tasks that have been linked to prefrontal cortex, such as the ability to identify a color type instead of reading a word (i.e., the Stroop task; 21), or even the ability to maintain fixation instead of making a saccade to a target (i.e., the anti-saccade task; 22). On the other hand, left pIFG may be a region of prefrontal cortex that is specialized for the selection of semantic knowledge, akin to theories that different portions of prefrontal cortex are specialized for maintaining different types of information (23). More research is needed to determine whether left pIFG subserves a general selection function or a specific semantic selection function.
In either case, we suggest that left pIFG subserves a mechanism that is similar to other operations performed by prefrontal cortex, which can be described as the selection of a response among competing information, rather than a specialized semantic retrieval mechanism. The ability to select only that information which is currently relevant may be a central function of prefrontal cortex, as documented across a wide variety of tasks both in humans (e.g., 21) and in nonhuman primates (e.g., 24). Recent models have described mechanisms which could enable prefrontal cortex to make flexible, context-sensitive responses (25), perhaps by the weighting of information in working memory (26). We believe these same mechanisms are at work in the verb generation task, particularly when there is increased competition among possible responses. Thus, although pIFG does in fact appear to be necessary for the verb generation task, we believe that it is not due to the semantic retrieval demands of the task but rather to a nonsemantic selection process that is engaged when there are many sources of competing information. Furthermore, this study illustrates the type of strong inference that can be drawn about the neural bases of cognition by combining neurophysiological and neuropsychological approaches.
Acknowledgments
We gratefully acknowledge the assistance of Elizabeth Wheeler in testing control subjects, Donatella Scabini in identifying and scheduling patients for this project, Clay Clayworth in preparing the lesion figures, and John Gabrieli and an anonymous reviewer for helpful comments on an earlier version of this manuscript. This research was supported by grants from the James S. McDonnell Foundation and National Institutes of Health Grants AG05743 to S.L.T.; DC03023 to D.S.; NS34030, AG14082, and AG00756 to M.J.F.; AG13483 and NS01762 to M.D.; NS21135 and P017778 to R.T.K.
ABBREVIATIONS
- IFG
inferior frontal gyrus
- pIFG
posterior region of the IFG
- aIFG
anterior portion of the IFG
- fMRI
functional MRI
- BA
Brodmann’s area
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Petersen S E, Fox P T, Posner M I, Mintun M A, Raichle M A. J Cognit Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 3.Martin A, Haxby J V, Lalonde F M, Wiggs C L, Ungerleider L G. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- 4.Raichle M E, Fiez J A, Videen T O, MacLeod A M, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Brain. 1991;114:1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- 6.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapur S, Craik F I, Tulving E, Wilson A A, Houle S, Brown G M. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrieli J D E, Desmond J E, Demb J B, Wagner A D, Stone M V, Vaidya C J, Glover G H. Psychol Science. 1996;6:76–82. [Google Scholar]
- 9.Goodglass H, Kaplan E. Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 10.Baldo J V, Shimamura A P. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- 11.Swick D, Knight R T. Neuropsychologia. 1996;34:1019–1028. doi: 10.1016/0028-3932(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 12.Saffran E M, Schwartz M F. In: Conscious and Nonconscious Information Processing: Attention and Performance XV. Umilta C, Moscovitch M, editors. Cambridge: MIT Press; 1994. pp. 507–534. [Google Scholar]
- 13.Hodges J R, Patterson K, Oxbury S, Funnell E. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 14.Hodges J R, Graham N, Patterson K. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- 15.Sarter M, Berntson G G, Cacioppo J T. Am Psychol. 1996;51:13–21. doi: 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Thompson-Schill S L, D’Esposito M, Aguirre G K, Farah M J. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner R L, Tulving E. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Vol. 10. Amsterdam: Elsevier; 1995. pp. 429–446. [Google Scholar]
- 18.Fiez J A. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- 19.Randolph C, Braun A R, Goldberg T E, Chase T N. Neuropsychology. 1993;7:82–88. [Google Scholar]
- 20.Robinson G, Blair J, Cipolotti L. Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Perret E. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 22.Guitton D, Buchtel H A, Douglas R M. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic P S. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 24.Miller, E. K. (in press) in Attention, Space and Action: Studies in Cognitive Neuroscience, eds. Humphreys, G. W., Duncan, J. & Treisman, A. M. (Oxford Univ. Press, Oxford).
- 25.Cohen J D, Servan-Schreiber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Kimberg D Y, Farah M J. J Exp Psychol Gen. 1993;112:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]