Abstract
Previous reports suggest that γ-aminobutyric acid type A (GABAA) receptors containing α1 subunits may play a pivotal role in mediating the discriminative stimulus effects of benzodiazepines (BZs). L-838,417 (7-tert-Butyl-3-(2,5-difluoro-phenyl)-6-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)-[1,2,4]triazolo[4,3-b]pyridazine) is a GABAA receptor modulator with intrinsic efficacy in vitro at α2, α3, and α5 subunit-containing GABAA receptors, and little demonstrable intrinsic efficacy in vitro at α1 subunit-containing GABAA receptors. The present study evaluated the discriminative stimulus effects of L-838,417 in order to determine the extent to which the α2, α3, and α5 subunit-containing GABAA receptors contribute to the interoceptive effects of BZ-type drugs. Squirrel monkeys (Saimiri sciureus) were trained to discriminate L-838,417 (0.3 mg/kg, i.v.) from vehicle under a 5-response fixed-ratio schedule of food reinforcement. Under test conditions, L-838,417 administration resulted in dose-dependent increases in drug lever responding that were antagonized by the BZ-site antagonist, flumazenil. Administration of non-selective BZs, compounds with 10-fold greater affinity for α1 subunit-containing GABAA receptors compared to α2, α3, and α5 subunit-containing GABAA receptors, barbiturates and ethanol (which modulate the GABAA receptor via a non-BZ site), all resulted in a majority of responses on the L-838,417-paired lever (65–100% drug-lever responding). βCCT, an antagonist that binds with 20-fold greater affinity for α1 subunit-containing GABAA receptors relative to α2, α3, and α5-containing GABAA receptors, had no significant effect on the discriminative stimulus effects of L-838,417 or the L-838,417-like effects of diazepam or zolpidem. These data suggest that efficacy at α2, α3, and/or α5 subunit-containing GABAA receptors likely are sufficient for engendering BZ-like discriminative stimulus effects.
1. Introduction
Benzodiazepines (BZs) commonly are prescribed for treating anxiety, sleep, and seizure disorders despite undesirable side effects such abuse and dependence liability (Korpi et al., 1997; Verster et al., 2002). BZs and related drugs exert their behavioral effects by acting as positive allosteric modulators of the γ-aminobutyric acid type A (GABAA) receptor. Moreover, multiple subtypes of the GABAA receptor exist, and recent research has focused on understanding the role of BZ-sensitive GABAA receptors (i.e., receptors containing α1, α2, α3, and α5 protein subunits) in the behavioral effects of BZ-type drugs (cf. Ator, 2005; Rowlett et al., 2005).
The abuse-related effects of drugs are influenced to a large degree by their interoceptive effects, which often are modeled in the laboratory using drug discrimination procedures. In general, drugs that share discriminative stimulus effects with the training drug (i.e. substitute for the training drug) likely share similar mechanisms of action (Lelas et al., 2000b; 2001a; Ator, 2005). Drug discrimination studies in which BZ-type drugs were used as the training drug suggest that GABAA receptors containing 1 subunits (α1GABAA receptors) may play a key role in mediating the interoceptive effects of BZs (e.g., Lelas et al., 2000b; Kohut and Ator, 2008). For instance, zolpidem, a compound with 10-fold greater affinity for α1 subunit-containing GABAA receptors compared to α2, α3, and α5 subunit-containing GABAA receptors, shared discriminative stimulus effects with non-selective BZs (e.g., Ator and Griffiths, 1989; Rowlett et al., 1999; Rush et al., 2000; Mirza et al., 2002). Moreover, antagonists that bind with approximately 20-fold greater affinity for α1 subunit-containing GABAA receptors relative to α2, α3, and α5 subunit-containing GABAA receptors blocked the discriminative stimulus effects of diazepam, triazolam, and zolpidem (e.g., Shannon et al., 1988; Lelas et al., 2002; Rowlett et al., 2003).
Other studies have suggested a more complex mechanism underlying the transduction of the BZ discriminative stimulus. For example, non-selective BZs substitute fully for zolpidem at low to intermediate training doses; however, these drugs did not substitute for higher training doses of zolpidem (Rowlett et al., 2000; 2003). In fact, only other compounds with relative selectivity for α1GABAA receptors substituted for the high-dose zolpidem discriminative stimulus, suggesting a training dose-related pharmacological specificity (Rowlett et al., 2000). These results raise the possibility that interoceptive effects mediated by the α1GABAA receptor may be unique compared with those of non-selective BZs. A possible implication of this observation is that under certain conditions, GABAA receptor subtypes other than the α1GABAA subtype may contribute to the discriminative stimulus effects of BZs.
In order to investigate the role of different GABAA receptor subtypes to the discriminative stimulus effects of BZ-type drugs, we took the approach of training monkeys to discriminate L-838,417, a compound with “functional selectivity” for α2GABAA, α3GABAA, and α5GABAA receptor subtypes. L-838,417 binds with equal affinity to α1GABAA, α2GABAA, α3GABAA, or α5GABAA receptor subtypes, but not to those GABAA receptors containing α4 or α6 subunits (McKernan et al., 2000). Electrophysiological studies with cloned GABAA receptors have demonstrated that L-838,417 is essentially an antagonist (i.e., has limited demonstrable intrinsic efficacy) at α1GABAA receptors, but is a partial agonist with equal efficacy at the α2GABAA, α3GABAA, and α5GABAA receptor subtypes. Thus, effects of L-838,417 might be attributed to α2GABAA, α3GABAA, and/or α5GABAA receptor subtypes, but not α1GABAA, receptors.
Consistent with the unique in vitro pharmacology of L-838,417, this compound has a unique profile of behavioral effects in both rodents and non-human primates (McKernan et al., 2000; Rowlett et al., 2005). In this regard, L-838,417 engendered anxiolytic activity in the absence of significant motor effects (McKernan et al., 2000; Rowlett et al., 2005). Considerable evidence suggests that the anxiolytic-like effect of BZ-type ligands is mediated by α2 and/or α3GABAA receptors (Löw et al., 2000; Collins et al., 2002; Atack et al., 2005; Dias et al., 2005), while the motor-impairing effects are mediated primarily by α1GABAA receptors (Rudolph et al., 1999; McKernan et al., 2000; Rudolph et al., 2001).
In the present report, we first established L-838,417 as a discriminative stimulus in squirrel monkeys using procedures described previously (e.g., Spealman, 1985; Lelas et al., 2002; Rowlett et al., 1999; 2003) in order to facilitate comparisons with the earlier data sets. The effects of L-838,417 dose, time after infusion, and sensitivity to the BZ-site antagonist flumazenil were assessed next. In order to determine the extent to which the L-838,417 stimulus was similar to that of conventional BZs, we evaluated the ability of representative BZs to engender L-838,417-like discriminative stimulus effects. Next, we evaluated the L-838,417-like discriminative stimulus effects of non-BZs, including agonists with preferential activity at α1GABAA receptors (zaleplon, zolpidem, and CL 218,872). These compounds were employed specifically to evaluate the hypothesis that agonists with α1GABAA selectivity would not share discriminative stimulus effects with L-838,417. The final series of experiments assessed the extent to which an antagonist with ~20-fold greater affinity for α1GABAA receptors, β-carboline-3-carboxylate-t-butyl ester (βCCT), blocked the discriminative stimulus effects of L-838,417 as well as the L-838,417-like effects of representative compounds.
2. Materials and Methods
2.1 Subjects
Seven adult squirrel monkeys (Saimiri sciureus), weighing 800 g to 950 g, were studied in daily sessions (Monday through Friday). Monkeys were maintained at 90–95% of their free-feeding body weight by adjusting their access to food in the home cage (Teklad Monkey Diet, supplemented with fresh fruit). Between sessions, all monkeys were housed individually and maintained under a 12 hr light/dark cycle in a temperature- and humidity-controlled room. All procedures were conducted with the approval and under the supervision of the Harvard University Institutional Animal Care and Use Committees. Animals in this study were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for Care and Use of Laboratory Animals” National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85–23, revised 1996.
2.2. Surgical Procedure
Monkeys were prepared with a chronic indwelling venous catheter (polyvinyl chloride; i.d., 0.38 mm; o.d., 0.76 mm) using the general surgical procedures described by Platt et al. (2005a). Under isoflurane anesthesia and aseptic conditions, one end of a catheter was passed to the level of the right atrium by way of a femoral or jugular vein. The distal end of the catheter was passed subcutaneously and exited in the mid-scapular region. Catheters were flushed daily with saline and were sealed with stainless steel obturators when not in use. Monkeys wore custom-made nylon-mesh jackets (Lomir Biomedical, Toronto, ON, Canada) at all times to protect the catheter.
2.3. Apparatus
Experimental sessions were conducted in ventilated and sound-attenuating chambers (Med Associates; Georgia, VT). Monkeys were seated in primate chairs facing a panel that included two response levers, lights, and a food tray. Each press of a lever with a minimum downward force of approximately 0.25 N produced an audible click and was recorded as a response. The colored lights mounted above the levers could be illuminated to serve as visual stimuli. Food pellets (Bioserve Precision pellets, 190 mg; Bioserve, Frenchtown, NJ) were delivered to the tray located between the levers.
2.4. Drug Discrimination Training
Squirrel monkeys were trained to discriminate L-838,417 from saline using the procedures described previously (Lelas et al., 2001a; 2002). Monkeys initially were trained to respond on each of two levers under an FR 5 schedule of food reinforcement. Once consistent lever pressing was established, the monkeys were implanted with intravenous catheters, and drug discrimination training was started 5 days after recovery from surgery. After an i.v. injection of the training dose of L-838,417 (0.3 mg/kg), 5 consecutive responses on one lever produced a food pellet, whereas after an i.v. injection of saline, 5 consecutive responses on the other lever produced a pellet. For three of the monkeys, responding on the right lever after an injection of L-838,417 resulted in pellet delivery. For the other monkeys, responding on the left lever after injection of L-838,417 resulted in pellet delivery. Delivery of each pellet was followed by a 10-s timeout period. Responses on the incorrect lever (e.g., the saline-appropriate lever after L-838,417 injection) reset the FR requirement.
Training sessions consisted of a variable number of components (n = 1 – 4) of the FR schedule. The number of components per session was randomized from day-to-day with the restriction that each number occurred equally often within a block of 20 sessions. Each component ended after 10 food pellets had been delivered or after 5 min had elapsed, whichever occurred first. A 10-min timeout period, during which the lights were off and responses had no programmed consequences, preceded each component. During most training sessions, saline was injected during timeout periods preceding the first n − 1 components, and L-838,417 was injected before the nth component of the session. Periodically, saline was injected before all components of a training session to prevent an invariant association between the fourth component and L-838,417 injection. Injections of L-838,417 or saline were administered from outside the chamber via a catheter extension during the 5th min of the 10-min timeout periods. Each injection was followed by a 1-ml infusion of saline to flush the catheter of any residual drug solution.
2.5. Testing
Test sessions were conducted once or twice per week with training sessions scheduled on intervening days. Test sessions were conducted only if ≥80% of responses were made on the injection-appropriate lever during at least four of the preceding five training sessions. In general, test sessions consisted of four FR components, each preceded by a 10-min timeout period. During each component, completion of 10 consecutive responses on either lever produced food. Dose-response functions were determined for test drugs using a cumulative dosing procedure. The drugs studied using this procedure were L-838,417, the non-selective BZ receptor ligands triazolam, lorazepam, midazolam, diazepam, chlordiazepoxide, and flumazenil, the α1GABAA ligands zaleplon, zolpidem, CL 218,872, and βCCT, and the reference compounds amobarbital, pentobarbital (barbiturates), ethanol (GABAA receptor modulator), muscimol (direct-acting GABAA agonist), and morphine (mu opioid agonist). Under the cumulative dosing procedure, incremental doses of each drug (1/4 – 1/2 log increments) were injected i.v. during timeout periods that preceded sequential FR components, permitting a four-point cumulative dose-response function to be determined in a single session. When warranted, five or more different doses of a drug were studied by administering overlapping ranges of cumulative doses during test sessions on different days. The effects of most doses were determined twice, although low doses that were found to be inactive and high doses that produced adverse effects usually were studied only once in each subject.
Antagonism studies were carried out with the modestly α1GABAA-preferring antagonist βCCT and the non-selective BZ antagonist flumazenil. Studies with βCCT and flumazenil were conducted by administering βCCT (3.0 mg/kg, i.v.) or flumazenil (1.0 mg/kg, i.v.) immediately before the session, followed by cumulative doses of L-838,417, diazepam, or zolpidem as described above. The doses of βCCT and flumazenil were chosen on the basis of earlier studies that found them to be effective at antagonizing the DS effects of zolpidem and diazepam in squirrel monkeys when administered using procedures similar to those described here (Rowlett et al., 1999; Lelas et al., 2001a; 2002).
2.6. Analysis of drug effects
Percentage of L-838,417-lever responding was computed for individual subjects in each component of a test session by dividing the number of responses on the L-838,417 lever by the total number of responses on both levers and multiplying by 100. Percentage of L-838,417-lever responding was calculated for an individual monkey only if the response rate was > 0.1 responses/s during the component. Mean percentage of L-838,417-lever responding and standard error of the mean (SEM) were then calculated for the group of monkeys at each dose. A drug was considered to substitute fully for L-838,417 if the maximum mean percentage of drug-lever responding was ≥ 80%. The doses of drug estimated to engender 50% L-838,417-appropriate responding (ED50) were determined for individual subjects by linear regression analysis in cases where the log dose-response function was defined by at least three data points or by linear interpolation in cases where the dose-response function was defined best by two points.
The overall rate of responding in each component was computed by dividing the total number of responses in a component (regardless of lever) by the total component duration. Responding during the timeout, as well as the timeout duration, was not used in the calculation of rate of responding. Rate of responding was converted to percentage of control by dividing an individual animal’s rate after drug by the animal’s average response rate during the saline training components before the test (the previous 3 saline training components were used), and multiplying by 100. Mean response rate (percentage of control SEM) then was calculated for the group at each dose.
The effects of each drug on response rate were analyzed by separate repeated measures ANOVAs. Further analysis was performed using Dunnett’s q statistic, which compares the effects of different doses of each drug with vehicle control. The significance of the βCCT-induced shifts in the L-838,417, diazepam, and zolpidem dose-response functions was evaluated using separate repeated measures two-way ANOVAs followed by Bonferroni t-tests. Finally, in the few instances when only two treatments were being compared (see Results), one-sample t-tests were used. The alpha level for all statistical tests was p<0.05.
2.7. Drug Preparation
The base forms of L-838,417 (7-tert-Butyl-3-(2,5-difluoro-phenyl)-6-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)-[1,2,4]triazolo[4,3-b]pyridazine); Merck, Sharp, and Dohme Research Laboratories; Harlow Essex, UK), triazolam, zolpidem, flumazenil (Sigma; St. Louis, MO), lorazepam (Wyeth-Ayerst; St. Davids, PA), and diazepam (Hoffman-La Roche; Nutley, NJ) were prepared in a vehicle of 50% propylene glycol (Fisher Scientific; Suwanee, GA) and 50% saline. βCCT (synthesized at the University of Wisconsin, Milwaukee, WI), zaleplon, CL 218,872 (Sigma), and bretazenil (Hoffman-La Roche) were prepared in a vehicle of 80% propylene glycol/10% ethanol/10% saline. Chlordiazepoxide, midazolam (Research Biochemicals; Natick, MA), muscimol (Hoffman-La Roche), and morphine (Sigma) were prepared in 0.9% saline. Amobarbital and pentobarbital (Sigma) were dissolved in sterile water. Ethanol (95%) (Pharmco Products; Brookfield, CT) was diluted with 0.9% saline solution to a concentration of 0.5 g/ml before injection. All drugs were injected in a volume of 0.1–1.0 ml/kg, depending on the dose and solubility, and were prepared the day of a test session.
3. Results
3.1. L-838,417 Discrimination
The average number of sessions required to establish reliable stimulus control by L-838,417 (0.3 mg/kg) was 105 (± 18.48 SEM; range 37–177). During training sessions throughout the course of the study the rates of responding following an injection of L-838,417 were the same as those observed following injections of saline (0.36 ± 0.03).
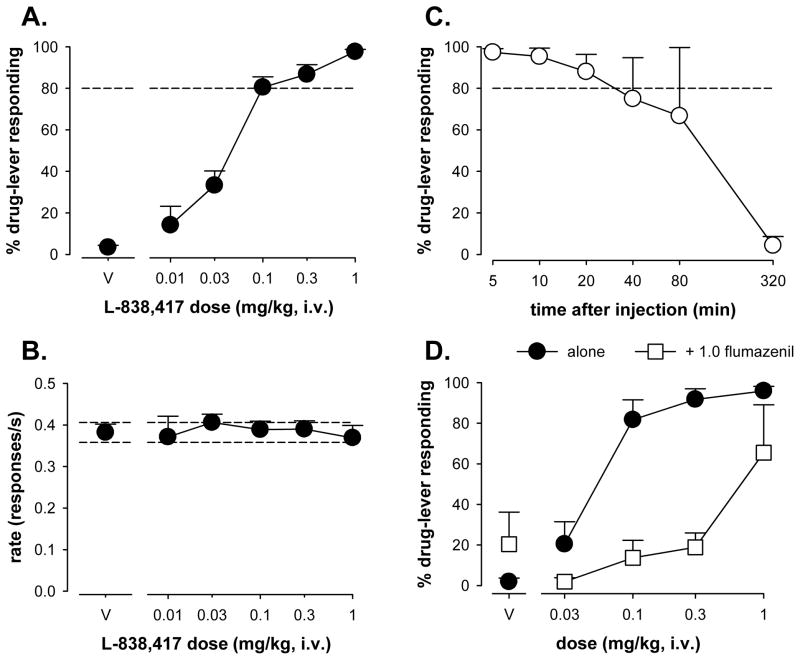
Under test conditions, cumulative doses of L-838,417 (0.01–1 mg/kg) engendered dose-dependent increases in the percentage of responding on the L-838,417-associated lever (Fig. 1A). Doses on the lower range of the dose-response function (0.01–0.03 mg/kg) engendered responding predominantly on the saline-associated lever, whereas doses ≥0.1 mg/kg engendered L-838,417-lever responding almost exclusively (≥80%). The mean ED50 was calculated to be 0.052 (SEM= 0.01; Table 1). As shown in Fig. 1B, administration of increasing doses of L-838,417 had no effects on the average rates of responding.
Figure 1.
A. Percentage of drug-lever responding (± SEM) for L-838,417 and B. effects on response rate (± SEM) for increasing doses of L-838,417 in squirrel monkeys (N=7) trained to discriminate L-838,417 (0.3 mg/kg) from vehicle. C. Percentage of drug-lever responding (± SEM) engendered by the training dose of L-838,417 (0.3 mg/kg) as a function of pretreatment time (N=3). D. Percentage of drug-lever responding (± SEM) for L-838,417 in the presence and absence of the benzodiazepine receptor antagonist flumazenil. Flumazenil was administered i.v. immediately before sessions in which cumulative doses of L-838,417 were tested.
Table 1.
Potencies of test compounds to engender discriminative stimulus effects (mean % L-838,417-appropriate responding ±SEM) and suppression of rates of responding (mean response rate ±SEM) in squirrel monkeys trained to discriminate L-838,417 (0.3 mg/kg, i.v.) from saline. Data are from N=3–7 monkeys.
| % L-838,417- appropriate responding | Response rate (responses/s) | ||
|---|---|---|---|
| Compound | Mechanism | ED50 (mg/kg, i.v.) | |
| L-838,417 | α2, α3, α5GABAA partial agonist/α1GABAA antagonist (training compound) | 0.052 ± 0.010 | NE (3.0)* |
| triazolam | Non-selective benzodiazepine (BZ) | 0.0043 ± 0.0001 | 0.060 ± 0.023 |
| lorazepam | Non-selective BZ | 0.035 ± 0.011 | NE (1.0) |
| midazolam | Non-selective BZ | 0.081 ± 0.034 | 0.32 ± 0.15 |
| diazepam | Non-selective BZ | 0.20 ± 0.043 | 1.1 ± 0.41 |
| chlordiazepoxide | Non-selective BZ | 1.31 ± 0.089 | NE (10.0) |
| zaleplon | α1GABAA-preferring agonist | 0.085 ± 0.040 | 0.12 ± 0.010 |
| zolpidem | α1GABAA-preferring agonist | 0.21 ± 0.083 | 0.15 ± 0.050 |
| CL 218,872 | α1GABAA-preferring partial agonist | 2.22 ± 1.27 | NE (10.0) |
| bretazenil | Non-selective BZ partial agonist | NC** | NE (1.0) |
| flumazenil | BZ antagonist (weak partial agonist) | 1.19 ± 0.68 | NE (3.0) |
| amobarbital | Barbiturate | 4.86 ± 1.93 | NE (10.0) |
| pentobarbital | Barbiturate | 2.51 ± 0.54 | NE (5.6) |
| ethanol | GABAA modulator | 0.77 ± 0.32 | NE (1.25 g/kg) |
| morphine | Opioid agonist | NE (1.0) | 0.50 ± 0.021 |
| muscimol | GABAA direct-acting agonist | NE (0.3)*** | NE (0.3)*** |
NE, Not effective up to the dose shown in parenthesis (dose in mg/kg, unless otherwise stated).
NC, Not calculated due to most subjects not clearly showing effects above and below 50%.
Higher doses induce seizures in squirrel monkeys.
In the experiment in which the pretreatment time for L-838,417 was varied, the maximal percentage of drug-lever responding occurred when the training dose of L-838,417 (0.3 mg/kg) was administered 5 min before the session (97%, SEM=1.8, Fig. 1C). The mean percentage of L-838,417-lever responding decreased at all subsequent pretreatment times; however, responding was at or near 80% for up to 40 min after the injection (mean = 75%, SEM= 19.80) and responding remained on the L-838,417-appropriate lever at 80 min after the injection (66.7%, SEM= 33). L-838,417-lever responding was comparable to that engendered by saline by 320 min (4%, SEM= 4).
Pretreatment with flumazenil (1.0 mg/kg) prior to re-determination of the L-838,417 dose-response function resulted in a shift to the right in the dose-response function for discriminative stimulus effects (Fig. 1D). There was a 14-fold rightward shift in the ED50 values when comparing the mean ED50 (±SEM) for L-838,417 alone (0.052 ± 0.02) to that following pretreatment with 1.0 mg/kg flumazenil (0.73 ± 0.34). Rates of responding were not affected by pretreatment with flumazenil (data not shown).
3.2. Effects of Non-Selective Benzodiazepine Type Compounds and Reference Drugs
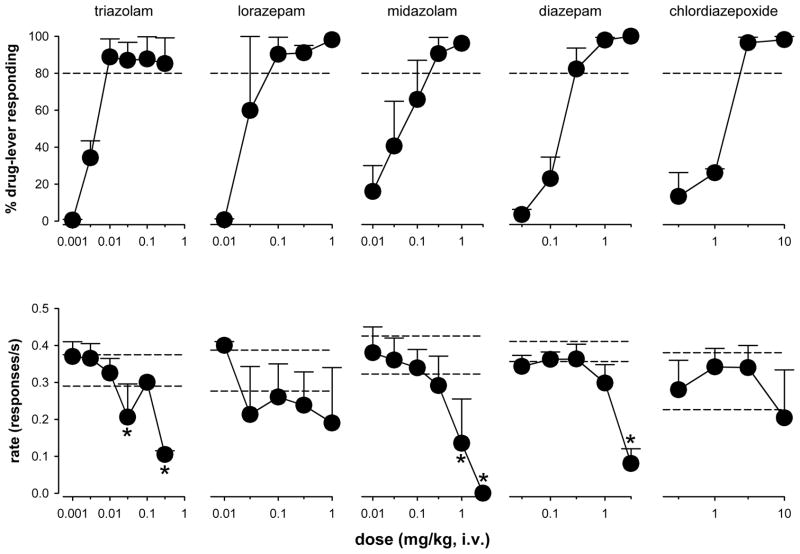
Dose-related increases in mean L-838,417-lever responding and accompanying decreases in mean rates of responding were observed following administration of cumulative doses of several non-selective BZs (Fig. 2, Table 1). Triazolam, lorazepam, midazolam, diazepam, and chlordiazepoxide all fully reproduced the discriminative stimulus effects of L-838,417 (≥80% drug-lever responding) at a minimum of 2 doses tested (Fig. 2, top). All of these compounds decreased mean rates of responding to different extents (Fig. 2, bottom). Triazolam [F(6,14)=19.16, p< 0.001], midazolam [F(6,13)=12.41, p< 0.001], and diazepam [F(5,20)=17.13, p< 0.001] decreased rates of responding significantly. Lorazepam and chlordiazepoxide did not decrease rates of responding; however, there were trends toward a decrease in responding for both drugs.
Figure 2.
Percentage of drug-lever responding (± SEM; top) and effects on response rate (± SEM; bottom) of the non-selective benzodiazepine receptor agonists triazolam, lorazepam, midazolam, diazepam, and chlordiazepoxide in squirrel monkeys (N=3–6) trained to discriminate L-838,417 (0.3 mg/kg) from saline. Dose-response functions were determined via cumulative dosing procedures.
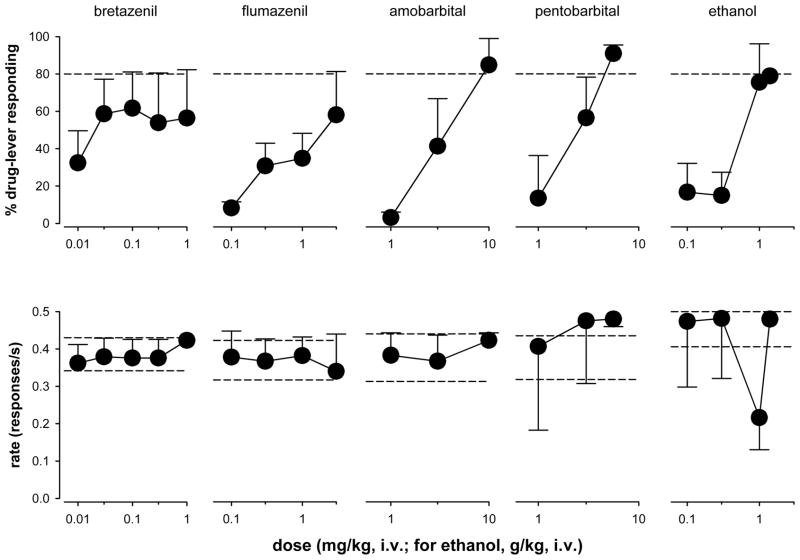
Fig. 3 shows the mean percentage of L-838,417-lever responding following administration of the reference compounds, bretazenil, amobarbital, pentobarbital, and ethanol; ED50 values are presented in Table 1. The non-selective partial BZ agonist bretazenil partially reproduced the DS effects of L-838,417. The maximum level of L-838,417-appropriate responding was observed at 0.1 mg/kg (62%, SEM=19), and a 10-fold higher dose engendered a similar degree of L-838,417-appropriate responding. The ED50 value for bretazenil could not be calculated due to a lack of consistent effects at the lower dose range. As with L-838,417, bretazenil had no significant effects on mean rates of responding. Flumazenil, which is typically classified as a non-selective BZ antagonist but has been shown to possess weak partial agonist effects (e.g., Weiss et al., 2002; Rowlett et al., 2005), engendered an average maximum of 58% (SEM=19) L-838,417-lever responding at the highest dose tested (3.0 mg/kg), with no significant effects on mean rates of responding. The ED50 value calculated for flumazenil was 1.19 mg/kg (SEM= 0.68). Solubility limits precluded evaluating higher doses of flumazenil.
Figure 3.
Percentage of drug-lever responding (± SEM; top) and effects on response rate (± SEM; bottom) of bretazenil, flumazenil, amobarbital, pentobarbital, and ethanol in squirrel monkeys trained to discriminate L-838,417 (0.3 mg/kg) from vehicle. Dose-response functions were determined via cumulative dosing procedures.
Cumulative dosing of the representative barbiturate amobarbital produced a clear dose-dependent increase in responding on the L-838,417-associated lever such that the highest dose tested (10 mg/kg) resulted in a mean of 85% L-838,417-lever responding (SEM=14, Fig. 3), with an ED50 of 4.86 mg/kg (SEM=1.93). Similarly, the representative barbiturate pentobarbital induced a clear dose-dependent increase in responding on the L-838,417-associated lever, with maximal responding of 91% L-838,417-associated responding (SEM=4.5, Fig. 3). Ethanol, which is a known modulator of GABAA receptors, engendered a dose-dependent increase in L-838,417-associated responding, with a maximum of 79% (Fig. 3). Like L-838,417, bretazenil, flumazenil, amobarbital, pentobarbital, and ethanol had no consistent effect on mean rates of responding at any of the doses tested.
Neither the direct-acting GABAA-site agonist muscimol nor the μ-opioid receptor agonist morphine engendered appreciable L-838,417-lever responding (Table 1). Muscimol engendered less than 10% drug-lever responding up to the dose of 0.3 mg/kg. Higher doses were not tested due to seizure-like activity that already has been demonstrated using procedures similar to those used in this study (Spealman, 1985). Administration of morphine resulted in an average maximum of 24% L-838,417-lever responding (SEM=18), and dose-dependently decreased rates of responding over the dose range tested [F(4,12)=3.79, p=0.05].
3.3. Effects of the relatively α1GABAA-Selective Agonists Zaleplon, Zolpidem, and CL 218,872
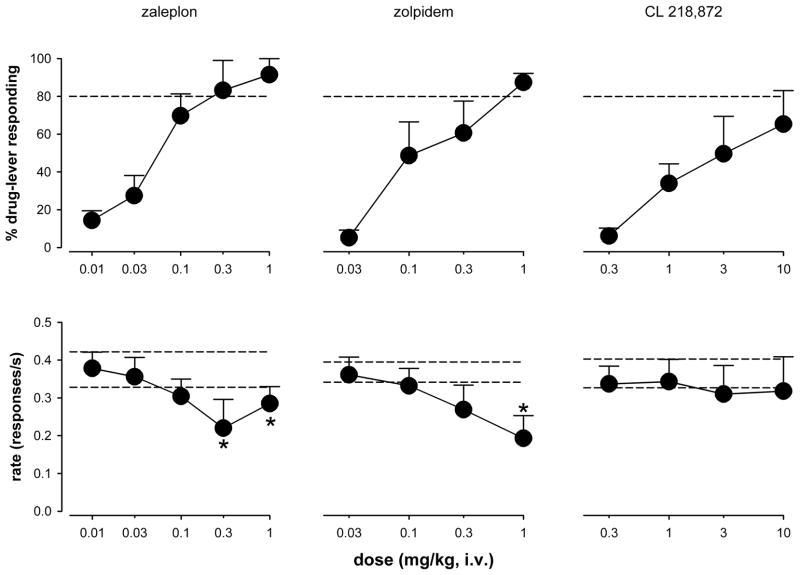
Increasing cumulative doses of the relatively α1GABAA receptor-selective agonists zaleplon (0.01–1 mg/kg), zolpidem (0.03–1 mg/kg), and CL 218,872 (0.3–10 mg/kg) engendered dose-related increases in average L-838,417-lever responding (Fig. 4, top; Table 1). Both zaleplon (ED50= 0.085 mg/kg, SEM= 0.04) and zolpidem (ED50= 0.21, SEM= 0.08) engendered full substitution at the highest doses tested. Both zaleplon [F(5,13)=7.63, p=0.002] and zolpidem [F(4,15)=6.87, p=0.002] also decreased mean rates of responding. CL 218,872 produced an average maximum of 65% L-838,417-lever responding (ED50 value= 2.22, SEM= 1.27), and had little or no effect on response rate (Fig. 4, bottom). Solubility limits precluded evaluating higher doses of CL 218,872.
Figure 4.
Percentage of drug-lever responding (± SEM; top) and effects on response rates (±SEM; bottom) of the α1GABAA-selective benzodiazepine site agonists zaleplon, zolpidem, and CL 218, 872 in squirrel monkeys (N=4–5) trained to discriminate L-838,417 (0.3 mg/kg) from vehicle. Dose-response functions were determined via cumulative dosing procedures.
3.4. βCCT antagonism
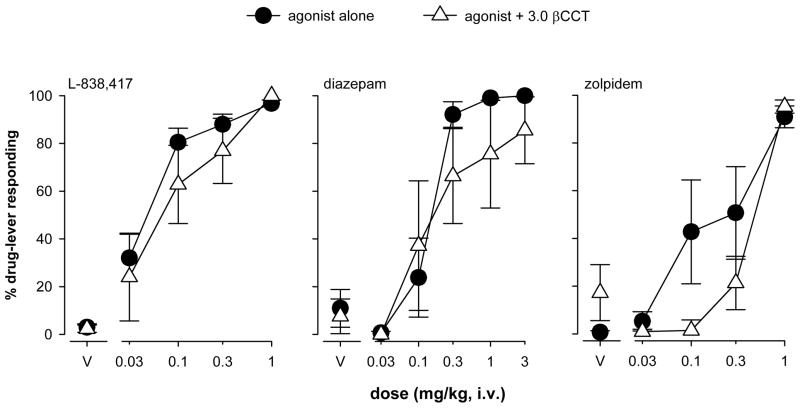
When tested alone, 3.0 mg/kg, i.v., of the relatively α1GABAA-selective antagonist βCCT engendered predominantly saline-lever responding (data not shown). In contrast to flumazenil, pretreatment with βCCT (3.0 mg/kg) had little effect on the discriminative stimulus effects of L-838,417 or the L-838,417-like effects of diazepam and zolpidem (Fig. 5). Based on the ED50 values, the dose-response function for L-838,417 was shifted a maximum of 2-fold to the right, while dose-response functions for both diazepam and zolpidem were shifted 1.5- to 2-fold to the right. None of these shifts achieved statistical significance (paired t-tests, P>0.05). Finally, βCCT had no effect on mean rates of responding, either alone or combined with L-838,417, diazepam, or zolpidem (data not shown).
Figure 5.
Percentage of drug-lever responding (± SEM) for L-838,417 in the presence and absence of the α1GABAA-selective benzodiazepine antagonist βCCT in squirrel monkeys (N=4–5) trained to discriminate L-838,417 (0.3 mg/kg) from vehicle. βCCT was administered i.v. immediately before sessions in which cumulative doses of L-838,417, diazepam, or zolpidem were tested.
4. Discussion
In the present study, the BZ-site ligand L-838,417 was established as a discriminative stimulus in squirrel monkeys to evaluate the discriminative stimulus effects of non-selective BZs, BZ agonists with selectivity for α1GABAA receptors, and compounds that modulate GABAA receptors via a non-BZ site. L-838,417 was found to be active for at least 80 minutes following i.v. injection, and to engender discriminative stimulus effects that were sensitive to antagonism by flumazenil, the latter finding consistent with this compound’s action at BZ-sensitive GABAA receptors. Similar to non-selective BZs, the α1GABAA–preferring agonists, as well as the barbiturates and ethanol, substituted for L-838,417.
Our previous research has suggested that the α1GABAA receptor subtype plays a key role in the discriminative stimulus effects of conventional BZs (for review, see Lelas et al., 2000). Despite L-838,417’s lack of demonstrable intrinsic efficacy at α1GABAA receptors in vitro (McKernan et al., 2000), non-selective BZs produced L-838,417-like effects at doses that did not modify response rate. These results do not appear to support our initial hypothesis, and potential alternative hypotheses include: (1) stimulation of α2GABAA, α3GABAA, and/or α5GABAA receptors is sufficient for the transduction of BZ-like discriminative stimulus effects; (2) because L-838,417 is a partial agonist at α2GABAA, α3GABAA, and α5GABAA receptors, the discriminative stimulus “signal” might be relatively weak, resulting in a non-specific generalization profile; and (3) a common, down-stream mechanism underlies the discriminative stimulus effects of BZ ligands.
Because of the relatively broad generalization profile of L-838,417 under the training conditions used in this study, our first proposed hypothesis is that stimulation of α2GABAA, α3GABAA, and/or α5GABAA receptors produces interoceptive effects that are shared with a range of BZ-type drugs. Although the α1GABAA–preferring agonists zaleplon, zolpidem, and CL 218,872 engendered full, or nearly full, substitution for L-838,417, these compounds only have modest selectivity (~10-fold) for α1GABAA receptors vs. α2GABAA and α3GABAA receptors in vitro; therefore, they may produce their L-838,417-like discriminative stimulus effects via binding to other GABAA receptor subtypes, particularly if the doses of drug are sufficiently high. This idea is supported by our findings with the α1GABAA-preferring antagonist βCCT. In this regard, a dose of βCCT (3.0 mg/kg, i.v.) that antagonized the discriminative stimulus effects of triazolam and zolpidem (≥7-fold shift that was statistically significant; Lelas et al., 2002; Rowlett et al., 2003) did not block the discriminative stimulus effects of L-838,417 or the L-838,417-like effects of diazepam and zolpidem (2-, 1.5-, and 2-fold shifts, respectively —these shifts were not statistically reliable). However, while it appears as though the α2GABAA, α3GABAA, and/or α5GABAA receptors may be more important for mediating the discriminative stimulus effects of BZ-type drugs, a recent rodent drug discrimination study demonstrated an inability to establish reliable stimulus control with TPA023B, a drug similar to L-838,417 with respect to its partial agonist activity at α2GABAA, α3GABAA, and α5GABAA receptors in vitro (Kohut and Ator, 2008). Species differences or differences in the pharmacokinetic profiles between TPA023B and L-838,417 may explain the discrepancy in the ability to train these drugs as discriminative stimuli, but our results are consistent with the idea that α1GABAA receptors are not involved in the discriminative stimulus effects of L-838,417 itself. Furthermore, these results suggest that the L-838,417-like discriminative stimulus effects of diazepam, and most strikingly, zolpidem, may not involve stimulation of α1GABAA receptors.
Another possible explanation for the generalization profile of L-838,417 may be related to its intrinsic efficacy. L-838,417 is a partial modulator of GABAA receptors containing α2, α3, and α5 subunits (McKernan et al., 2000). Consistent with its efficacy profile in vitro, the nonselective BZ partial agonist bretazenil (Facklam et al., 1992; Smith et al., 2001) engendered a relatively high degree of substitution for L-838,417, as did flumazenil which has been shown to have very weak partial agonist effects in some procedures (e.g., Haefely, 1988; Cook et al., 2005; Rowlett et al., 2006). L-838,417 failed to induce drug-lever responding in squirrel monkeys trained to discriminate triazolam from vehicle; and, in fact, antagonized the discriminative stimulus effects of triazolam (Rowlett et al., 2005). L-838,417 also has been shown to attenuate the discriminative stimulus effects of other higher efficacy BZ agonists (McMahon and France, 2006). These findings raise the possibility that the L-838,417 discriminative stimulus reflects this compound’s low efficacy, rather than its functional selectivity (although they are not mutually exclusive). In other words, L-838,417 engenders a relatively weak discriminative stimulus “signal” that psychophysically lacks specificity, akin to a relatively low training dose of a high efficacy agonist (cf. Rowlett et al. 2000; 2003). The primary evidence against this interpretation is βCCT’s ineffectiveness at blocking the discriminative stimulus effects of L-838,417. If the relatively broad generalization profile of L-838,417 reflected solely its partial agonist profile, one would expect βCCT to attenuate the discriminative stimulus effects of L-838,417 as well as the other agonists tested. Nonetheless, low intrinsic efficacy as a key determinant for L-838,417’s discriminative stimulus effects cannot be ruled out (one method for evaluating this hypothesis would be to train a high efficacy agonist with functional selectivity for α2GABAA, α3GABAA, and α5GABAA receptors).
Our third proposed hypothesis for the lack of specificity of the discriminative stimulus effects of L-838,417 is that downstream signaling events may serve as a common substrate for the effects of BZ ligands in the L-838,417-trained monkeys. At present, there is a paucity of data on intracellular signaling adaptations induced by BZ-type ligands, although acute exposure to diazepam recently has been shown to down-regulate transcripts of genes involved in regulating synaptic function and plasticity (e.g., calcium/calmodulin-dependent kinase IIα Huopaniemi et al., 2004; for review, see Licata and Rowlett, 2008). Differences between non-selective BZs and functionally selective compounds in mediating intracellular signaling are unexplored at present, and consequently, the contribution of downstream events to behavioral effects of BZ-type ligands are unknown.
Based on our earlier research using similar procedures, an unexpected finding in the present study was that the barbiturates amobarbital and pentobarbital fully substituted for L-838,417. Previously, barbiturates failed to demonstrate BZ-like discriminative stimulus effects when midazolam (Spealman, 1985), zolpidem (Rowlett et al., 1999), or triazolam (Lelas et al., 2001a) were the training drugs in squirrel monkeys following i.v. administration. Ethanol also engendered primarily L-838,417-lever respondin and this finding complements an earlier study suggesting that α1GABAA receptors do not have a primary role in the discriminative stimulus effects of ethanol (Platt et al., 2005b). In contrast, neither the direct GABAA agonist muscimol nor the non-GABAergic opioid agonist morphine induced significant L-838,417-lever responding. Overall, these findings suggest that the discriminative stimulus effects of L-838,417 are relatively specific to positive allosteric modulators of GABAA receptors, although within this class there was no distinction among modulators with different mechanisms of action (e.g., BZ site vs. barbiturate site).
Taken together, the results of the present study and those from earlier reports may provide a framework for understanding the role of specific GABAA receptor subtypes in the discriminative stimulus effects of GABA ligands under the conditions used in this study and our early studies. On one end of a continuum, a compound with limited or no efficacy at α1GABAA receptors may engender the most inclusive discriminative stimulus among BZ ligands. In other words, lacking α1GABAA receptor activity may result in a stimulus that is sensitive to all GABAA positive modulators. By “increasing” the contribution of the α1GABAA receptor, discriminative stimulus effects for non-selective BZs and relatively selective α1GABAA-preferring agonists persist but barbiturates and ethanol no longer share stimulus effects with BZs. In other words, increasing α1GABAA receptor agonist activity increases the specificity of the discriminative stimulus to include only BZ-type drugs. At the other end of the continuum, a discriminative stimulus effect based predominantly on α1GABAA receptor activation (e.g., i.v. high-dose zolpidem) results in a stimulus that is exclusive for compounds with relatively selective α1GABAA activity. It should be noted that at present, there is no clear mechanism of action for such a phenomenon. Nevertheless, evaluation of predictions based on this model, as well as establishing how other GABAergic compounds with different selectivity profiles fit into this model, should provide a conceptual platform for understanding the distinctive interoceptive effects of these clinically important drugs.
Acknowledgments
We thank Laura Paes Teixeira, Lindsay Kettinger, and Brett Lewis-DeWeese for expert technical assistance. This work was supported by U.S. Public Health Services Grants DA11792, DA18473, AA16179, MH46851, and RR00168.
Abbreviations
- BZ
benzodiazepine
- βCCT
β-carboline-3-carboxylate-t-butyl ester
- FR
fixed-ratio
- L-838
417, (7-tert-Butyl-3-(2,5-difluoro-phenyl)-6-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)-[1,2,4]triazolo[4,3-b]pyridazine)
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford KA, McKernan RM, Dawson GR. Anxiogenic properties of an inverse agonist selective for alpha3 subunit-containing GABA A receptors. Br J Pharmacol. 2005;144:357–366. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA. High-dose discrimination training with midazolam: context determines generalization profile. Pharmacol Biochem Behav. 1999;64:237–243. doi: 10.1016/s0091-3057(99)00050-7. [DOI] [PubMed] [Google Scholar]
- Ator NA. Contributions of GABAA receptor subtype selectivity to abuse liability and dependence potential of pharmacological treatments for anxiety and sleep disorders. CNS Spectr. 2005;10:31–39. doi: 10.1017/s1092852900009883. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Asymmetrical cross-generalization with lorazepam and pentobarbital training conditions. Drug Dev Res. 1989;16:355–364. [Google Scholar]
- Collins I, Moyes C, Davey WB, Rowley M, Bromidge FA, Quirk K, Atack JR, McKernan RM, Thompson SA, Wafford K, Dawson GR, Pike A, Sohal B, Tsou NN, Ball RG, Castro JL. 3-Heteroaryl-2-pyridones: Benzodiazepine site ligands with functional selectivity for α2/α3-subtypes of human GABAA receptor-ion channels. J Med Chem. 2002;45:1887–1900. doi: 10.1021/jm0110789. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Out FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Foster KL, Eiler WJ, 2nd, McKay PF, Woods J, 2nd, Harvey SC, Garcia M, Grey C, McCane S, Mason D, Cummings R, Li X, Cook JM, June HL. Selective GABAA alpha5 benzodiazepine inverse agonist antagonizes the neurobehavioral actions of alcohol. Alcohol Clin Exp Res. 2005;29:1390–1401. doi: 10.1097/01.alc.0000175073.94575.86. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;46:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam M, Schoch P, Haefely WE. Relationship between benzodiazepine receptor occupancy and potentiation of gamma-aminobutyric acid-stimulated chloride flux in vitro of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther. 1992;261:1106–1112. [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- Haefely W. The preclinical pharmacology of flumazenil. Eur J Anaesthesiol Suppl. 1988;2:25–36. [PubMed] [Google Scholar]
- Kohut SJ, Ator NA. Novel discriminative stimulus effects of TPA023B, a subtype-selective γ-aminobutyric-acidA/benzodiazepine modulator: Comparisons with zolpidem, lorazepam, and TPA023. Pharmacol Biochem Behav. 2008;90:65–73. doi: 10.1016/j.pbb.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Mattila MJ, Wisden W, Lüddens H. GABAA-receptor subtypes: clinical efficacy and selectivity of benzodiazepine site ligands. Ann Med. 1997;29:275–282. doi: 10.3109/07853899708999348. [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. Discriminative-stimulus effects of triazolam and midazolam in rhesus monkeys. Behav Pharmacol. 1999;10:39–50. doi: 10.1097/00008877-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. Antagonism of the discriminative stimulus effects of positive gamma-aminobutyric acid(A) modulators in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther. 2000a;294:902–908. [PubMed] [Google Scholar]
- Lelas S, Spealman RD, Rowlett JK. Using behavior to elucidate receptor mechanisms: a review of the discriminative stimulus effects of benzodiazepines. Exp Clin Psychopharm. 2000b;8:294–311. doi: 10.1037//1064-1297.8.3.294. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. Triazolam discrimination in squirrel monkeys distinguishes high-efficacy agonists from other benzodiazepines and non-benzodiazepine drugs. Psychopharmacology. 2001a;154:96–104. doi: 10.1007/s002130000615. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. Isobolographic analysis of chlordiazepoxide and triazolam combinations in squirrel monkeys discriminating triazolam. Psychopharmacology. 2001b;158:181–189. doi: 10.1007/s002130100868. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD, Cook JM, Ma C, Li X, Yin W. Role of GABAA/benzodiazepine receptors containing α1 and α5 subunits in the discriminative stimulus effects of triazolam in squirrel monkeys. Psychopharmacology. 2002;161:180–188. doi: 10.1007/s00213-002-1037-y. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Differential behavioral effects of low efficacy positive GABAA modulators in combination with benzodiazepines and a neuroactive steroid in rhesus monkeys. Br J Pharmacol. 2006;147:260–268. doi: 10.1038/sj.bjp.0706550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Rodgers RJ, Mathiasen LS. Comparative cue generalization profiles of L-838,417, SL651498, zolpidem, CL218872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination. J Pharmacol Exp Ther. 2006;316:1291–1299. doi: 10.1124/jpet.105.094003. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivan J, editors. Current Protocols in pharmacology. Wiley; New York: 2005a. pp. 9.21.1.1–9.21.15. [Google Scholar]
- Platt DM, Duggan A, Spealman RD, Cook JM, Li X, Yin W, Rowlett JK. Contribution of α1GABAA and α5GABAA receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys. J Pharmacol Exp Ther. 2005b;313:658–667. doi: 10.1124/jpet.104.080275. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD, Lelas S. Discriminative stimulus effects of zolpidem in squirrel monkeys: comparison with conventional benzodiazepines and sedative-hypnotics. J Pharmacol Exp Ther. 1999;291:1233–1241. [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Spealman RD. Transduction of the discriminative stimulus effects of zolpidem by GABAA/α1 receptors. Eur J Pharmacol. 2000;406:9–10. doi: 10.1016/s0014-2999(00)00669-5. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Spealman RD, Lelas S, Cook JM, Yin W. Discriminative stimulus effects of zolpidem in squirrel monkeys: role of GABAA/α1 receptors. Psychopharmacology. 2003;165:209–215. doi: 10.1007/s00213-002-1275-z. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology. 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW, Rowlett JK. Discriminative-stimulus effects of zolpidem, triazolam, pentobarbital, and caffeine in zolpidem-trained humans. Exp Clin Psychopharmacol. 2000;8:22–36. doi: 10.1037//1064-1297.8.1.22. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Hagen TJ, Guzman F, Cook JM. Beta-carbolines as antagonists of the discriminative stimulus effects of diazepam in rats. J Pharmacol Exp Ther. 1988;246:275–281. [PubMed] [Google Scholar]
- Spealman RD. Discriminative-stimulus effects of midazolam in squirrel monkeys: Comparison with other drugs and antagonism by Ro 15-1788. J Pharmacol Exp Ther. 1985;235:456–462. [PubMed] [Google Scholar]
- Verster JC, Volkerts ER, Verbaten MN. Effects of alprazolam on driving ability, memory functioning and psychomotor performance: a randomized, placebo-controlled study. Neuropsychopharmacology. 2002;27:260–269. doi: 10.1016/S0893-133X(02)00310-X. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ, Kemp JA. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant gamma-aminobutyric acidA receptor subtypes. Mol Pharmacol. 1993;43:240–244. [PubMed] [Google Scholar]
- Weiss M, Tikhonov D, Buldakova S. Effect of flumazenil on GABAA receptors in isolated rat hippocampal neurons. Neurochem Res. 2002;27:1605–1612. doi: 10.1023/a:1021674708556. [DOI] [PubMed] [Google Scholar]