Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is linked to mutations in the cardiac ryanodine receptor (RyR2) or calsequestrin. We recently found that the drug flecainide inhibits RyR2 channels and prevents CPVT in mice and humans. Here we compared the effects of flecainide and tetracaine, a known RyR2 inhibitor ineffective in CPVT myocytes, on arrhythmogenic Ca2+ waves and elementary sarcoplasmic reticulum (SR) Ca2+ release events, Ca2+ sparks. In ventricular myocytes isolated from a CPVT mouse model, flecainide significantly reduced spark amplitude and spark width, resulting in a 40% reduction in spark mass. Surprisingly, flecainide significantly increased spark frequency. As a result, flecainide had no significant effect on spark-mediated SR Ca2+ leak or SR Ca2+ content. In contrast, tetracaine decreased spark frequency and spark-mediated SR Ca2+ leak, resulting in a significantly increased SR Ca2+ content. Measurements in permeabilized rat ventricular myocytes confirmed the different effects of flecainide and tetracaine on spark frequency and Ca2+ waves. In lipid bilayers, flecainide inhibited RyR2 channels by open state block, whereas tetracaine primarily prolonged RyR2 closed times. The differential effects of flecainide and tetracaine on sparks and RyR2 gating can explain why flecainide, unlike tetracaine, does not change the balance of SR Ca2+ fluxes. We suggest that the smaller spark mass contributes to flecainide's antiarrhythmic action by reducing the probability of saltatory wave propagation between adjacent Ca2+ release units. Our results indicate that inhibition of the RyR2 open state provides a new therapeutic strategy to prevent diastolic Ca2+ waves resulting in triggered arrhythmias, such as CPVT.
Keywords: Calcium sparks, flecainide, tetracaine, RyR2, catecholaminergic polymorphic ventricular tachycardia
Introduction
In cardiac excitation-contraction coupling, transmembrane Ca2+ flux via L-type Ca2+ channels triggers Ca2+ release from RyR2 Ca2+ release channels located in the junctional SR.[1] The SR Ca2+ content is determined by the net balance of efflux via RyR2 channels and Ca2+ uptake via the SR Ca2+ ATPase (SERCA), also referred to as leak-pump balance.[2] Agents that increase RyR2 channel open probability and Ca2+ efflux (e.g. caffeine) cause a decrease in SR Ca2+ content, whereas RyR2 inhibitors (e.g. tetracaine) increase SR Ca2+ content in intact myocytes during steady-state pacing.[3, 4] However, despite these changes in SR Ca2+ content, agents that alter the open probability of RyR2 typically have no effect on the steady-state Ca2+ transient due to compensatory changes in fractional Ca2+ release.[5]
Mutations in RyR2 or calsequestrin (Casq2), the major SR Ca2+ binding protein, can cause an inherited arrhythmia syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT).[6, 7] Ventricular myocytes isolated from mouse models of CPVT exhibit catecholamine-induced premature SR Ca2+ release and spontaneous Ca2+ waves that trigger delayed afterdepolarizations and premature beats.[8, 9] These Ca2+ waves are likely responsible for triggering ventricular arrhythmias in vivo.[10] We recently found that the anti-arrhythmic drug flecainide blocks RyR2 channels, reduces Ca2+ wave frequency in Casq2-/- cardiomyocytes and prevents CPVT in mice and humans.[11] In contrast, the RyR2 channel inhibitor, tetracaine, was ineffective and did not suppress Ca2+ waves in Casq2-/- myocytes during prolonged exposure.[11] Surprisingly, unlike tetracaine, flecainide did not cause the increase in SR Ca2+ content expected for RyR2 channel inhibitors, in apparent violation of the principle of SR Ca2+ flux balance.[12, 13]
Here we test the hypothesis that flecainide prevents arrhythmogenic Ca2+ waves by altering the properties of elementary SR Ca2+ release events, Ca2+ sparks.[14] We found that in contrast to tetracaine, flecainide reduced spark mass but increased spark frequency. As a result, flecainide had no net effect on spark mediated Ca2+ leak, which would explain why flecainide does not affect SR Ca2+ leak pump balance or the SR Ca2+ content. The different effects of flecainide and tetracaine on Ca2+ sparks are likely the result of differential block of RyR2 channels: flecainide only inhibits open RyR2 channels and reduced RyR2 channel open duration, whereas tetracaine blocks RyR2 channels by reducing the rate of RyR2 channel openings, but has no effect on open RyR2 channels. Thus, our data suggest that the mode of RyR2 channel inhibition (e.g. open channel versus closed channel block) determines the anti-arrhythmic efficacy of RyR2 inhibitors.
Methods
The use of animals in this study was approved by the Animal Care and Use Committees of Vanderbilt University in the USA, University of Leeds in the UK, and University of Newcastle in Australia.
Experiments on intact myocytes from Casq2-/- mice
Ventricular myocytes from 3-4 month old Casq2-/- mice were isolated by a modified collagenase/protease method as previously described.[8] All chemicals, unless otherwise specified were obtained from Sigma (St. Louis, MO). All the experiments were conducted in Tyrode's solution containing (in mM): NaCl 134, KCl 5.4, MgCl2 1, glucose 10, and HEPES 10, pH adjusted to 7.4 with NaOH. For experiments using field-stimulation, ventricular myocytes were loaded with Fura-2AM, and cytosolic [Ca2+] estimated from the ratio of fluorescence emitted at 340 and 380 nm excitation (Fratio) as described.[8] For Ca2+ spark measurement, myocytes were incubated in Tyrode's solution containing 1 mM Ca2+, 6.6 μM fluo-4 acetoxymethyl ester (fluo-4 AM) and 0.16 % Pluronic F127 for 20 minutes at room temperature to load the indicator in the cytosol. The supernatant was removed and myocytes were washed once with 1 mM Ca2+ Tyrode's solution. A minimum of 30 minutes were allowed for de-esterification of the indicator before imaging the cells. Aliquots of fluo-4 loaded myocytes were then incubated for 10 min in petri-dishes containing one of three experimental solutions: vehicle (0.1 vol% ethanol), flecainide (6 μM) or tetracaine (50 μM). Flecainide and tetracaine were dissolved in ethanol stock solutions to achieve a final ethanol concentration of 0.1 vol%. All experimental solutions contained 2 mM Ca2+ Tyrode's solution and 100 nM isoproterenol (ISO). In all experiments on mouse cells, Ca2+ sparks were detected in line scan mode using a Zeiss LSM 510 microscope, with the scan line positioned along the longitudinal axis of each cell. A subset of cells was rapidly exposed to 10 mM caffeine to assess the SR Ca2+ content under each condition. Cells were illuminated at 488 nm and emitted fluorescence was measured at >515 nm. Scan lines were 512 μm in length and collected at 1.60 ms intervals.
Experiments on permeabilized rat myocytes
Ventricular myocytes isolated from adult Wistar rats (150-200 g) were permeabilized by exposure to saponin (10 μg/ml) in a mock intracellular solution for 6 minutes, before centrifugation and re-suspension. Unless otherwise stated, chemicals used following permeabilization were obtained from the Sigma Chemical Corporation, Dorset, UK. Permeabilized cells were perfused with weakly Ca2+ -buffered solutions approximating to the intracellular milieu and SR Ca2+ release was detected using fluo-3. The basic solution contained (mM): KCl, 100; HEPES, 25; EGTA, 0.05-0.36; phosphocreatine 10; ATP, 5 and fluo-3 (pentapotassium) salt, 0.002, pH 7.1, 22 °C. MgCl2 was added (from 1 M stock solution) to produce a free [Mg2+] concentration of 1.0 mM. The free [Ca2+] was adjusted by addition of CaCl2.
The apparatus used for [Ca2+] measurement in permeabilized cells has been described previously.[15] Briefly, permeabilized cells were placed in a cylindrical bath (5 mm diameter) in a Perspex block. The bottom of the bath was formed by attaching a coverslip to the underside of the block. A drop of solution containing cells was placed in the bath and a tightly fitting Perspex column inserted into the well until the lower surface was close to myocytes resting on the coverslip. Perfusion was achieved by pumping solution (0.3 ml/min) down a narrow bore running longitudinally through the column. After obtaining line scans in vehicle-containing solutions, myocytes were exposed for 2 min to flecainide (25 μM) or tetracaine (50 μM) and the line scans repeated. In permeabilized cells, the drug can enter rapidly and therefore, the exposure times were shorter than in the experiments with intact myocytes. Flecainide accumulates in the heart[11], which explains why higher drug concentrations were required to achieve a significant effect permeabilized myoyctes compared to intact myocytes.
The chamber was placed on the stage of a Nikon Diaphot Eclipse TE2000 inverted microscope and cells were viewed and illuminated using a confocal laser-scanning unit (Microradiance, Bio-Rad, Herts, UK) via a 60X water immersion lens (Plan Apo,NA 1.2). The dye was excited at 488 nm and emitted fluorescence was measured at >515 nm.
Analysis of confocal images
In both intact and permeabilized cell experiments, confocal data were analyzed using ImageJ (NIH, USA, http://rsbweb.nih.gov/ij/). The automated detection of Ca2+ sparks and the measurement of temporal and spatial spark properties was carried out using the “SparkMaster” plugin for ImageJ with human verification of spark identification. The detection criteria were set to 3.8; i.e., the threshold for the detection of events was 3.8 times the standard deviation of the background noise divided by the mean.[16]
Single RyR2 channel measurements
SR vesicles containing RyR2 channels were obtained from sheep hearts and were reconstituted into artificial lipid bilayers as previously described.[17] Lipid bilayers were formed from phosphatidylethanolamine and phosphatidylcholine (8:2 wt/wt, Avanti Polar Lipids, Alabaster, AL) in n-decane, (50 mg/ml, ICN Biomedicals) across an aperture of 150–250 μm diameter in a Delrin cup which separated two baths (cis and trans). During SR-vesicle incorporation, the cis (cytoplasmic) bath contained (in mM) 250 Cs+ (230 CsCH3O3S, 20 CsCl), 1.0 CaCl2 and 500 mannitol, while the trans (luminal) solution contained 50 Cs+ (30 CsCH3O3S, 20 CsCl2) and 1.0 CaCl2. After detection of channels in the bilayer the compositions of the cis and trans solutions were changed as follows. The [CsCH3O3S] in the trans solution was increased to 230 mM (i.e. establishing 250 mM Cs+ in both cis and trans baths) by means of aliquot addition of 4 M stock. The cytoplasmic solution was changed to one containing 2 mM ATP, 0.1 μM free Ca2+ by adding aliquots of ATP and BAPTA (4.5 mM) to the cis bath. The caesium salts were obtained from Aldrich Chemical Company, mannitol was obtained from Ajax chemicals and CaCl2 from BDH Chemicals. Solutions were pH-buffered with 10 mM N-tris [Hydroxymethyl]methyl-2-aminoethanesulfonic acid (TES, ICN Biomedicals) and solutions were titrated to pH 7.4 using CsOH (optical grade, ICN Biomedicals) and were redox buffered with 5 mM glutathione (ICN Biomedicals). Flecainide (acetate salt) and tetracaine (hydrochloride) were obtained from Sigma.
Bilayer potential was controlled using an Axopatch 200B amplifier (Axon Instruments). Electrical potentials are expressed using standard physiological convention (i.e., cytoplasmic side relative to the luminal side at virtual ground). Single channel recordings were obtained using bilayer potential difference of +40 mV. The current signal was digitized at 10 kHz and low-pass filtered at 2 kHz with a Gaussian digital filter. Open probability (Po) as well as open and closed durations was measured by the 50% threshold detection method. The burst properties of RyR2 openings (i.e. mean burst duration, intraburst Po and interburst gap durations) were derived from exponential fits to dwell-time distributions of channel open and closured intervals. Analysis was carried out using Channel2 software (P.W. Gage and M. Smith, Australian National University, Canberra).
Statistical analysis
All experiments were done in random sequence with respect to the drug, and measurements were taken by a single observer who was blinded to the drug treatment. Differences between groups were assessed using a one-way analysis of variance (ANOVA). If statistically significant differences were found, individual groups were compared with Student's t-test. Results were considered statistically significant if the p-value was less than 0.05. Unless otherwise indicated, results are expressed as arithmetic means ± standard error of mean.
Results
Flecainide prevents premature Ca2+ waves without altering SR Ca2+ content
Upon stimulation with the β-adrenergic receptor agonist ISO, field-stimulated Casq2-/- myocytes exhibit spontaneous Ca2+ release resulting in Ca2+ waves that occur prior to the next pacing stimulus (Fig. 1A, arrows, top panel). Such premature Ca2+ waves have been the characteristic finding in cardiomyocytes isolated from murine CPVT models[8, 9, 18] and are thought to be the mechanism responsible for triggering premature beats and ventricular tachycardia in vivo.[19] We previously reported that flecainide reduces the frequency of Ca2+ waves, but this was tested only in quiescent Casq2-/- cardiomyocytes.[11] We find that flecainide was even more effective in preventing premature Ca2+ waves in field-stimulated myocytes (Fig. 1A, lower panel). Compared to 90% of vehicle-treated myocytes, only 38% of flecainide-treated myocytes exhibited Ca2+ waves during the ∼60 s pacing train. In myocytes where Ca2+ waves were still present, flecainide significantly reduced wave frequency by more than 50% compared to vehicle-treated myocytes (Fig. 1B). At the same time, flecainide had no significant effect on diastolic [Ca2+] (vehicle: 1.98±0.06 Fratio, n=27, flecainide 1.91±0.07 Fratio, n=26, p=0.5) or on SR Ca2+ content (Fig. 1C), which was estimated by rapid caffeine application after the pacing train (Fig. 1A). We next tried tetracaine, a RyR2 channel blocker which does not prevent Ca2+ waves in quiescent Casq2-/- myocytes during prolonged exposure.[11] Unfortunately, 50 μM tetracaine rendered myocytes unresponsive to field stimulation, making a direct comparison with flecainide impossible.
Fig. 1.
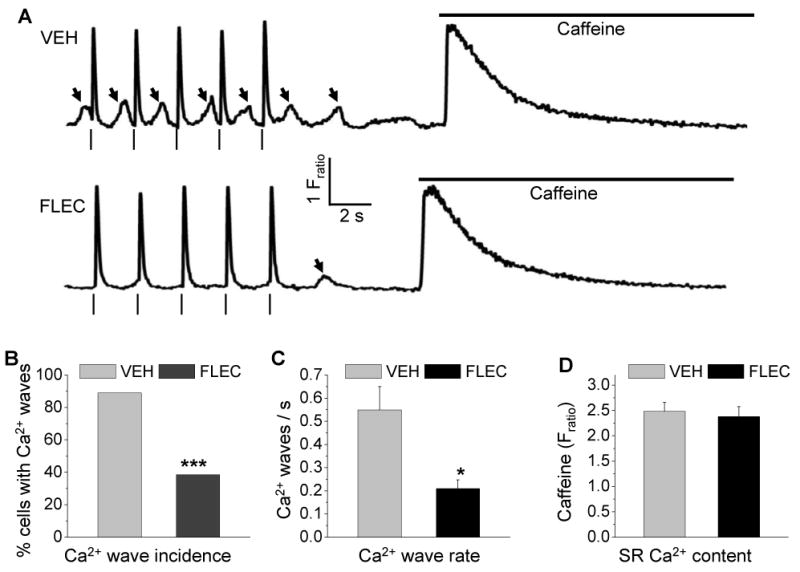
Flecainide prevents premature Ca2+ waves.
(A) Representative examples of cytosolic Ca2+ fluorescence recordings from field-stimulated Casq2-/- myocytes loaded with the fluorescent indicator fura2AM. Arrhythmogenic Ca2+ waves (arrows) were induced by 1 μM ISO in the bath solution. SR Ca2+ content was quantified by rapid caffeine (10 mM) 0.5 Hz pacing train. In vehicle treated cells (VEH), spontaneous Ca2+ waves followed each application following the paced beat (|). Pretreatment with flecainide (FLEC, 6 μM) prevented the generation of Ca2+ waves during the pacing train.
(B) Fraction of Casq2-/- myocytes that exhibit ISO-induced Ca2+ waves during the pacing train. VEH n=27, FLEC n=26, ***p<0.001 by Fisher-exact test.
(C) Comparison of average rate of Ca2+ waves during pacing train. Only myocytes that exhibited Ca2+ waves were included in the analysis. VEH n=24, FLEC n=10, *p<0.05, ***p<0.001.
(D) Comparison of average SR Ca2+ content after the pacing train. VEH n=27, FLEC n=26 myocytes.
Flecainide reduces Ca2+ spark mass but increases Ca2+ spark frequency
We next compared the effect of flecainide and tetracaine on Ca2+ sparks (Fig. 2A) and the SR Ca2+ content, measured by rapid caffeine application (Fig. 2B). Compared to vehicle or tetracaine, flecainide significantly reduced the amplitude, duration and spatial width of Ca2+ sparks in Casq2-/- myocytes (Table 1 and Fig. 2A). On the other hand, tetracaine had no significant effect on spark amplitude or duration (Fig. 2A and Table 1). Interestingly, flecainide and tetracaine had the opposite effect on the frequency of spark occurrence: Flecainide significantly increased spark frequency, whereas tetracaine decreased it (Fig. 2C). Spark mass (calculated as amplitude × 1.206 × FWHM3)[20] was significantly reduced by flecainide but not by tetracaine (Fig. 2D). As a result, the estimated spark-mediated leak was unchanged by flecainide, but significantly reduced by tetracaine (Fig. 2E). At the same time, flecainide had no significant effect on SR Ca2+ uptake rates (estimated from the decay time constant (τ) of field-stimulated Ca2+ transients (Vehicle 0.32 ± 0.08 s, n=19, vs flecainide 0.35 ± 0.10 s, n=20, p = n.s.). Ca2+ transient decline in mice predominantly depends on SERCA function.[21] Consistent with their differential effect on Ca2+ spark mediated leak, tetracaine increased SR Ca2+ content, whereas flecainide did not (Fig. 2F).
Fig. 2.
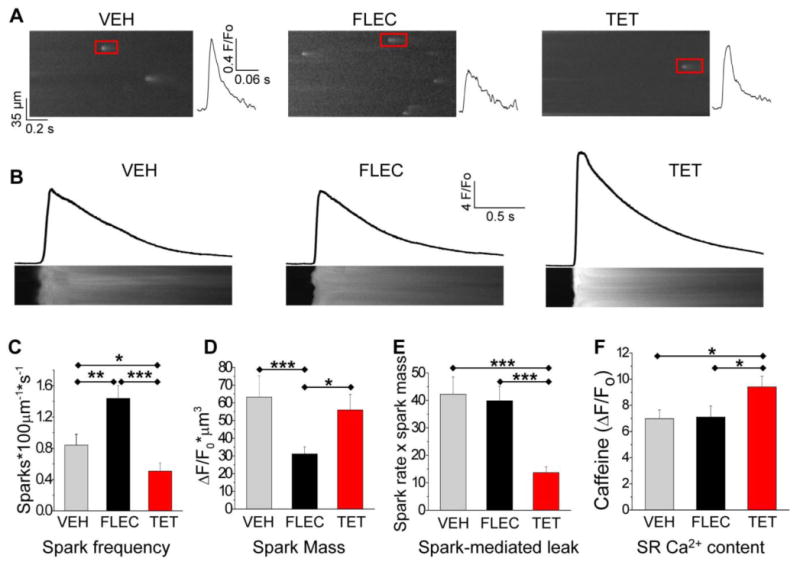
Differential effect of flecainide and tetracaine on Ca2+ sparks and SR Ca2+ content in intact Casq2-/- myocytes.
(A) Representative line scans and individual Ca2+ sparks (red box) of quiescent Casq2-/- myocytes loaded with Fluo-4AM. To avoid excessive Ca2+ wave generation, ISO concentration was reduced to 0.1 μM in the bath solution. Myocytes were incubated for 10 min with either vehicle (VEH), flecainide (FLEC, 6 μM) or tetracaine (TET, 50 μM) before obtaining the confocal images. Red box: spark plot to the right
(B) Representative line scans during rapid caffeine application. The amplitude of the caffeine transient was used as an estimate of SR Ca2+ load.
(C-F) Comparison of average spark frequency (C), spark mass (D), spark mediated SR Ca2+ leak (=spark mass × spark frequency) and SR Ca2+ content (F). n=77-97 myocytes per group, *p<0.05, ***p<0.001
Table 1.
Ca2+ spark parameters in intact Casq2-/- myocytes
| Parameters | Vehicle (n = 87) |
Flecainide (n = 78) |
Tetracaine (n = 84) |
ANOVA P-value |
|---|---|---|---|---|
| Spark Frequency (Sparks·100μm-1·s-1) |
0.84±0.14 | 1.44±0.16#* | 0.48±0.10# | <0.001 |
| Amplitude (ΔF/F0) | 0.89±0.04 | 0.71±0.02#* | 0.89±0.02 | <0.001 |
| FWHM (μm) | 3.35±0.05 | 2.93±0.04#* | 3.13±0.09# | <0.001 |
| FDHM (ms) | 79.5±2.5 | 67.3±1.8#* | 78.5±4.6. | <0.001 |
| Full Width (μm) | 5.52±0.12 | 4.76±0.09#* | 5.31±0.16 | <0.001 |
| Full Duration (ms) | 131±4.0 | 110±3.0#* | 128±5.8 | <0.001 |
| Time to Peak (ms) | 33.9±1.8 | 28.2±1.4 | 32.3±2.9 | 0.053 |
| Maximum Steepness of Upstroke (Δ(F/Fo)/s) | 57.5±2.1 | 53.1±1.7 | 58.8±3.1 | 0.266 |
| Tau (ms) | 91.6±4.9 | 113.0±22.1 | 87.9±6.0 | 0.583 |
| Spark Mass ((ΔF/F0) μm3) | 63.3±8.8 | 31.1±4.0#* | 56.4±12.9 | <0.001 |
All myocytes were bathed for 10 min in experimental solutions containing either vehicle (0.01% ethanol), flecainide (6 μM) or tetracaine (50 μM) prior to recording line-scans. FWHM: spark full width at half maximum amplitude, FDHM: spark duration at half maximum amplitude; data are mean ± S.E.M.; p-values of post-hoc Student's T-test:
p < 0.05 vs. vehicle;
p < 0.05 flecainide vs. tetracaine; number of sparks analyzed per group: vehicle = 294, flecainide = 461, tetracaine = 155
Flecainide prevents Ca2+ waves in permeabilized rat myocytes
The data from intact Casq2-/- myocytes suggest that flecainide reduced the probability of propagated waves by reducing spark Ca2+ mass. To test this hypothesis more directly, and at the same time exclude the possibility that the flecainide effect is limited to mouse Casq2-/- myocytes stimulated with ISO, we next compared flecainide and tetracaine in permeabilized rat ventricular myocytes, where there are no contributions from ion transport across the sarcolemma, cytosolic [Ca2+] can be controlled experimentally, and ISO is not needed to induce Ca2+ waves. In the presence of 200 nM Ca2+ and 0.05 mM EGTA, permeabilized myocytes exhibited regular spontaneous Ca2+ waves (Fig. 3A), comparable with those observed in intact cells in the presence of ISO (Fig. 2A). Under these conditions, introduction of 25 μM flecainide (upper) or 50 μM tetracaine (lower) reduced the frequency of spontaneous Ca2+ waves by 42.8 ± 6.2 % (n=9) and 18 ± 4.6 % (n=6), respectively. However, in the presence of tetracaine, the amplitude of the spontaneous Ca2+ waves increased by 17.9 ± 7.1 % (n=7), while in the presence of flecainide, the amplitude decreased by 21.4 ± 2.1 (n=9). We next assessed the effects of flecainide and tetracaine on the SR Ca2+ content at the point of spontaneous SR Ca2+ release: In cells exhibiting spontaneous Ca2+ release at regular intervals, the frequency was monitored over 3-4 Ca2+ release and re-uptake cycles. Caffeine (20 mM) was then rapidly applied to maximally deplete SR Ca2+ at the point in the cycle when a spontaneous Ca2+ release would otherwise have occurred. The amplitude of the caffeine-induced Ca2+ transient was used as an assay of the SR Ca2+ content under control conditions, or in the presence of flecainide or tetracaine (Fig. 3B). Tetracaine significantly increased the Ca2+ content of the SR at the point of spontaneous Ca2+ release, whereas flecainide had no significant effect.
Fig. 3.
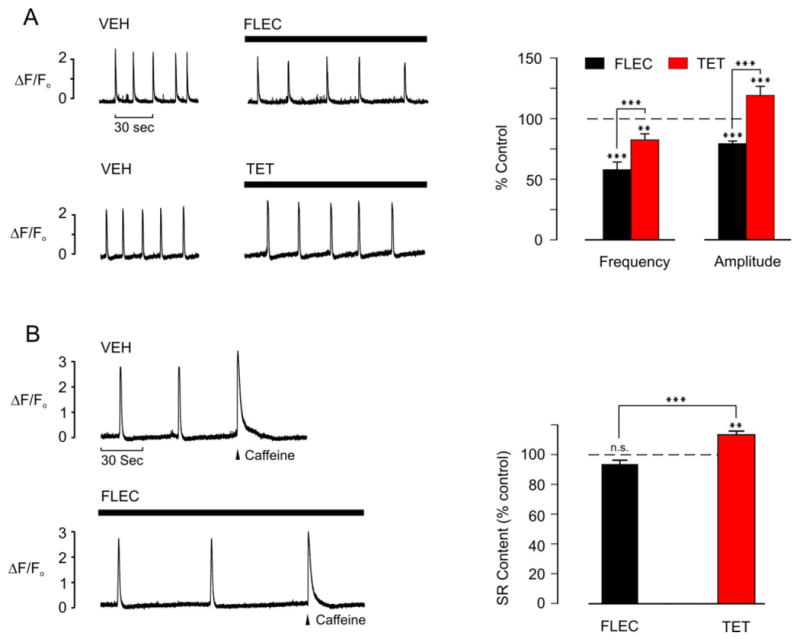
Effects of flecainide or tetracaine on Ca2+ waves and the SR Ca2+ content in permeabilized rat myocytes
(A) Representative integrated line-scan images (left) showing spontaneous Ca2+ waves in permeabilized ventricular myocytes during exposure to vehicle (VEH) and after 2 min exposure to either 25 μM flecainide (FLEC) or 50 μM tetracaine (TET). Average data (right) comparing the effects of flecainide and tetracaine on Ca2+ wave amplitude and frequency. FLEC n = 9, TET n = 7, **p<0.01, ***p<0.001.
(B) Representative integrated line scan images (left) showing spontaneous Ca2+ waves in permeabilized ventricular myocytes in the presence of vehicle or 2 min after introduction of 25 μM FLEC. In both cases, the wave frequency was monitored and 10 mM caffeine rapidly applied when a wave would otherwise have occurred (arrowhead). The amplitude of the caffeine-induce Ca2+ transient was used as an index of the SR Ca2+ content in each case. Average data comparing the effects of flecainide and tetracaine on the SR Ca2+ content under these conditions (right). FLEC n = 6, TET n = 6, **p<0.01, ***p<0.001, n.s. = not significant.
In rat myocytes exhibiting repeated spontaneous Ca2+ waves, flecainide increased the frequency of Ca2+ sparks immediately before each release, although the events appeared of smaller amplitude and width (Fig 4A). In order to investigate the effects of flecainide on spontaneous Ca2+ sparks under more controlled conditions, the [EGTA] of the bathing solution was increased to a level that prevents propagation of Ca2+ waves (0.36 mM), without affecting spontaneous Ca2+ sparks.[22] Under these conditions, flecainide significantly increased spark frequency but decreased the spark mass by 45%, such that that there was no significant change in the spark mediated Ca2+ leak (Fig. 4B). The decrease in spark mass caused by flecainide was predominantly due to a decrease in spark width (Fig. 4B). In contrast to the effects of flecainide, tetracaine significantly decreased spark frequency, while spark width was unaffected. The mean spark amplitude also decreased slightly, resulting in a modest overall decrease in spark mass of ∼12%, while the Ca2+ leak decreased by ∼37% (Fig. 4).
Fig. 4.
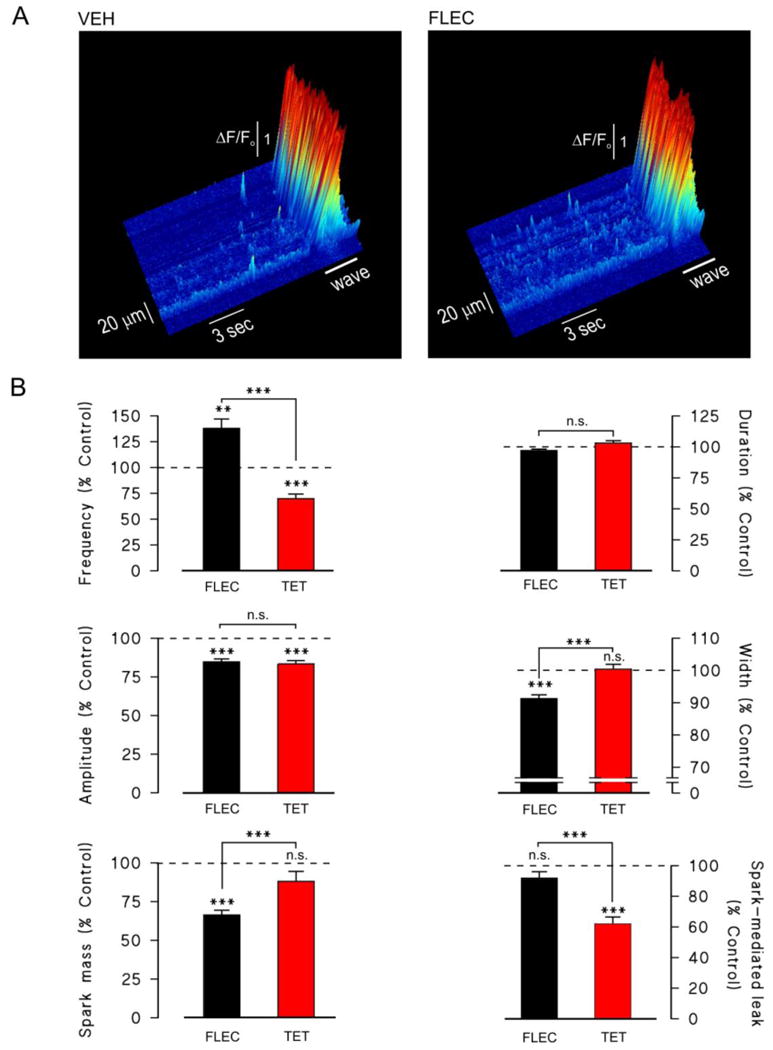
Effects of flecainide and tetracaine on spontaneous Ca2+ sparks in permeabilized myocytes
(A) Surface plots of raw line scan images obtained from a permeabilized rat ventricular myocyte during spontaneous Ca2+ wave generation, in the presence of vehicle (left) or after introduction flecainide (right). Note the increased spark frequency in the myocyte exposed to FLEC. The cell was bathed in weakly Ca2+ -buffered solutions (0.05 mM EGTA) approximating to the intracellular milieu, with a free [Ca2+] of ∼200 nM.
(B) Cumulative data showing the relative changes in Ca2+ spark frequency, amplitude, mass, duration and width following introduction of 25 μM flecainide or 50 μM tetracaine. Spark data was obtained in more strongly Ca2+ buffered solutions (0.36 mM) to prevent Ca2+ wave propagation. **p<0.01, ***p<0.001, n.s. = not significant. 160-341 sparks from n = 25-33 myocytes per group.
Flecainide inhibits open RyR2 channels
To test the hypothesis that differential modulation of RyR2 channel gating is responsible for their different effects on Ca2+ sparks, we next compared the effect of flecainide and tetracaine on RyR2 channels in lipid bilayers. Under control conditions (1 mM luminal Ca2+, 0.1 μM cytoplasmic Ca2+), RyR2 had an open probability (Po) of 0.15 ± 0.04, mean open time (τo) of 46 ± 11 ms and mean closed time (τc) of 441 ± 111 ms, (n=14). The addition of flecainide to the cytoplasmic bath induced brief channel closures with a mean duration of ∼1 ms to a substate of ∼20% of the maximal channel conductance (Fig. 5A). These closures caused the conversion of channel long-lasting openings present under control conditions to bursts of openings (Fig. 5A). Although flecainide and tetracaine both inhibited RyR2, they did so with different mechanisms of action. Unlike flecainide, tetracaine did not induce short closures of the channel (Fig 5B). Moreover, tetracaine did not shorten the burst duration, as illustrated by the example in Fig 5B (right panel), where the mean burst duration was essentially equal to the mean open time.
Fig. 5.
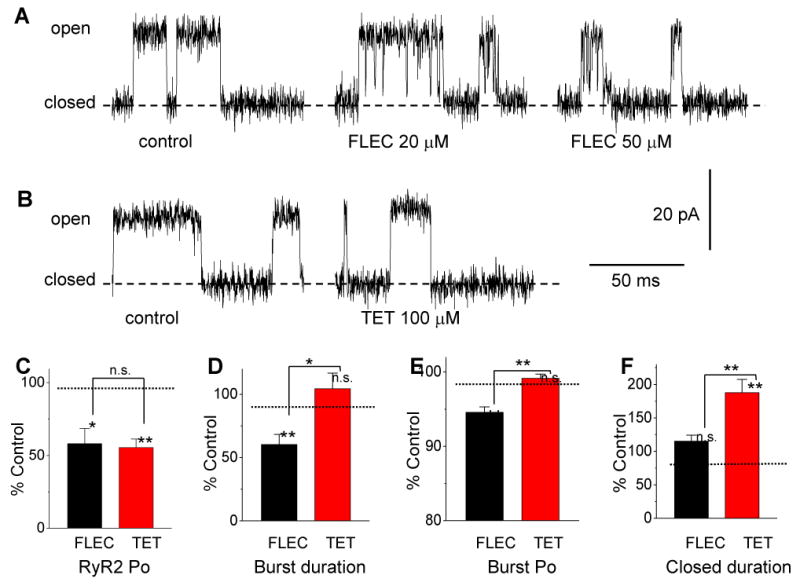
Flecainide and tetracaine block single RyR2 channels by different mechanisms.
(A,B) Records are representative examples of single channel activity of RyR2 in lipid bilayers. Control conditions were 1 mM luminal Ca2+ (trans bath), 0.1 μM cytoplasmc Ca2+ plus 2 mM ATP (cis bath). Bilayer potential was 40 mV (relative to trans bath as ground). Relatively high concentrations of flecainide (FLEC) and tetracaine (TET) were used to better illustrate their differential effect on RyR2 channel gating.
(A) Single experiment where flecainide was added to the cytoplasmic bath. Under control conditions, this channel had an open probability (Po) of 0.25, mean open time (τo) of 83 ms and mean closed time (τc) of 228 ms. The full duration of typical closed periods is not seen on this time scale. Addition of flecainide introduced short (∼ 1 ms) closures to a substate at ∼20% of the full channel conductance which lead to bursts of short channel openings.
(B) Different experiment where tetracaine was added to the bath. Under control conditions, this channel had Po = 0.11, τo = 33 ms and τc = 237 ms. Addition of 100 μM tetracaine increased τc to 913 ms but had little effect on τo (τo =27 ms).
(C) The effects of flecainide and tetracaine on burst parameters were compared at concentrations that reduced RyR2 Po by approximately 50% (10 μM and 50 μM, respectively).
(D-F) Comparison of average burst parameters derived from burst analyses of single channel recordings. The data are expressed as burst properties relative to control before addition of FLEC or TET (control conditions are given in methods). Note that FLEC inhibits RyR2 by reducing burst duration (D) and intraburst Po (E), whereas TET increased the interburst closed duration (F). *p<0.05, **p<0.01, n=10 per group
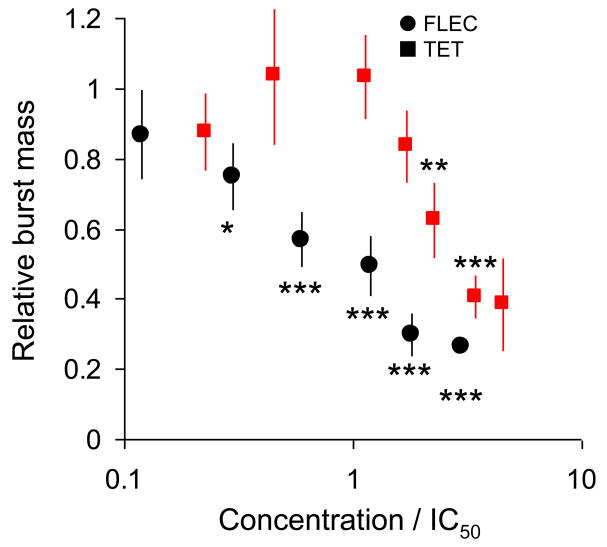
Flecainide inhibited the Po with an IC50 of 16 ± 3 μM (Hill coefficient of 1.3 ± 0.8) whereas tetracaine inhibited Po with an IC50 of 44 ± 4 μM (Hill coefficient of 1.4 ± 0.5, Supplemental Fig. 1A). We next quantified the concentration dependence of the effect of flecainide and tetracaine on the closed time between bursts (Supplemental Fig. 1B), length of bursts (Supplemental Fig. 1C), and the open probability within bursts (Supplemental Fig. 1D). Flecainide was a more potent inhibitor of burst duration and burst Po compared with tetracaine. Both drugs had about equal potency in increasing interburst gap duration. To determine the mechanism responsible for the inhibition of RyR2, we next compared these effects of flecainide and tetracaine at drug concentrations that produced an approximately equal reduction in RyR2 Po (Fig 5C-F). For flecainide, the decrease in RyR2 Po was due to a 47% reduction in burst duration (Fig. 5D) and a 6% reduction in burst Po (Fig. 5E), whereas interburst closed duration (Fig. 5F) was not significantly different from control. Thus, the main inhibitory effect of flecainide was a shortening of the mean burst duration, as illustrated in Fig. 5A, right panel. For tetracaine, the decreased Po was mainly the result of increasing the mean closed duration (a 104% increase in the duration of interburst closures, Fig. 5F). Tetracaine had no significant effects on burst duration (Fig. 5D) and burst Po (Fig. 5E). Thus, flecainide inhibited RyR2 primarily by reducing mean burst duration, whereas tetracaine inhibited RyR2 by increasing the mean time channels remained closed between bursts. The different effects of tetracaine and flecainide on RyR2 channels are best illustrated by comparing burst mass (Fig. 6). Burst mass is the product of burst duration and open probability within bursts, analogous to calculating spark mass in myocytes. At concentrations below ∼2 × IC50, tetracaine has no significant effect on burst mass whereas flecainide significantly reduced burst mass (Fig. 6).
Fig. 6.
Flecainide and tetracaine block single RyR2 channels by different mechanisms. The concentration dependence of the effects of flecainide (●) and tetracaine (■) on the total RyR2 activity within bursts (burst mass). For ease of comparison, drug concentrations are expressed as a ratio of concentration and the IC50 values (flecainide 16 μM, tetracaine 44 μM). Burst mass was calculated from the product of the burst duration (Supplemental Fig. 1C) and the open probability within bursts (Supplemental Fig. 1D) normalized to values obtained in the absence of drug. *p<0.05, **p<0.01, ***p<0.001 compared to control by paired t-test.
Discussion
In this study we have compared the actions of two RyR2 blockers that had very different effects on Casq2-/- myocytes, a model of CPVT: Flecainide induced a sustained decrease in the frequency of spontaneous Ca2+ waves, whereas tetracaine was ineffective.[11] Here we demonstrate that tetracaine and flecainide have different inhibitory actions on single RyR2 channels, which result in markedly different effects on Ca2+ sparks, Ca2+ waves and the balance of SR and sarcolemmal Ca2+ fluxes. The mechanism of flecainide action is an open channel block. Flecainide consistently reduces spark mass in different species (mouse, rat). Flecainide exerts analogous effects in intact and permeabilized myocytes regardless of whether cells were treated with ISO or not, suggesting that the state of RyR2 phosphorylation is not a major determinant of flecainide action.
Regulation of RyR2 and the balance of SR and sarcolemmal Ca2+ fluxes
In paced ventricular myocytes, increasing the open probability of RyR2 with a low concentration of caffeine (∼0.2 mM) initially increases the amplitude of the whole cell Ca2+ transient.[3, 23] However, the increased Ca2+ transient amplitude results in a greater efflux of Ca2+ from the cell, which progressively depletes SR Ca2+ until Ca2+ transient return to control levels.[3, 23] In the new steady state the SR Ca2+ content is decreased and fractional SR Ca2+ release is increased, reflecting the continuing effect of caffeine on RyR2. A decrease in the open probability of RyR2 induced by application of tetracaine produces essentially the opposite effect. Decreased Ca2+ transient amplitudes and reduced Ca2+ extrusion from the cell cause a progressive increase in the SR Ca2+ content until the Ca2+ transient amplitude is restored.[3] This tendency for SR Ca2+ release to “autoregulate” with changes in SR content is also apparent with spontaneous Ca2+ sparks[24] and reflects the effect of SR luminal Ca2+ on RyR2 open probability.[25]
Flecainide apparently violates these principles of SR autoregulation: In contrast to tetracaine,[26] RyR2 inhibition with flecainide suppressed spontaneous SR Ca2+ waves without causing a compensatory increase in SR load in paced ventricular myocytes (Fig. 1). At the same time, flecainide increased Ca2+ spark frequency in intact (Fig. 2) and permeabilized myocytes (Fig. 4), a result usually seen with RyR2 channel activators such as caffeine.[24] However, despite increasing spark frequency, flecainide significantly reduced Ca2+ wave frequency (Fig. 1+3), which is the opposite effect of caffeine.[27]
Unique regulation of RyR2 channels by flecainide
The paradoxical effects of flecainide on myocyte Ca2+ handling are likely the result of flecainide's unique action on RyR2 channels. Flecainide does not alter the RyR2 closed times (Fig. 5), which is in stark contrast to the effects of caffeine or tetracaine, the two compounds used most often by investigators studying the effect of RyR2 channel modulation on myocyte Ca2+ handling.[13, 24, 26-30] Both caffeine and tetracaine modulate RyR2 open probability by acting on closed RyR2 channels,[28, 31] which likely explains their effect on spark frequency.[24] Although tetracaine can also decrease RyR2 open times in the presence of high cytoplasmic [Ca2+],[11] at more physiological cytoplasmic [Ca2+] examined here (0.1 μM Ca2+) and previously by Gyorke and co-workers (3 μM Ca2+),[28] tetracaine acts primarily by stabilizing closed channels (Fig. 5). Caffeine also does not alter RyR2 open times.[31]
In contrast, flecainide acts mainly as an open channel blocker. Flecainide decreases RyR2 open times and reduces RyR2 conductance by causing brief closures to a subconductance state (Fig. 5). As a result, flecainide is much more potent than tetracaine in reducing burst mass (Fig. 6). The reduction in RyR2 burst mass can explain flecainide's effect of decreasing spark mass in intact and permeabilized myocytes (Figs. 2+4). On the other hand, it is much less clear why flecainide increased spontaneous Ca2+ spark frequency (Fig. 2). We previously found that flecainide reduced both open and closed times of RyR2 channels activated by high cytosolic (100 μM) Ca2+,[11] which could explain the dual effect of reduced spark mass and increased spark frequency (Fig. 2). However, flecainide had no effect on closed times of RyR2 channels under experimental conditions that mimic spontaneous sparks in myocytes studied here (Fig. 5). Alternatively, the increased spark frequency may be the consequence of flecainide's effect on spark mass. Because spark mass is reduced, each spark results in a smaller reduction in SR luminal [Ca2+]. The effect of luminal Ca2+ on RyR2 gating[25] is then analogous to a pressure overflow valve, triggered at a set pressure: In the presence of flecainide, smaller spontaneous sparks must now occur more frequently to match the luminal Ca2+ control signal, dictated by constant Ca2+ influx via SR Ca2+ ATPase.
Regardless of the underlying mechanism, flecainide had no effect on SR Ca2+ leak due to its opposing influence on spark frequency and spark mass, and SR Ca2+ content remained unchanged. The characteristic changes in Ca2+ spark properties induced by flecainide may also underlie its potency as an inhibitor of diastolic Ca2+ waves: The pronounced decrease in spark mass induced by flecainide likely reduces the probability of saltatory propagation between neighboring couplons. In that regard it is interesting to note that sparks measured in Casq2-/- myocytes (table 1) were about 50% wider and longer than those reported in the literature.[16] While the bigger spark mass can be in part explained by the presence of ISO,[32] we cannot exclude that loss of Casq2 also contributed to the large spark mass found in Casq2-/- myocytes.
Implications for therapeutic actions of RyR2 inhibitors
Our data suggests that the beneficial actions of flecainide stem from its peculiar ability to block RyR2 channels only while they are open, to cause blocking events with a sufficient frequency to reduce mean open times to ∼1 ms, and sufficient brevity to avoid an increase in the interburst intervals. While many compounds are known to inhibit RyR2 activity,[33] relatively few of them have been studied at the single channel level. Among those, even fewer act as open channel blockers. For example, ryanoids (ryanodine analogues) are highly specific open channel blockers, but RyR2 inhibition is much slower than flecainide and occurs on the second timescale.[34] Polyamines such as neomycin are open channel blockers that operate by a “blocker-stopper” mechanism with fast kinetics.[35] Unfortunately, in the presence of diastolic Ca2+ levels, neomycin also acts as a closed channel blocker by competing with Ca2+ for a channel activation site.[36] Finally, quaternary ammonium ions have been shown to plug the RyR2 pore and act as fast open channel blockers.[37] However, the block has a relatively low affinity (∼1 mM) and these compounds are well known K+ channel blockers. Thus, the properties of flecainide represent a unique mechanism of modulating RyR2 channels, which is ideally suited to suppress arrhythmogenic Ca2+ waves without causing compensatory increases in SR Ca2+ content.
In summary, our results suggest that sustained inhibition of catecholamine-induced diastolic Ca2+ waves in CPVT myocytes requires a drug that interacts primarily with RyR2 in their open state to reduce spark mass, but without affecting the overall balance of SR Ca2+ fluxes. In the absence of changes in net Ca2+ fluxes or the SR Ca2+ content, slow compensatory changes in RyR2 function via autoregulation do not occur. This results in a sustained therapeutic action which is reflected by a reduced probability of saltatory wave propagation due to a smaller spark mass. Our data suggests a new paradigm for anti-arrhythmic drug development targeting RyR2 channels.
Supplementary Material
Acknowledgments
This work was supported in part by the US National Institutes of Health grants HL88635 and HL71670 (to BCK) and DK53434 (to DWP), by the American Heart Association Established Investigator Award 0840071N (to BCK), by British Heart Foundation grants (to ZY and DSS), and by an NSW Health infra structure grant (DRL). We wish to thank Paul Johnson, Divya Mehra and Meegan Jones at the University of Newcastle for their assistance with the single channel recording.
Footnotes
Disclosure Statement: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nabauer M, Callewaert G, Cleemann L, Morad M. Regulation of calcium release is gated by calcium current, not gating charge, in cardiac myocytes. Science. 1989 May 19;244(4906):800–3. doi: 10.1126/science.2543067. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000 Aug 18;87(4):275–81. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 3.Overend CL, O'Neill SC, Eisner DA. The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes. J Physiol. 1998 Mar 15;507(Pt 3):759–69. doi: 10.1111/j.1469-7793.1998.759bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neill SC, Donoso P, Eisner DA. The role of [Ca2+]i and [Ca2+] sensitization in the caffeine contracture of rat myocytes: measurement of [Ca2+]i and [caffeine]i. J Physiol. 1990 Jun;425:55–70. doi: 10.1113/jphysiol.1990.sp018092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000 Jan 15;522(Pt 2):259–70. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106(1):69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 7.Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, et al. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002 Oct 18;91(8):e21–6. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- 8.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006 Sep;116(9):2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, et al. Arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia: insights from a RyR2 R4496C knock-in mouse model. Circ Res. 2006 Aug 4;99(3):292–8. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 10.Liu N, Rizzi N, Boveri L, Priori SG. Ryanodine receptor and calsequestrin in arrhythmogenesis: what we have learnt from genetic diseases and transgenic mice. J Mol Cell Cardiol. 2009 Feb;46(2):149–59. doi: 10.1016/j.yjmcc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009 Apr;15(4):380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008 Jan 15;77(2):285–92. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 13.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002 Oct 4;91(7):594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–4. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Steele DS. Effects of cytosolic ATP on spontaneous and triggered Ca2+-induced Ca2+ release in permeabilised rat ventricular myocytes. J Physiol. 2000 Feb 15;523(Pt 1):29–44. doi: 10.1111/j.1469-7793.2000.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007 Sep;293(3):C1073–81. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 17.Chopra N, Laver D, Davies SS, Knollmann BC. Amitriptyline activates cardiac ryanodine channels and causes spontaneous sarcoplasmic reticulum calcium release. Mol Pharmacol. 2009 Jan;75(1):183–95. doi: 10.1124/mol.108.051490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008 Aug 1;103(3):298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- 19.Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature. 2008 Feb 21;451(7181):929–36. doi: 10.1038/nature06799. [DOI] [PubMed] [Google Scholar]
- 20.Hollingworth S, Peet J, Chandler WK, Baylor SM. Calcium sparks in intact skeletal muscle fibers of the frog. J Gen Physiol. 2001 Dec;118(6):653–78. doi: 10.1085/jgp.118.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998 Apr;274(4 Pt 2):H1335–47. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- 22.Lukyanenko V, Gyorke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J Physiol. 1999 Dec 15;521(Pt 3):575–85. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trafford AW, Diaz ME, Eisner DA. Stimulation of Ca-induced Ca release only transiently increases the systolic Ca transient: measurements of Ca fluxes and sarcoplasmic reticulum Ca. Cardiovasc Res. 1998 Mar;37(3):710–7. doi: 10.1016/s0008-6363(97)00266-6. [DOI] [PubMed] [Google Scholar]
- 24.Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys J. 2001 Aug;81(2):785–98. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyorke S, Gyorke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci. 2002 Jun 1;7:d1454–63. doi: 10.2741/A852. [DOI] [PubMed] [Google Scholar]
- 26.Venetucci LA, Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Reducing ryanodine receptor open probability as a means to abolish spontaneous Ca2+ release and increase Ca2+ transient amplitude in adult ventricular myocytes. Circ Res. 2006 May 26;98(10):1299–305. doi: 10.1161/01.RES.0000222000.35500.65. [DOI] [PubMed] [Google Scholar]
- 27.Nieman CJ, Eisner DA. Effects of caffeine, tetracaine, and ryanodine on calcium-dependent oscillations in sheep cardiac Purkinje fibers. J Gen Physiol. 1985 Dec;86(6):877–89. doi: 10.1085/jgp.86.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyorke S, Lukyanenko V, Gyorke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997 Apr 15;500(Pt 2):297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol. 1997 Aug 1;502(Pt 3):471–9. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neary P, Duncan AM, Cobbe SM, Smith GL. Assessment of sarcoplasmic reticulum Ca(2+) flux pathways in cardiomyocytes from rabbits with infarct-induced left-ventricular dysfunction. Pflugers Arch. 2002 Jun;444(3):360–71. doi: 10.1007/s00424-002-0794-0. [DOI] [PubMed] [Google Scholar]
- 31.Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990 Apr;423:425–39. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, et al. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not {beta}-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res. 2009 Jul 15; doi: 10.1093/cvr/cvp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol. 1994 Jun;266(6 Pt 1):C1485–504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- 34.Tanna B, Welch W, Ruest L, Sutko JL, Williams AJ. The interaction of a neutral ryanoid with the ryanodine receptor channel provides insights into the mechanisms by which ryanoid binding is modulated by voltage. J Gen Physiol. 2000 Jul 1;116(1):1–9. doi: 10.1085/jgp.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mead FC, Williams AJ. Electrostatic mechanisms underlie neomycin block of the cardiac ryanodine receptor channel (RyR2) Biophys J. 2004 Dec;87(6):3814–25. doi: 10.1529/biophysj.104.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laver DR, Hamada T, Fessenden JD, Ikemoto N. The ryanodine receptor pore blocker neomycin also inhibits channel activity via a previously undescribed high-affinity Ca(2+) binding site. J Membr Biol. 2007 Dec;220(13):11–20. doi: 10.1007/s00232-007-9067-3. [DOI] [PubMed] [Google Scholar]
- 37.Tinker A, Williams AJ. Measuring the length of the pore of the sheep cardiac sarcoplasmic reticulum calcium-release channel using related trimethylammonium ions as molecular calipers. Biophys J. 1995 Jan;68(1):111–20. doi: 10.1016/S0006-3495(95)80165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.