Abstract
The congenital long QT syndrome (LQTS) is a heritable arrhythmia in which mutations in genes coding for ion channels or ion channel associated proteins delay ventricular repolarization and place mutation carriers at risk for serious or fatal arrhythmias. Triggers and therapeutic management of LQTS arrhythmias have been shown to differ in a manner that depends strikingly on the gene that is mutated. Additionally, beta-blockers, effective in the management of LQT-1, have been thought to be potentially proarrhythmic in the treatment of LQT-3 because of concomitant slowing of heart rate that accompanies decreased adrenergic activity. Here we report that the beta-blocker propranolol interacts with wild type (WT) and LQT-3 mutant Na+ channels in a manner that resembles the actions of local anesthetic drugs. We demonstrate that propranolol blocks Na+ channels in a use-dependent manner; that propranolol efficacy is dependent on the inactivated state of the channel; that propranolol blocks late non-inactivating current more effectively than peak sodium current; and that mutation of the local anesthetic binding site greatly reduces the efficacy of propranolol block of peak and late Na+ channel current. Furthermore our results indicate that this activity, like that of local anesthetic drugs, differs both with drug structure and the biophysical changes in Na+ channel function caused by specific LQT-3 mutations.
Keywords: Long QT syndrome, Na+ channels, Beta-blockers, Local anesthetics
1. Introduction
The long QT syndrome (LQTS) is a rare inherited disorder that is associated with an increased propensity to arrhythmogenic syncope, polymorphous ventricular tachycardia, and sudden cardiac death. To date at least 11 genes have been discovered that, when mutated, lead to LQTS, but LQTS variants 1–3 comprise the majority of documented genotyped LQTS to date [1–3]. LQT-1 and LQT-2, which are due to mutations in the potassium channel alpha subunits KCNQ1 and hERG respectively, make up approximately 80–90% of the genotyped cases whereas LQT-3, the variant due to mutations in SCN5A, the gene encoding the alpha subunit of the primary heart voltage-gated sodium channel, accounts for 5–8% of the known cases [4,5]. In the absence of genetic information, beta-blockers are the first line treatment for this disorder [6]. However, because the triggers for cardiac events in LQTS have been shown to be gene and mutation specific, and because the biophysical mechanisms underlying different variants of LQTS have been shown to be diverse, the therapeutic strategies for managing LQTS in genotyped patients have also emerged using a gene-specific approach [1].
In the case of LQT-1, life-threatening events occur most frequently during periods of sympathetic activation, allowing this subset of patients to be effectively protected by the use of anti-adrenergic therapies including beta-blockers [7,8]. Clinical studies thus far have shown that beta-blocker therapy is less effective in the treatment of LQT-2 and LQT-3 patients [6].
The prototypical LQT-3 mutation is characterized by a disruption in Na+ channel inactivation and a subsequent increase in inward current during the critical plateau phase of the cardiac action potential [9]. This mutation dependent inward current, termed late non-inactivating sodium current (INaL), has been shown to increase when channels are opened at slower frequencies [10]. Consistent with this idea, LQT-3 patients have an increased risk of fatal arrhythmia during periods of slow heart rate such as sleep due, at least in part, to a further increase in APD when heart rate slows [10,11].
Na+ channel blockers such as mexiletine and flecainide, which are members of the local anesthetic (LA) family of drugs, are currently believed to be the most effective treatment for LQT-3 patients due to preferential inhibition of INaL [1,12,13]. Given LQT-3 patients increased risk of arrhythmia during periods of slowed heart rate, beta-blockers would seem to be a potentially harmful course of treatment because of the slowing of heart rate that accompanies reduced adrenergic input. As early as the 1960s, beta-blockers have been thought to have LA-like activity [14–16]. This property of beta-blockers, particularly propranolol, has been further supported by its anti-arrhythmic efficacy [17]. However, while this LA activity has long been appreciated its molecular basis as well as the potential impact of propranolol treatment in LQT-3 patients, particularly via block of mutation--altered INaL, have been largely unexamined. Recent computational modeling work has suggested that a closer experimental examination of beta-blocker activity on LQT-3 patients may be necessary [18].
Here we show that the beta-blockers propranolol and carvedilol, but not metoprolol, block sodium current in a manner similar to the blocking of LA drugs. We examine closely the effects of propranolol on WT NaV1.5 channels as well as a series of mutant channels. We demonstrate that propranolol efficacy is dependent on the inactivated state of the channel; that propranolol blocks late non-inactivating current more effectively than peak sodium current; and that mutation of the LA binding site greatly reduces the efficacy of propranolol block of NaV1.5 channels. Our results reveal the molecular basis of beta-blocker modulation of heart Na+ channels and indicate that, like local anesthetic drugs, beta-blocker effects on Na+ channels differ with drug structure and the biophysical properties of inactivation that depend on specific LQT-3 mutations.
2. Methods
2.1. Electrophysiology
Site-directed mutagenesis was done on NaV1.5 in pcDNA3.1 using the Quik Change site-directed mutagenesis kit (Stratagene). Whole cell recordings were made on Human Embryonic Kidney (HEK) 293 cells expressing WT and mutant NaV1.5 channels along with hβ1 subunits (Lipofectamine, Invitrogen) [19].
Patch clamp procedures were used with the following internal solution (in mM): 50 aspartic acid, 60 CsCl, 5 Na2ATP, 11 EGTA, 10 HEPES, 4.27 CaCl2 (resulting in a final [Ca2+]i of 100 nM), and 1 MgCl2, pH 7.4 adjusted with CsOH. The external solutions for measurement of all Na+ channel activity contained (in mM): 130 NaCl, 2 CaCl2, 5 CsCl, 1.2 MgCl2, 10 HEPES, and 5 glucose, pH 7.4 adjusted with NaOH. The voltage dependence of inactivation was determined by measuring current at −10 mV after application of conditioning pulses (−130 mV to −20 mV for 5 s) applied once every 15 s. Currents were normalized to currents measured after the −130 mV conditioning pulse. Late non-inactivated sodium current (INaL) was measured as the tetrodotoxin (TTX; 50 μM)-sensitive current measured at 200 ms during depolarization to −10 mV. INaL was normalized to peak TTX-sensitive Na+ channel current measured at −10 mV and plotted as percentage of peak current in relevant figures. Steady-state UDB was reached in response to trains of 200 pulses with a pulse duration of 25 ms at frequencies indicated in the figure legends. UDB was measured as the ratio of peak current at −10 mV after and before application of a conditioning train and is reported as the percentage block of peak current. The drug block data was also normalized to the steady-state accumulation of inactivated channels at the end of 200 pulses in the absence of drug.
Mexiletine, propranolol (+/−), carvedilol, and metoprolol tartrate were purchased from Sigma (St Louis, Missouri). TTX was purchased from Ascent Scientific (UK). Drugs were applied locally to the outside of the cell being patched via homemade perfusion system using microfluidic valves (Lee Co, Essex, CT). Currents were measured at room temperature (~23 °C). Pipettes were borosilicate from VWR (West Chester, PA). Typical pipette resistance was between 1.5 and 3 MΩ. After whole cell configuration is achieved only cells with access resistance less than 7 MΩ are recorded. Membrane currents were measured with Axopatch 200B amplifiers (Axon Instruments, Foster City, CA). Capacitance and series resistance compensation were carried out using analog techniques according to the amplifier manufacturer (Axon Instruments, Foster City, CA). Only cells with access resistance and peak current that, after compensation, have voltage errors less than ~5 mV are used for analysis. PClamp8 (Axon Instruments) was used for data acquisition and initial analysis. Analysis was carried out in Excel (Microsoft), Origin 7.0 (Microcal Software, Northampton, MA), and programs written in Matlab (The Mathworks, Natick, MA). Analyzed data are shown as mean +/− S.E. M. Statistical significance was tested using Student’s t test; p<0.05 was considered statistically significant.
3. Results
3.1. Propranolol blocks wild type Na+ channels in a use-dependent manner: similarity to effects of local anesthetic drugs
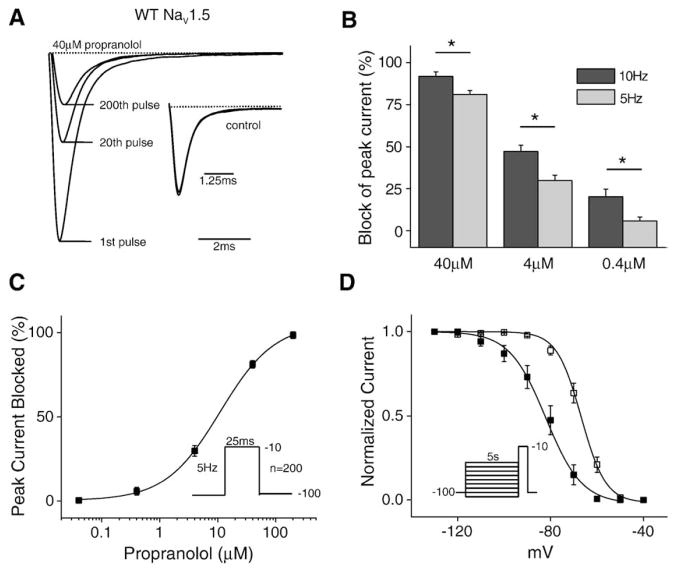
In order to determine whether beta-blockers such as propranolol, block Na+ channel currents similarly to LAs, we first investigated the effects of propranolol on Na+ channels expressed in human embryonic kidney (HEK) cells transfected with wild type (WT) channels and the beta subunit hβ1 using whole cell patch clamp procedures. To facilitate the comparison of the properties of propranolol on sodium channels with those of previously studied LA molecules, we chose first to examine the concentration dependence of use-dependent block (UDB) of the channel. Fig. 1A illustrates the average current traces measured in the absence (inset) and presence of 40 μM propranolol in response to a train of depolarizing voltage pulses applied at a stimulation frequency of 5 Hz after a stimulus-free period at the −100 mV holding potential. The arrows indicate the 1st, 20th, and 200th (final) pulses in the train. In the absence of drug (inset) there is a little change in current amplitude during the conditioning train, but in the presence of propranolol, block clearly develops in a pulse-dependent manner. The block reached steady state well in advance of the final trace in all cases. As is the case for LA drugs, increasing pulse frequency during the conditioning train increases UDB (Fig. 1B). Plotting the total block at 5 Hz vs. drug concentration reveals the IC50 (10.94 μM) for propranolol block of peak sodium current at a 5 Hz stimulation frequency (Fig. 1C).
Fig. 1.
Propranolol blocks cardiac sodium channels in a manner similar to that of LAs. (A) Average currents recorded in the presence and absence (inset) of 40 μM propranolol (n=8). Currents are normalized to the peak current in control conditions. The inset shows the accumulation of inactivated channels at the same three pulses (1st, 20th, and 200th), of which there is almost none, in response to the 5 Hz stimulation protocol. (B) Block of peak current at two stimulation frequencies (5 and 10 Hz) in the presence of three propranolol concentrations (n=4–8 cells). (C) UDB at a fixed pulse frequency (5 Hz) over a range of propranolol concentrations (40 nM to 200 μM). The smooth curves are the best fits of the Hill equation 1/{1+[(drug)/IC50]n} to the data. The estimated IC50 and n values obtained from the fit are 10.94 μM and 1.17 (n=4–8 cells). (D) Steady-state inactivation was measured with a 5 s conditioning pulse followed by a brief −10 mV test pulse. Propranolol caused a significant hyperpolarizing shift (15.3 +/− 3.5 mV, n=5) in the voltage-dependence of steady-state inactivation in the presence of 40 μM propranolol.
In addition to use-dependent block, another hallmark of lipophilic LA molecules is the ability to access the drug binding site, within the pore of the channel, without the channel opening. As a result, these drugs are able to bind the channel at voltages where the channel transitions from the closed to the inactive state without moving through the open state and alter the relationship between channel availability and voltage (steady-state inactivation). Fig. 1D shows the steady-state inactivation curve of WT channels in the presence and absence of 40 μM propranolol. Propranolol alters channel availability after conditioning pulses to voltages negative to −80 mV, voltages over which channels do not open. Thus, propranolol, like lipophilic LAs, interacts with the channel at hyperpolarized potentials, potentially by moving through the membrane, and shifts the channel availability curve to the left (control V1/2 = − 66.8 +/− 1.4 mV, k=5.3 +/− 0.28; drug V1/2 = − 82.1 +/− 3.2 mV, k=7.8 +/− 0.7). Fig. 1D shows that only ~85% of channels are available at −100 mV, and since the currents in Fig. 1A are the result of pulses to −10 mV from a holding potential of −100 mV, the decrease in channel availability at −100 mV contributes to the overall reduction in sodium current seen when the channel is exposed to propranolol.
3.2. The voltage-dependence of closed state Na+ channel inactivation affects propranolol efficacy
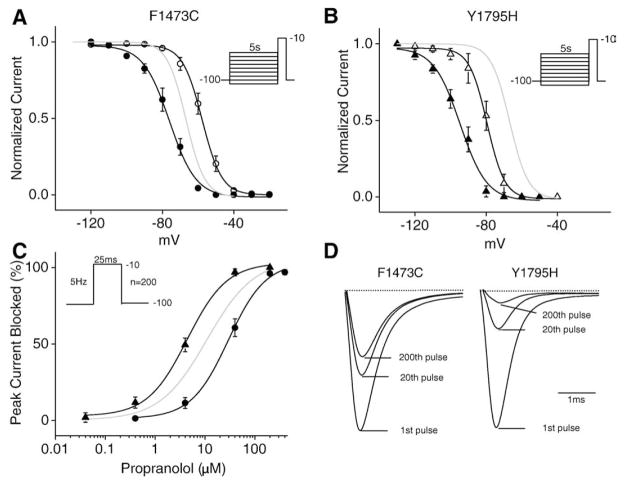
Previous work has shown that the efficacy of the LA drugs flecainide and mexiletine is modulated by inherited disease-related mutations that alter the voltage-dependence of steady-state Na+ channel inactivation. For example, mutations that shift the steady-state inactivation curve in the hyperpolarizing direction enhance Na+ channel block of channels by LAs [20]. Here we examine the effect of propranolol on two previously described Na+ channel mutations in order to determine whether propranolol efficacy is also affected by shifts in the voltage dependence of Na+ channel inactivation. We chose to investigate the F1473C [21] and Y1795H [22] mutations because, while both have previously been reported to increase INaL, the mutations have opposite effects on the voltage-dependence of steady-state inactivation. The effects of each mutation and propranolol on steady-state inactivation are illustrated in Figs. 2A and B. In each panel a solid grey curve represents WT steady-state inactivation; inactivation for each mutant is shown as open (control) and filled (propranolol) symbols. We confirm the previously-reported right shift in the availability curve caused by the F1473C mutation (V1/2 = −57.7 +/− 1.7 mV, k=5.5 +/− 0.2) and show that, in agreement with our WT data in Fig. 1D, and similar to the previously-reported effects of mexiletine and ranolazine, 40 μM propranolol shifts the closed state inactivation voltage range back towards the range of WT channels (Fig. 2A, F1473C in drug V1/2 = −71.8 +/− 6.7 mV, k=4.0 +/− 0.7). The Y1795H mutation shifts steady-state inactivation in the negative direction (V1/2 = −80.8 +/− 2.8 mV, k=6.2 +/− 0.8), and propranolol further left shifts this curve (Fig. 2B, Y1795H in drug V1/2 = −95.1 +/− 2.5 mV, k=7.5 +/− 0.4). From this, we would predict that, if propranolol were acting like an LA drug, the shifts in the voltage dependence of channel availability of these two mutants would have significant, but opposing, effects of the efficacy of drug block of the Na+ channel. The experiments summarized in Fig. 2C confirm this prediction: the IC50 value of propranolol UDB is critically dependent on the voltage dependence of inactivation (Fig. 2C; Y1795H IC50 =4.4 μM and F1473C IC50 =29.4 μM). Y1795H channels are more sensitive, while F1474C channels are less sensitive, than wild type channels (grey curve in Fig. 2C) to inhibition by propranolol. Average current traces shown in Fig. 2D illustrate the impact of these mutations on propranolol UDB block of measured currents.
Fig. 2.
Propranolol block of sodium channels is affected by the inactivated state of the channel. (A) F1473C causes a depolarizing shift (9.1 +/− 2.2 mV) in the steady-state availability curve (open circles, n=7). The addition of propranolol (40 μM) causes a 14.1 +/− 6.9 mV hyperpolarizing shift in the steady-state availability curve (filled circles, n=4). (B) The Brugada syndrome mutation Y1795H results in a hyperpolarizing shift (14.0 +/− 3.1 mV) in the steady-state availability curve (open triangles, n=4). Addition of propranolol (40 μM) causes a 14.3 +/− 3.8 mV hyperpolarizing shift in the steady-state inactivation curve (filled triangles, n=4). For both (A) and (B) the light grey line represents the steady-state inactivation curve for WT channels in the control solution. (C) UDB at 5 Hz over a range of propranolol concentrations (40 nM to 400 μM) for both Y1795H (filled triangles) and F1473C (filled circles) channels. The estimated IC50 and n values obtained from the fit to the Hill equation are 29.4 μM and 1.1 for F1473C channels and 4.4 μM and 1.2 for Y1795H channels (n=3–7 cells). (D) Average current recordings of 25 ms pulses to −10 mV at 5 Hz recorded in the presence of 40 μM propranolol for both F1473C and Y1795H channels (n=4).
3.3. Propranolol blocks late non-inactivating current more effectively than peak sodium current
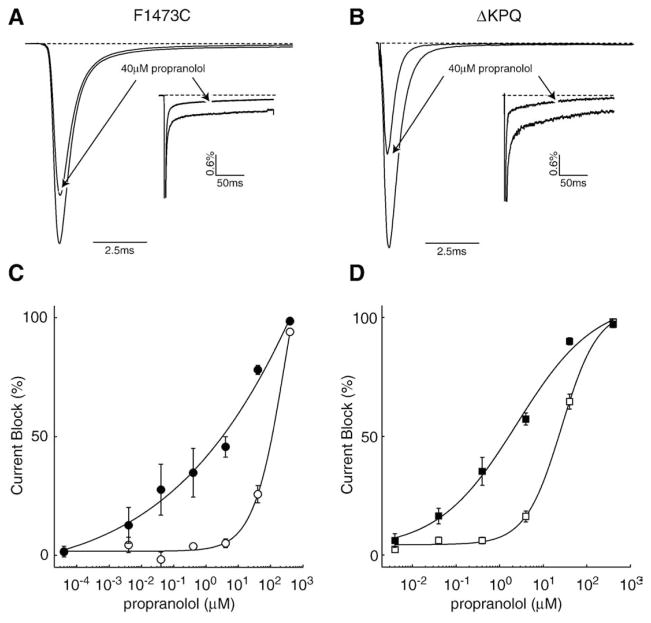
The therapeutic utility of LA drugs such as flecainide and mexiletine in the treatment of LQT-3 mutation carriers results from the demonstrated selectivity of these drugs for late non-inactivating Na+ channel activity compared with the effects of these drugs on peak Na+ channel currents [1,13]. Here we show, using both the F1473C LQT-3 mutation as well as the canonical LQT-3 mutation (ΔKPQ), that propranolol also shares this important hallmark of LAs. We chose these mutations because each has a large component of INaL that is frequently the cause of APD prolongation in LQT-3 patients. We detected preferential propranolol inhibition of INaL vs. peak current (Fig. 3A, B) in both mutants, which, when measured over a broad concentration range (Figs. 3C and D), indicates a roughly 10 fold difference in the IC50 of late vs. peak currents (F1473C: IC50-peak = 91.3 μM, IC50late = 10.3 μM; ΔKPQ: IC50peak = 28 μM, IC50late=2.4 μM), a difference that resembles those of drugs that interact with the LA receptor such as mexiletine, flecainide, and ranolazine [13,21,23]. The substantially lower IC50 value for peak block of the F1473C mutant channel shown here as compared to the data in Fig. 2C is also consistent with the use-dependent nature of propranolol. The high frequency stimulation protocol results in higher efficacy of block. These results suggest that the effects of propranolol may be additive with effects of LAs on INaL, a suggestion confirmed in supplemental data. We examined whether or not propranolol together with the local anesthetic mexiletine had an additive effect on the block of INaL. We showed that at a single dose for each drug, the combination of blockers was more effective at inhibiting INaL than either of the drugs alone (Supplemental Figs. 1A and B).
Fig. 3.
Propranolol preferentially blocks late non-inactivating current of LQT-3 mutant channels. Averaged currents from F1473C (A) and ΔKPQ (B) channels evoked by 200 ms depolarizing currents to −10 mV at 0.5 Hz in the presence and absence of 40 μM propranolol (n=4). An arrow indicates traces in the presence of drug. INaL measurements for each condition are normalized to the peak current of the respective condition. Peak current measurements are normalized to the peak of the control recording. Block of F1473C (C) and ΔKPQ (D) currents, over a wide range of propranolol concentrations (40pM to 400 μM), evoked by 200 ms depolarizing currents to −10 mV at 0.5 Hz. Open symbols show block of peak current using this protocol and filled symbols show block of INaL. The estimated IC50 and n values are 91.3 μM and 1.4 for F1473C and 28 μM and 0.48 for ΔKPQ for block of peak current and 10.3 μM and 0.3 for F1473C and 2.4 μM and 0.48 for ΔKPQ for block of INaL (n =4–5 cells).
3.4. Mutation of the local anesthetic binding site greatly reduces propranolol efficacy in block of NaV1.5 channels
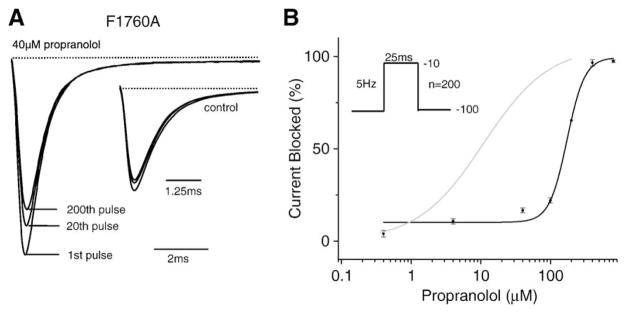
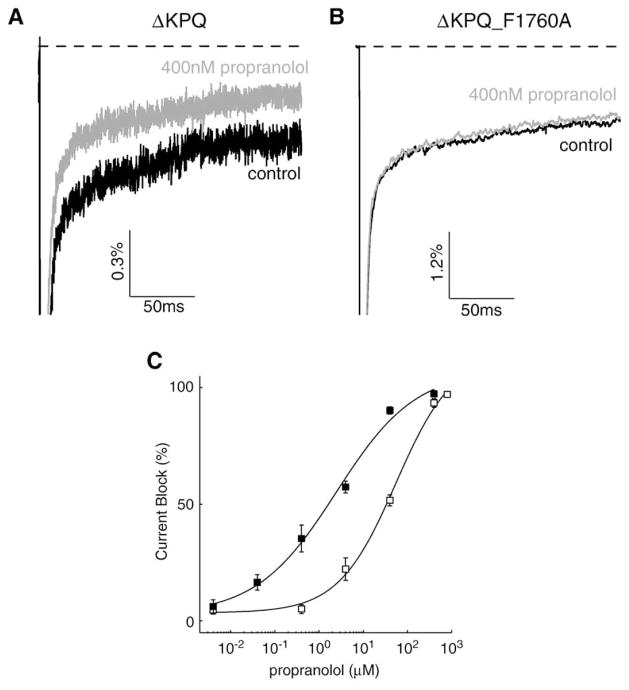
LA molecules have been shown to bind sodium channels in the inner mouth of the pore, and alanine scanning mutagenesis has identified key residues in this region that coordinate LA binding. These studies point to a phenylalanine in domain IV S6 at NaV1.5 residue 1760 (or equivalent positions in other Na+ channel isoforms) as a critical molecular determinant for LA block; mutation of this residue to an alanine (F1760A) greatly inhibits LA channel block [23–25]. Fig. 4A shows representative recordings from cells expressing F1760A channels in the presence and absence (inset) of 40 μM propranolol. As is the case for LA molecules, the F1760A mutation results in a marked reduction in the efficacy of propranolol block of peak current conducted by the channel. Fig. 4B summarizes the effect of propranolol on F1760A channels over a range of concentrations. The IC50 of the resulting curve reveals a greater than 15-fold reduction the drug efficacy (IC50 =172.7 μM). These data indicate that the F1760A mutation greatly reduces propranolol block of NaV1.5 channels and suggest that the effects of propranolol are mediated by the cardiac Na+ channel LA receptor binding site.
Fig. 4.
Mutation of the local anesthetic receptor residue (F1760A) reduces UDB of peak sodium channel current by propranolol. (A) Shown are averaged peak Na+ recordings F1760 channels in the presence and absence (inset) of 40 μM propranolol. Currents are normalized to the peak current in control conditions. (B) UDB at a fixed pulse frequency (5 over a range of propranolol concentrations (40 nM to 400 μM). Again the curves are the best fits of the Hill equation (see previous) to the data. The estimated IC50 and n obtained from the fit are 172.7 μM and 3.4 (n=4–5 cells).
In addition to disrupting peak current block of the sodium channel, mutation at F1760 also has been shown to reduce LA block of INaL [23]. Figs. 5A and B show block of ΔKPQ channels using 400 nM propranolol vs. block of channels that harbor both the ΔKPQ mutation as well as the F1760A LA binding site mutation. The double mutation results in a clear shift in the concentration range (Fig. 5C) over which propranolol is effective at blocking INaL (ΔKPQ: IC50late=2.4 μM; ΔKPQ_F1760A: IC50late=59.9 μM). These results demonstrate that propranolol interacts with the LA binding site and disruption of this interaction greatly reduces the efficacy of propranolol block for both peak current and non-inactivating late current.
Fig. 5.
Mutation at the LA receptor site (F1760A) also reduces block of INaL. Averaged currents from ΔKPQ (A) and ΔKPQ_F1760A (B) channels evoked by 200 ms depolarizing currents to −10 mV at 0.5 Hz in the presence and absence of 400 nM propranolol (n=4). (C) Block of INaL in ΔKPQ (filled squares) and ΔKPQ_F1760A (open squares) channels over a wide range of propranolol concentrations. The estimated IC50 and n values obtained are 2.4 μM and 0.48 for ΔKPQ and 59.9 μM and 0.57 for ΔKPQ_F1760A (n=4 cells).
3.5. Commonly used beta-blockers metoprolol and carvedilol show different efficacies for block of WT NaV1.5
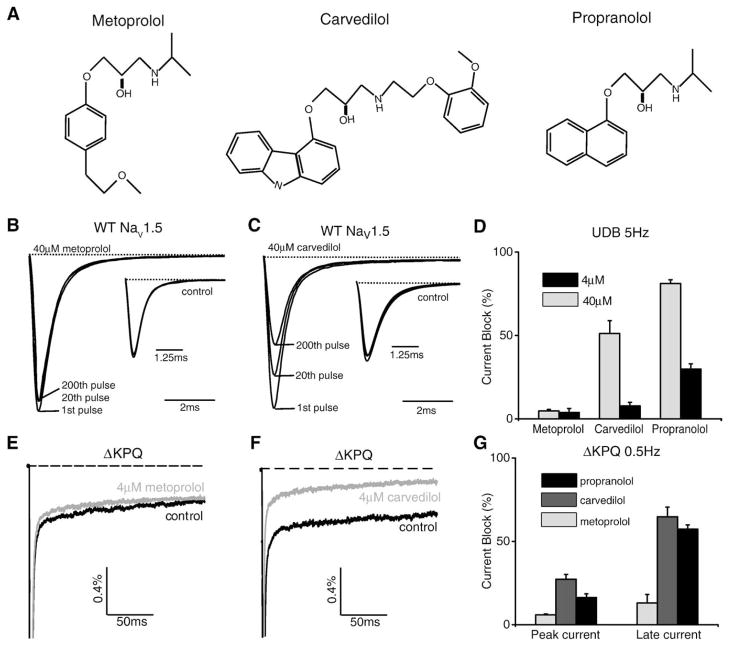
While propranolol is the most commonly prescribed beta-blocker for LQTS, other beta-blockers are used to treat patients for other more commonly occurring cardiovascular pathologies including congestive heart failure, hypertension, and atrial fibrillation. In order to see if LA-like activity is a universal effect of beta-blockers, we tested whether two commonly prescribed beta-blockers, metoprolol and carvedilol also block peak and late current through NaV1.5 channels. Fig. 6A shows the drug structures of the three compounds used in this study. In response to a 5 Hz stimulation train, carvedilol, but not metoprolol, blocks sodium channels in a use-dependent manner (Figs. 6B and C). In Fig. 6D the percent block at the end of the pulse train at both 4 and 40 μM is shown for each drug. The data show that the affinity of carvedilol for the Na+ channel is slightly lower than that of propranolol (block at 40 μM=51.2 +/− 7.7% for carvedilol and 81.2 +/− 2.2% for propranolol, block at 4 μM=7.8 +/− 2.0% for carvedilol and 29.8 +/− 3.2% for propranolol), but metoprolol has little LA activity even at 40 μM. Since INaL is critical to the pathology of LQT-3 as well as potentially important to other disease pathogenesis, such as ischemia-reperfusion injury and heart failure [26,27], we examined the block of INaL that results from application of 4 μM carvedilol and metoprolol (Figs. 6E and F). Carvedilol blocked the mutation induced INaL (Fig. 6G: 64.8 +/− 5.8% at 4 μM) whereas metoprolol had almost no effect (Fig. 6G: 13 +/− 5.2% at 4 μM). These concentrations are in excess of the likely therapeutic doses of these drugs. Previous work has suggested blood plasma concentrations that are around 50–200 nM for carvedilol and 200–600 nM for metoprolol [28,29]. Nevertheless, the data here provides additional biophysical evidence that beta-blockers are able to block disease causing INaL, but that the block will depend critically on drug structure. A more detailed comparison of these two drugs, and perhaps additional beta-blockers, may be interesting but is beyond the scope of this study.
Fig. 6.
Carvedilol, but not metoprolol, block sodium channel currents in a use-dependent manner. (A) Structural comparison of three beta-blockers metoprolol, carvedilol, and propranolol. (B, C) Average sodium currents recorded at 5 Hz stimulation in the presence and absence (inset) of 40 μM metoprolol and 40 μM carvedilol. The control recording in carvedilol (inset) is in 0.1% DMSO. (D) Plot shows the percent block of all three beta-blockers at both 4 μM and 40 μM (n=3–5). (E, F) Average sodium currents recorded from ΔKPQ channels at 0.5 Hz stimulation for 200 ms in the presence and absence of 4 μM metoprolol and carvedilol. The control recording in carvedilol is in 0.1% DMSO. (G) Plot shows the percent block of peak current vs. late current in ΔKPQ channels for all three beta-blockers at 4 μM (n=4–5).
4. Discussion
Beta-blockers have become first-line prophylactic therapy for the management of LQTS. While this approach is highly successful for the treatment of patients with LQT-1, clinical evidence suggests that patients with mutations in SCN5A show little reduction in the frequency of cardiac events when treated with beta-blockers [6,30]. In addition, there is significant evidence both at the channel level as well as the whole animal level that slow heart rates present an increased risk for potentially fatal arrhythmias [10,31]. Previous work has shown local anesthetic like activity of beta-blockers at doses higher than those required to reach maximal beta-blockade. The data we present here demonstrate that beta-blockers likely act at the local anesthetic binding site and block sodium channels with properties similar to those of local anesthetics: they block late current preferentially over peak current and the interaction depends critically on the inactivated state of the channel. The data presented here provide a biophysical and molecular explanation for the LA-like effect of beta-blockers that has been observed in many experimental setups over the last four decades. In addition, these results present interesting possibilities for the effects of beta-blocker therapy on LQT-3 patients.
Investigation of the biophysics and pharmacology of disease-associated mutant Na+ channels in heterologous expression systems as well as in genetically altered mice has shown the importance of mutation induced INaL in the pathophysiology of LQT-3. Pharmacological blockade of INaL using local anesthetics has become one of the most frequently used approaches for the management of LQT-3. Importantly, we have shown here that propranolol and carvedilol share this critical property of LA molecules. Our data indicate that the beta-blockers propranolol and carvedilol, block INaL current preferentially over peak Na+ current at an approximately 10–1 ratio, consistent with previous reports of preferential block of INaL using LAs [23].
Propranolol blocks Na+ channel activity in a manner that resembles LA block of Na+ channels: block is use-dependent and depends critically on the inactivated state of the channel. This observation, coupled with the preferential block of INaL over peak current suggested that the drug might interact with the channel through the same site as LA molecules. Previous studies have demonstrated that high-affinity binding of the LAs to the inactivated state of the Na+ channel relies on two critical amino acid residues, Phe-1760 and Tyr-1767, located in the IVS6 transmembrane segment of the voltage-gated Na+ channels [23,24,32]. We show that mutation of the critical phenylalanine at 1760 reduces the IC50 of the drug for the channel by more than an order of magnitude indicating that beta-blockers share a common site for drug block of sodium channels. Both the block of peak current as well as the block of INaL are affected by mutation at F1760. Recent work has shown that cationic LAs, which posses a titratable amine that carries positive charge at neutral pH, interact with the critical phenylalanine, F1759, in the case of rNaV1.4, via an electrostatic attraction to the negative electrostatic potential on the face of pore-lining aromatic side chains [33]. The presence of this secondary amine group (Fig. 6A) suggests an intriguing possibility for how these drugs interact with the sodium channel at the LA binding site.
In addition to a dramatic decrease in IC50 that results from mutation of the phenylalanine at 1760, the Hill coefficient increases approximately 3 fold (see figure captions of Figs. 1 and 4). While this may suggest cooperativity, care must be shown in interpreting these data: mutation of the LA site at 1760 may reveal multiple binding sites for propranolol. However, the F1760A mutation also influences channel gating and hence conformational changes of the NaV1.5 protein. This too may affect the steepness of the concentration-response curve as well as possible cooperativity among binding sites [34].
The potential therapeutic benefit for propranolol on LQT-3 patients may merit further study. Our data indicate that propranolol displays a higher potency for blocking INaL current over peak Na+ current in two LQT-3 mutant channels (ΔKPQ and F1473C) that promote INaL and are causally linked to LQT-3 in patients [9,21]. The preferential inhibition of late current occurs over a concentration range from 40 nM to 40 μM. Optimal doses for effects on beta-receptors have been shown to be in the 50–500 nM range [17]. While peak current would not be affected over this concentration range, inhibition of INaL does occur. This raises the possibility that propranolol could have an impact on patients harboring mutations that cause INaL even at relatively low doses of propranolol that are designed for optimal beta-blockade.
However, linking our results with the clinical effects of propranolol treatment must be made with caution. Propranolol, like many other anti-arrhythmic drugs, may have a nonspecific pharmacological profile and consequently will display complex effects in the myocardium. Previous studies have shown that hERG, a frequent target of many drugs, is blocked by propranolol with an IC50 of between 10 μM and 53 μM [35,36]. In addition, little is known about its direct effects on other channels such as the L-type calcium channel or Kir2.1. Another complicating feature of propranolol treatment in LQT-3 patients stems from the fact that it has been shown to have a complicated pharmacokinetic interaction with some local anesthetics [37]. It has been shown that, in rats, lidocaine concentrations in serum were significantly increased after co-administration of propranolol. Nevertheless, our finding that beta-blockers preferentially inhibit INaL, suggests that this action could act to offset potential proarrhythmic actions of these agents in treatment of LQT-3.
In addition to the treatment of LQT-3, beta-blockers are used to treat a large number of varied conditions, many of which are thought to involve sodium channels. There is some evidence that epilepsy, a condition that causes abnormal, excessive or synchronous neuronal activity in the brain, can be helped by administration of propranolol in conjunction with anti-convulsants [38,39]. This reduction in seizures is thought to be due to the LA-like properties of propranolol. Also, chronic heart failure increases late non-inactivating sodium current in ventricular myocytes leading to a greater sodium influx that contributes to abnormal repolarization and potential life-threatening arrhythmias [40]. Standard treatment for heart failure involves administrations of beta-blockers that are thought to exert their beneficial effects through beta-receptor antagonism, but any effect these drugs might have on normalizing late sodium current may help rescue normal repolarization and action potential duration in patients with chronic heart failure.
In conclusion, we find that the beta-blocking drugs such as propranolol inhibit cardiac Na+ channels in a use-dependent manner and preferentially inhibit late vs. peak Na+ channel currents. These effects, which are inhibited by mutation of the binding site for LA drugs, depend on both drug structure and the voltage-dependence of Na+ channel inactivation, which is altered differentially by disease causing mutations.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (1R01 HL-56810-15 to RSK and TG HL087745 support for JRB).
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.yjmcc.2009.05.012.
References
- 1.Kass RS, Moss AJ. Mutation-specific pharmacology of the long QT syndrome. Handb Exp Pharmacol. 2006;171:287–304. doi: 10.1007/3-540-29715-4_11. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007 Dec 19; doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006 Nov 14;114(20):2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 4.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000 Sep 5;102(10):1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, et al. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. Jama. 2005 Dec 21;294(23):2975–80. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000 Feb 15;101(6):616–23. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 7.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, et al. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation. 2009 Jan 20;119(2):215–21. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype–phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001 Jan 2;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 9.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995 Aug 24;376(6542):683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 10.Clancy CE, Tateyama M, Kass RS. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J Clin Invest. 2002 Nov;110(9):1251–62. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995 Dec 15;92(12):3381–6. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, et al. Safety and efficacy of flecainide in subjects with Long QT-3 syndrome (DeltaKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol. 2005 Oct;10(4 Suppl):59–66. doi: 10.1111/j.1542-474X.2005.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagatomo T, January CT, Makielski JC. Preferential block of late sodium current in the LQT3 DeltaKPQ mutant by the class I(C) antiarrhythmic flecainide. Mol Pharmacol. 2000 Jan;57(1):101–7. [PubMed] [Google Scholar]
- 14.Morales Aguilera A, Vaughanwilliams EM. The effects on cardiac muscle of beta-receptor antagonists in relation to their activity as local anaesthetics. Br J Pharmacol Chemother. 1965 Apr;24:332–8. doi: 10.1111/j.1476-5381.1965.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray KT, Reilly C, Koshakji RP, Roden DM, Lineberry MD, Wood AJ, et al. Suppression of ventricular arrhythmias in man by d-propranolol independent of beta-adrenergic receptor blockade. J Clin Invest. 1990 Mar;85(3):836–42. doi: 10.1172/JCI114510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sada H, Ban T. Frequency-dependent block of nerve conduction by beta-adrenergic blocking agents. Arch Int Pharmacodyn Ther. 1981 Nov;254(1):134–44. [PubMed] [Google Scholar]
- 17.Duff HJ, Mitchell LB, Wyse DG. Antiarrhythmic efficacy of propranolol: comparison of low and high serum concentrations. J Am Coll Cardiol. 1986 Oct;8(4):959–65. doi: 10.1016/s0735-1097(86)80441-7. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens-Nicklas RC, Clancy CE, Christini DJ. Re-evaluating the efficacy of {beta}-adrenergic agonists and antagonists in long QT-3 syndrome through computational modelling. Cardiovasc Res. 2009 Mar 24; doi: 10.1093/cvr/cvp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abriel H, Cabo C, Wehrens XH, Rivolta I, Motoike HK, Memmi M, et al. Novel arrhythmogenic mechanism revealed by a long-QT syndrome mutation in the cardiac Na(+) channel. Circ Res. 2001 Apr 13;88(7):740–5. doi: 10.1161/hh0701.089668. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Tateyama M, Clancy CE, Abriel H, Kass RS. Channel openings are necessary but not sufficient for use-dependent block of cardiac Na(+) channels by flecainide: evidence from the analysis of disease-linked mutations. J Gen Physiol. 2002 Jul;120(1):39–51. doi: 10.1085/jgp.20028558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankston JR, Yue M, Chung W, Spyres M, Pass RH, Silver E, et al. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS ONE. 2007;2(12):e1258. doi: 10.1371/journal.pone.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P, et al. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem. 2001 Aug 17;276(33):30623–30. doi: 10.1074/jbc.M104471200. [DOI] [PubMed] [Google Scholar]
- 23.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006 May;148(1):16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994 Sep 16;265(5179):1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na(+) channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121(3):199–214. doi: 10.1085/jgp.20028723. 2003/03// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005 Mar;38(3):475–83. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium–calcium overload. Heart. 2006 Jul;92(Suppl 4):iv1–5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenero DM, Henderson LS, Baidoo CA, Harter AH, Campanile AM, Danoff TM, et al. Pharmacokinetic properties of a new controlled-release formulation of carvedilol. Am J Cardiol. 2006 Oct 2;98(7A):5L–16L. doi: 10.1016/j.amjcard.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Talbert RL. Pharmacokinetics and pharmacodynamics of beta blockers in heart failure. Heart Fail Rev. 2004 Apr;9(2):131–7. doi: 10.1023/B:HREV.0000046368.08825.20. [DOI] [PubMed] [Google Scholar]
- 30.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. Jama. 2004 Sep 15;292(11):1341–4. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 31.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, et al. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001 Sep;7(9):1021–7. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 32.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahern CA, Eastwood AL, Dougherty DA, Horn R. Electrostatic contributions of aromatic residues in the local anesthetic receptor of voltage-gated sodium channels. Circ Res. 2008 Jan 4;102(1):86–94. doi: 10.1161/CIRCRESAHA.107.160663. [DOI] [PubMed] [Google Scholar]
- 34.Colquhoun D. Binding, gating, affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998 Nov;125(5):924–47. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupuis DS, Klaerke DA, Olesen SP. Effect of beta-adrenoceptor blockers on human ether-a-go-go-related gene (HERG) potassium channels. Basic Clin Pharmacol Toxicol. 2005 Feb;96(2):123–30. doi: 10.1111/j.1742-7843.2005.pto960206.x. [DOI] [PubMed] [Google Scholar]
- 36.Yao X, McIntyre MS, Lang DG, Song IH, Becherer JD, Hashim MA. Propranolol inhibits the human ether-a-go-go-related gene potassium channels. Eur J Pharmacol. 2005 Sep 20;519(3):208–11. doi: 10.1016/j.ejphar.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Saranteas T, Mourouzis C, Koumoura F, Tesseromatis C. Effects of propranolol or paracetamol on lidocaine concentrations in serum and tissues. J Oral Maxillofac Surg. 2003 May;61(5):604–7. doi: 10.1053/joms.2003.50090. [DOI] [PubMed] [Google Scholar]
- 38.Mayer T, Specht U. Propranolol in startle induced epileptic seizures. J Neurol Neurosurg Psychiatry. 1995 Mar;58(3):382–3. doi: 10.1136/jnnp.58.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renshaw PF, Ford HE, Brotman AW. Possible synergistic anticonvulsant effect of propranolol and carbamazepine. Am J Psychiatry. 1990 Dec;147(12):1687–8. doi: 10.1176/ajp.147.12.1687b. [DOI] [PubMed] [Google Scholar]
- 40.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999 Mar;55(3):494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.