Abstract
Objective
To estimate patients' elasticity of demand, willingness to pay, and consumer surplus for five high-cost specialty medications treating metastatic disease or hematologic malignancies.
Data Source/Study Setting
Claims data from 71 private health plans from 1997 to 2005.
Study Design
This is a revealed preference analysis of the demand for specialty drugs among cancer patients. We exploit differences in plan generosity to examine how utilization of specialty oncology drugs varies with patient out-of-pocket costs.
Data Collection/Extraction Methods
We extracted key variables from administrative health insurance claims records.
Principal Findings
A 25 percent reduction in out-of-pocket costs leads to a 5 percent increase in the probability that a patient initiates specialty cancer drug therapy. Among patients who initiate, a 25 percent reduction in out-of-pocket costs reduces the number of treatments (claims) by 1–3 percent, depending on the drug. On average, the value of these drugs to patients who use them is about four times the total cost paid by the patient and his or her insurer, although this ratio may be lower for oral specialty therapies.
Conclusions
The decision to initiate therapy with specialty oncology drugs is responsive to price, but not highly so. Among patients who initiate therapy, the amount of treatment is equally responsive. The drugs we examine are highly valued by patients in excess of their total costs, although oral agents warrant further scrutiny as copayments increase.
Keywords: Willingness to pay, economic analysis, cost–benefit analysis, oncology
The high cost of specialty medications has been intensely scrutinized by lawmakers, patient activists, and the popular press (Kolata and Pollack 2008; Lee and Emanuel 2008; Willey et al. 2008;). Both supply- and demand-side factors push up the cost of these drugs (Goldman et al. 2006). Most are biologics, which are more costly to produce than traditional medications and are administered via infusion or direct injection, both of which involve substantial costs. On the demand side, many of these drugs provide treatments for disabling (e.g., arthritis, multiple sclerosis) or life-threatening diseases (e.g., cancer) that respond to few therapeutic substitutes. As a result, the willingness to pay for these drugs may be high.
Among physicians, there is widespread belief that the benefits of specialty medications are low relative to their costs, with only 25 percent of oncologists believing that bevacizumab (Avastin), a recently developed therapy for metastatic colorectal cancer, offers a “good value” (Nadler, Eckert, and Neumann 2006). This skepticism is also shared by many public payers. For example, the United Kingdom's National Health Service (NHS) recently declined to cover bevacizumab after concluding that the documented improvement in median survival—about four and a half months, according to one major study (Hurwitz et al. 2004)—did not justify the drug's high cost, reportedly as much as U.S.$100,000/year (Kolata and Pollack 2008).
We quantify the overall price and income responsiveness by estimating demand and income elasticities. We also use the estimated relationship between out-of-pocket costs, income, and utilization to determine how much (in dollar terms) individuals value the drugs in question, not simply how responsive they are to changes in out-of-pocket costs and income. Comparing patients' willingness to pay to the cost of the treatments allows us to estimate consumer surplus: the benefit to patients net of the amount paid. As Jena and Philipson (2007) observe, a drug's cost-effectiveness and consumer surplus are closely linked: the greater the consumer surplus, the higher the cost-effectiveness.
We report three main findings. First, the decision to initiate therapy with these drugs is somewhat, but not highly, responsive to patient out-of-pocket costs. Second, among those who initiate therapy, the amount of use is fairly unresponsive to patient out-of-pocket costs. Third, the value of the drugs to users is on average equal to four times their total cost to payers and patients, although there is some evidence to suggest lower benefit ratios for oral drugs. Inference for the latter group of drugs, however, is complicated by much lower mean and maximum rates of cost sharing, which decrease the precision and generalizability of our findings. This suggests one limitation of the revealed preference approach in this context.
METHODS
To analyze how the demand for specialty drugs for cancer varies with patient income and the out-of-pocket payments patients face, we utilize an extensive set of deidentified administrative insurance claims data drawn from a nonrandom sample of more than 50 private health plans offered by 15 employers. These data cover roughly 10.8 million beneficiary years from 1997 to 2005. For each medical and pharmacy claim in the data, detailed information exists on health plan and patient out-of-pocket spending, diagnostic codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and procedure codes recorded under the Current Procedural Terminology (CPT) or Health Care Financing Agency Common Procedure Coding System (HCPCS). Limited demographic information on the claimant is also available; namely, age, gender, an indicator for urban residence, and 2000 Census median income in the zip code of residence.
We focus our attention on five relatively recent treatments spanning a broad range of systemic, proliferative diseases:
Bevacizumab (Avastin, Genentech, South San Francisco, CA) in metastatic colorectal cancer (ICD-9-CM diagnosis codes of 153, 154.0, or 154.1)
Trastuzumab (Herceptin, Genentech, South San Francisco, CA) for metastatic breast cancer (ICD-9-CM diagnosis code of 174)
Rituximab (Rituxan, Genentech, South San Francisco, CA and Biogen Idec, Cambridge, MA) for non-Hodgkin's lymphoma (NHL) (ICD-9-CM diagnosis codes of 200 or 202)
Erlotinib (Tarceva, Genentech, South San Francisco, CA, and OSI Pharmaceuticals, Melville, NY) for metastatic nonsmall lung cancer (ICD-9-CM diagnosis code of 162) and pancreatic cancer (ICD-9-CM diagnosis code of 157)
Imatinib mesylate (Gleevec, Novartis AG, Basel, Switzerland) for Philadelphia chromosome positive chronic myeloid leukemia (CML) (ICD-9-CM diagnosis code of 205.1)
Our sample consists of cancer patients who are eligible for these treatments. These are generally patients with metastatic disease (or hematologic malignancies) corresponding to one of the cancers listed above. We identified these patients as those with (a) one or more of the ICD-9-CM diagnosis codes for the forms of cancer noted above and (b) at least two principal or secondary ICD-9-CM diagnosis codes indicating metastatic disease (196–199), separated by 30 days or more. The second criterion was added so as to exclude cases in which metastatic disease is under evaluation, that is a “rule-out” diagnosis code. This method for identifying metastatic cancer patients has been used in other studies (Rao, Kubisiak, and Gilden 2004; Jacobson et al. 2006; Krupski et al. 2006; Pelletier et al. 2008;) and validated in a study using Medicare claims data compared with a gold standard of prospectively collected clinical trial data (Lamont et al. 2006). However, in the case of non-Hodgkin's lymphoma and chronic myeloid leukemia, we did not restrict ourselves to metastatic patients, because the treatments corresponding to these (liquid tumor) diseases are indicated for all patients.
Use of each of the five drugs is identified in one of two ways. For the two orally administered products, all pharmacy claims are flagged that include any of the national drug codes (NDCs) for either imatinib mesylate or erlotinib. Because some claims include prescriptions written for multiple months, we redefine usage into 30-day equivalents (e.g., a 90-day prescription counts as three 30-day-equivalents). For the four other products, we use medical claims data to identify use of each product from physicians' offices, home care agencies, and outpatient facilities such as outpatient hospital clinics. Claims for a particular product are identified based on the CPT or HCPCS codes associated with that product (e.g., J9355 refers to an administration of bevacizumab). Because some of the treatments are indicated for diseases other than cancer, we only included treatment claims that contain a diagnostic code for the corresponding cancer. Thus, claims for other potential uses were included only if they mentioned both the cancer and the alternative indication, an extremely rare occurrence. For example, none of the rituximab claims with a diagnosis code for non-Hodgkin's lymphoma had a diagnosis code for rheumatoid arthritis, which is another of its indications.
Using the large variation in out-of-pocket spending per claim generated by differences in plan benefit design, we estimate demand in two steps. First, we estimate how the probability of initiating therapy responds to out-of-pocket costs per claim and income among individuals utilizing any of the five medications considered. Specifically, we estimate the following linear probability model:
| (1) |
In the above equation, Useijt, is a dummy variable which is equal to 1 if an eligible patient j used drug i in year t; fi is a drug fixed-effect; δt is a year effect; Xjt is a vector of individual characteristics: Region (Midwest, South, West, Northeast), Urban residence (1=urban; 0 otherwise), Age, Gender; OOPijt is the average out-of-pocket costs per claim for the given drug, which is calculated as the average out-of-pocket amount per claim among all persons using drug i in individual j 's plan at year t; incomejt is individual j 's income in year t, proxied by the median 2,000 income of his three-digit zip code of residence; eijt is the error term, which we cluster at the plan-year level, since the variation in out-of-pocket costs is at the plan level.
We weight the regression by the annual number of claims for the drug in a given plan. We use β1 and β2 to calculate arc elasticities for out-of-pocket cost and income. To do so, we used our estimates of the parameters in equation (1) to project the change in probability of utilization associated with a 25 percent decrease in out-of-pocket costs or income. The arc price elasticity for an individual is then calculated as
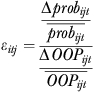 |
(2) |
where Δprobijt is the estimated change in probability, 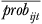 is the average of the initial and new probability of utilization, ΔOOPijt is the absolute change in out-of-pocket costs, and
is the average of the initial and new probability of utilization, ΔOOPijt is the absolute change in out-of-pocket costs, and 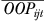 is the average of the initial and new out-of-pocket costs. Equation (2) represents the elasticity for an individual; we calculate and report the average out-of-pocket cost elasticity by calculating the elasticity for each individual and averaging over the population. A similar approach is used to calculate the average income elasticity. Standard errors for both elasticities are calculated using the delta method.
is the average of the initial and new out-of-pocket costs. Equation (2) represents the elasticity for an individual; we calculate and report the average out-of-pocket cost elasticity by calculating the elasticity for each individual and averaging over the population. A similar approach is used to calculate the average income elasticity. Standard errors for both elasticities are calculated using the delta method.
The use of average plan-level out-of-pocket costs addresses the potential problem of reverse causality: individuals may have lower out-of-pocket costs because they use more therapy, which may drive them above deductibles or out-of-pocket maximums.
The inclusion of drug fixed-effects in this specification means that the model does not make use of variation in out-of-pocket costs across drugs, only across insurance plans. This approach is robust to unobserved drug attributes correlated with out-of-pocket costs and quantity. For example, if users of erlotinib have unobservably higher incomes than users of imatinib mesylate, a standard demand specification would incorrectly attribute the difference in incomes to the drug itself. Including drug fixed effects eliminates this type of variation. Drug fixed effects also account for drug-specific characteristics, such as the differences between oral and injectable agents.
We also include year fixed effects to control for common temporal factors, such as changes in practice patterns or reimbursement that influence all drugs. In addition, our proxy for individual income uses the median income of an individual's zip code of residence, as reported in the 2000 Census. As aggregate income varies over the time period in our study, the use of year effects allows us to control for secular trends in this income measure, such as those due to economic growth and inflation.
Because some of the drugs in our sample are used by only a small number of patients, estimating equation (1) separately for each drug could lead to imprecise estimates for the lightly utilized drugs. “Stacking” the regression datesets for each individual drug and estimating a common demand equation allows more precise estimates, at the cost of imposing additional structure. Because rituximab accounts for nearly three quarters of the observations in our sample, we estimate equation (1) for rituximab alone. For the remaining drugs, we estimated a pooled model, but with drug fixed effects that allow the intercept term to vary across drugs.
After estimating the probability of initiation, we estimate the following equation among users of these drugs to determine the effect of out-of-pocket costs on therapy continuation:
| (3) |
In the equation above, Claimsitj is the number of claims for drug i filed by individual j in year t. All the other variables are the same as above. As with equation (1), we estimated equation (3) separately for rituximab and then pooled the regressions for the remaining drugs. In addition, we cluster standard errors at the plan-year level and report the average out-of-pocket cost and income elasticities for the population, using methods similar to those previously described. We use the results of equation (3) to calculate how much (in dollar terms) patients in our sample using the medications value them. Specifically, as equation (3) represents a linear demand curve for each treatment, a given individual's willingness-to-pay for Q claims of a drug is1
| (4) |
We then calculated the consumer surplus for each patient as the willingness to pay for the number of claims administered in a given year, as shown in equation (4), divided by the total amount spent by the patient and his or her insurer for those claims.
RESULTS
Table 1 presents descriptive statistics for the sample as a whole and separately for each of the five drugs considered.
Table 1.
Sample Summary Statistics
| Annual Out-of-Pocket Costs (Cost Sharing, %)* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | No. Eligible Persons | No. Person Years | No. Users* | No. Person Years (Users)* | Total Claims* | Mean | Median | 75th percentile | 90th percentile |
| Bevacizumab | 201 | 201 | 29 | 29 | 125 | 1,799 | 338 | 2,251 | 4,739 |
| (12.8) | (1.98) | (18.4) | (48.1) | ||||||
| Trastuzumab | 1,335 | 2,026 | 75 | 100 | 1,156 | 3,085 | 93 | 1,155 | 7,609 |
| (10.4) | (0.924) | (10.0) | (44.7) | ||||||
| Rituximab | 26,618 | 36,912 | 1,445 | 1,885 | 9,326 | 4,294 | 431 | 2,073 | 14,599 |
| (12.7) | (1.69) | (12.9) | (51.1) | ||||||
| Imatinib mesylate | 689 | 1,149 | 288 | 581 | 5,581 | 879 | 134 | 470 | 2,113 |
| (3.61) | (0.632) | (1.92) | (13.2) | ||||||
| Erlotinib | 548 | 586 | 81 | 83 | 365 | 609 | 112 | 470 | 990 |
| (6.10) | (1.16) | (2.37) | (15.5) | ||||||
| All | 29,391 | 40,874 | 1,918 | 2,678 | 16,533 | 3,445 | 319 | 1,470 | 9,642 |
| (10.4) | (1.14) | (8.67) | (44.1) | ||||||
Dollar amounts shown are in year 2008 dollars. Parentheses show cost sharing, calculated as out-of-pocket costs divided by total spending by the patient and the insurer.
Our sample consists of 29,539 patients eligible for one of the treatments we consider, with 41,874 person-years between them. Only a small proportion of these patients—<10 percent—used one or more of the five drugs in our sample. Limiting the sample to patients who used one or more of these drugs, there are 2,678 person-years and 16,533 claims in total. Patients using rituximab account for three-fourths of the users in our sample, followed by patients using imatinib mesylate (about 15 percent of the users in the sample). Taken together, bevacizumab, trastuzumab, and erlotinib account for about 10 percent of the user base. This is not that surprising, because bevacizumab and erlotinib were both approved in 2004, just 1 year after the end of our sample window.
Across drugs, the range in out-of-pocket spending is large. The orally administered products imatinib mesylate and erlotinib cost patients on average U.S.$609–879/year,2 while average cost for the injected products range from U.S.$1,799/year (bevacizumab) to U.S.$15,074/year (trastuzumab). Even among patients receiving the same drug, there is substantial variation in out-of-pocket costs, reflecting variation in health plan generosity. For example, the median annual out-of-pocket cost for patients receiving bevacizumab is just U.S.$338/year, but patients in the 75th percentile pay U.S.$2,251/year and patients in the 90th percentile pay U.S.$4,739 annually.
Table 1 also shows the levels of cost sharing for each drug, which is calculated as out-of-pocket costs divided by the total spending of insurer and patient. The share of cost paid out-of-pocket differs substantially across injectables and orals. At the 90th percentile, for instance, cost sharing is about 50 percent for the injectables (bevacizumab, trastuzumab, and rituximab), but just 15 percent for the orals (imatinib mesylate and erlotinib). The lower rate of cost sharing complicates our inferences about WTP for oral specialty products, as there is a narrower distribution of out-of-pocket costs across which to identify the full demand curve.
We first consider how the probability of initiating therapy among all eligible patients (N=40,874 person-years) responds to changes in out-of-pocket cost and income. With demographic controls (our preferred specification), the estimated elasticity of demand in the therapy-initiation equation is −0.258 (p<.05) for rituximab and −0.189 for the remaining specialty drugs. This means that a 25 percent reduction in out-of-pocket cost leads to a relative 6.4 percent increase in the probability of initiating therapy for rituximab and a relative 4.7 percent increase in the probability of initiating therapy for other drugs, although the latter value is not statistically different from zero. By contrast, the estimated income elasticity is statistically indistinguishable from zero.
Next we restrict the sample to metastatic cancer patients (N=1,918) who used one or more of the five specialty oncology medications (N=2,678 user-years) and consider how the annual number of claims responds to changes in out-of-pocket cost and income. With demographic controls, the estimated elasticity of demand is −0.0367 (p<.01) for rituximab and −0.108 (p<.05) for the remaining specialty drugs. Thus, a 25 percent reduction in out-of-pocket expenses reduces the number of claims, on average, by 0.917 percent for rituximab and 2.7 percent for the remaining drugs. The estimated income elasticity once again is statistically indistinguishable from zero. These regression results, along with results of models with alternative specifications, are shown in Table 2.
Table 2.
Effect of Cost Sharing on Therapy Initiation and Number of Claims
| Effect of Cost Sharing on Therapy Initiation |
Effect of Cost Sharing on No. of Claims |
|||||
|---|---|---|---|---|---|---|
| DV=1 if Ever Used Drug in Given Year | DV=No. of Claims in Given Year | |||||
| Rituximab | ||||||
| Demand elasticity with respect to: | ||||||
| Out-of-pocket costs | −0.0129 | −0.0235 | −0.258* | −0.0391** | −0.0402** | −0.0367* |
| (0.0335) | (0.0436) | (0.117) | (0.0148) | (0.0145) | (0.0141) | |
| Income | −0.860** | −0.909 | −1.08 | 0.0720 | 0.116 | 0.103 |
| (0.189) | (0.231) | (0.633) | (0.116) | (0.113) | (0.119) | |
| Year fixed effects | N | Y | Y | N | Y | Y |
| Demographic characteristics | N | N | Y | N | N | Y |
| N | 36,912 | 36,912 | 36,912 | 1,885 | 1,885 | 1,885 |
| R2 | 0.0044 | 0.0073 | 0.0222 | 0.0032 | 0.0068 | 0.0076 |
| Other drugs (bevacizumab, trastuzumab, imatinib mesylate, erlotinib) | ||||||
| Demand elasticity with respect to: | ||||||
| Out-of-pocket costs | −0.211** | −0.0984 | −0.189 | −0.0749 | −0.0912* | −0.108** |
| (0.0551) | (0.277) | (0.150) | (0.0462) | (0.0383) | (0.0410) | |
| Income | −0.492* | 0.275 | 0.0847 | 0.336 | 0.169 | 0.227 |
| (0.210) | (4.38) | (5.90) | (0.505) | (0.556) | (0.569) | |
| Drug fixed effects | N | Y | Y | N | Y | Y |
| Year effects | N | Y | Y | N | Y | Y |
| Demographic characteristics | N | N | Y | N | N | Y |
| N | 3,962 | 3,962 | 3,962 | 793 | 793 | 793 |
| R2 | 0.0168 | 0.2301 | 0.2351 | 0.0054 | 0.1716 | 0.2060 |
Notes. Out-of-pocket cost is the average out-of-pocket cost for each plan (see “Methods”). Out-of-pocket cost and income elasticities are arc elasticities, computed at the individual level and averaged across the sample. Standard errors clustered at the plan-year level are shown in parentheses; therapy-continuation regression excludes users who do not have metastatic disease.
p<.05.
p<.01.
DV, dependent variable.
Other studies (Contoyannis et al. 2005; Liu and Chollet 2006;) find demand elasticities for prescriptions drugs ranging from −0.10 to −0.60, although Gaynor, Li, and Vogt (2006) estimate elasticities ranging from −0.6 to −0.8 and Goldman et al. (2006) find elasticities of demand for specialty drugs to range from −0.01 to −0.21. Overall, our estimates are consistent with and at the lower end of these findings.
Next we infer the dollar willingness to pay among users of the drugs in our sample by examining the estimated utilization–out-of-pocket cost relationship in equation (4). We then calculate the ratio between dollar willingness to pay and the total cost of the drug—to the patient and all payers. This ratio greatly exceeds 1 for the vast majority of patients on injectables, indicating substantial consumer surplus. In other words, the value of these treatments to the patients who receive them typically is much higher than the amount paid out of pocket by the patient, but it is also substantially more than the total cost paid for the drug (patient and insurance cost combined). For the specialty orals, however, results are mixed.
As Table 3 shows, the net benefit ratio (of aggregate willingness to pay to aggregate spending) is 4.04 for the sample as a whole. In aggregate, the total value of the drugs for the patients in our sample to be U.S.$164 million, against a total cost of U.S.$72.2 million. We find that willingness to pay exceeds aggregate spending for more than 98 percent of patients on rituximab. The same is true for more than 75 percent of patients on other injectables.
Table 3.
Annual Net Benefit Ratio for Specialty Oncology Drugs, Stratified by Drug Type
| Net Benefit Ratio (Annual WTP Divided by Total Spending) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Percentile |
||||||||
| Drug | N (Person-Years) | % of Patients for Whom Net Benefit Ratio <1.0 | Range | Mean | 25th | 50th | 75th | 90th |
| Rituximab | 1,885 | 1.6 | 0.200–61.6 | 4.59 | 2.23 | 3.31 | 4.84 | 6.72 |
| Nonrituximab (injectables) | 129 | 23.3 | 0.200–70.2 | 3.06 | 1.20 | 2.25 | 3.58 | 6.30 |
| Nonrituximab (orals) | 664 | 55.0 | 0.166–57.1 | 1.10 | 0.739 | 0.963 | 1.20 | 1.51 |
| All | 2,678 | 26.1 | 0.166–70.2 | 4.04 | 1.72 | 2.90 | 4.46 | 6.21 |
For oral therapies, we find that only 45 percent of patients on erlotinib and imatinib mesylate value the drug in excess of costs. It is important to note though that oral agents are typically covered under the prescription drug benefits, with much less cost sharing, as shown in Table 1. Because virtually none of the patients in our sample faces high cost sharing on these drugs, the slope, and (more importantly) the intercept of the demand curve are based primarily on the region where consumers are facing lower costs, relative to the total price. If consumers are more price sensitive when utilization is higher, this can produce misleading results. There are other important differences for orals: the physician takes a less active role in ensuring compliance and has fewer incentives to do so; and the patient's nonpecuniary costs of self-administration might also play a role. With the recent emergence of new tiering arrangements for expensive oral agents, there will be an opportunity to study this issue in the future as patients face much higher cost sharing for these products.
Setting these issues aside, Table 3 suggests that, on average, oral agents are valued in excess of costs, and that the median patient values them very close to cost. It is less clear that marginal patient populations derive values equal to cost for the orals.
Table 4 provides the ratio of aggregate willingness to pay to aggregate spending for demographic subgroups.
Table 4.
Annual Net Benefit Ratio for Specialty Oncology Drugs, Stratified by Demographic Characteristics
| Percentile |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | Range | Mean | 25th | 50th | 75th | 90th |
| Age | |||||||
| ≤50 | 295 | 0.166–11.9 | 3.02 | 1.03 | 2.15 | 4.24 | 6.25 |
| 51–64 | 845 | 0.200–25.2 | 3.50 | 1.29 | 2.98 | 4.69 | 6.59 |
| ≥65 | 1,538 | 0.233–70.2 | 4.24 | 1.81 | 2.90 | 4.39 | 6.03 |
| Gender | |||||||
| Male | 1,468 | 0.285–70.2 | 3.75 | 1.55 | 2.81 | 4.09 | 8.29 |
| Female | 1,210 | 0.166–61.6 | 4.35 | 1.87 | 3.04 | 4.76 | 6.85 |
| Income* | |||||||
| ≤U.S.$40,000 | 387 | 0.200–53.1 | 4.13 | 1.73 | 2.95 | 4.42 | 5.75 |
| U.S.$40,000+ | 2,291 | 0.166–70.2 | 4.02 | 1.71 | 2.87 | 4.49 | 6.25 |
Note. The overall mean net benefit ratio in our sample is 4.04.
Proxied by median income in 2,000 in patient's three-digit zip code of residence.
While these ratios of willingness to pay to amount paid may seem high, they are consistent with the observed (low) elasticities of demand for these drugs. Moreover, it is important to realize that it is not unusual for willingness to pay to exceed the average amount actually paid per claim, in the same way that the average individual's willingness to pay for any product may often exceed the average out-of-pocket cost actually paid. Take, for example, overall food consumption. Few would argue that individuals would not be willing to spend vast amounts of their income to consume any food at all—yet, as a share of income, food consumption remains below 10 percent of individual income in the United States. The reason is not that individuals do not value food highly, but that competition in the food industry drives out-of-pocket costs down to near cost so that the actual willingness to pay for food overall substantially exceeds the amount actually paid. In fact, for drugs used to treat HIV/AIDS, Philipson and Jena (2006) find exactly this effect—individuals value these drugs at nearly 10–20 times their price.
DISCUSSION
In previous work (Goldman et al. 2006), we found that use of a broad class of specialty drugs for many conditions is insensitive to changes in out-of-pocket cost. This paper extends this analysis by focusing specifically on specialty drugs aimed at treating metastatic disease or hematologic malignancies, and by examining the economic value of these drugs. Overall, we find that while therapy initiation is sensitive to out-of-pocket cost for these five products, therapy continuation, as measured by annual number of claims for users, is largely insensitive to out-of-pocket cost. In addition, our results suggest that generally, the value of these five therapies is high and exceeds the total costs to payers and patients, although the evidence for specialty orals (imatinib mesylate and erlotinib) is less clear. While caution may be warranted in interpreting the findings for the oral molecules due to much narrower dispersion of out-of-pocket costs, these results suggest that further efforts to elucidate the cost-effectiveness of the few marketed specialty oral drugs may be useful. The recent emergence of high cost-sharing tiers in pharmacy benefit designs should provide such an opportunity.
This study has several important limitations. First, because this study is based on administrative claims data rather than medical records, we had to rely on ICD-9-CM codes to construct our sample of eligible patients. As a result, our sample of eligibles includes patients who had certain characteristics that made them unlikely to receive any of the drugs in our sample. For example, trastuzumab (Herceptin) generally is given to Her-2 positive breast cancer patients, but current ICD-9-CM diagnosis codes do not provide information about Her-2 test results. In the absence of these data, our sample of eligibles is larger than otherwise would be the case; the take-up rate is lower; and our estimated demand and income elasticities in the therapy-initiation equation are likely be biased toward zero. Accordingly, we are more confident about the estimates in the therapy-continuation equation (which includes only patients who used the drugs in question) than in the therapy-initiation equation. Nonetheless, it is useful to point out that our estimates of how cost sharing affects therapy initiation may represent a lower bound on the actual effect.
Second, we were not able to accurately measure patients' personal income. Instead, we imputed to each patient the median income of his or her three-digit zip code of residence. The variation in patient incomes is less than the variation in zip code incomes, which means our estimated income elasticities may be biased toward zero. Because of this, we are more confident about how the demand for specialty oncology drugs responds to out-of-pocket cost than income.
Third, our use of out-of-pocket cost differences across health plans to estimate demand assumes that patients do not select into plans in any predictable way that is correlated with plan generosity. This assumption may be reasonable as it may be quite difficult for individuals to infer how generously a plan will cover specialty drugs simply by observing its stated medical or pharmacy benefits, since deductibles, benefit caps, and out-of-pocket maximums complicate these calculations. The out-of-pocket cost a patient will pay for a given drug will depend not only on its tier but also on where it is dispensed (e.g., mail-order or at a bricks-and-mortar store) and at what time of the year (e.g., before or after the patient has reached his or her deductible). For biologics, this issue is further complicated by the fact that many are administered clinically and paid for as part of medical services. If sicker patients were choosing to enroll in plans with more generous coverage, we would see a disproportionate number of cancer patients in plans with low cost sharing. That is not the case in our sample, which suggests that this limitation may not be of much importance in practice.
Fourth, our assumption of a linear demand curve may lead to bias in the estimation of patients' willingness to pay, particularly since few patients face full cost sharing, so that we are required to estimate out-of-sample to infer the nature of the demand curve for high out-of-pocket costs. To the degree that the true elasticity of demand is higher (lower) than predicted with our linear demand curve, our results will overstate (understate) patients' willingness to pay. In general, it seems likely that patient will have more elastic demand when out-of-pocket costs are higher, a property which holds true with a linear demand curve. Thus, any biases in our estimation of patients' willingness to pay will only occur to the degree that the elasticity of demand is increasing with price at a faster rate than predicted by a linear demand curve. This may be a particular concern with oral agents, however.
It is well documented that a high proportion of health care spending occurs at the end of life (Lubitz and Riley 1993). The high costs of end-of-life care have been a source of consternation to many observers who argue that such care is not cost effective in the sense of extending quality-adjusted life-years at a reasonable price. In the United States, it is often said that any treatment that costs more than U.S.$50,000–100,000 per quality-adjusted life-year does not provide good value (Neumann et al. 2000). This rule of thumb apparently is based at least in part on Medicare coverage of end-stage renal disease and dialysis, which once had a cost-effectiveness threshold in that range (Russell et al. 1996). The current cost-effectiveness ratio for renal dialysis, however, is at least U.S.$129,000 per quality-adjusted life-year, according to a recent study (Lee, Chertow, and Zenios 2009), suggesting that current cost-effectiveness threshold limits should be raised to take into account societal preferences and medical inflation (Ubel et al. 2003).
This issue is highly salient for specialty oncology drugs, many of which have cost-effectiveness ratios that exceed U.S.$100,000 per quality-adjusted life-year (Tappenden et al. 2007). However, the argument that such drugs are cost ineffective appears hard to reconcile with the observed behavior of patients in our sample—patients for whom the value of these drugs greatly exceeds the total amount paid by the patient and his insurer. Should drugs be considered cost ineffective if patients using their own money reveal willingness to pay more than the total cost?
A recent working paper by Becker, Murphy, and Philipson (2007) argues that treatments that increase life expectancy in the final months of life (e.g., specialty oncology drugs that shrink tumors) are regarded by consumers very differently than treatments that lower mortality risks only incrementally throughout an individual's entire adult life (e.g., statins that reduce cholesterol). For the former, individuals diagnosed with terminal cancer may plausibly be willing to spend their entire remaining wealth to live a few weeks longer, because the value of their wealth (to the individual) may lose much (or all) of its value at death. This almost certainly is not the case for a treatment that reduces the probability of death by 1 in 500 over a lifetime, even if the latter treatment improves life expectancy by the same amount, on average, as the former. People who face imminent death, in short, may place a much higher value on life-extending treatments than people for whom death is merely a remote risk. This study of specialty oncology medications provides evidence in support of that argument.
Given the high value of these drugs relative to their costs, this study suggests that payers should find ways to increase, rather than decrease, access to specialty oncology drugs. As our results find little evidence that use of these specialty drugs is sensitive to costs, moral hazard for these drugs appears to be quite low, suggesting that reducing cost sharing for these drugs could appropriately increase access. In addition, alternative design of insurance benefits, such as reducing cost sharing for high-value treatments, may enhance patient welfare (Chernew, Rosen, and Fendrick 2007). It should be borne in mind, however, that this study assesses ex post willingness to pay for treatment rather than ex ante willingness to pay for insurance. While these two concepts are related, they are distinct (Neumann and Johannesson 1994; Dolan et al. 2003;). Moreover, the willingness to pay of healthy people imagining what they would pay for life-extending cancer treatments may differ dramatically from that of actual cancer patients confronted with imminent death (Nord 1999). Future research should examine whether healthy people place a different value on life-extending cancer treatments than actual cancer patients and, if so, how such discrepancies should be factored into health insurance coverage decisions.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was sponsored by Amgen Incorporated and the Bing Center for Health Economics at RAND. The opinions expressed here are solely those of the authors.
Disclosures: None.
Disclaimers: None.
NOTES
Note that since the error term is unobserved, equation (4) represents the expected willingness to pay, given the patient's characteristics. This is calculated by integrating the area under the demand curve given in equation (3).
All dollar values are reported in 2008 terms, deflated using the consumer price index.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Becker GS, Murphy KM, Philipson TJ. The Value of Life Near Its End and Terminal Care. Cambridge, MA: National Bureau of Economic Research; 2007. [Google Scholar]
- Chernew ME, Rosen AB, Fendrick AM. Value-Based Insurance Design. Health Affairs. 2007;26(2):w195–203. doi: 10.1377/hlthaff.26.2.w195. [DOI] [PubMed] [Google Scholar]
- Contoyannis P, Hurley J, Grootendorst P, Jeon S-H, Tamblyn R. Estimating the Price Elasticity of Expenditure for Prescription Drugs in the Presence of Non-linear Price Schedules: An Illustration from Quebec, Canada. Health Economics. 2005;14(9):909–23. doi: 10.1002/hec.1041. [DOI] [PubMed] [Google Scholar]
- Dolan P, Olsen JA, Menzel P, Richardson J. An Inquiry into the Different Perspectives That Can Be Used When Eliciting Preferences in Health. Health Economics. 2003;12(7):545–51. doi: 10.1002/hec.760. [DOI] [PubMed] [Google Scholar]
- Gaynor M, Li J, Vogt WB. Is Drug Coverage a Free Lunch? Cross-Price Elasticities and the Design of Prescription Drug Benefits. National Bureau of Economic Research Inc., NBER Working Paper No. 12758. Cambridge, MA: National Bureau of Economic Research.
- Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit Design and Specialty Drug Use. Health Affairs. 2006;25(5):1319–31. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab Plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. New England Journal of Medicine. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jacobson M, O'Malley AJ, Earle CC, Pakes J, Gaccione P, Newhouse JP. Does Reimbursement Influence Chemotherapy Treatment for Cancer Patients? Health Affairs. 2006;25(2):437–43. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- Jena AB, Philipson T. Cost-Effectiveness as a Price Control. Health Affairs. 2007;26(3):696–703. doi: 10.1377/hlthaff.26.3.696. [DOI] [PubMed] [Google Scholar]
- Kolata G, Pollack A. Cost Cancer Drug Offers Hope, but Also a Dilemma. New York Times.
- Krupski TL, Saigal CS, Hanley J, Schonlau M, Litwin MS. Patterns of Care for Men with Prostate Cancer after Failure of Primary Treatment. Cancer. 2006;107(2):258–65. doi: 10.1002/cncr.21981. [DOI] [PubMed] [Google Scholar]
- Lamont EB, Herndon JE, II, Weeks JC, Henderson IC, Earle CC, Schilsky RL, Christakis NA. Measuring Disease-Free Survival and Cancer Relapse Using Medicare Claims from CALGB Breast Cancer Trial Participants (Companion to 9344) Journal of the National Cancer Institute. 2006;98(18):1335–8. doi: 10.1093/jnci/djj363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Chertow GM, Zenios S. An Empiric Estimate of the Value of Life: Updating the Renal Dialysis Cost-Effectiveness Standard. Value in Health. 2009;12(1):80–7. doi: 10.1111/j.1524-4733.2008.00401.x. [DOI] [PubMed] [Google Scholar]
- Lee TH, Emanuel EJ. Tier 4 Drugs and the Fraying of the Social Compact. New England Journal of Medicine. 2008;359(4):333–5. doi: 10.1056/NEJMp0804261. [DOI] [PubMed] [Google Scholar]
- Liu S, Chollet D. Price and Income Elasticity of the Demand for Health Insurance and Health Care Services: A Critical Review of the Literature. Washington, DC: Mathematica Policy Research Inc; 2006. [Google Scholar]
- Lubitz JD, Riley GF. Trends in Medicare Payments in the Last Year of Life. New England Journal of Medicine. 1993;328(15):1092–6. doi: 10.1056/NEJM199304153281506. [DOI] [PubMed] [Google Scholar]
- Nadler E, Eckert B, Neumann PJ. Do Oncologists Believe New Cancer Drugs Offer Good Value? The Oncologist. 2006;11(2):90–5. doi: 10.1634/theoncologist.11-2-90. [DOI] [PubMed] [Google Scholar]
- Neumann PJ, Johannesson M. The Willingness to Pay for In Vitro Fertilization: A Pilot Study Using Contingent Valuation. Medical Care. 1994;32(7):686–99. doi: 10.1097/00005650-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are Pharmaceuticals Cost-Effective? A Review of the Evidence. Health Affairs. 2000;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- Nord E. Cost-Value Analysis in Health Care. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Pelletier EM, Shim B, Goodman S, Amonkar MM. Epidemiology and Economic Burden of Brain Metastases among Patients with Primary Breast Cancer: Results from a US Claims Data Analysis. Breast Cancer Research and Treatment. 2008;108(2):297–305. doi: 10.1007/s10549-007-9601-0. [DOI] [PubMed] [Google Scholar]
- Philipson TJ, Jena AB. Who Benefits from New Medical Technologies? Estimates of Consumer and Producer Surpluses for HIV/AIDS Drugs. Forum for Health Economics and Policy. 2006;9(2):1–31. [Google Scholar]
- Rao S, Kubisiak J, Gilden D. Cost of Illness Associated with Metastatic Breast Cancer. Breast Cancer Research and Treatment. 2004;83(1):25–32. doi: 10.1023/B:BREA.0000010689.55559.06. [DOI] [PubMed] [Google Scholar]
- Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The Role of Cost-Effectiveness Analysis in Health and Medicine. Panel on Cost-Effectiveness in Health and Medicine. Journal of American Medical Association. 1996;276(14):1172–7. [PubMed] [Google Scholar]
- Tappenden P, Jones R, Paisley S, Carroll C. The Cost-Effectiveness of Bevacizumab in the First-Line Treatment of Metastatic Colorectal Cancer in England and Wales. European Journal of Cancer. 2007;43(17):2487–94. doi: 10.1016/j.ejca.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What Is the Price of Life and Why Doesn't It Increase at the Rate of Inflation? Archives of Internal Medicine. 2003;163(14):1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- Willey VJ, Pollack MF, Lednar WM, Yang WN, Kennedy C, Lawless G. Costs of Severely Ill Members and Specialty Medication Use in a Commercially Insured Population. Health Affairs. 2008;27(3):824–34. doi: 10.1377/hlthaff.27.3.824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


