Abstract
Adult outbred Sprague-Dawley rats can be classified as either low or high cocaine responders (LCRs or HCRs, respectively). Importantly, LCRs and HCRs are distinguished by their differential responsiveness to acute cocaine-induced (but not baseline) locomotor activity, inhibition of the dopamine transporter (DAT) and resulting extracellular DA (HCR>LCR), as well as by repeated cocaine-induced locomotor sensitization and measures of cocaine’s rewarding and reinforcing effects (LCR>HCR). Curiously, 30 min after acute cocaine HCRs exhibit greater DAT-mediated [3H]DA uptake into striatal synaptosomes than LCRs. To investigate this finding further, we measured locomotor activity, striatal [3H]DA uptake kinetics and DAT cell surface expression in LCRs and HCRs over an extended period (25 – 180 min) after a single relatively low-dose of cocaine (10 mg/kg, i.p.). HCRs exhibited the “predicted” locomotor response: a marked initial activation that returned to baseline by 120 min post-injection. While LCRs exhibited a >50% lower maximal locomotor response, this increase was sustained, lasting ~33% longer than in HCRs. At 25 min post-cocaine, maximal velocity (Vmax) of [3H]DA uptake was significantly higher by 25% in HCRs than LCRs, with no difference in affinity (Km). Despite the DAT Vmax difference, however, DAT surface expression did not differ between LCRs and HCRs. There was a similar trend (HCR>LCR) for DAT Vmax at 40 min, but not at 150 or 180 min. These findings suggest that, compared to LCRs, HCRs have an enhanced ability to rapidly up- regulate DAT function in response to acute cocaine, which may contribute to their more “normal” cocaine-induced locomotor activation.
Keywords: Individual differences, cocaine, rats, dopamine transporter function, rapid-regulation
1. Introduction
Cocaine addiction remains a significant public health problem. Importantly, however, it is estimated that only 10–15% of initial cocaine users will become addicted (Gawin, 1991). Individual variability in cocaine’s CNS effects likely contributes to these differences. Indeed, studies of individual differences in humans have found that greater positive subjective responses following initial cocaine use predicts greater lifetime use and dependence (Davidson et al., 1993; Lambert et al., 2006). Thus, identifying factors that contribute to individual differences in susceptibility to cocaine addiction could help in development of effective cocaine addiction treatments and prevention strategies.
Previously, we have shown that adult outbred Sprague-Dawley rats, classified as either low or high cocaine responders (LCRs or HCRs, respectively), are a useful animal model for studying individual differences to cocaine (Allen et al., 2007; Briegleb et al., 2004; Gulley et al., 2003; Mandt et al., 2009; Mandt et al., 2008; Nelson et al., 2009; Sabeti et al., 2002, 2003). Classification is based on the magnitude of locomotor activity induced during the first 30-min after an acute, relatively low-dose of cocaine (10 mg/kg, i.p.); LCRs fall below the group median and HCRs above. Importantly, LCRs and HCRs do not differ in brain cocaine levels (Gulley et al., 2003). Although HCRs exhibit greater initial cocaine-induced activation, with repeated cocaine administration LCRs exhibit greater cocaine-induced locomotor sensitization, cocaine conditioned place preference (CPP) and motivation to self-administer cocaine (Allen et al., 2007; Mandt et al., 2009; Mandt et al., 2008; Nelson et al., 2009; Sabeti et al., 2002, 2003). Thus, LCRs appear to represent a phenotype for increased susceptibility to the rewarding and reinforcing properties of cocaine.
Cocaine inhibits dopamine transporter (DAT)-mediated uptake of DA, and striatal DATs are critically involved in mediating the rewarding and reinforcing effects of cocaine (Chen et al., 2006; Ritz et al., 1987; Thomsen et al., 2009). DATs can also be rapidly regulated following exposure to either substrates or inhibitors (see Gulley and Zahniser, 2003; Williams and Galli, 2006). Cocaine increases DAT function and surface expression after both in vitro and in vivo exposure (Daws et al., 2002; Little et al., 2002).
Acute cocaine inhibits in vivo DAT-mediated striatal DA clearance to a greater extent and results in higher extracellular DA levels in HCRs than LCRs (Nelson et al., 2009; Sabeti et al., 2002). Since cocaine’s potency to inhibit DAT-mediated [3H]DA uptake is inversely related to DAT expression level (Chen and Reith, 2007), HCRs would be expected to have fewer DATs than LCRs; and this was recently found to be the case (Nelson et al., 2009). Surprisingly, however, uptake measured ex vivo with a single sub-saturating [3H]DA concentration in washed striatal synaptosomes 30 min post-cocaine is greater in HCRs than LCRs (Briegleb et al., 2004). One possibility is that the greater uptake in HCRs reflects a compensatory increase in DAT function and/or surface expression. Additionally, cocaine-induced locomotor activity and DA clearance have been monitored in LCRs and HCRs for only 60 min post-cocaine, making a longer post-cocaine time course of interest (Gulley et al., 2003; Sabeti et al., 2002). Therefore, here we measured locomotor activity in LCRs and HCRs, along with [3H]DA uptake kinetics and DAT cell surface expression in striatal synaptosomes, up to 180 min after acute cocaine administration (10 mg/kg, i.p.).
2. Materials and methods
2.1. Drugs
Dopamine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA), (−)-cocaine hydrochloride was generously provided by the National Institute on Drug Abuse (Research Triangle Institute International, Research Triangle Park, NC, USA) and saline (0.9% sodium chloride) was purchased from Baxter Healthcare Corporation (Deerfield, IL, USA). Cocaine was dissolved in saline at a concentration of 10 mg/ml and injected i.p. in a volume of 1 ml/kg. This single dose of cocaine was chosen because it has previously been used to classify and study LCRs and HCRs (Allen et al., 2007; Briegleb et al., 2004; Gulley et al., 2003; Mandt et al., 2009; Mandt et al., 2008; Nelson et al., 2009; Sabeti et al., 2002, 2003). Drug weight refers to the salt. Saline was injected i.p. in a volume of 1 ml/kg as the vehicle (veh) control. [3H]DA (specific activity 39–60 Ci/mmol) was purchased from Perkin-Elmer Life Sciences (Boston, MA, USA). EZ-Link sulfo-NHS-biotin and immobilized monomeric avidin beads were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). Antibodies used included: mouse monoclonal anti-protein phosphatase 2A catalytic α subunit (PP2Aα) primary antibody from BD Transduction Laboratories (San Diego, CA, USA); mouse monoclonal anti-DAT primary antibody, a generous gift from Dr. Roxanne Vaughan (University of North Dakota, Grand Forks, ND, USA; Gaffaney and Vaughan, 2004); and goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody from Bio- Rad Laboratories (Hercules, CA, USA). All other chemicals were purchased from either Sigma-Aldrich or Thermo Fisher Scientific.
2.2. Animals
Separate groups of outbred male Sprague-Dawley rats (200–300 g) were purchased from Charles Rivers Laboratories (Wilmington, MA) for use in the behavioral/uptake experiments at four post-cocaine time-points (25-min group n = 24; 40-min group n = 27; 150-min group n = 27; 180-min group n = 34) and the behavioral/biotinylation experiments at two post-cocaine time-points (30-min group n = 36; 180-min group n = 24). In addition, a separate group of rats (n = 5) was used to assess vehicle-induced locomotor activity over the 180 min post-saline injection. Two cocaine-treated rats and one vehicle-treated control rat were used in each daily experiment, with the exception of one group of rats in the 180-min post-cocaine condition (n = 10 of the 34 listed above), which did not include a vehicle-treated group. Rats were housed four per cage with ad libitum access to food and water in the animal care facility at the University of Colorado Denver (UCD) – Anschutz Medical Campus for one week before being used in an experiment. This facility is on a 12-h light/dark cycle (lights on at 0600 h), and all testing was conducted during the light cycle. All animal care and use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCD Institutional Animal Care and Use Committee.
2.3. Locomotor activity testing
Locomotor activity testing was conducted as previously described (Gulley et al., 2003). Rats were taken to the behavioral testing room in their home cage and allowed to habituate for 45 – 60 min. At the start of the behavioral recording session, rats were placed in open-field activity chambers consisting of acrylic boxes (16″ × 16″ × 15″) fitted with a photo beam frame (eight beams per dimension 0.5″ from the floor; San Diego Instruments, San Diego, CA, USA). After acclimation to the novel environment for 90 min, rats were injected with either vehicle or cocaine and returned to the chamber for various times depending on the experiment. For the uptake experiments, cocaine-induced locomotor activity was recorded for 20, 30, 140 or 170 min post-injection. For the biotinylation experiments, cocaine-induced locomotor activity was recorded for either 25 or 170 min post-injection. The time difference between locomotor activity recordings and biochemical assays (e.g. locomotor activity over 170 min and uptake assay at 180 min) is a result of the time required for tissue preparation. Vehicle-treated control and cocaine-treated rats were placed in identical chambers; but with the exception of one vehicle group, locomotor activity was not recorded for the controls. Locomotor activity was quantified using the automated consecutive horizontal photo beam interruptions converted to distance traveled (cm) per 10-min bins. The sum of distance traveled over 30 min post-cocaine was used to determine the median split for all rats within each group and to categorize the rats as either LCRs or HCRs, with two exceptions: the sum of distance traveled over 20 min and 25 min post-cocaine was used for LCR/HCR classification of rats in the 25-min uptake and 30-min biotinylation groups, respectively.
2.4. [3H]DA uptake assays
The bilateral striata (primarily dorsal striata) of LCR, HCR and control rats were rapidly dissected on ice and homogenized with a glass homogenization tube and Teflon pestle (Wheaton Science Products, model # 358029; Millville, NJ, USA) in ice cold phosphate buffer (3.3 mM NaH2PO4 + 12.7 mM Na2H PO4, pH 7.4) containing 0.32 M sucrose. The tissue homogenate was centrifuged at 1000 g for 12 min at 4 °C, and all subsequent centrifugations also occurred at 4 °C. The supernatant was centrifuged at 12,500 g for 15 min to isolate the synaptosomal P2 pellet, which was resuspended at 50 mg wet weight/ml in assay buffer (134 mM NaCl, 240 mM KCl, 65mM CaCl2, 70 mM MgSO4, 3.3 mM NaH2PO4, 12.7 mM Na2H PO4, 11 mM glucose, and 1 mM ascorbic acid, pH 7.4). Next, synaptosomal tissue (40 μl) was preincubated with assay buffer containing 1 μM pargyline for 10 min at 37 °C with gentle shaking. The preincubation step was followed by the addition of [3H]DA (5 nM); the uptake assays were performed for 3 min at 37 °C. The single [3H]DA concentration specific uptake assay was used only to assess viability of the synaptosomes used in the cell-surface biotinylation assays (see below). Analysis of DAT kinetic parameters was conducted using saturation uptake assays with seven concentrations of unlabeled DA (0, 5 nM, 10 nM, 50 nM, 0.1 μM, 0.5 μM, and 1 μM) added concomitantly with the [3H]DA (0.5 nM). In all [3H]DA uptake assays, non-specific uptake was determined with 100 μM cocaine. All samples were assayed in duplicate, and all uptake assays were halted by placing samples on ice, followed by three washes each with 3 ml of ice-cold 0.32 M sucrose and filtration through Whatman GF/C filters with a cell harvester (Brandel, model # M-24). Radioactivity in the filters was determined using liquid scintillation spectrometry. Protein levels were determined using the Bradford method (Bradford, 1976) with bovine serum albumin (BSA) as the standard.
2.5. Cell surface biotinylation
Cell-surface biotinylation assays were conducted as previously described (Hoover et al., 2007; Richards and Zahniser, 2009; Zhu et al., 2005b). Following behavioral recording, synaptosomes were prepared as described above, with the exception that tissue was resuspended at 100 mg wet weight/ml in Krebs assay buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 0.045 mM Na-EDTA, 1.7 mM CaCl2, 25mM NaHCO3, 10 mM dextrose, 0.1 mM pargyline, 0.1 mM ascorbic acid, pH 7.4; saturated with 95% O2/5% CO2). An aliquot of tissue was removed for [3H]DA uptake measurement (see above). The remaining tissue was centrifuged at 8000 g for 4 min at 4 °C, and the resulting pellet was resuspended at 100 mg wet weight/ml in phosphate buffered saline (PBS)/Ca2+/Mg+ buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1mM MgCl2, 0.1 mM CaCl2, pH 7.3) containing 2 mg/ml sulfo-NHS-biotin and incubated for 1 hr at 4 °C with gentle agitation. All subsequent centrifugations also occurred at 4 °C. The biotinylation reaction was terminated by addition of 1 ml 1.0 M glycine in PBS/Ca2+/Mg+ buffer for 10 min on ice. Tissue was then centrifuged at 8000 g for 4 min, and the pellet was resuspended in 1 ml of 0.1 M glycine in PBS/Ca2+/Mg+ buffer. This step was repeated twice, followed by incubation of tissue in 1 ml of 0.1 M glycine in PBS/Ca2+/Mg+ buffer for 30 min at 4 °C with gentle agitation. Following incubation, tissue was centrifuged at 8000 g for 4 min followed by three washes in 1 ml of PBS/Ca2+/Mg+ buffer. The final pellet was resuspended in 300 μl of 1% Triton X-100 buffer containing protease inhibitors (10 mM Tris, 150 mM NaCl, 1mM EDTA, 1% Triton X-100, 0.1% SDS, 1% deoxycholic acid, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 0.1 mmol/L aminoethylbenzenesulfonyl (AEBSF), pH 7.4) and subjected to 2 – 4 sec of sonication, before a 30-min incubation at 4 °C with gentle agitation. Tissue was then centrifuged at 20,000 g for 30 min, and 75 μl of the supernatant was removed for analysis of the “total sample”. The remaining supernatant was incubated with 100 μl of freshly prepared monomeric avidin beads for 15 – 16 hr at 4 °C with gentle agitation. Samples were then centrifuged at 17,700 g for 4 min; and 75 μl of supernatant were removed, representing the “non-biotinylated intracellular fraction”. The remaining supernatant was discarded, and the biotinylated protein/avidin bead complex was washed three times with 1 ml of Triton X-100 buffer. Beads were then incubated with 100 μl of Laemmli buffer containing protease inhibitors (62.5 mM Tris, 20% glycerol, 2% SDS, 5% β-mercaptoethanol, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 0.1 mmol/L AEBSF, pH 6.8) for 1 hr at room temperature with gentle agitation. Samples were centrifuged a final time at 17,700 g for 4 min; and the supernatant was removed, representing the “biotinylated, cell-surface fraction”.
2.6. Western blot analysis
Biotinylated samples were subjected to SDS-PAGE on a 7.5% gel before transfer to PVDF membranes. Equal volumes (10 μl) of total and biotinylated sample were loaded on each gel; however, it should be noted this resulted in loading a greater percentage of the biotinylated sample (10%), relative to the total sample (2.5%). Non-biotinylated samples were collected for measurement of PP2Aα, as a control for intracellular contamination of the synaptosomes, but were not analyzed between LCRs and HCRs. Samples were run in duplicate, and each gel contained samples from three rats (1 LCR, 1 HCR and 1 control). After transfer, blots were blocked in Tris buffered-saline/Tween-20 (TBST; 140 mM NaCl, 20 mM Tris, pH 7.6, 0.01% Tween-20) containing 5% non-fat dried milk for 1 hr at room temperature. Blots probed for DAT were incubated with DAT primary antibody (1:10,000 dilution) in TBST containing 3% BSA. As a control for intracellular contamination, blots were probed for PP2Aα (1:5000 dilution) in TBST. All incubations with primary antibody occurred at 4 °C for 15 – 16 hr. Following incubation with primary antibody, blots were incubated for 2 hr at room temperature with HRP-conjugated secondary antibody. DAT and PP2Aα immunoreactive bands were visualized with enhanced chemiluminescence on x-ray film and analyzed with the program ImageJ, version 1.40g (Wayne Rasband, National Institute of Health, USA, http://rsb.info.nih.gov/ij/). As mentioned previously, a greater percentage of the biotinylated sample was loaded relative to the total sample. Consequently, total sample immunoreactive values were normalized to biotinylated sample values by multiplying by a correction factor of 4. DAT surface levels were calculated as a percentage of the ratio of the biotinylated DAT signal to total DAT signal for each LCR, HCR and control rat.
2.7. Data analysis
Data are presented as mean values ± SEM, with n = the number of animals. Statistical analysis was conducted using SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism, version 5.0 (GraphPad Software, La Jolla, CA, USA). All two-way ANOVA analyses were conducted using SPSS. The sum of locomotor activity over the first 20, 25 or 30 min post-cocaine was used for classification of LCRs/HCRs, as described above. In addition, locomotor activity of the LCRs and HCRs was summed into 30-min bins for analysis of the baseline and 170-min post-cocaine time course (0, 30, 60, 90, 120, 150 and 170 min post-cocaine, with the exception of the 170-min bin that consists of only 20 min of locomotor activity) with two-way RMANOVA. When appropriate, locomotor activity post hoc comparisons were conducted with one-way RMANOVA and Dunnett’s multiple comparisons test comparing cocaine-induced locomotor activity to baseline locomotor activity values. Baseline values did not differ between LCRs and HCRs; thus, to normalize and compare % cocaine-induced increases in locomotor activity over baseline between LCRs and HCRs, baseline was defined as the average of the LCRs’ and HCRs’ activity during the 30-min pre-injection period. When the assumption of sphericity was violated for a particular repeated-measures analysis, as revealed by Mauchly’s test statistic, tests of significance were based on the more conservative Huynh-Feldt corrected degrees of freedom, as determined with SPSS. The symbol, a, indicates Huynh-Feldt corrected values throughout the text. One group of LCRs (n = 5) and HCRs (n = 5) in the 170-min group was included in the locomotor activity analysis, but not in the [3H]DA uptake analysis because they were not tested in conjunction with vehicle-treated control rats.
The maximal velocity (Vmax) and affinity (Km) of DAT-mediated specific [3H]DA uptake were determined for the data from each individual rat using non-linear curve fitting analysis based on the equation for a rectangular hyperbola (GraphPad Prism). In addition to these absolute values, [3H]DA uptake values for LCRs and HCRs were also calculated as a percent of their respective control rat’s value. [3H]DA uptake (% control) for LCRs and HCRs over the entire 180-min time course was compared with two-way ANOVA. When warranted, post hoc comparisons were conducted using Student’s independent samples t-test. Correlation analysis was conducted using GraphPad Prism. Statistical outliers were determined according to Chauvenet’s criterion. Absolute Vmax values were used to determine statistical outliers in the uptake experiments and resulted in exclusion of 1 rat (HCR) from the 25-min group. Similarly, surface DAT values determined to be statistical outliers from the group were not included in analysis, resulting in exclusion of 2 rats (1 LCR and 1 HCR) in the 30-min group. An additional two rats were not included in the 30-min biotinylation analysis because following behavioral recording their biotinylation samples were compromised, preventing analysis.
3. Results
3.1. Time-course of cocaine-induced locomotor activity
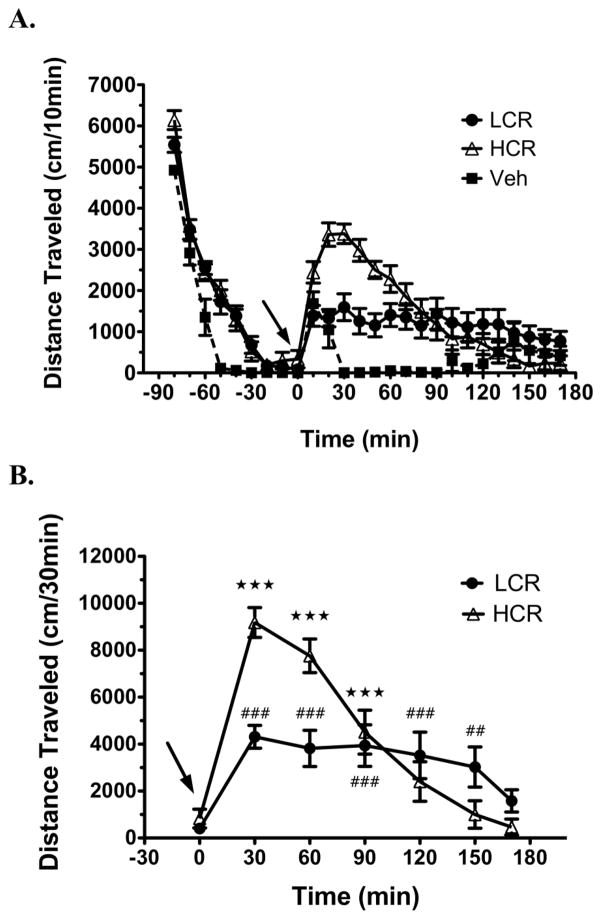
Analyzing cocaine-induced locomotor activity for 170 min after an acute 10 mg/kg i.p. injection revealed new differences between LCRs and HCRs. The detailed time-course (10-min bins) of pre- and post-injection locomotor activity in LCRs, HCRs and vehicle-treated rats completing the entire 170-min post-injection period is presented in Fig. 1A. In all of the rats, following the initial exploratory phase, locomotor activity rapidly declined and stabilized at a minimal level for the last 30 min prior to injection. After injection, their activity again increased. This increase lasted less than 20 min in the vehicle-injected rats but was more pronounced and sustained in the cocaine-injected rats. Cocaine-injected rats were subsequently divided into LCRs and HCRs, based on the group median split (medians for the two groups of rats used = 7597 cm and 5126 cm; see Methods) of their cocaine-induced locomotor activity during the first 30 min post-injection. For statistical analysis, locomotor activity of the LCRs and HCRs was summed into 30-min bins for the final 30-min pre-injection period (baseline) and the 170-min post-injection period (Fig. 1B). Analysis with two-way RMANOVA revealed a significant time × classification interaction [aF(7.3, 103.2) = 9.1, p < 0.001]. Subsequent analysis with one-way RMANOVA comparing cocaine-induced locomotor activity to baseline locomotor activity revealed significant effects for LCRs [F(6, 72) = 8.8, p < 0.0001] and HCRs [F(6, 72) = 37.2, p < 0.0001].
Fig. 1.
Distinct locomotor activity profiles in LCRs and HCRs during the 170 min after an acute injection of cocaine (10 mg/kg, i.p.; arrow). A Locomotor activity of LCRs (n = 13), HCRs (n = 13) and vehicle (veh)-treated control rats (n = 5) summed into 10-min bins over the 90-min pre-injection and 170-min post-injection period. B LCR/HCR locomotor data from (A) summed into 30-min bins over the 30-min pre-injection (baseline; time 0) and 170-min post-cocaine period for statistical analysis. Values are presented as mean ± SEM. ★★★ p < 0.001, HCR; ##p < 0.01, ###p < 0.001, LCR; all values compared to baseline.
LCRs and HCRs were not significantly different in baseline locomotor activity levels (Student’s t-test, p = 0.33); and as such, the average of their activity during this time was defined as baseline (618 cm) for determining the % increase in cocaine-induced locomotor activity. For HCRs, cocaine-induced locomotor activity was significantly increased over baseline maximally by ~1400 %, but only remained elevated during the first 90 min post-injection (Fig. 1B.). While LCRs exhibited >50% lower maximal level of cocaine-induced locomotor activity (~600 % over baseline) relative to HCRs, this cocaine-induced increase was more stable and persisted for 150 min post-injection, before returning to non-significant levels by 170 min post-injection (Fig. 1B).
3.2. Striatal [3H]DA uptake kinetic parameters in LCRs and HCRs
In order to assess DAT function in LCRs and HCRs over the same extended time period post-cocaine (10 mg/kg, i.p.), saturation kinetics for specific [3H]DA uptake were measured ex vivo at 25, 40, 150 and 180 min in washed striatal synaptosomes following behavioral recording at each of the aforementioned times. Analysis of Vmax values for LCRs and HCRs (% Veh control; see below) at the different time-points using two-way ANOVA revealed a significant time × classification interaction [F(3, 59) = 4.7, p = 0.008]. Analysis of Km values with two-way ANOVA revealed a significant effect of time [F(3, 90) = 33.9, p < 0.001]. However importantly, no differences in Km were revealed between LCRs and HCRs at any time point. These results and subsequent post hoc comparisons between LCRs and HCRs conducted using Student’s independent samples t-test are presented below with behavioral results from each time-point.
3.3. Locomotor activity and striatal [3H]DA uptake at 25 min post-cocaine
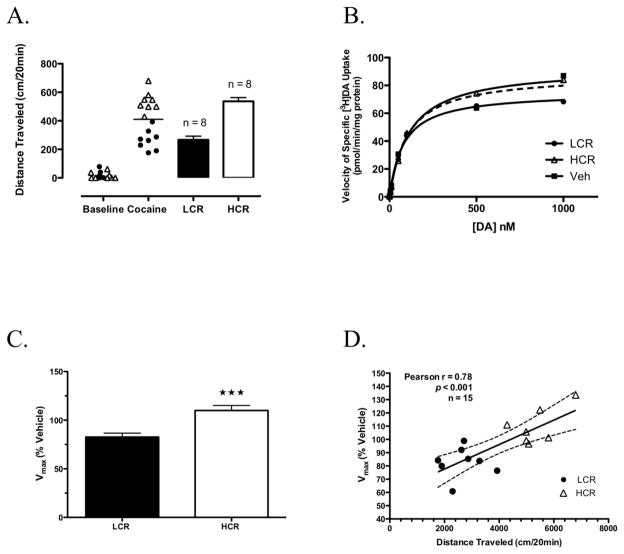
The distributions of baseline (30 min pre-injection) and cocaine-induced locomotor activity (20 min post-injection; the shortest time at which LCRs and HCRs could be classified reliably) for the group of cocaine-treated rats in which striatal DAT kinetics were measured ex vivo at 25 min post-cocaine are shown in Fig. 2A. Rats were classified as either LCRs or HCRs based on the group median split of cocaine-induced locomotor activity, with HCRs traveling ~twice the distance of LCRs (Fig. 2A). To control for day-to-day variability (as much as 20%) in absolute values of striatal [3H]DA uptake, a vehicle-treated control rat was assayed each day, and its Vmax value was used to normalize the Vmax values from the two cocaine-treated rats from that day as % control. The relationship for specific uptake (absolute values) over the range of [3H]DA concentrations used are shown from a representative experiment for an LCR, HCR and vehicle-treated rat in Fig. 2B. Kinetic parameters for saturation of specific [3H]DA uptake determined for each group from these analyses were as follows: LCRs Vmax = 62 ± 3 pmol/min/mg, Km = 171 ± 8 nM, n = 8; HCRs Vmax = 74 ± 3 pmol/min/mg, Km = 161 ± 11 nM, n = 7; and vehicle-treated rats Vmax = 73 ± 4 pmol/min/mg, Km = 180 ± 17 nM, n = 8.
Fig. 2.
Classification of LCRs and HCRs and kinetic analysis of DAT-mediated uptake in striatal synaptosomes prepared 25 min post-cocaine from these rats. A Distribution of baseline and the initial 20 min of cocaine-induced locomotor activity in rats subsequently classified as either LCRs or HCRs (bars). The solid horizontal line represents this group’s median cocaine-induced locomotor activity. B Representative results for specific [3H]DA saturation uptake for an LCR, HCR and veh-treated rat, assayed on the same day, in the presence of 0.5 nM [3H]DA and increasing concentrations of unlabeled DA (0, 5 nM, 10 nM, 50 nM, 0.1 μM, 0.5 μM, and 1 μM). C [3H]DA uptake Vmax values for all LCRs (n = 8) and HCRs (n = 8) presented as a percentage of their respective veh-treated control. D Correlation of distance traveled 20 min post-cocaine and Vmax values at 25 min post-cocaine for all cocaine-treated rats. Bars in (A) and (C) are means ± SEM. ★★★ p < 0.001, LCR vs. HCR.
Vmax values for LCRs and HCRs are presented as a percentage of their respective vehicle-treated control in Fig. 2C. Similar to previous findings for specific uptake of a single sub-saturating concentration of [3H]DA at 30 min post-cocaine (Briegleb et al., 2004), these results showed that 25 min after the acute injection of cocaine, HCRs exhibited significantly greater velocity of [3H]DA uptake into washed striatal synaptosomes than LCRs. Specifically, Vmax in HCRs (109 ± 5%; n = 7) was 25% greater than Vmax in LCRs (82 ± 3%; n = 8; Student’s t-test, p = 0.001; Fig. 2C), with no differences in Km. In addition, in all of the cocaine-treated rats and regardless of LCR/HCR classification, at this time point Pearson correlation analysis revealed a significant positive correlation between distance traveled in the 25 min after cocaine injection and DAT Vmax (Pearson’s r = 0.78, p < 0.001; Fig. 2D).
3.4. Locomotor activity and striatal DAT cell surface expression at 30 min post-cocaine
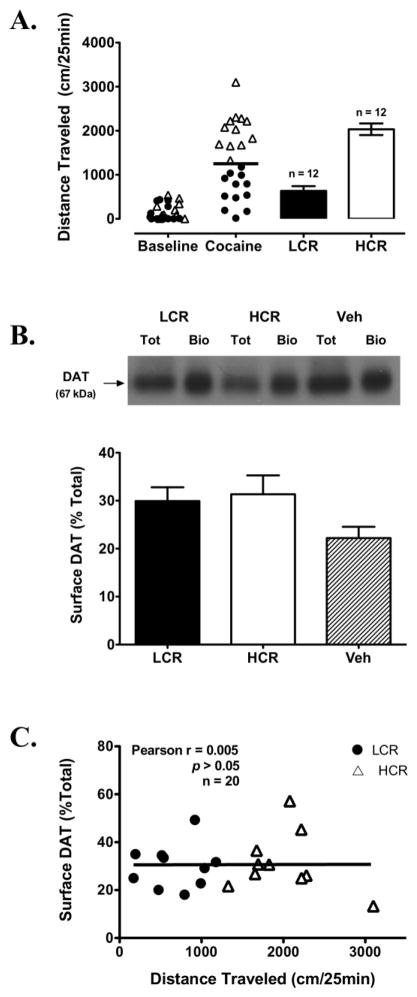
The difference observed in [3H]DA uptake Vmax, but not Km, between LCRs and HCRs at 25 min post-cocaine is consistent with altered DAT cell surface expression. Therefore, in order to assess this possibility directly, striatal DAT cell-surface biotinylation assays were conducted at 30 min post-cocaine. The distributions of baseline and cocaine-induced locomotor activity (25 min post-injection) for this group of rats and mean cocaine-induced locomotor activity values for rats classified as LCRs and HCRs are shown in Fig. 3A. Despite the LCR/HCR Vmax differences at 25 min post-cocaine, however, no differences were observed in DAT surface expression between LCRs (29 ± 2%; n = 10), HCRs (31 ± 3%; n = 10) and vehicle-treated control rats (22 ± 2%; n = 11) at 30 min post-cocaine (Fig. 3B). No appreciable levels of PP2Aα were detected in the biotinylated fractions of any group, suggesting that the synaptosomes were not “leaky” and these fractions were free from intracellular contamination with biotin (data not shown). In contrast, PP2Aα was detected strongly in the total and non-biotinylated fractions (data not shown). Further, DAT surface expression and distance traveled 30 min post-cocaine in all of the cocaine-treated rats were not correlated (Pearson’s r = 0.005, p > 0.05; Fig. 3C).
Fig. 3.
Classification of LCRs and HCRs and cell surface DATs in striatal synaptosomes prepared 30 min post-cocaine in these rats. A Distribution of baseline and the initial 25 min of cocaine-induced locomotor activity in rats subsequently classified as either LCRs or HCRs. The solid horizontal line represents this group’s median cocaine-induced locomotor activity. B Representative Western blot for DAT in the total (tot) and biotinylated (bio) fractions from an LCR, HCR and veh-treated rat. Bottom panel: Surface DAT values for all LCRs (n = 10), HCRs (n = 10) and veh-treated rats (n = 11), expressed as a percentage of their respective tot value. C Correlation of distance traveled 25 min post-cocaine and surface DAT values 30 min post-cocaine for all cocaine-treated rats. Bars in (A) and (B) are means ± SEM.
3.5. Locomotor activity and striatal [3H]DA uptake at 40 min post-cocaine
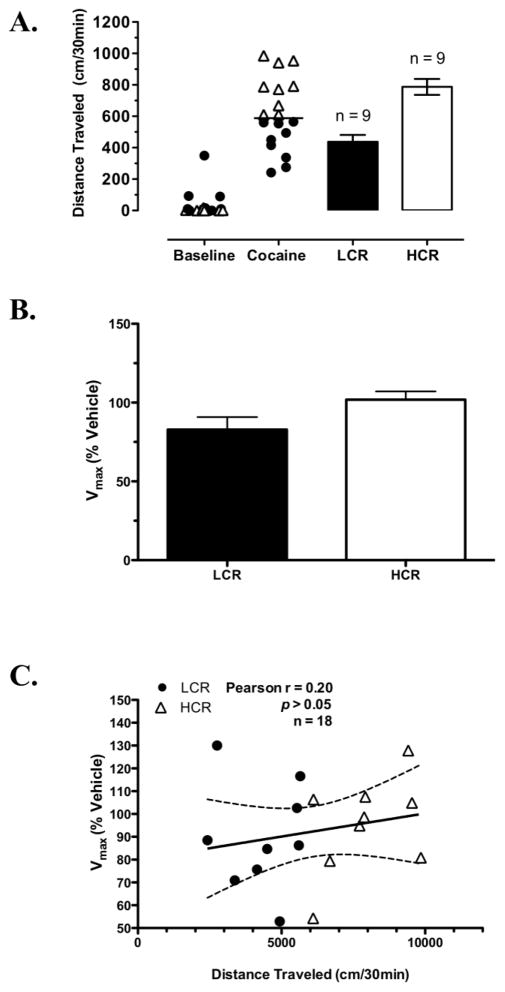
The distributions of baseline and cocaine-induced locomotor activity (30 min post-injection) for this group of rats in which striatal DAT kinetics were measured ex vivo at 40 min post-cocaine and mean cocaine-induced locomotor activity values for LCRs and HCRs are shown in Fig. 4A. Kinetic parameters for specific [3H]DA uptake determined for each group from these analyses were as follows: LCRs Vmax = 63 ± 6 pmol/min/mg, Km = 91 ± 6 nM, n = 9; HCRs Vmax = 57 ± 6 pmol/min/mg, Km = 82 ± 6 nM, n = 9; and vehicle-treated rats Vmax = 68 ± 9 pmol/min/mg, Km = 76 ± 9 nM, n = 9. Expressed as % respective control, kinetic parameters for [3H]DA uptake at 40 min post-cocaine no longer differed significantly between LCRs and HCRs; however, there was a statistical trend for greater Vmax values in HCRs (101 ± 5 %, n = 9) compared to LCRs (82 ± 7 %, n = 9; Student’s t-test, p = 0.06; Fig. 4B). Similar to 25 min post-cocaine, Km values did not differ between LCRs and HCRs. However, unlike 25 min after cocaine, in this case a significant correlation between cocaine-induced distance traveled in all of the rats (30 min post-cocaine) and DAT Vmax values (40 min post-cocaine) was not detected (Pearson’s r = 0.20, p = 0.40; Fig. 4C).
Fig. 4.
Classification of LCRs and HCRs and kinetic analysis of DAT-mediated uptake in striatal synaptosomes prepared 40 min post-cocaine from these rats. A Distribution of baseline and the initial 30 min of cocaine-induced locomotor activity in rats subsequently classified as either LCRs or HCRs (bars). The solid horizontal line represents this group’s median cocaine-induced locomotor activity. B [3H]DA uptake Vmax values for all LCRs (n = 9) and HCRs (n = 9) presented as a percentage of their respective veh-treated control; p = 0.06 for LCR vs. HCR. C Correlation of distance traveled 30 min post-cocaine and Vmax values at 40 min post-cocaine for all cocaine-treated rats. Bars in (A) and (B) are means ± SEM.
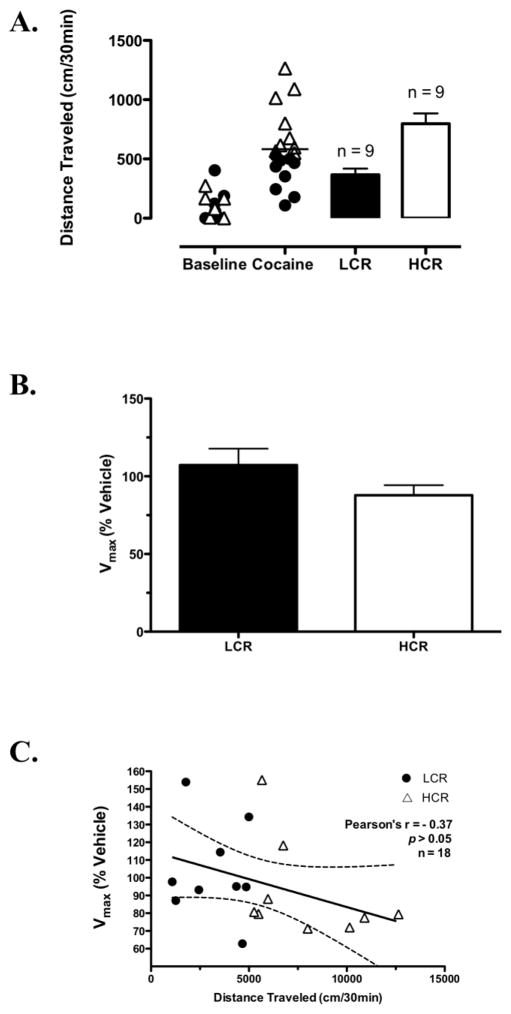
3.6. Locomotor activity and striatal [3H]DA uptake at 150 min post-cocaine
By 150 min after cocaine injection, the locomotor activity relationship between LCRs and HCRs had switched, with only LCRs still showing cocaine-induced activation (Fig. 1B). Thus, we were also interested in determining DAT kinetics in LCRs and HCRs at later post-cocaine time points. The distributions of baseline and cocaine-induced locomotor activity (30 min post-injection) for the group of rats in which striatal DAT kinetics were measured ex vivo at 150 min post-cocaine and mean cocaine-induced locomotor activity values for LCRs and HCRs in this group are shown in Fig. 5A. Kinetic parameters for specific [3H]DA uptake determined for each group from these analyses were as follows: LCRs Vmax = 50 ± 5 pmol/min/mg, Km = 186 ± 22 nM, n = 9; HCRs Vmax = 45 ± 6 pmol/min/mg, Km = 132 ± 22 nM, n = 9; and vehicle-treated rats Vmax = 52 ± 7 pmol/min/mg, Km = 154 ± 23 nM, n = 9. Expressed as % respective control, Vmax values were not significantly different between LCRs (107 ± 10%, n = 9) and HCRs (87 ± 6%, n = 9; Fig. 5B) at 150 min post-cocaine. Similar to the earlier time points, there were also no significant LCR/HCR differences in Km. Further, no correlation was detected between distance traveled 30 min post-cocaine and DAT Vmax values at this time point in all of the cocaine-treated rats (Pearson’s r = −0.37, p > 0.05; Fig. 5C).
Fig. 5.
Classification of LCRs and HCRs and kinetic analysis of DAT-mediated uptake in striatal synaptosomes prepared 150 min post-cocaine from these rats. A Distribution of baseline and the initial 30 min of cocaine-induced locomotor activity in rats subsequently classified as either LCRs or HCRs (bars). The solid horizontal line represents this group’s median cocaine-induced locomotor activity. B [3H]DA uptake Vmax values for all LCRs (n = 9) and HCRs (n = 9) presented as a percentage of their respective veh-treated control. C Correlation of distance traveled 30 min post-cocaine and Vmax values at 150 min post-cocaine for all cocaine-treated rats. Bars in (A) and (B) are means ± SEM.
3.7. Locomotor activity and striatal [3H]DA uptake at 180 min post-cocaine
Striatal DAT [3H]DA uptake kinetics were also determined for LCRs and HCRs at a time point (180 min post-injection) when neither LCRs nor HCRs exhibited a cocaine-induced increase in locomotor activity. Kinetic parameters for specific [3H]DA uptake determined for each group from these analyses were as follows: LCRs Vmax = 62 ± 3 pmol/min/mg, Km = 43 ± 3 nM, n = 8; HCRs Vmax = 77 ± 6 pmol/min/mg, Km = 55 ± 4 nM, n = 8; and vehicle-treated rats Vmax = 64 ± 7 pmol/min/mg, Km = 44 ± 6 nM, n = 8. Expressed as % respective control at 180 min post-cocaine, neither Vmax values (LCRs 104 ± 4.6%, n = 8; HCRs 112 ± 7%, n = 8) nor Km values differed between LCRs and HCRs. Further, no correlation was detected between distance traveled 30-min post-cocaine and DAT Vmax values at this time point (Pearson’s r = −0.08, p > 0.05).
3.8. Locomotor activity and striatal DAT cell surface expression at 180 min post-cocaine
To assess striatal DAT cell surface expression in LCRs and HCRs at a later time after cocaine administration, cell-surface biotinylation assays were also conducted 180 min post-cocaine. Similar to 30 min post-cocaine, at this later time ~30% of the DATs were detected on the surface of the striatal synaptosomes and no significant differences were observed in DAT surface expression between LCRs (29 ± 2%, n = 6), HCRs (31 ± 2%, n = 6) and vehicle-treated rats (31 ± 2%, n = 6). Again, no appreciable levels of PP2Aα were detected in the biotinylated fractions, indicating these samples were free of intracellular biotin contamination; and high levels of PP2Aα were detected in the total and non-biotinylated fractions (data not shown). Further, no correlation was observed between distance traveled 30 min post-cocaine and DAT surface expression at 180 min post-cocaine (Pearson’s r = 0.27, p > 0.05).
4. Discussion
Studying a more extended time course of cocaine-induced locomotor activity and conducting a detailed kinetic analysis of striatal DATs revealed additional important LCR/HCR differences. While the large initial increase in cocaine-induced locomotor activity in HCRs dissipated by 120 min post-injection, the >50% lower level of maximal cocaine-induced activity in LCRs persisted for ~33% longer. The identification of LCR/HCR differences in cocaine’s effects on DATs was further expanded to include [3H]DA uptake Vmax values. While at 25 min post-cocaine HCRs exhibited significantly greater DAT Vmax than LCRs, this difference was a statistical trend by 40 min post-cocaine and completely absent by 150 and 180 min post-injection. Although increases in Vmax without changes in Km are consistent with altered DAT surface expression, no LCR/HCR differences were detected with cell surface biotinylation assays conducted at 30 min post-cocaine.
Previous LCR/HCR studies recorded cocaine-induced locomotor activity for as long as 60 min post-cocaine (Gulley et al., 2003; Sabeti et al., 2002, 2003). In the first 30 min marked differences were observed in cocaine-induced locomotor activity, with HCRs exhibiting ≥two-fold greater activity than LCRs. These behavioral profiles began to converge around 60 min. However, when we recorded locomotor activity up to 170 min after the same dose of cocaine (10 mg/kg, i.p.), we observed additional LCR/HCR differences. The large initial increase in cocaine-induced activation in HCRs returned to baseline by 120 min post-injection. This behavioral profile is typically reported following 10 mg/kg i.p. cocaine administration and is consistent with cocaine pharmacokinetics (Broderick et al., 1993; Kalivas and Duffy, 1993; Lau et al., 1991). Interestingly, in contrast to HCRs, the lower level of cocaine-induced activation in LCRs was more long-lasting and did not return to baseline until 170 min post-injection, resulting in a reversal of the more active phenotype after 90 min (i.e. LCRs>HCRs).
In a previous study using a single non-saturating concentration of [3H]DA to measure uptake 30 min after acute cocaine, uptake into striatal synaptosomes was increased in HCRs, compared to LCRs; and distance traveled and uptake in individual rats were positively correlated over the same time (Briegleb et al., 2004). Here, examination of a similar time point (25 min post-cocaine) confirmed these findings and extended them by showing that an increased Vmax, without changes in Km, explained the greater DAT activity observed in HCRs. We also found a significant positive correlation between cocaine-induced distance traveled and Vmax values during this time, suggesting that the magnitudes of cocaine-induced locomotor activity and striatal DAT function are related in outbred male Sprague-Dawley rats.
Importantly, here we also found that ex vivo DAT function of LCRs and HCRs paralleled their cocaine-induced locomotor activity over a much longer period post-cocaine. When HCRs exhibited the largest increase in cocaine-induced locomotor activity compared to LCRs, they also displayed their fastest DAT velocity (see Fig. 2). However, as cocaine-induced locomotor activity decreased in HCRs over time, the LCR/HCR differences in DAT function also disappeared. Notably, these results appear to provide an example of a role for rapid-regulation of DAT activity in controlling the acute behavioral effects of cocaine.
The question is, however, whether the higher striatal DAT Vmax in HCRs, measured 25 min post-cocaine, represents a baseline or a cocaine-induced difference. We favor the latter explanation for several reasons. First, no baseline LCR/HCR differences in locomotor activity, striatal DA clearance or extracellular DA levels have been observed; these differences are only revealed following cocaine injection (Gulley et al., 2003; Nelson et al., 2009; Sabeti et al., 2002). Second, HCRs have significantly fewer total DAT binding sites than LCRs in dorsal striatum, as measured with [3H]WIN 35,428 at 35 min post-cocaine (Nelson et al., 2009). Thus, the significant increase in DAT-mediated activity observed in HCRs, relative to LCRs, at 25 min post-cocaine is not likely due to baseline differences. Third, others have shown that acute in vivo exposure to higher dose cocaine can rapidly and markedly up-regulate DAT function in synaptosomes prepared from rat nucleus accumbens (Daws et al., 2002). Thus, following cocaine’s significant initial inhibition of the DAT and increase in extracellular DA in HCRs (Nelson et al., 2009; Sabeti et al., 2002), striatal DAT function may be rapidly up-regulated to compensate for these differential effects of cocaine in HCRs. The need for increased DAT function would be expected to decrease over time as cocaine’s effects decline. Indeed, when HCRs’ activity did not differ from baseline, their Vmax values were no longer increased relative to LCRs. In contrast, the magnitude of cocaine’s inhibition of the DAT and/or the increase in extracellular DA levels in LCRs may be insufficient (or LCRs may be unable) to trigger this same type of regulatory response.
The LCR/HCR differences in [3H]DA uptake Vmax observed here were suggestive of altered DAT surface expression. However, cell surface biotinylation assays did not reveal any differences. Acute exposure to cocaine has been shown to increase DAT function ex vivo in rat nucleus accumbens synaptosomes and DAT surface expression in vitro in heterologous cell systems (Daws et al., 2002; Little et al., 2002). However, increased surface DATs were not observed following in vitro exposure of striatal synaptosomes to cocaine (Chi and Reith, 2003). Thus, cocaine-induced increases in surface DATs in striatal synaptosomes have not been observed following in vitro drug exposure and have not been explored using the ex vivo model. Potential reasons that we did not detect a cocaine-induced increase in surface DAT expression include the relatively low dose of cocaine administered, use of synaptosomes (vs. intact cells) and/or the sensitivity of the biotinylation assays, as well as the possibility that cell surface DATs were unchanged. An alternative approach would be to measure DAT uptake kinetics and cell surface expression in a slice preparation using electrochemical methods and biotinylation assays, respectively. However, to assess post-cocaine changes accurately, slices would have to be assayed immediately, without any time for recovery of their energy stores.
The transiently higher striatal DAT Vmax observed in HCRs could be explained entirely by kinetic alterations in the DAT. Others have reported rapid trafficking-independent increases in DAT function. For example, acute nicotine administration increased [3H]DA uptake Vmax in striatal synaptosomes without changing Km; and this increase was not paralleled by altered DAT surface expression detected by either cell-surface biotinylation or sub-cellular fractionation assays (Middleton et al., 2007). Interestingly, in this study nicotine increased Vmax by 25%, which is the same magnitude of increase seen here in HCRs over LCRs. Furthermore, trafficking-independent alterations in function have been reported for the related NET and SERT, suggesting this may be a common mechanism for rapid, transient regulation of the monoamine transporter family (Apparsundaram et al., 2001; see Steiner et al., 2008; Zhu et al., 2005a; Zhu et al., 2004). Trafficking-independent alterations in DAT function might result from post-translational modification, such as phosphorylation, or from changes in Na+/Cl− binding to the DAT. Regardless, determining the molecular mechanisms mediating these differences in DAT function between LCRs and HCRs following cocaine administration will be critical directions for future experiments.
This study revealed additional differences in cocaine-induced locomotor activity and DAT function between LCRs and HCRs over a more prolonged time post-cocaine. These findings suggest that HCRs, as compared to LCRs, exhibit the more “normal” marked and short-lived locomotor response to acute administration of lower dose cocaine, as well as an increased ability to rapidly up-regulate striatal DAT function. Importantly, overall changes in cocaine-induced locomotor activation and striatal DAT Vmax in HCRs paralleled one another, providing evidence for a role of rapid DAT regulation in controlling the behavioral response to acute cocaine. It will be important in the future to determine if differences in rapid regulation of DAT function persist in LCRs and HCRs following repeated cocaine and if this is one of the mechanisms contributing to the increased susceptibility of LCRs to the rewarding and reinforcing effects of cocaine that we have observed in previous studies.
Acknowledgments
This work and the authors were supported by NIH grants R37 DA004216, F31 DA023343, T32 GM007635 and K05 DA015050. We thank Dr. Toni Richards-Winters for her assistance with the biotinylation experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Briegleb SK, Gulley JM, Hoover BR, Zahniser NR. Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats: contribution of the dopamine transporter. Neuropsychopharmacology. 2004;29:2168–2179. doi: 10.1038/sj.npp.1300536. [DOI] [PubMed] [Google Scholar]
- Broderick PA, Kornak EP, Jr, Eng F, Wechsler R. Real time detection of acute (IP) cocaine-enhanced dopamine and serotonin release in ventrolateral nucleus accumbens of the behaving Norway rat. Pharmacol Biochem Behav. 1993;46:715–722. doi: 10.1016/0091-3057(93)90567-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem. 2007;101:377–388. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Gaffaney JD, Vaughan RA. Uptake inhibitors but not substrates induce protease resistance in extracellular loop two of the dopamine transporter. Mol Pharmacol. 2004;65:692–701. doi: 10.1124/mol.65.3.692. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur J Pharmacol. 2003;479:139–152. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257:444–456. [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61:436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Allen RM, Zahniser NR. Individual differences in initial low-dose cocaine-induced locomotor activity and locomotor sensitization in adult outbred female Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;91:511–516. doi: 10.1016/j.pbb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology (Berl) 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, Dwoskin LP. Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol. 2007;554:128–136. doi: 10.1016/j.ejphar.2006.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Larson GA, Zahniser NR. Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J Pharmacol Exp Ther. 2003;305:180–190. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Galli A. The dopamine transporter: a vigilant border control for psychostimulant action. Handb Exp Pharmacol. 2006:215–232. doi: 10.1007/3-540-29784-7_11. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005a;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005b;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]