Abstract
Contingency management (CM) treatments are usually applied individually for drug abstinence, but CM can also be targeted toward health behaviors and implemented in groups. HIV-positive patients with cocaine or opioid use disorders (n = 170) were randomized to weekly CM or 12 step (TS) groups for 24 weeks (mean (SD) attendance was 10.8 ± 8.1 sessions for CM participants and 9.0 ± 6.9 session for TS participants). During the treatment period, both groups received compensation for attendance ($10 per session) and submission of urine samples (about $2 per sample). In addition, participants received $25 for submitting samples and completing evaluations at months 1, 3, 6, 9, and 12, with 65 to 75 of the 81 participants assigned to TS and 71 to 80 of the 89 participants assigned to CM completing these evaluations. During the treatment period, patients in the CM group only received chances to win prizes contingent upon completing health activities and submitting substance-free specimens (mean = $260, SD = $267). CM participants submitted a significantly greater number of consecutive drug-free specimens than TS participants (5.2 ± 6.0 versus 3.7 ± 5.6), but proportions of negative samples did not differ between groups during treatment or at follow-up evaluations. From pre- to post-treatment, CM participants showed greater reductions in viral loads and HIV-risk behaviors than TS participants, but these effects were not maintained throughout the follow-up period. These data suggest the efficacy of group-based CM in HIV-positive substance abusers, but more research is needed to extend the long-term benefits.
Keywords: contingency management, group therapy, cocaine abuse, heroin abuse, HIV, AIDS, community settings
Contingency management (CM) interventions are efficacious in enhancing participation in substance abuse treatment and reducing drug use. CM re-arranges the environment to frequently detect behaviors targeted for change and provides tangible reinforcers upon objective evidence of desired behaviors. Reinforcers are usually vouchers, exchangeable for retail goods and services (Higgins et al., 1994) or chances to win prizes (Petry, Martin, Cooney, & Kranzler, 2000). CM is efficacious in a range of clinical populations and settings (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006).
Providing tangible reinforcement for evidence of abstinence via CM procedures reduces drug use. In meta-analyses (Lussier et al., 2006; Prendergast et al., 2006), CM interventions increase durations of abstinence, with estimated overall effect sizes across studies ranging from d = 0.32 to 0.42. Further, durations of abstinence achieved in treatment are strong predictors of long-term abstinence (Higgins, Badger, & Budney, 2000; Petry, Martin, & Simcic, 2005).
CM is not only efficacious for increasing durations of abstinence, but it can also be targeted toward other behaviors. Building on the Community Reinforcement Approach (CRA) (Sisson & Azrin, 1989), some CM interventions reinforce completion of treatment-related goals (Iguchi, Belding, Morral, & Lamb, 1997). For example, if patients desire to improve their health, they may schedule or attend a doctor's appointment, or closely monitor their blood sugar levels or medication adherence. If they successfully accomplish these activities as noted by objective verification, they earn reinforcement. Combined CM treatments, reinforcing both abstinence and activity completion, reduce drug use (Bickel, Amass, Higgins, Badger, & Esch, 1997; Petry et al., 2000, 2004; Petry, Alessi, Marx, Austin, & Tardif, 2005).
Most CM studies provide reinforcement solely on an individualized basis, but the vast majority of substance abuse treatment is group-based. Studies have begun integrating aspects of CM in groups (Alessi, Hanson, Wieners, & Petry, 2007; Ledgerwood, Alessi, Hanson, Godley, & Petry, 2008; Sigmon & Stitzer, 2005). A pilot study assessing CM for activity completion in group therapy found it was efficacious in increasing attendance (Petry, Martin, & Finocche, 2001), and patients completed over 65% of activities when reinforced for doing so.
The purpose of this study was to evaluate the efficacy of a group-based CM intervention that addresses both health and substance use behaviors in HIV-positive individuals attending a drop-in center. HIV drop-in centers are common in urban areas and serve an important resource for the HIV-positive community (Arno, 1986; Mor, Fleishman, Piette, & Allen, 1993; Wolcott, Namir, Fawzy, Gottlieb, & Mitsuyasu, 1986). They provide meal services, recreation, health and nutrition education, and peer counseling. Most individuals attending drop-in centers are active substance abusers. While many drop-in centers offer substance abuse treatment, engagement rates are low (Petry, Martin, & Finocche, 2001). As substance abuse is related to poor adherence to medical treatment (Cohen et al., 2004; Tucker, Burnam, Sherbourne, Kung, & Gifford, 2003), adverse health consequences (Arnsten et al., 2002; Miguez, Shor-Posner, Morales, Rodriguez, & Burbano, 2003), and transmission of HIV to the community via high risk sex and drug use practices (Ehrenstein, Horton, & Samet, 2004; Metzger et al., 1993; Woody et al., 1999), methods to improve engagement and health and drug use outcomes of HIV-positive individuals are needed.
This study arranged for contingent reinforcement for completing health and other goal-related activities in weekly group therapy sessions in the CM condition; abstinence was also reinforced in this condition, but on an individual basis to ensure confidentiality of results. The CM intervention was compared to a standardized 12-step (TS) oriented therapy. To encourage equal therapy participation and submission of samples in the two groups, participants in both groups received modest compensation for attending groups and providing samples. We hypothesized that CM would result in greater numbers of consecutive negative samples submitted, higher proportions of negative samples, and reduced viral loads. Based on prior literature (Hanson, Alessi, & Petry, 2008), we also anticipated that CM would decrease HIV-risk behaviors thereby potentially reducing the spread of HIV. The primary analyses focused on during treatment effects. Secondary analyses examined effects throughout a one-year follow-up and also evaluated predictors of abstinence at the one-year follow-up.
Method
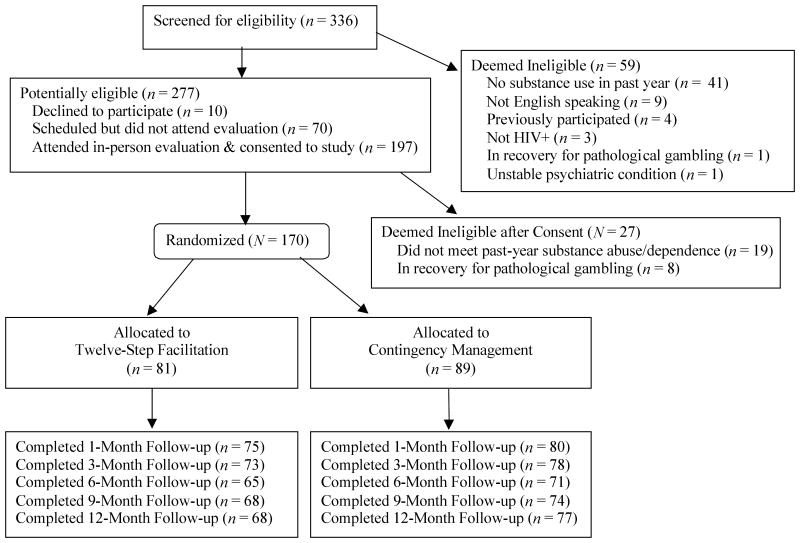
Participants were 170 individuals attending an HIV drop-in center in Hartford, CT, between March 2003 and January 2007. The sample size of about 85 per group was estimated from effect sizes of studies employing similar CM procedures (Petry et al., 2004; Petry, Alessi, et al., 2005), and was sufficient to show medium effect sizes in drug use and medical outcomes (Cohen, 1988). Patients were study eligible if they were HIV-positive, met past-year Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (American Psychiatric Association, 1994) criteria for cocaine or opioid abuse or dependence, and were members of the drop-in center. Membership was free and required only the completion of a brief demographics form and verification of HIV status. Patients were excluded if they were unable to comprehend the study, demonstrated severely disruptive behaviors, or were in recovery for pathological gambling, because the CM condition has an element of chance (but see Petry et al., 2006). Study criteria were not restrictive to increase generalization. Participants provided written informed consent, as approved by the University's Institutional Review Board. As shown in Figure 1, of 250 potentially eligible participants, 170 (68%) were randomized to a treatment condition for 24 weeks.
Figure 1.
Flow chart of participants in study.
Procedures
Following informed consent, participants completed a 2-hour interview. Demographic data were collected, and modules from the Structured Clinical Interview for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) evaluated drug use diagnoses. The National Opinion Research Center's DSM Scale assessed pathological gambling (Gerstein et al., 1999).
The Addiction Severity Index (ASI) (McLellan et al., 1985), a reliable and valid instrument (Bovasso, Alterman, Cacciola, & Cook, 2001; Kosten, Rounsaville, & Kleber, 1983; Leonard, Mulvey, Gastfreind, & Shwartz, 2000), examined severity of psychosocial problems in medical, employment, family, legal, drug, alcohol, and psychiatric domains. Composite scores are derived for each domain, ranging from 0 to 1, with higher scores reflecting greater problems.
Past 3-month versions of the HIV Risk Behavior Scale (Darke, Hall, Heather, Ward, & Wodak, 1991) were administered. The HRBS has 6 questions about injection drug use and 5 about sexual behaviors. Responses are coded on a 6-point scale, with higher values indicating greater risk. An overall summary score, and drug and sexual behavior subscale scores, are computed by summing ordinal values of responses. Scores range from 0 to 55 on the total scale, and 0 to 30 and 0 to 25 on the drug use and sexual risk scales, respectively. The mean total score in this sample was 5.1, reflecting behaviors such as using needles more than once but less than daily in the past 3 months and having 2 sexual partners in that timeframe but always using condoms (or one sexual partner and “often” using condoms). The HRBS has established psychometric properties for measuring HIV risk including internal reliabilities of 0.82 to 0.77 and test-retest reliabilities of 0.86 to 0.90 for lifetime and recent versions (Darke et al., 1991; Kelley & Petry, 2000; Petry, 2001). High agreement is observed between substance abusers and their sexual partners regarding injection and sexual behaviors (Darke et al., 1991).
The past 3-month version of the HRBS was re-administered at follow-ups scheduled for months 3, 6 (post-treatment), 9, and 12. At each follow-up, urine and breath samples were also collected and screened for substances as described below. Participants received $15 for completing baseline and $25 for other interviews. Follow-up rates did not differ by group (Figure 1; ps > .3), with only 3 participants failing to participate in any post-baseline evaluations.
We attempted to obtain viral load results at these same follow-up intervals. After signing a release of information, participants asked physicians to send copies of their most recent viral load tests. For those with no regular physician, viral load testing was arranged at our university hospital at no cost. Participants received $25 each time the viral load results were returned. Of those assigned to 12-Step, a minimum of 50.6% of participants, and a maximum of 79.0%, provided viral load results at any one assessment point. For those assigned to CM, rates ranged from 50.6% to 82.0%. While relatively few participants returned viral loads at all 5 assessment points, only 18 participants (7 from TS and 11 from CM) did not provide any viral load results. Availability of viral load tests did not vary by treatment condition at any point, ps > .30.
Treatment group assignment
After completing the baseline evaluation, participants were randomly assigned to one of two treatments and were scheduled to attend their first group session within a one-week period. A computerized urn randomization procedure (Stout, Wirtz, Carbonari, & Del Boca, 1994) balanced treatment groups on race, baseline urinalysis results, and whether the participant reported taking any HIV medications (Yes/No).
Participants in both groups met with a research assistant weekly to submit breath samples that were screened for alcohol using an Alco-sensor IV Alcometer (Intoximeters, St. Louis, MO), and urine specimens that were screened for opioids and cocaine using OnTrak TesTstiks (Varian, Inc., Walnut Creek, CA). To encourage submission during the treatment period (weeks 1 to 24), participants drew from a prize bowl until they drew a winning card for each set of breath and urine samples submitted, regardless of results.
The prize bowl contained 500 cards. Of them, 250 stated, “Good job,” but did not result in a prize; 224 were small prizes worth up to $1 (choice of $1 food coupons, toiletries, or bus tokens), 25 were large prizes worth up to $20 (CDs, phone cards, walkman, watches, etc.), and one was a jumbo prize worth up to $100 (stereo, DVD, television). Cards were replaced after each draw, so chances remained constant, and participants were aware of probabilities.
Each treatment group met once weekly for 60 minutes. To encourage equal attendance at the two types of groups, participants received $10 vouchers to the retail store of their choice (Sears, CVS, Dunkin Donuts, etc.) for each session attended.
Twelve-step facilitation was based on a manualized intervention (Nowinski, Baker, & Carroll, 1992) designed to encourage engagement in 12-step fellowships (Alcoholic Anonymous, Narcotics Anonymous) and understanding of 12-step principles. In each session, the therapist introduced a chapter from the manual and solicited discussions. Because of the 24 week treatment duration, most chapters and topics were reviewed twice. No contingencies on urinalysis results were in effect in this condition.
CM group sessions focused on determining weekly treatment goals, and reinforcing completion of activities related to those goals. One goal for all participants was improving health, and examples of specific health activities included: scheduling or attending a medical or nutritionist appointment, obtaining medications, recording medication or food consumption daily, or working out at a gym. Other goal area(s) were individually determined and consisted of sobriety, recreational, transportation, housing, legal, education/employment, psychiatric, or personal improvement. Popular activities (and their goal areas) were: attending 12-step meetings (sobriety), going bowling (recreational), or creating a resume (employment). In conjunction with the therapist and group members, each participant decided upon one health and one other activity to complete each week, and wrote these activities on an activity contract, along with an objective form of verification (e.g., signed slip, receipt; see Petry, Tedford, & Martin, 2001).
At the beginning of sessions, the therapist verified completion of activities from the prior week's activity contracts. Participants earned at least one draw from the prize bowl described earlier for each activity completed and verified. They also earned bonus draws, starting at 1 and increasing by 1 (up to a maximum of 10) for each consecutive week in which 2 activities were completed. If a participant did not complete both activities in a given week, bonus draws were reset to 1 the next week both activities were accomplished. Draws were conducted in group, and participants could earn up to 243 draws (about $330) for completing 48 activities over 24 weeks.
Following awarding of activity draws, the therapist initiated a discussion of new activities that could be completed within the upcoming week to improve health and other psychosocial functioning. Participants individually decided upon activities they wished to undertake and signed activity contracts, listing forms of verification, with the therapist.
As in the TS condition, participants earned the opportunity to draw from the prize bowl until they won a prize for each set of urine and breath samples submitted, regardless of results. CM participants earned additional draws for submitting negative samples. They earned 1 additional draw if the breath sample was negative for alcohol and the urine sample tested negative for either cocaine OR opioids, and 2 draws if they tested negative for all 3 substances. Bonus draws were awarded for each week in a row samples tested negative for all substances. Bonus draws started at 1 and increased by 1 for each consecutive week in which all negative samples were submitted, up to a maximum of 10. Number of draws reset to 1 with submission of a positive sample and refusal or failure to submit a sample. Participants could earn up to 243 draws (about $330) for submitting negative samples, and 486 draws in total (about $660).
Therapy supervision
Post-doctoral fellows (MWL and JW) administered each of the two interventions, and a licensed clinical psychologist provided weekly supervision and reviewed group therapy tapes. A modified version of the Yale Adherence Competence Scale (Carroll et al., 2000) assessed TS, CM, and general therapy skills on 1 to 7 likert scales for both types of treatments, with the expectations that little use of TS strategies would occur in CM groups and that little use of CM strategies would occur in TS groups. For CM sessions, mean ratings and standard deviations of TS strategies were 1.89 ± 0.19, reflecting minimal use of these strategies, and 5.78 ± 0.25 for CM strategies, reflecting “good” use. In TS sessions, mean ratings were 4.78 ± 0.60 and 1.09 ± 0.27 for TS and CM strategies, respectively. Mean ratings for general therapy skills and empathy were 5.95 ± 0.26 and 5.71 ± 0.21 for CM and TS groups, respectively.
Data analyses were conducted on an intent-to-treat basis. Between group differences in demographics and substance use were examined using analysis of variance for continuous variables, Chi-square tests for nominal variables, and Kruskal-Wallis tests for continuous variables that could not be normalized.
Univariate analyses compared groups on treatment engagement and the primary drug use dependent measures: longest consecutive number of negative samples submitted, and proportion of negative samples submitted. Proportions of negative samples were calculated with submitted samples in the denominator, thereby unaffected by missing samples, while the longest number of consecutive negative samples was broken if a scheduled sample was not provided. These analyses controlled for gender, baseline severity of drug use (ASI-drug scale scores) and age, as these variables were associated with primary outcome measures. In addition, we also present exploratory analyses related to the greatest number of consecutive negative samples submitted and percentage of samples negative for each drug separately.
For long-term outcomes, logistic regression analysis was used to predict submission of a negative sample (for cocaine, opioids and alcohol) at month 12. Independent variables included in the first step were gender, age, and baseline ASI-drug scores; treatment condition and greatest number of consecutive negative samples during treatment were added to the model in step 2. Odds ratios (OR) and 95% confidence intervals (CI) are presented. Analyses were conducted twice-- both excluding non-followed up participants and including them as non-abstinent.
We also evaluated viral loads. Viral loads, available from 162 participants, were first log transformed, with values of 2.3 included for non-detectable viral loads (Gulick et al., 1997). Hierarchical linear modeling (HLM) examined changes over time and by group over time for log-transformed viral loads; these analyses take into account all available data and estimate missing data based on individual and group trajectories. For the primary analyses, HLM assessed during treatment effects (baseline to month 6), and secondary analyses evaluated effects throughout the study period (baseline to month 12).
Changes in HRBS scores were also evaluated from baseline to post-treatment (primary analyses) and from baseline through month 12 (secondary analyses) using univariate analysis of variance, with gender, age, and treatment condition included as independent variables. The non-normal distribution of these data prevented analyses using HLM.
All analyses other than HLM were performed on SPSS for Windows. A two-tailed alpha < .05 was considered significant.
Results
Demographics and baseline characteristics are reported in Table 1. No statistically significant differences were evident between groups, although males were somewhat overrepresented in the TS group.
Table 1.
Demographic and baseline characteristics
| Variable | 12-Step (N=81) | Contingency management (N=89) | Significance test value (df or N) | p-value |
|---|---|---|---|---|
| Age | 43.1 ±7.1 | 42.6 ± 6.7 | t (167) = 0.46 | .64 |
| Male, % (N) | 67.9 (55) | 55.1 (49) | χ2 (1) = 2.95 | .08 |
| Years of education | 11.2 ± 2.4 | 11.5 ± 2.0 | t (168) = -0.83 | .41 |
| Marital and living status | χ2 (3) = 4.82 | .19 | ||
| Never married, % (N) | 45.7 (37) | 59.6 (53) | ||
| Separated/divorced, % (N) | 35.8 (29) | 31.5 (28) | ||
| Married or living w/partner, %(N) | 11.1 (9) | 4.5 (4) | ||
| Widowed, % (N) | 7.4 (6) | 4.5 (4) | ||
| Employed full or part time | 37.0 (30) | 29.5 (26) | χ2 (1) = 1.18 | .28 |
| Annual incomea | $8,156 ± 6,771 | $8,964 ± 9,565 | U (165) = 2987.5 | .19 |
| Ethnicity | χ2 (3) = 2.03 | .57 | ||
| European American, % (N) | 14.8 (12) | 15.7 (14) | ||
| African American, % (N) | 45.7 (37) | 41.6 (37) | ||
| Hispanic American, % (N) | 33.3 (27) | 30.3 (27) | ||
| Other, % (N) | 6.2 (5) | 12.4 (11) | ||
| Viral load (logged) | 3.2 (1.1) | 3.4 (1.1) | t (134) = 0.89 | .38 |
| Past year substance dependence | ||||
| Alcohol, % (N) | 31.3 (25) | 23.6 (21) | χ2 (1) = 1.25 | .26 |
| Cocaine, % (N) | 75.0 (60) | 77.5 (69) | χ2 (1) = 0.15 | .70 |
| Heroin, % (N) | 55.0 (44) | 50.6 (45) | χ2 (1) = 0.33 | .56 |
| In methadone treatment, % (N) | 38.8 (31) | 33.7 (30) | χ2 (1) = 0.46 | .50 |
| Addiction Severity Index Scores | ||||
| Medicala | 0.44±0.66 | 0.50±0.75 | U (N=170) = 3246.0 | .26 |
| Employmenta | 1.00±0.25 | 1.00±0.25 | U (N=170) = 3441.5 | .56 |
| Alcohola | 0.00±0.17 | 0.01±0.18 | U (N=170) = 3460.5 | .63 |
| Drug | 0.14±0.11 | 0.16±0.11 | t (168) = 1.32 | .18 |
| Legala | 0.00±0.12 | 0.00±0.00 | U (N=170) = 3445.0 | .51 |
| Family/sociala | 0.03±0.37 | 0.10±0.31 | U (N=170) = 3522.5 | .79 |
| Psychiatric | 0.30±0.22 | 0.28±0.22 | t (168) = 0.54 | .59 |
Note. All values are means and standard deviations unless otherwise noted.
Values represent medians and interquartile ranges.
CM=Contingency management
Retention
No statistically significant differences emerged in number of sessions attended by condition, F(1, 168) = 2.71, p = .10, with means (SD) of 9.0 ± 6.9 and 10.8 ± 8.1 sessions (of 24 possible), for TS and CM conditions, respectively. Throughout the study period, mean (SD) group size was 4.0 ± 3.0 for TS and 4.5 ± 2.6 for CM groups, F(1, 330) = 2.54, p = .11.
Drug abstinence during treatment
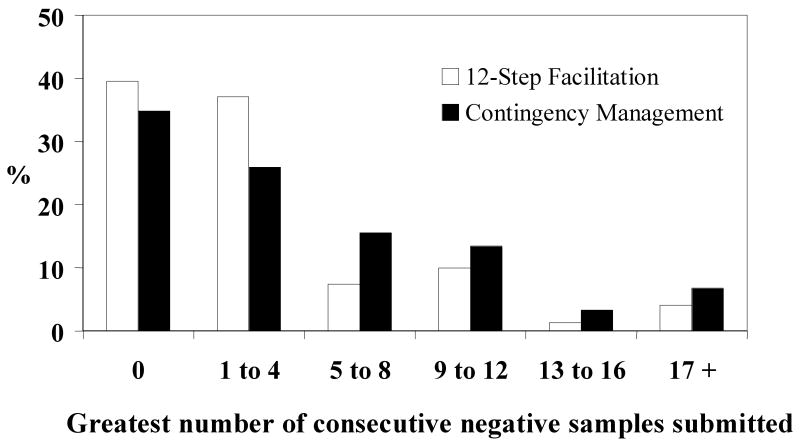
Number of samples submitted did not vary between groups, with means of 12.5 ± 7.3 and 14.0 ± 7.3 for TS and CM groups, respectively, F(1, 168) = 1.70, p = .20. However, as shown in Table 2, longest number of consecutive negative samples submitted for all three substances differed significantly between groups. Figure 2 depicts percentages of participants in each group who submitted various consecutive numbers of samples negative for cocaine, opioids and alcohol.
Table 2.
Substance use treatment outcomes during the 24-week treatment period.
| Outcome measure | 12-Step | Contingency management | F2(164) | p-value |
|---|---|---|---|---|
| N | 81 | 89 | ||
| Longest number of consecutive samples submitted negative for cocaine, opioids, and alcohol (weeks) | 3.7±5.6 | 5.2±6.0 | 98.37 | <.002 |
| From cocaine | 4.2±6.0 | 5.9±6.6 | 54.12 | <.05 |
| From opioids | 4.9±5.9 | 7.0±6.7 | 22.53 | .11 |
| From alcohol | 5.6±5.9 | 8.3±7.0 | 46.89 | .07 |
| Proportion of samples negative for cocaine, opioids, and alcohol | 59.6±37.4 | 59.0±38.3 | 0.01 | .96 |
| For cocaine | 63.8±38.7 | 60.7±38.3 | 0.19 | .74 |
| For opioids | 79.2±33.8 | 86.0±27.2 | 14.59 | .14 |
| For alcohol | 99.0±15.9 | 99.3±7.2 | 0.05 | .86 |
Values represent means and standard deviations.
Figure 2.
Proportion of patients submitting various numbers of consecutive samples negative for alcohol, opioids, and cocaine during the 24-week treatment period. Participants assigned to the 12-step facilitation condition (n = 81) are shown in empty bars, and those assigned to contingency management (n = 89) are shown in filled bars.
Most of the differences were attributed to refraining from cocaine, as numbers of consecutively negative cocaine samples differed significantly between groups (Table 2). Only trends were noted with respect to numbers of consecutively negative opioid and alcohol samples. There was no effect of treatment group on proportion of samples submitted that were negative for all three drugs simultaneously, or for any substance independently (Table 2).
Post-treatment drug use
No group differences were noted in percentages of participants who provided negative samples at the 12-month follow-up. Rates of negative samples were 55.6% (n = 34 of 62) in the TS and 58.2% (n = 39 of 67) in the CM condition. Logistic regression analyses evaluated predictors of abstinence at month 12. Step 1 with age, gender and baseline ASI-drug scores entered was not significant, χ2(n = 129, df = 3) = 2.77, p = .43. Step 2 with treatment group and greatest number of consecutive negative samples (for cocaine, opioids and alcohol) submitted during treatment improved the model, with both the step, χ2(n = 129, df = 2) = 13.24, p < .001, and model significant, χ2 (n = 129, df = 5) = 16.00, p < .01. Only greatest number of consecutive negative samples during treatment was significantly predictive of abstinence at month 12, Beta = 0.38 (standard error (SE) = 0.04), Wald = 9.67 (df = 1), p < .01, OR = 1.14 and 95 % CI = 1.05 to 1.24, with each consecutive negative sample submitted during treatment associated with a 14% increased probability of abstinence at month 12.
Results were similar when participants with missing samples were considered positive. Step 2 was significant, χ2 (n = 170, df = 2) = 14.00, p < .001, as was the model, χ2(n = 170, df = 5) = 17.72, p < .01. Only greatest number of consecutive negative samples was associated with abstinence at month 12, Beta = 0.11 (SE = 0.03), Wald = 11.62 (df = 1), p < .001, OR = 1.12 and 95% CI = 1.05 - 1.19.
Viral loads
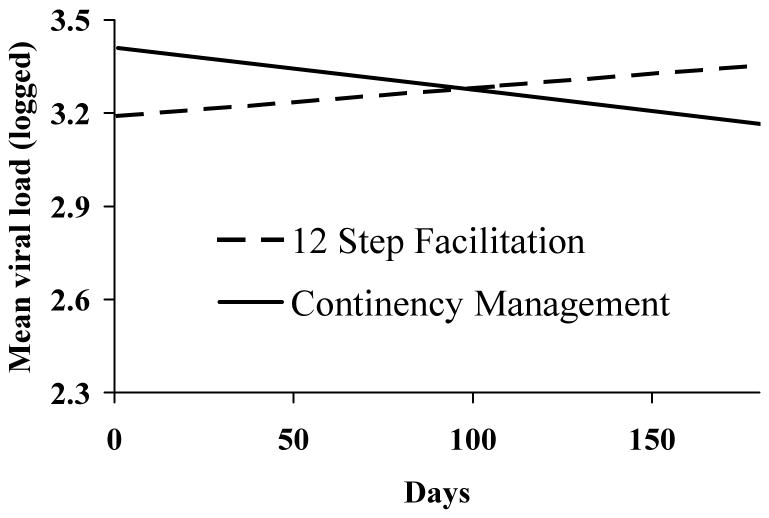
Examining data between baseline and month 6, HLM analyses of viral loads found significant treatment by time effects as shown in Figure 3. Effects of time were significant, χ2(n = 162, df = 112) = 159.56, p < .002, and the group by time interaction was also significant, χ2(n = 162, df = 146) = -2.66, p < .01, with a reduction in viral loads occurring in CM and an increase in TS participants over time.
Figure 3.
Viral loads results between baseline and month 6. Data represent estimated means from hierarchical linear models of participants assigned to 12-step treatment (dashed lines; n = 81) or contingency management treatment (solid lines; n = 89) and take into account data collected between baseline and month 6 (end of treatment). The lower bound on the y-axis of 2.3 reflects non-detectable viral loads as data were log-transformed, and values of 2.3 were included for non-detectable viral loads.
Despite changes in viral loads between groups over time, groups did not differ with respect to proportions taking HIV medications. At baseline, 63.5% of participants in the CM and 72.0% in the TS group reported taking HIV medications, χ2(n = 170, df = 2) = 1.30, p > .25. These proportions did not change by group or over time (range: 59.2% - 69.0%, all ps > .36).
When viral load data throughout the 12-month follow-up period were included in the secondary HLM analyses, group by time effects were no longer statistically significant (p > .70). Effects of time remained significant, χ2(n = 162, df = 127) = 166.09, p < .02, with viral loads generally decreasing slightly over time amongst both groups (data not shown).
HIV risk behavior change scores
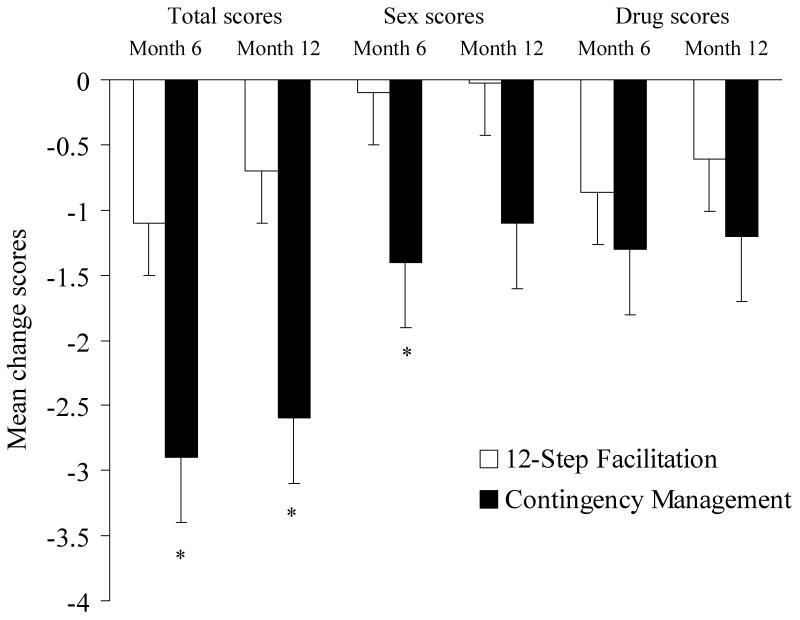
Participants assigned to the CM group reported significantly reduced HIV risk behaviors over time relative to participants assigned to the TS group. As shown in Figure 4, between baseline and month 6, the change in past 3-month HRBS total scores dropped significantly more in the CM compared to the TS condition, F (1, 133) = 4.75, p < .05. In examining subscale scores, changes in drug scores did not differ significantly between groups (p > .35), but sexual risk behaviors decreased among those assigned to the CM group relative to the TS group, F(1, 131) = 4.38, p < .04. The item on which the greatest change occurred related to use of condoms with regular sexual partners in the past month, χ2 (n = 136, df = 2) = 7.32, p < .05. Of participants assigned to TS, 25.8% (n = 17 of 66) used condoms less frequently with regular sexual partners in the past month at the post-treatment evaluation compared to baseline. Another 51.5% (n = 34 of 66) of those assigned to TS used condoms with the same frequency as at baseline, and 22.7% (N = 15 of 66) used condoms at a greater frequency. In contrast, compared to baseline, only 8.6% (n= 6 of 70) of participants assigned to CM reduced frequency of condom use with regular sex partners at the post-treatment evaluation, while 60.0% (n = 42 of 70) showed no change and 31.4% (n= 22 of 70) used condoms more often at the post-treatment assessment.
Figure 4.
Changes in HIV-Risk Behavior Scale scores between baseline and month 6 and between baseline and month 12. Changes are shown for total scores, as well as for the sexual and drug risk behavioral sub-scores. Participants assigned to the 12-step facilitation condition are shown in empty bars, and those assigned to contingency management are shown in filled bars. The asterisks indicate statistically significant differences between conditions based on univariate analysis of variance (p<.05).
In a secondary analyses evaluating post-treatment effects, reductions in HRBS total scores were also significantly greater among CM-treated participants between baseline and month 12, F(1, 139) = 5.23, p < .05. Decreases in sexual risk behavior scores approached significance between groups between these time periods, F(1, 140) = 2.79, p = .09, but changes in drug scale scores were similar between groups between baseline and month 12 (p > .35).
Contingent reinforcement earned
In the CM group, average (SD) number of activities that participants agreed to undertake was 31.5 ± 23.4, with 21.5 ± 20.2 completed. Participants earned an average (SD) of 75.0 ± 82.5 draws for completing activities, and 74.3 ± 77.1 for submitting negative samples. In total, CM participants earned $260 ± $267 worth of prizes contingent on these behaviors.
In addition to these reinforcers, CM participants received on average (SD) $109.0 ± $81.8 for attending groups sessions and $53.0 ± $18.3 for submitting urine and breath samples; TS participants earned averages (SD) of $89.7 ± $69.2 and $31.3 ± $18.3 for attendance and sample submission, respectively. These values did not differ significantly between groups, ps>.10.
Serious adverse events
Four participants (2 from CM and 2 from TS) died of HIV-related causes during the study period, and one (CM group) from an overdose/suicide. No deaths were deemed to be study related, and no study-related serious adverse events were noted.
Discussion
This study found that, during the active treatment period, a CM intervention increased number of consecutive negative samples provided relative to a TS treatment in HIV-positive substance abusers, and it also resulted in reductions in viral loads and HIV risk behaviors. Results on drug use outcomes are similar in magnitude to other CM studies (Higgins et al., 1994, 2003; Higgins, Wong, Badger, Ogden, & Dantona, 2000; Petry et al., 2000, 2004; Petry, Alessi, et al., 2005; Petry, Martin, et al., 2005). Typically, CM extends durations of abstinence achieved, but not proportions of negative samples in outpatient psychosocial settings, most likely because participants who continue using cease coming to treatment and submitting urine samples for toxicology testing (Petry et al., 2004; Petry, Alessi, et al., 2005). This study extends the benefits of CM to polydrug using HIV-positive patients in a group treatment setting. Anecdotally, patients in the CM treatment enjoyed doing the draws in groups, and were supportive and encouraging of one another. Provision of group-based CM is relevant clinically, as most treatment is provided in a group context.
Importantly, the CM intervention also significantly reduced viral loads during the treatment period. Prior studies successfully utilized CM techniques to reinforce adherence to HIV medications (Rigsby et al., 2000; Rosen et al., 2007; Sorensen et al., 2007) with some, but not all, trials finding improvements in viral loads (Rosen et al., 2007; Sorensen et al., 2007). In the present study, nearly half the participants were not taking HIV medications, yet benefits of CM were noted in terms of engendering lower viral loads. These effects did not appear to be moderated by HIV medication, as few participants initiated HIV medications during the treatment period and inclusion of medication status in the analyses was not significant, F(1, 63) = 0.05, p = .83 (data not shown).
This CM intervention also reduced behaviors that spread HIV. Only two other studies have examined the impact of CM on HIV risk behaviors. Schroeder, Epstein, Umbricht, and Preston (2006) failed to find any benefits of CM relative to standard methadone treatment on HIV risk behaviors during methadone initiation, when injection risk behaviors generally decline compared to pre-methadone treatment (Lott, Strain, Brooner, Bigelow, & Johnson, 2006; Metzger et al., 1993). However, Hanson and colleagues (2008) demonstrated benefits of CM on decreasing HIV-risk behaviors in methadone patients stabilized on methadone. In the present study, overall HIV-risk behaviors also decreased, along with specific reductions in sexual risk behaviors among participants in the CM condition. Thus, this CM intervention may have had benefits not only for improving health of the participants themselves by reducing contraction of other infectious diseases, but decreasing the spread of HIV to others. These are clinically significant findings, especially given the personal and societal costs associated with HIV.
These findings should be interpreted in the context of several limitations. Although follow-up rates for interviews were high, biological outcome measures were often missing, especially at longer-term evaluations. We analyzed missing urine toxicology screens at follow-up by including missing samples as positive and found similar results as when only submitted samples were utilized. At some time points, up to half the participants failed to provide viral load results, yet we were able to obtain viral loads from over 89.4% of the sample for at least some assessments. HLM analyses accommodate for missing samples and base results on actual dates samples were provided.
The mechanisms of action whereby CM engendered this improvement remain unclear. Because many different forms of HIV medications were prescribed to participants in this study, we were unable to evaluate the effects of specific medications on outcomes. Other health-related activities may have played a role in treatment-related changes noted in viral loads, but no clear pattern emerged with respect to specific health activities that were commonly completed, and the correlation of activities completed and changes in viral load was not significant, r = .17, p = .27. On the other hand, greatest consecutive number of negative samples submitted was moderately correlated with changes in viral loads, r = .20, p = .06, and drug abstinence may have partly contributed to these benefits.
In this study, participants in both conditions received payment for attending therapy and providing samples so differences in attendance and sample submission would not confound treatment outcomes. While these efforts were somewhat successful in that the groups did not differ significantly with respect to attendance or samples submitted, participants on average complied with the therapy and testing regimen roughly half the time. This low rate of engagement speaks to the difficulties inherent in engaging substance abusers, and especially HIV drop-in center attendees, into substance abuse treatment (Appel, Ellison, Jansky, & Oldak, 2004; Booth, Kwiatkowski, Iguchi, Pinto, & John, 1998; Petry, Martin, et al., 2001; Substance Abuse and Mental Health Services Administration, 2006). While helpful in equalizing attendance rates, this procedure reduces generalization of the findings, as clinics are unlikely to compensate patients for attending treatment sessions.
CM improved abstinence and reduced viral loads during the period in which it was in effect, but few benefits persisted during the follow-up period. Nevertheless, greatest consecutive number of negative samples submitted was a strong and consistent predictor of long-term abstinence in this, and other, CM studies (Higgins, Badger, et al., 2000; Higgins, Wong, et al., 2000; Petry, Alessi, Hanson, & Sierra, 2007; Petry, Alessi, Hanson, et al., 2005; Petry, Alessi, Marx, et al., 2005; Petry, Martin, et al., 2005). While additional research is needed to determine how best to extend CM's benefits, these data show promise of this CM intervention in this important patient population.
Other limitations to this study are that more sophisticated analytical techniques were not used to account for the hierarchical nature of the group-based intervention. Instead, we analyzed outcomes as though they were independent among individuals enrolled in the groups. In addition, urine samples were collected only once a week in this study, so not all instances of drug use may have been detected.
Strengths of the study include the relatively large sample size, broad inclusion criteria, use of group-based treatment, and monitoring multiple outcome measures in a particularly important and underserved patient population. Benefits of a CM intervention that reinforced both abstinence and adherence with health-related goals were noted on drug abuse and viral loads, along with decreases in behaviors that spread HIV and other infectious diseases to the community at large. The CM condition, however, engendered only acute abstinence and reductions in viral loads and HIV risk behaviors, and its beneficial effects were not maintained at long-term follow-ups. Nevertheless, any changes in health behaviors in this population are important. Most notably, this study demonstrated a decrease in HIV viral load, an objective indicator of improved health status. Future research should focus on methods to improve engagement of HIV-positive patients in substance use treatment and to extend the long-term benefits, as these data indicate they are responsive to CM interventions.
Acknowledgments
This research was funded by NIH grants R01-DA14618 and M01-RR06192. Preparation of this report was also supported in part by NIH grants R01-DA13444, R01-DA018883, R01-DA016855, R01-DA021567, R01-DA022739, RO1-DA024667, P60-AA03510, P50-DA09241, and T32-AA07290. We thank Damaris Hernandez, Ellen Ciesielski, Jen Ayers, Tressa Hanson, and Drs. Susan Sampl and Elise Kabela for assistance with the conduct of this trial.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/ccp/
References
- Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: delivering incentives partially in group therapy. Experimental and Clinical Psychopharmacology. 2007;15(3):293–300. doi: 10.1037/1064-1297.15.3.293. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Appel PW, Ellison AA, Jansky HK, Oldak R. Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. American Journal of Drug and Alcohol Abuse. 2004;30(1):129–53. doi: 10.1081/ada-120029870. [DOI] [PubMed] [Google Scholar]
- Arno PS. The nonprofit sector's response to the AIDS epidemic: community-based services in San Francisco. American Journal of Public Health. 1986;76(11):1325–30. doi: 10.2105/ajph.76.11.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17(5):377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, Esch R. Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting and Clinical Psychology. 1997;65(5):803–810. doi: 10.1037//0022-006x.65.5.803. [DOI] [PubMed] [Google Scholar]
- Booth RE, Kwiatkowski C, Iguchi MY, Pinto F, John D. Facilitating treatment entry among out-of-treatment injection drug users. Public Health Reports. 1998;113 1:116–28. [PMC free article] [PubMed] [Google Scholar]
- Bovasso GB, Alterman AI, Cacciola JS, Cook TG. Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behaviors. 2001;15(3):171–176. [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57(3):225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral science. 2nd. Hilsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. American Journal of Public Health. 2004;94(7):1147–1151. doi: 10.2105/ajph.94.7.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5(2):181–85. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Ehrenstein V, Horton NJ, Samet JH. Inconsistent condom use among HIV-infected patients with alcohol problems. Drug and Alcohol Dependence. 2004;73(2):159–166. doi: 10.1016/j.drugalcdep.2003.10.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version. Washington, DC: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- Gerstein DR, Volberg RA, Toce MT, Harwood H, Johnson RA, Buie T, et al. Gambling Impact and Behavior Study: Report to the National Gambling Impact Study Commission. Chicago, IL: National Opinion Research Center; 1999. [Google Scholar]
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with Indinavir, Zidovudine, and Lamivudine in Adults with Human Immunodeficiency Virus Infection and Prior Antiretroviral Therapy. New England Journal of Medicine. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- Hanson T, Alessi SM, Petry NM. Contingency management reduces drug-related human immunodeficiency virus risk behaviors in cocaine-abusing methadone patients. Addiction. 2008;103(7):1187–97. doi: 10.1111/j.1360-0443.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer-term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8(3):377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60(10):1043–52. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68(1):64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Belding MA, Morral AR, Lamb RJ. Reinforcing operants other than abstinence in drug abuse treatment: An effective alternative for reducing drug use. Journal of Consulting and Clinical Psychology. 1997;65(3):421–428. doi: 10.1037//0022-006x.65.3.421. [DOI] [PubMed] [Google Scholar]
- Kelley JL, Petry NM. HIV risk behaviors in male substance abusers with and without antisocial personality disorder. Journal of Substance Abuse Treatment. 2000;19(1):59–66. doi: 10.1016/s0740-5472(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Concurrent validity of the Addiction Severity Index. Journal of Nervous and Mental Disease. 1983;171(10):606–10. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Hanson T, Godley M, Petry NM. Contingency management for attendance to group substance abuse treatment administered by clinicians in community clinics. Journal of Applied Behavior Analysis. 2008;41(4):617–22. doi: 10.1901/jaba.2008.41-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index: A field study of internal consistency and validity. Journal of Substance Abuse Treatment. 2000;18(2):129–35. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Lott DC, Strain EC, Brooner RK, Bigelow GE, Johnson RE. HIV risk behaviors during pharmacologic treatment for opioid dependence: a comparison of levomethadyl acetate, buprenorphine, and methadone. Journal of Substance Abuse Treatment. 2006;31(2):187–94. doi: 10.1016/j.jsat.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173(7):412–23. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Metzger DS, Woody GE, McLellan AT, O'Brien CP, Druley P, Navaline H, et al. Human immunodeficiency virus seroconversion among intravenous drug users in- and out-of-treatment: an 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes. 1993;6(9):1049–1056. [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addiction Biology. 2003;8(1):33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Mor V, Fleishman JA, Piette JD, Allen SM. Developing AIDS community service consortia. Health Affairs. 1993;12(1):186–199. doi: 10.1377/hlthaff.12.1.186. [DOI] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Twelve-step facilitation therapy manual: a clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 1. Rockville, MD: Institute on Alcohol Abuse and Alcoholism; 1992. DHHS Publication No. ADM 92-1893 Project MATCH Monograph Series. [Google Scholar]
- Petry NM. Reliability of drug users' self-reported HIV risk behaviors using a brief, 11-item scale. Substance Use and Misuse. 2001;36(12):1731–47. doi: 10.1081/ja-100107576. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. Journal of Consulting and Clinical Psychology. 2007;75(6):983–91. doi: 10.1037/0022-006X.75.6.983. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73(6):1005–14. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kolodner KB, Li R, Peirce JM, Roll JM, Stitzer ML. Prize-based contingency management does not increase gambling. Drug and Alcohol Dependence. 2006;83(3):269–73. doi: 10.1016/j.drugalcdep.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Variable-ratio contingency management for treatment of alcohol dependence. Journal of Consulting and Clinical Psychology. 2000;68(2):250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Finocche C. Contingency management in group treatment: A demonstration project in an HIV drop-in center. Journal of Substance Abuse Treatment. 2001;21(2):89–96. doi: 10.1016/s0740-5472(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F. Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73(2):354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treatment of cocaine abusers: How low can we go, and with whom? Addiction. 2004;99(3):349–60. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Martin B. Reinforcing compliance with non-drug related activities. Journal of Substance Abuse Treatment. 2001;20(1):33–44. doi: 10.1016/s0740-5472(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rigsby MO, Rosen MI, Beauvais JE, Cramer JA, Rainey PM, O'Malley SS, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. Journal of General Internal Medicine. 2000;15(12):841–47. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MI, Dieckhaus K, McMahon TJ, Valdes B, Petry NM, Cramer J, et al. Improved adherence with contingency management. AIDS Patient Care and STDS. 2007;21(1):30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Epstein DH, Umbricht A, Preston KL. Changes in HIV risk behaviors among patients receiving combined pharmacological and behavioral interventions for heroin and cocaine dependence. Addictive Behaviors. 2006;31(5):868–879. doi: 10.1016/j.addbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Stitzer ML. Use of a low-cost incentive intervention to improve counseling attendance among methadone-maintained patients. Journal of Substance Abuse Treatment. 2005;29(4):253–258. doi: 10.1016/j.jsat.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sisson RW, Azrin NH. The Community Reinforcement Approach. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. Vol. 157. Elmsford, NY: Pergamon Press; 1989. pp. 242–258. (Pergamon general psychology). [Google Scholar]
- Sorensen JL, Haug NA, Delucchi KL, Gruber V, Kletter E, Batki SL. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug and Alcohol Dependence. 2007;88(1):54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RL, Wirtz RW, Carbonari J, Del Boca FK. Ensuing balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol Supplement. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National survey on drug use and health. 2006 Retreived August, 10, 2008, from http://www.oas.samhsa.gov/NSDUH/2k6nsduh/tabs/Sect5peTabs54to56.pdf.
- Tucker JS, Burnam A, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. American Journal of Medicine. 2003;114(7):573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- Wolcott DL, Namir S, Fawzy FI, Gottlieb MS, Mitsuyasu RT. Illness concerns, attitudes towards homosexuality, and social support in gay men with AIDS. General Hospital Psychiatry. 1986;8(6):395–403. doi: 10.1016/0163-8343(86)90019-8. [DOI] [PubMed] [Google Scholar]
- Woody GE, Donnell D, Seage GR, Metzger D, Marmor M, Koblin BA, et al. Non-injection substance use correlates with risky sex among men having sex with men: data from HIVNET. Drug and Alcohol Dependence. 1999;53(3):197–205. doi: 10.1016/s0376-8716(98)00134-3. [DOI] [PubMed] [Google Scholar]