Abstract
Highly active antiretroviral therapy (HAART) is often associated with endothelial dysfunction and cardiovascular complications. In this study, we determined whether HIV non-nucleoside reverse transcriptase inhibitor efavirenz (EFV) could increase endothelial permeability. Human coronary artery endothelial cells (HCAECs) were treated with EFV (1, 5 and 10 µg/ml) and endothelial permeability was determined by a transwell system with a fluorescence-labeled dextran tracer. HCAECs treated with EFV showed a significant increase of endothelial permeability in a concentration-dependent manner. With real time PCR analysis, EFV significantly reduced the mRNA levels of tight junction proteins claudin-1, occludin, zonula occluden-1 and junctional adhesion molecule-1 compared with controls (P < 0.05). Protein levels of these tight junction molecules were also reduced substantially in the EFV-treated cells by western blot and flow cytometry analyses. In addition, EFV also increased superoxide anion production with dihydroethidium and cellular glutathione assays, while it decreased mitochondrial membrane potential with JC-staining. Antioxidants (ginkgolide B and MnTBAP) effectively blocked EFV-induced endothelial permeability and mitochondrial dysfunction. Furthermore, EFV increased the phosphorylation of MAPK JNK and IκBα, thereby increasing NFκB translocation to the nucleus. Chemical JNK inhibitor and dominant negative mutant JNK and IkBa adenoviruses effectively blocked the effects of EFV on HCAECs. Thus, EFV increases endothelial permeability which may be due to the decrease of tight junction proteins and the increase of superoxide anion. JNK and NFκB activation may be directly involved in the signal transduction pathway of EFV action in HCAECs.
Keywords: Efavirenz, HAART, endothelial permeability, tight junction protein, oxidative stress, antioxidant, ginkgolide B, MnTBAP, JNK, NFκB
1. Introduction
Highly active antiretroviral therapy (HAART) is the combination of several antiretroviral drugs used to control HIV infection [1]. So far, this treatment offers the best chance of preventing HIV from multiplying. The goal of antiretroviral therapy is to reduce the viral load to a level that can no longer be detected with current blood tests. These antiretroviral drugs include nucleoside reverse transcriptase inhibitors (NRTIs) such as zidovudine; nonnucleoside reverse transcriptase inhibitors (NNRTIs) such as efavirenz (EFV); protease inhibitors (PIs) such as ritonavir; and fusion inhibitors such as enfuvirtide.
Although HAART therapy has significantly improved the prognosis of HIV-1-infected patients, it is associated with side effects such as diabetes, atherosclerosis, and cardiovascular complications [2–4]. Long term exposure to HAART results in endothelial oxidative stress and activation of mononuclear cell recruitment, an early event in atherosclerosis. HAART drug combinations, consisting of zidovudine, EFV, and either of the indinavir or nelfinavir increased reactive oxygen species (ROS) formation in human aortic endothelial cells (HAECs).
EFV is one of NNRTIs, and used with other HAART drugs to treat HIV infection in patients with or without acquired immunodeficiency syndrome (AIDS). Both NRTIs and NNRTIs inhibit the same target, the reverse transcriptase enzyme, an essential viral enzyme which transcribes viral RNA into DNA. Unlike NRTIs, which bind at the enzyme's active site, NNRTIs bind within the NNRTI pocket of the enzyme. Clinical studies have indicated that exposure to HAART including EFV is associated with increased fasting low-density lipoprotein cholesterol (LDL-c) levels, which is a well known risk factor for cardiovascular disease [5,6]. EFV has been shown to increase plasma F2 isoprostane concentrations, which indicate oxidative stress [7]. However, it is not clear whether EFV could affect endothelial functions.
Endothelial barrier function is established and maintained mainly by endothelium-endothelium junctional structures such as adherens junctions and tight junctions including claudins, occludins, zona occludens-1 (ZO-1), junctional adhesion molecule-1 (JAM-1) and VE-cadherin. Increased vascular permeability is a common feature in many pathophysiological conditions such as obstruction of pulmonary airways [8], circulatory collapse in sepsis, cancer growth and metastatic spread, and many inflammatory conditions [9]. Increased vascular permeability is also associated with the development of atherosclerosis [10]. Several inflammation cytokines such as tumor necrosis factor-alpha (TNF-α) can significantly induce endothelial permeability [11].
In the current study, we determined whether EFV could affect endothelial monolayer permeability. Human coronary artery endothelial cells (HCAECs) were treated with EFV, and endothelial monolayer permeability was investigated. In addition, potential molecular mechanisms, including role of endothelial junction molecules, oxidative damage, change of mitochondrial membrane potential, and signal transduction molecules mitogen-activated protein kinases (MAPKs) and transcriptional factor NFκB were studied. This study provides us a better understanding of the molecular mechanism of HAART-associated vascular complications and the possibility using new strategies to combat the HAART-associated side effects.
2. Materials and Methods
Detail methods are provided in online supplemental materials.
2.1. Endothelial permeability
Pure EFV was obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Primary HCAECs were obtained from Lonza Walkersville Inc (Walkersville, MD). Paracellular permeability through the endothelial monolayer was studied in a Coaster Transwell system as previously described [12].
2.2. Western blot
Equal amounts of proteins (40 µg) were loaded onto 7% or 10% SDS PAGE gel and western blotting experiment was performed as previously described [13]. Protein levels of ZO-1, occludin, claudin-1, JAM-1, IκBα, phosphorylated IκBα, and NFκB (p65) were determined by western blot. β-actin or laminin were also detected for loading controls. Selected protein bands were subjected to density analysis using ImageJ software and normalized to β-actin as the fold of control samples.
2.3. Real-time RT-PCR analysis
Primers and methods for detecting the mRNA levels of ZO-1, claudin-1, occludin, JAM -1 and VE-cadherin were described in our previous publications [12–14].
2.3. Flow cytometry
Flow cytometry analyses for the expression of juctional molecules, superoxide anion generation with DHE staining, and mitochondrial membrane potential with JC-1 staining were done as described in our previous publications [12–19].
2.4. Cellular GSH assay
For GSH-mediated quantification of ROS, HCAECs were treated with either EFV (10 µg/ml,) or pretreated with MnTBAP for 30 min followed by EFV treatment for 45 min. Cellular superoxide anion was indirectly measured as per manufacture’s instructions by following a GSH-Glo Glutathione assay kit (Promega, Madison, WI).
2.5. Bio-plex luminuoassay
The phosphorylated and total MAPK (ERK1/2, p38, and JNK) and IkBα (phospho-IκBα and total IκBα) were detected by Bio-Plex phosphoprotein and total target assay kits [12–14].
2.6. Statistical analysis
Data were expressed as the mean ± SD. Comparisons were made using the Student’s t-test. A P value <0.05 was considered statistically significant.
3. Results
3.1. EFV increases endothelial monolayer permeability in HCAECs
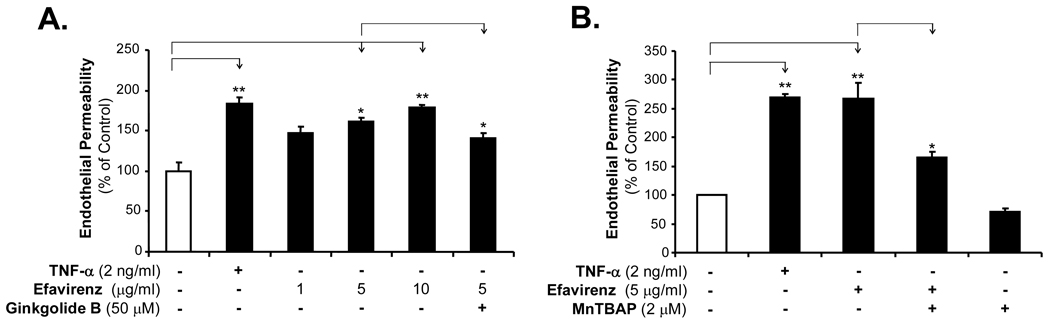
To determine whether EFV could affect the paracellular permeability of the endothelial monolayer, HCAECs were treated with EFV, and endothelial permeability was analyzed by the Costar transwell system using a Texas-Red-labeled dextran tracer. Treatment of HCAEC monolayer with increasing concentrations of EFV for 24 hrs significantly increased endothelial permeability by 75% compared with untreated cells (Fig. 1A, n = 3, P < 0.05). TNF-α (2 ng/ml) was used as a positive control since it can significantly increases HCAEC monolayer permeability [15]. Interestingly, antioxidants (ginkgolide B and MnTBAP) effectively blocked EFV-induced permeability in HCAECs compared with EFV treatment (Fig. 1A and 1B, n = 3, P < 0.05).
Fig. 1.
Effects of EFV, gingkolide B and MnTBAP on endothelial monolayer permeability in HCAECs. Endothelial monolayer permeability was tested with a transwell system and a Texas Red labeled dextran tracer. (A) Concentration-dependent study. HCAECs were treated with increasing concentrations of EFV (1, 5 and 10 µg/ml) or with TNF-α (2 ng/ml) as a positive control for 24 hrs. Ginkgolide B (50 µM) was used to block EFV-induced endothelial permeability. (B) MnTBAP (a SOD mimetic antioxidant) effectively blocked EFV-induced endothelial permeability. HCAECs were treated with 5 µg/ml EFV in the presence of 2 µM MnTBAP for 24 hrs. n = 3, *P < 0.05. **P < 0.01.
3.2. EFV decreases the expression of endothelial tight junction proteins ZO-1, claudin-1, occludin and JAM-1 in HCAECs
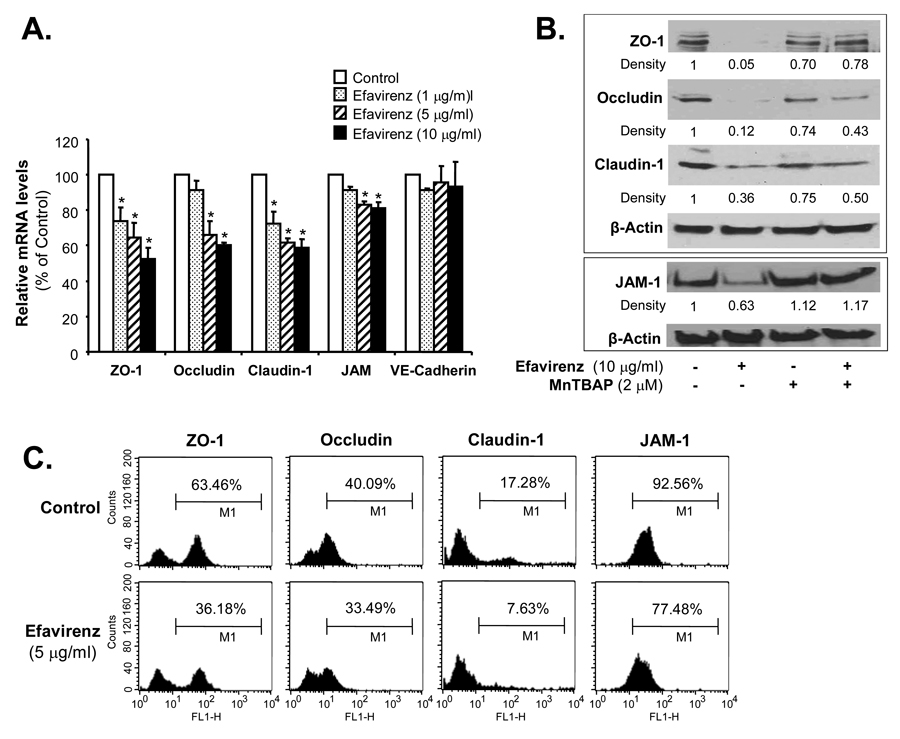
To determine whether EFV could affect the expression of endothelial junctional molecules especially the tight junction molecules at both mRNA and proteins levels. Concentration-dependent treatment (1, 5 and 10 µg/ml, respectively) of HCAECs with EFV for 24 hrs significantly decreased mRNA levels of tight junction molecules ZO-1, claudin-1, occludin and JAM-1. For examples, the treatment of EFV at 10 µg/ml significantly reduced mRNA levels of ZO-1 by 45%, occludin by 40%, claudin-1 by 35%, and JAM-1 by 12% (Fig. 2A, n = 3, P < 0.05). To further confirm gene expression effects, protein levels of tight junction proteins were determined by western blot and flow cytometric analyses, which showed that EFV treatment substantially decreased protein levels of ZO-1, claudin-1, occludin and JAM-1, respectively, in HCAECs (Fig. 2B and C). Antioxidant MnTBAP effectively blocked EFV-induced decrease in these molecules at the protein levels (Fig. 2B).
Fig. 2.
Effects of EFV and MnTBAP on the expression of junctional molecules in HCAECs. HCAECs were treated with serial concentrations of EFV (1, 5 and 10 µg/ml) for 24 hrs. (A) The mRNA levels of ZO-1, claudin-1, occludin, JAM-1 and VE cadherin were analyzed by real time PCR, and values were normalized to β-actin. n = 3. *P < 0.05. (B) The protein levels of ZO-1, occludin, claudin-1 and JAM-1 were analyzed by western blot analysis. Relative density of the expressed proteins was normalized to the corresponding β-actin band as the fold of the control. (C) Histogram of flow cytometry analysis showing protein levels of ZO-1, claudin-1, occludin and JAM-1 under control and EFV treated conditions. HCAECs were treated with 5 µg/ml EFV for 24 hrs.
3.3. EFV increases superoxide anion production in HCAECs
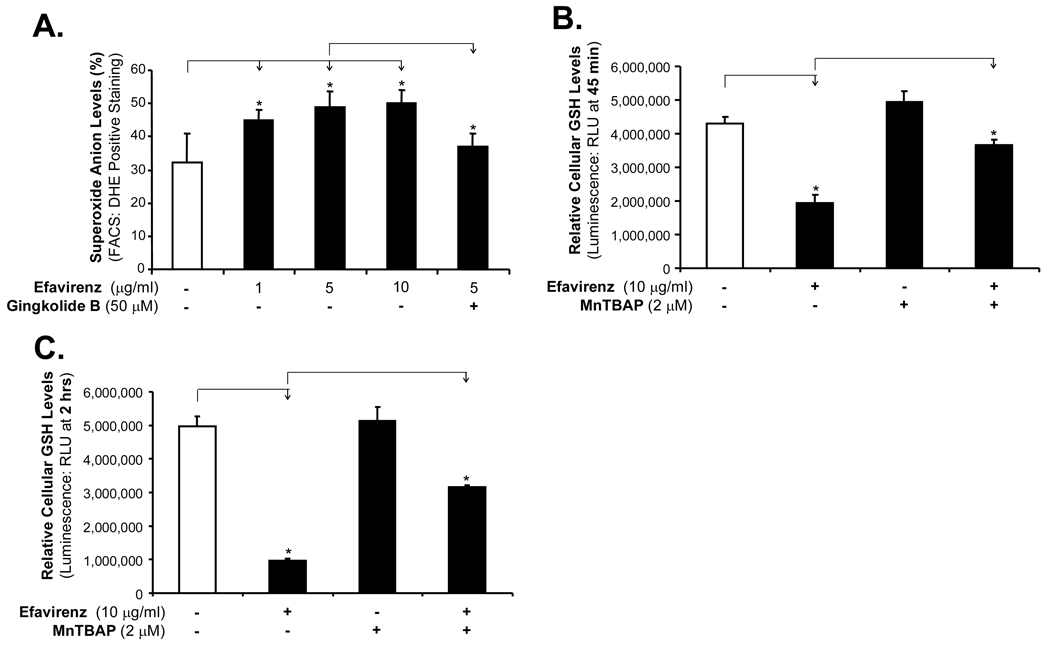
Since oxidative stress due to inflammation and other conditions is involved in increase of endothelial permeability [3, 16], we investigated whether EFV could increase superoxide anion (the major ROS) production in HCAECs. Cellular superoxide anion levels were determined by staining with fluorescent dye DHE followed by flow cytometric analysis. Cytosolic DHE displays blue fluorescence, whereas after oxidation by oxidants such as superoxide anion and H2O2 it becomes 2-hydroxyethidium and ethidium, displaying red fluorescence. Study indicates that 2-hydroxyethidium, but not ethidium, correlates with supperoxide production [19]. Treatment of HCAECs with increasing concentrations of EFV (1, 5 and 10 µg/ml) for 24 hrs significantly increased the superoxide anion production by 50%, 60% and 70%, respectively, compared with controls, while EFV and gingkolide B co-treatment significantly decreased superoxide anion production compared with EFV treatment alone (Fig. 3A, n = 3, P < 0.05).
Fig. 3.
Effects of EFV, ginkgolide B and MnTBAP on superoxide anion production in HCAECs. (A) Superoxide anion levels were determined by fluorescent dye DHE staining followed by flow cytometry analysis. HCAECs were treated with different concentrations of EFV (1, 5 and 10 µg/ml, respectively) for 24 hrs, and then stained with DHE (3 µM, 20 min). n = 3, *P < 0.05. Co-treatment of HCAECs with ginkgolide B and EFV for 24 hrs significantly reduced DHE-positively stained cells. HCAECs were treated with either EFV (10 µg/ml,) or pretreated with MnTBAP for 45 min (B) and 2 hrs (C). Cellular superoxide anion was indirectly measured. Treatment of HCAECs with antioxidant MnTBAP significantly reduced EFV-induced ROS generation. n = 3, *P < 0.05. Error bars represent standard deviations (SD).
Furthermore, cellular superoxide levels were indirectly measured by GSH assay. Superoxide can be converted to H2O2 by superoxide dismutase (SOD), and then H2O2 can be rapidly removed by catalase or peroxidases such as the GSH peroxidases, which use reduced GSH as the electron donor. Thus, cellular GSH levels are negatively correlated to ROS levels. EFV treatment of HCAECs for either 45 min or 2 hrs, significantly reduced the GSH levels, while MnTBAP partially blocked EFV-induced reduction in GSH levels in HCAECs (Fig. 3B and 3C, n = 3, P < 0.05).
3.4. EFV decreases mitochondrial member potential in HCAECs
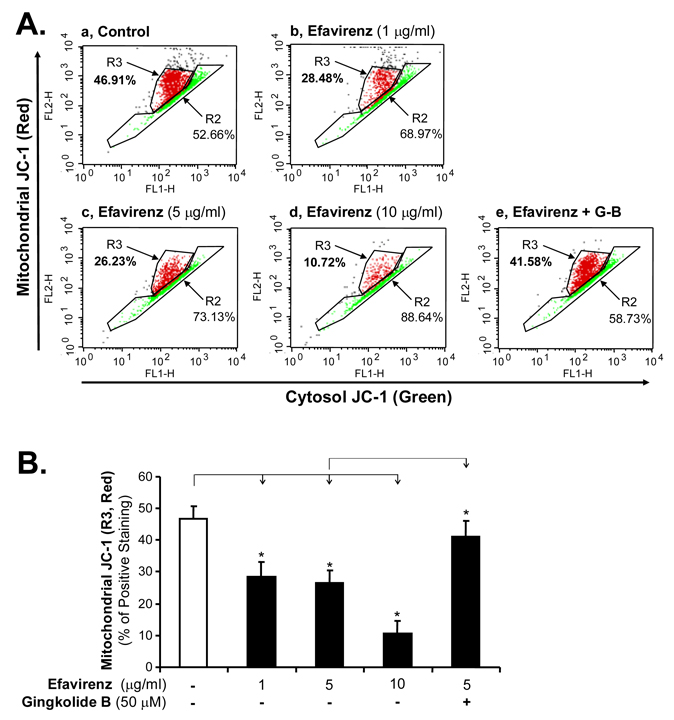
To determine the potential source of superoxide anion production, we investigated whether EFV could have any effect on mitochondrial membrane potential since increased cellular level of ROS has been associated with loss of mitochondrial membrane potential [17]. JC-1 mitochondrial membrane potential detection kit was used. JC-1 is a cationic dye that indicates mitochondrial polarization by shifting its fluorescence emission from green (~525 nm) to red (~590 nm). This potential-sensitive color shift is due to concentration-dependent formation of red fluorescent JC-1 aggregates. A shift from red to green indicates membrane depolarization. HCEACs were treated for 24 hrs with increasing concentrations (1, 5 and 10 µg/ml, respectively) of EFV and then a flow cytometric analysis was performed. EFV reduced the JC-1 aggregate formation in HCAECs in a concentration-dependent manner as indicated by the reduction of red JC-1 formation. Treatment of HCAECs with 10 µg/ml EFV decreases red fluorescent JC-1 aggregates to 10.72% compared with the control value of 46.91% (Fig. 4A and B, n = 3, P < 0.05). Co-treatment of HCAECs with EFV and gingkolide B for 24 hrs blocked EFV-induced depolarization (Fig. 4A and B).
Fig. 4.
Effects of EFV and ginkgolide B on mitochondrial membrane potential in HCAECs. (A) Histogram of flow cytometry analysis showing the distribution of JC-1 aggregates (red) and JC-1 monomer in the mitochondrial membrane and cytoplasm, respectively. Normal HCAECs without EFV treatment served as controls. HCAECs were treated with different concentrations of EFV (1, 5 and 10 µg/ml) for 24 hrs and then stained for the JC-1 followed by flow cytometry analysis. (B) Bar graph showing JC-1 (red) positive cells in control and EFV treated cells. Addition of ginkgolide B in EFV-treated cells for 24 hrs restored the JC-1 aggregate formation almost similar to control levels. n = 3, *P < 0.05, Error bars represent SD.
3.5. EFV increases the phosphorylation of JNK and IκBα in HCAECs
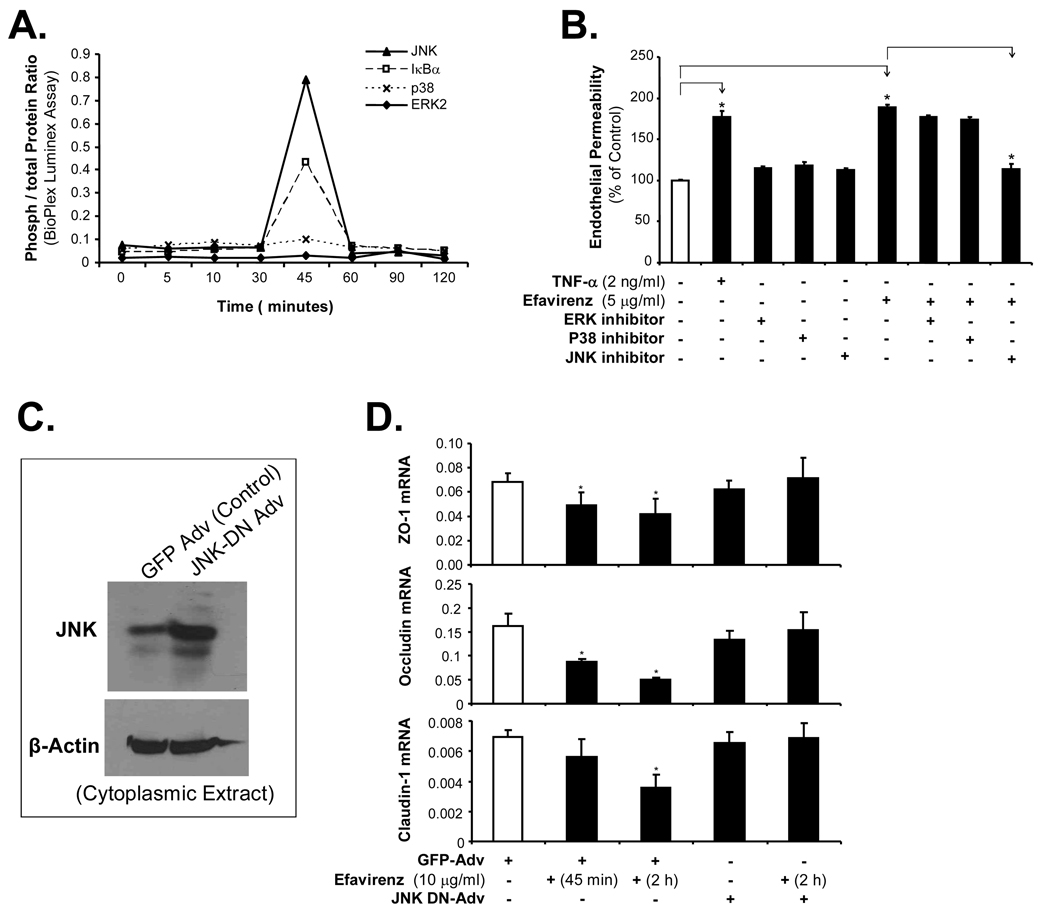
To determine the involvement of signal transduction pathway in the EFV-induced permeability increase in HCAECs, the total and phosphorylated IκBα, JNK, p38 and ERK1/2 were measured by Bio-Plex luminuoassay. The activation of JNK and IκBα was indicated by the increase of JNK and IκBα phosphorylation. Ratios of phosphorylated to total protein levels for JNK and IκBα, but not p38 and ERK1/2, were substantially increased by about 8-fold at 45 min in EFV-treated (5 µg/ml) HCAECs (Fig. 5A). To further confirm the functional role of MAPKs in EFV-induced HCAEC permeability increase, specific inhibitors for JNK (SP600125 at 25 µM), ERK1/2 (PD98059 at 50 µM), and p38 (SB203580 at 10 µM) were used in HCAEC cultures with the transwell system. HCAECs were pretreated with inhibitors for ERK1/2, p38, or JNK for 30 min, and then the cells were treated with EFV (5 µg/ml) for 24 hrs. The inhibitor for JNK, but not for ERK1/2 and p38, effectively blocked EFV-induced HCAEC permeability increase (Fig. 5B). These data are consistent with MAPK activation detected by Bio-Plex immunoassay described above (Fig. 5A). Furthermore, we infected the cells with a dominant negative JNK adenovirus (JNK-DN-Adv). This virus expresses a mutant form of JNK which can not be phosphorylated and then checked the expression of tight junction molecules by real time PCR under EFV treatment. Infection of JNK-DN-Adv significantly blocked the EFV-induced decrease of tight junction molecule expression in HCAECs including ZO-1, occludin and claudin-1 (Fig. 5C and D). Thus, activation of MAPK JNK is important for EFV-mediated decrease of tight junction proteins in HCAECs.
Fig. 5.
Roles of MAPKs in the EFV-induced endothelial permeability in HCAECs. (A) Bio-Plex luminescence assay. HCAECs were treated with EFV (5 µg/ml) for different time points (1, 5, 10, 30, 45, 60, 90 and 120 min). The phosphorylated and total proteins of each MAPK and IκBα were detected. (B) Effects of MAPK inhibitors on EFV-induced endothelial permeability in HCAECs. HCAECs were treated with EFV (5 µg/ml) alone or pretreated with each specific inhibitor of MAPKs (SP600125 for JNK; PD98059 for ERK1/2; and SB203580 for p38). Monolayer permeability was detected by a transwell system with a fluorescence-labeled dextran tracer. n = 3. *P < 0.05. (C) HCAECs were infected with an adenovirus containing a dominant negative form of c-jun N Terminal kinase (JNK) (JNK-DN-Adv) for 48 hrs. Adenovirus encoding green fluorescence protein (GFP-Adv) was used as a negative control. Expression of JNK-DN-Adv was determined by western blot analysis using an antibody against JNK. Loading efficiencies were determined by reprobing the blot with the antibody against β-actin. (D) Effect of JNK-DN-Adv on the EFV-mediated expression of tight junction molecules in HCAECs. HCAECs with JNK-DN-Adv or GFP-Adv infection were treated with EFV (10 µg/ml) for indicated time points and mRNAs were isolated from the cells. The mRNA levels of ZO-1, occludin and claudin-1 were analyzed by real time PCR, and values were normalized to GAPDH. n = 3. *P < 0.05.
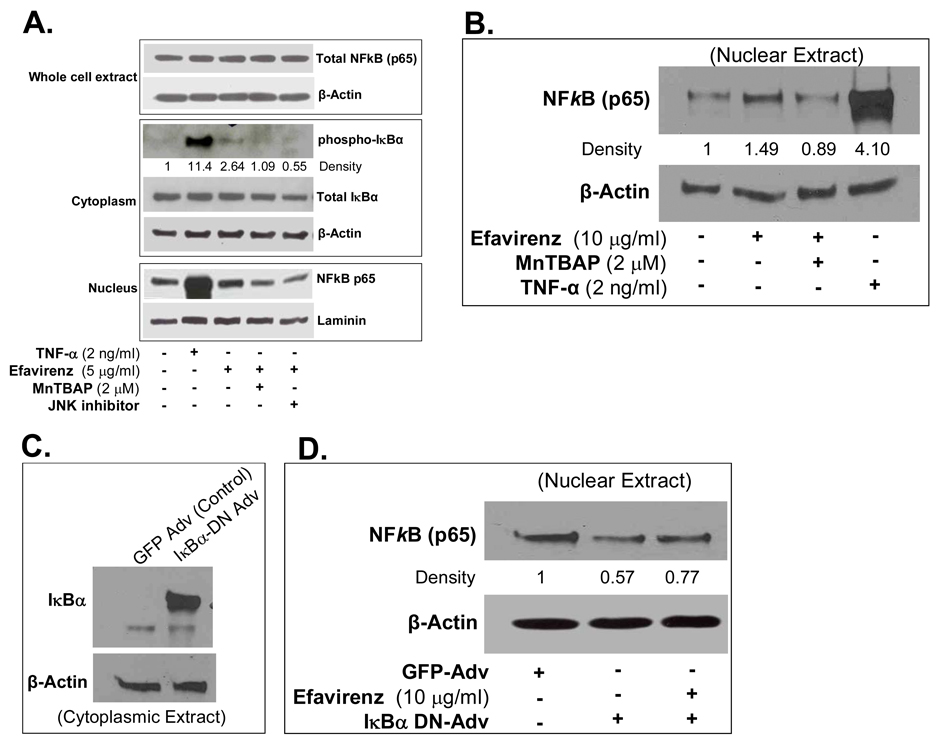
Western blot also confirmed the increase in phospho-IκBα in cytoplasmic extracts of EFV-treated cells compared with controls. EFV treatment increased the NFκB translocation from the cytoplasm to the nucleus. Use of antioxidant MnTBAP or JNK inhibitor effectively blocked the phospho-IκBα levels and inhibited NFκB nucleus translocation in EFV-treated HCAECs (Fig. 6A and B). In order to directly confirm the functional role of the activation of NFkB in EFV-mediated endothelial permeability increase, we infected HCAECs with an adenovirus expressing a mutant dominant negative form of IkBα. (IκBα-DN-Adv). This mutant form of IkBα can not be phosphorylated and hence inhibits translocation of NFkB into the nucleus. EFV treatment increased the nuclear translocation of NFkB in HCAECs at 45 min compared with untreated cells. Infection of cells with IκBα-DN-Adv reduced the NFkB translocation to the nucleus and EFV treatment failed to increase the NFkB nuclear translocation in IκBα-DN-Adv infected cells (Fig. 6C and D). Thus, NFkB may play an important role in the EFV-mediated signaling pathways in HCAECs.
Fig. 6.
Effects of EFV on IkBa phosphorylation and NFkB nuclear translocation. (A). The protein levels of total IκBα and phospho-IκBα in the cytoplasmic extract, and nucleus-translocated NFκB in the nucleus extract were determined by western blot analysis. Loading efficiencies for cytoplasmic extract and nuclear extract were determined by reprobing the blot with antibodies against β-actin and laminin, respectively. Specific antibody against NFκB subunit p65 was used to detect this protein in the whole cell extract. Relative density of the protein bands of phospho-IκBα was normalized to β-actin as the fold of the control. (B) The protein levels of NFkB in the nucleus extract were determined by western blot analysis. Loading efficiencies were determined by reprobing the blot with the antibody against β-actin. Specific antibody against NFkB subunit p65 was used to detect this protein in the nuclear extract. Antixodant MnTBAP blocked the effect of EFV. TNF-α was used as a positive control. Relative density of the protein bands of NFκB (p65) was normalized with corresponding β-actin bands. (C) HCAECs were infected with an adenovirus containing a dominant negative form of IκBα (IκBα-DN-Adv) for 48 hrs. Cytoplasmic extracts were purified using NE-PER Nuclear and Cytoplasmic Extraction Reagents. Expression of IkBα was determined by western blot analysis. Loading efficiencies were determined by reprobing the blot with the antibody against β-actin. (D) HCAECs were infected with IκBα-DN-Adv (inhibition of IκBα) or GFP-Ad-GFP as a negative control for 48 hrs and the nuclear extract was prepared. Nuclear NFkB (p65) levels were determined by western blot analysis. For EFV effects, HCAECs expressing IkBα-DN-Adv were treated with EFV (10 µg/ml) for 45 min. Loading efficiencies were determined by reprobing the blot with the antibody against β-actin. Relative density of the protein bands of NFκB (p65) was normalized with corresponding β-actin bands as the fold of control.
4. Discussion
In this study, we show that NNRTI EFV significantly increases the in vitro monolayer permeability of HCAECs. The molecular mechanism of EFV-induced permeability increase in HCAECs may be due to the decreased expression of tight junction proteins ZO-1, claudin-1, occludin and JAM-1. These effects are mediated by increased generation of ROS and activation of JNK and NFκB. Accordingly, inhibitors specific to JNK and antioxidants effectively blocked the EFV-induced permeability and oxidative stress in HCAECs. This study suggests a novel effect of EFV, which may contribute to HAART-associated cardiovascular complications in HIV-infected patients.
To determine the paracellular permeability through endothelial cells, we used a costar transwell permeability model. The model has been successfully used in this laboratory to test the effects of ritanovir [18], lysophosphatidylcholine [14], stanniocalcin-1 [15], and secretoneurin [12] on endothelial monolayer permeability. In the present study, this in vitro model was used to test the effect of EFV on paracellular permeability through HCAEC monolayer. HCAECs were grown to confluence on the transwell and then subjected to EFV treatment with different concentrations. Mature monolayer was stained with Calcein AM and checked under fluorescent microscope to make sure the monolayer is confluent and the permeability increase is not due to the leakage through subconfluent HCAECs.
Clinically recommended dose of EFV for adults and children weighing more than 40 kg (88 lbs) is between 100–600 mg once daily that is 2.5 mg to 15 mg/kg, which can generate 4.1±1.2 µg/ml maximal plasma concentration (Cmax) [20]. Keeping this in mind, we selected EFV concentrations between 1–10 µg/ml, which fall within the clinically relevant concentrations. Our results show that EFV treatment of HCAECs for 24 hrs significantly increases endothelial permeability up to a maximum of 75% compared with controls, and this effect of EFV is similar to that of HIV protease inhibitor ritanovir [17].
Intercellular junctional structures mediate adhesion and communication between adjoining endothelial monolayer and comprise of tight junction molecules including transmembrane proteins such as occludin, claudin and JAM-1, and intracellular proteins such as ZO-1 and cingulin; and adherens junction proteins including transmembrane protein VE-cadherin and intracellular protein β-catenin. Tight junctions serve the major functional purpose of providing a “barrier” and a “fence” within the membrane by regulating paracellular permeability and maintaining cell polarity. In this study, we show that EFV significantly decreases the expression of tight junction molecules ZO-1, occludin, claudin-1 and JAM-1 at both mRNA and protein levels. However, it has no effect on VE-cadherin expression. Other HAART drugs such as ritanovir also decrease the expression of tight junction proteins and thus increase endothelial permeability. Decreased levels of tight junction proteins may contribute to the damage of tight junction and barrier function of endothelial cells in response to EFV and thus may contribute to the development of arteriosclerosis. However, in the current study, we have not directly confirmed the functional roles of tight junction proteins in the EVF-induced endothelial permeability. In future investigation, the experiments including knockdown or overexpression of these molecules could be included.
It is well known that ROS play critical roles in cardiovascular disease. There are several sources of ROS generation in the human body including mitochondria, xanthine oxidase, cytochrome P450-based enzymes, NADPH oxidases, dysfunctional NO synthases, and infiltrating inflammatory cells [20]. ROS cause endothelial barrier dysfunction through alterations in the cytoskeleton and extracellular matrix [21]. ROS are known to quench NO [22]. NO synthesis inhibition can potentiate agonist-induced increases in vascular permeability or increase basal microvascular permeability via an alteration of endothelial actin cytoskeleton [23]. In the present study, we show a concentration-dependent increase of superoxide anion (a main species of ROS) production by EFV in HCAECs. To determine the possible source of superoxide anion production in EFV-treated cells, the change of mitochondrial membrane potential was investigated. As expected, EFV treatment caused a concentration-dependent decrease of mitochondrial membrane polarization resulting in the release of superoxide anion from the mitochondria into the cytosol. Thus intracellular ROS formation and loss of mitochondrial membrane potential might play an important role in EFV-induced endothelial permeability increase.
To further confirm the involvement of ROS, we used the two antioxidants ginkgolide B and MnTBAP, which effectively blocked the EFV-induced increase of vascular permeability and the decrease of tight junction protein expression. Antioxidant effects of ginkgolide B are well documented, and however, its mechanisms of action are largely unknown [24]. In addition, ginkgolide B has many other biological functions. For example, ginkgolide B can specifically inhibit effects of platelet activating factor (PAF) via competitively binding to PAF receptor [25]. In our study, we demonstrated that ginkgolide B effectively inhibited EFV-induced ROS production and endothelial permeability. However, we can not confirm whether ginkgolide A acts on superoxide anion. Therefore, we used a second antioxidant MnTBAP, which has a superoxide dismutase-like activity. Its blocking effects on EFV-induced endothelial permeability can confirm the critical role of superoxide anion in endothelial dysfunction. Both antioxidants also blocked the EFV-induced IκBα activation and NFκB nucleus translocation. Findings from the current study suggest that EFV-induced oxidative stress may be one of the molecular mechanisms involved in the damage of endothelial barrier function and the use of antioxidants may be a novel strategy in preventing HAART-associated cardiovascular complications in HIV-infected patients.
MAPKs (JNK, ERK1/2, and p38) play an important role in mediating cellular responses to a number of agents. In order to check the molecular mechanisms and signal transduction pathways of EFV-induced permeability increase in HCAECs, the involvement MAPKs as well as the role of NFκB were studied. NFκB plays a major role in TNF-α-mediated permeability increase in vascular cells during inflammation [15]. Our study clearly shows JNK MAPK as well as IκBα activation and NFκB nuclear translocation are involved in the EFV-induced endothelial permeability increase. To further confirm the functional significance of these molecular events, specific inhibitors for JNK, p38 and ERK1/2 were used. JNK inhibitor, but not p38 and ERk1/2 inhibitors, effectively blocked the EFV-induced HCAEC permeability increase. This important finding is also confirmed by infection of a dominant negative JNK adenovirus (JNK-DN-Adv). This virus expresses a mutant form of JNK which can not be phosphorylated. JNK-DN-Adv significantly blocked the EFV-induced decrease of tight junction molecule expression in HCAECs. Thus, the signal transduction mechanism of EFV-induced HCAEC permeability increase includes the activation of JNK.
NFkB is a crucial transcription factor that regulates many genes. Prior to cytokine stimulation, NFkB is restricted to the cytosol as an inactive complex with its inhibitors such as IκBα. Upon activation by cytokines, IkBα is phosphorylated, dissociates from the NFkB, and is subsequently ubiquitinated and degraded. This allows active NFkB to enter the nucleus and bind to the NFkB consensus regulatory elements in the promoters of many responsive genes. Consequently, NFkB could increase or decrease the expression of certain specific genes via direct or indirect mechanisms. In the present study, IκBα phosphorylation levels were increased and NFkB was translocated to nucleus 45 min after treatment with EFV, suggesting the activation of NFkB in EFV-treated HCAECs. When we blocked IkBα function in HCAECs by infection of recombinant adenovirus expressing a dominant negative mutant form of IκBα, NFkB translocation to nucleus was suppressed and EFV treatment could not further increase NFkB p65 nuclear translocation. Therefore, NFkB may play an important role in the EFV mediated signaling pathways in HCAECs. NFκB possibly binds to the promoters of the genes expressing tight junction proteins and negatively prevents their expression.
We previously reported that during TNF-α-induced permeability increase, phospho-IκBα is increased and there is also increased translocation of NFkB to the nucleus [15]. In the current study, TNF-α was used as a positive control to demonstrate the reliability of the cell culture system. We have noticed that TNF-α (2 ng/ml) and EFV (5 and 10 µg/ml) have a similar effect on HCAEC permeability, while having marked difference in the phosphorylation of IκBα and nuclear translocation of NFκB. This observation suggests that the contribution of IκBα/NFκB activation to EFV-induced endothelial permeability is different from TNF-α.
HAART drug combinations, consisting of zidovudine, a nucleoside reverse transcriptase inhibitor; EFV and either of the two protease inhibitors (PIs), indinavir or nelfinavir, increased mononuclear cell adhesion and intercellular adhesion molecule-1 (ICAM-1) gene expression and concomitant exposure to TNF-α further increased ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and endothelial-leukocyte adhesion molecule cell surface protein levels in human aortic endothelial cells [26]. ICAM-1 and VCAM-1 are particularly important for firm, integrin-mediated adhesion of leukocytes to endothelial cells and subsequent transendothelial migration [27]. Future investigations could focus on the potential posttranscriptional mechanism of EFV-mediated decrease of junctional proteins such as ROS-mediated phosphorylation of tight junction molecules and ubiquitin proteosome-mediated degradation of phosphyrylated proteins. It is also important to study the expression endothelial adhesion molecules and inflammatory effects such any transendothelial migration of leukocytes through endothelial cells in response to EFV treatment. Identifying these pathways should reveal new targets for therapeutic intervention that will be relevant for treating HAART-associated vascular complications, inflammatory diseases and the many pathologic situations where vascular permeability is adversely affected.
In conclusions, the present study demonstrates a new function of EFV, which increases vascular permeability in HCAECs. The underlying molecular mechanisms may involve down-regulation of tight junction proteins, oxidative stress, and activation of NFκB and MAPK JNK. The understanding of molecular mechanisms and development of new therapeutic strategies for EFV-induced endothelial dysfunction may contribute to the prevention or treatment of HAART-associated cardiovascular complications in HIV-infected patients. Reducing oxidative stress or inhibiting JNK activation may be new strategies for this purpose.
Supplementary Material
Acknowledgments
This work is partially supported by research grants from the National Institutes of Health (Yao: DE15543; and Chen: HL65916, HL72716), and by the Baylor College of Medicine, Houston, Texas. Authors have no conflict of interest. EFV was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Authors thank Dr. Shaoyu Yan for his technique assistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.EuroSIDA study group. Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Mu H, Chai H, Lin PH, Yao Q, Chen C. Current update on HIV-associated vascular disease and endothelial dysfunction. World J Surg. 2007;31:632–643. doi: 10.1007/s00268-006-0730-0. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–449. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 5.Parienti JJ, Massari V, Rey D, Poubeau P, Verdon R. SIROCCO study team. Efavirenz to nevirapine switch in HIV-1-infected patients with dyslipidemia: a randomized, controlled study. Clin Infect Dis. 2007;45:263–266. doi: 10.1086/518973. [DOI] [PubMed] [Google Scholar]
- 6.Squires K, Lazzarin A, Gatell JM, Powderly WG, Pokrovskiy V, Delfraissy JF, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36:1011–1019. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hulgan T, Morrow J, D'Aquila RT, Raffanti S, Morgan M, Rebeiro P, Haas DW. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–1717. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 8.Groeneveld AB. Vascular pharmacology of acute lung injury and acute respiratory distress syndrome. Vascul Pharmacol. 2002;39:247–256. doi: 10.1016/s1537-1891(03)00013-2. [DOI] [PubMed] [Google Scholar]
- 9.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381–5385. [PubMed] [Google Scholar]
- 10.Bonetti PO, Best PJ, Rodriguez-Porcel M, Holmes DR, Jr, Lerman LO, Lerman A. Endothelin type A receptor antagonism restores myocardial perfusion response to adenosine in experimental hypercholesterolemia. Atherosclerosis. 2003;168:367–373. doi: 10.1016/s0021-9150(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 11.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O’Donnell L, et al. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol Heart Circ Physiol. 1997;273:H286–H294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 12.Yan S, Wang X, Chai H, Wang H, Yao Q, Chen C. Secretoneurin increases monolayer permeability in human coronary artery endothelial cells. Surgery. 2006;140:243–251. doi: 10.1016/j.surg.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Mu H, Chai H, Liao D, Yao Q, Chen C. Human immunodeficiency virus protease inhibitor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am J Pathol. 2007;171:304–314. doi: 10.2353/ajpath.2007.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan S, Chai H, Wang H, Yang H, Nan B, Yao Q, Chen C. Effects of lysophosphatidylcholine on monolayer cell permeability of human coronary artery endothelial cells. Surgery. 2005;138:464–473. doi: 10.1016/j.surg.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Jamaluddin S, Yan S, Sheikh-Hamad D, Yao Q. Human stanniocalcin-1 blocks TNF-α-induced monolayer permeability in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:906–912. doi: 10.1161/ATVBAHA.108.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 17.Shah AM, Channon KM. Free radicals and redox signalling in cardiovascular disease. Heart. 2004;90:486–487. doi: 10.1136/hrt.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Lu XH, Yan S, Chai H, Yao Q. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem Biophys Res Commun. 2005;335:874–882. doi: 10.1016/j.bbrc.2005.07.155. [DOI] [PubMed] [Google Scholar]
- 19.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Developed by the Panel on Antiretroviral Guidelines for Adult and Adolescents. 2008 January 29; http://www.aidsinfo.nih.gov.
- 21.Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S, et al. Nitric oxide-dependent generation of reactive species in sickle cell disease: actin tyrosine nitration induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 22.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin AL, Thurston G, al Naemi H. Inhibition of nitric synthesis increases venular permeability and alters endothelial actin cytoskeleton. Am J Physiol. 1998;274:H1776–H1784. doi: 10.1152/ajpheart.1998.274.5.H1776. [DOI] [PubMed] [Google Scholar]
- 24.Hyun SK, Jung HA, Chung HY, Choi JS. In vitro peroxynitrite scavenging activity of 6-hydroxykynurenic acid and other flavonoids from Gingko biloba yellow leaves. Arch Pharm Res. 2006;29:1074–1079. doi: 10.1007/BF02969294. [DOI] [PubMed] [Google Scholar]
- 25.Grypioti AD, Kostopanagiotou G, Demopoulos CA, Roussos A, Mykoniatis M. Platelet activating factor (PAF) antagonism with ginkgolide B protects the liver against acute injury. Importance of controlling the receptor of PAF. Dig Dis Sci. 2008;53:1054–1062. doi: 10.1007/s10620-007-9982-2. [DOI] [PubMed] [Google Scholar]
- 26.Mondal D, Pradhan L, Ali M, Agrawal KC. HAART Drugs Induce Oxidative Stress in Human Endothelial Cells and Increase Endothelial Recruitment of Mononuclear Cells: Exacerbation by Inflammatory Cytokines and Amelioration by Antioxidants. Cardiovasc Toxicol. 2004;4:287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- 27.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.