Abstract
Background
This study examined the potential of an antidepressant drug, escitalopram, to improve depression, resilience to stress, and quality of life in family dementia caregivers in a randomized placebo-controlled double-blind trial.
Methods
Forty family caregivers (43–91 years of age, 25 children and 15 spouses; 26 women) who were taking care of their relatives with Alzheimer’s disease were randomized to receive either escitalopram 10 mg/day or placebo for 12 weeks. Severity of depression, resilience, burden, distress, quality of life, and severity of care-recipient’s cognitive and behavioral disturbances were assessed at baseline and over the course of the study. The Hamilton Depression Rating Scale (HDRS) scores at baseline ranged between 10–28. The groups were stratified by the diagnosis of major and minor depression.
Results
Most outcomes favored escitalopram over placebo. The severity of depression improved and the remission rate was greater with the drug compared to placebo. Measures of anxiety, resilience, burden and distress improved on escitalopram compared to placebo.
Discussion
Among caregivers, this small randomized controlled trial found that escitalopram use resulted in improvement in depression, resilience, burden and distress, and quality of life. Our results need to be confirmed in a larger sample.
Keywords: Dementia caregiver, stress, depression, resilience, quality of life, escitalopram
Recent estimates indicate that the prevalence of Alzheimer’s disease (AD) is increasing at an alarming rate; causing a dramatic upsurge in the cost of this disease to society. At least five million Americans provide care for someone with AD (1, 2), and the number of caregivers will increase proportionately to the number of new AD cases. These individuals provide extraordinary, uncompensated care, predominantly in the home setting, involving significant amounts of time and energy for months or years, and requiring the performance of tasks that may be physically, emotionally, socially, or financially demanding. The personal, social, and health impacts of informal dementia caregiving have been well documented in recent years (3–6).
Chronic severe stress puts caregivers at higher risk for depression, and declining resilience and quality of life. On average, 50% of family caregivers report various degrees of depression at any given time (7). The majority of AD caregivers are elderly, with women providing the highest levels of care. These caregivers are twice as likely to report physical strain and high levels of emotional stress as a direct result of caregiving responsibilities. Caregiver burden and depression are related to level of patient severity of dementia, disability, and behavioral disturbances. As a result of the impaired resilience to stress with advancing age(8, 9); and increased allostatic load, tolerance of stress may result in cardiovascular disease and decline of health and quality of life. In a longitudinal cohort study of 400 older spousal caregivers (1), caregivers who experienced mental or emotional strain related to caregiving had mortality risks 63% higher than non-caregiving controls. One of the proposed mechanisms of chronic caregiver stress health effects is related to declining immune function and increase in inflammation (10–15).
Despite widespread interest in the challenges facing family caregivers of people with dementia, the literature on empirically-validated treatments has grown slowly (16), with many of the existing treatment trials showing only modest benefits on caregiver outcomes.
Unfortunately, the majority of the literature suggests that the limitations in the research strategies used to test the effectiveness of mostly psychosocial interventions may have contributed to their limited findings of improvement. Although some studies included antidepressant use as an adjunct treatment in severely depressed caregivers undergoing psychosocial interventions, no prior studies tested the efficacy of antidepressant drugs for caregiver depression in a double blind placebo-controlled trial. Despite the fact that caregiver depression shares clinical features with other depressive disorders of multiple etiologies, it is unclear that antidepressant response will be similar since depressed caregivers represent a more homogeneous group with regard to shared life stressors. In fact, this question has not been examined yet in the literature. To-date, there have been no randomized placebo-controlled trials of the efficacy of antidepressant treatments for caregiver depression. Moreover, no studies have evaluated whether antidepressant treatment leads to improvement in coping with stress and daily functioning. Our study is the first to investigate the efficacy of an antidepressant drug, escitalopram, to improve depression, resilience to stress, and quality of life in depressed family dementia caregivers in a double-blind placebo-controlled trial. We report on the effect of an antidepressant on 1) depression; 2) anxiety; 3) resilience and coping; and 4) perceived burden.
Methods
Over a period of 24 months, we screened a total of 134 family caregivers, and recruited 40 family caregivers (45–91 years of age, 25 adult children and 15 spouses; 26 women) who were taking care of their relatives with Alzheimer’s disease and were willing to participate in the study. After completely describing the study to the subjects, written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board.
Potential subjects were first screened using the Center for Epidemiological Studies-Depression Scale (CES-D) (17). Those who had scores of 16 and above were invited to participate in the trial. The Structured Clinical Interview for the DSM-IVR (SCID) was used to establish a diagnosis of either major or minor depression. In case of minor depression, the research diagnostic definition of the DSM-IVr was used. All subjects met the following inclusion criteria: 1) Caregivers of patients with dementia who present for evaluation to the Alzheimer’s Disease Center for evaluation of dementia, cognitive impairment and/or co-existing behavioral disturbances. Adult or elderly caregivers were identified by the patient or the staff as the primary source of assistance and/or support, and were in contact with the patient at least three times per week. 2) Caregivers met the Diagnostic Statistical Manual (DSM-IVr) criteria for a current diagnosis of major depressive disorder, single episode or recurrent, without psychotic features, or for the DSM diagnosis of minor depression or depression not otherwise specified (NOS). Other inclusion criteria were a) score of 16 or higher on the Center for Epidemiological Studies-Depression scale (CES-D) and b) Score of 26 or higher on the Folstein Mini-mental State Examination (MMSE). Exclusion criteria were: 1) A history of other psychiatric illness or alcohol or substance abuse or dependence; 2) severe or acute medical illness; 3) acute suicidal or violent behavior; 4) any other CNS diseases or dementia.
Response was defined as remission or as the HDRS scores of 6 or less. In addition, we compared improvement in residual depressive symptoms in the two treatment groups over time using the HDRS scores.
All subjects were randomized using a computer-generated randomization table to receive either escitalopram 10 mg/day or placebo for 12 weeks. Escitalopram is one of the most effective currently available SSRIs (18, 19). In addition, escitalopram is very easy to use in a single effective dose of 10 mg, and generally, is well-tolerated by older patients (20, 21). The convenience of the use of escitalopram in the context of our proposal includes the lack of the dose titration and the lack of the waiting period after each increase in the dose to observe maximal effect. In addition, there is an evidence of a faster onset of antidepressant and anxiolytic action of escitalopram compared to citalopram in the adult and geriatric samples (20–23). The recommended dose of escitalopram for use in geriatric patients is 10 mg.
We assessed the severity of depression, anxiety, resilience, burden, distress, and severity of care-recipient’s cognitive and behavioral disturbances at baseline and at follow up. The HDRS scores at baseline ranged between 10–28. The groups were stratified by the diagnosis of major and minor depression. The assessments of depressive symptoms were repeated every two weeks throughout the study.
Procedures
All subjects underwent the Structured Clinical Interview for DSM-IV (SCID) (24) to diagnose major and minor depressive disorders as administered by one rater (HL). All subjects received baseline physical examinations and laboratory testing to rule out new-onset medical illnesses.
In addition to the treatment protocol, following randomization, all caregivers also received psychoeducation about the course and prognosis of Alzheimer’s disease and about caregiver health, which was intended to address identified shortcomings in the care of elderly patients with dementia. All subjects were encouraged to attend monthly caregiver support groups provided by the local chapters of the Alzheimer’s Association.
Study medications and treatment procedures
Caregivers were evaluated every two weeks for 12 weeks. Subjects received either 10 mg of escitalopram daily or placebo. The use of concomitant medications was restricted to the use of lorazepam up to 1 mg day for comorbid anxiety administered to some individuals with excessive anxiety in both groups. At the end of the trial, the decisions were made to continue the prescribed drug, or switch to another antidepressant based on treatment response and tolerability.
Assessment instruments
The Hamilton Rating Scale for Depression (HRSD-24 item) (25) was used as a primary outcome measure to quantify mood symptoms. The Connor-Davidson Resilience scale (CD-RISC), was used as a secondary outcome measure to quantify stress coping ability. The CD-RISC is comprised of 25 items, each rated on a 5-point scale (0–4), with higher scores reflecting greater resilience. The scale demonstrated good psychometric properties and factor analysis yields five factors. The CD-RISC has sound psychometric properties and distinguishes between those with greater and lesser resilience (26). The CD-RISC is an internally consistent scale for assessing resilience among older adults yielding four factors in older adults that reflected items involving: 1) personal control and goal orientation, 2) adaptation and tolerance for negative affect, 3) leadership and trust in instincts, and 4) spiritual coping (27). Specific to caregiver stress, the Revised Memory and Behavior Problems Checklist (RMBPC) (28) determines how frequently a patient with dementia engages in problematic behaviors and which problems are especially upsetting for family members. There are two parts to the RMBPC, and it consists of 24 items. The first part determines the frequency with which common problems have occurred, and the care recipient’s cognitive and behavior status is scored on a Likert scale of 0–4 (0, never happens; 4, happens every day). The timeframe used was one week and this was selected to minimize the recall task for informants. The second part of the RMBPC obtains the informant’s subjective appraisal of each problem and measures the degree to which behaviors ‘bothered or upset’ the caregiver. Side-effects were assessed by the UKU Side Effect Rating Scale (29). The Caregiver Burden Interview (BI) (30) is a 22-item questionnaire which was designed to assess the stress experienced by family caregivers of older people and disabled persons. Anxiety symptoms were assessed using the Hamilton Anxiety Rating scale (25).
The rest of the instruments were used to compare groups at baseline. Cognitive performance was measured by the Mini-Mental State Examination (MMSE). Medical co-morbidity was measured by the Cerebrovascular Risk Factor Prediction Chart (CVRF) (31) of the American Heart Association for rating cerebrovascular risk factors, including age, systolic blood pressure, antihypertensive medication use, history of diabetes, smoking, previous strokes, atrial fibrillation, and left ventricular hypertrophy. The Cumulative Illness Rating Scale (CIRS) (32) was used for rating global chronic medical illness burden.
Statistical analysis
All data were entered into the database at the time of their collection. Patients in the two treatment groups were compared on all demographic and clinical measures at baseline to assess the success of the randomization procedures using chi-square tests for the categorical measures and two-sided t-tests for the continuous measures. Safety analyses were performed using descriptive statistics and frequency distribution of dropouts. The proportion of subjects who achieved remission was analyzed using chi-square test. The primary outcome measure, namely the continuous HDRS score, was analyzed using a mixed effects random regression model using SAS v 9.1. Treatment group, time and the interaction term between time and treatment group were included in the model, with group as a between-subject effect and time as a continuous within-subject variable. We repeated the analysis co-varying for age, caregiver status (spouse or adult children), and baseline HDRS scores. For the secondary measures such as anxiety, resilience, distress and burden, change scores were analyzed using t-tests. We examined the relationship between the variables using Pearson correlation coefficients. The level of significance was set at the two-sided alpha level of p≤ 0.05.
Results
Table 1 presents the baseline demographic and clinical characteristics of the completers in the two subject groups. Figure 1 summarizes changes in all outcomes of interest. The groups did not differ on any of the baseline characteristics. On average, caregivers in our study scored higher on the distress measure (RMBP-distress) compared to other studies of caregiver stress. This may have to do with our sample that had levels of clinical depression due to a more prolonged and more complicated provision of care than in other samples (33, 34). Twenty eight subjects completed the trial: 14 subjects in each group, twelve subjects (30%) dropped out, and six subjects were in each treatment group. Dropouts in both groups occurred either due to emerging mild-to-moderate side-effects (i.e., anxiety, nausea, sleep problems), or due to the lack of efficacy, or inability to commit to the study schedule. The groups did not differ by the levels of side-effects, or by the time spent in support or psychoeducation groups.
Table 1.
Comparison of the baseline demographic and clinical characteristics in the two treatment groups.
| esCIT (N=20) Mean (SD) | PBO (N=20) Mean (SD) | Chi-square/t; p* | |
|---|---|---|---|
| Men | 5/20 | 9/20 | 1.7;0.2 |
| Number of depressive episodes | 1.9 (0.9) | 2.5 (1.5) | 1.5;0.1 |
| Chronic depression | 11/20 | 12/20 | 0.1;0.8 |
| Major depression | 13/20 | 11/20 | 0.4:0.5 |
| Age | 60 (9.4) | 63.3 (13.4) | 1.3;0.2 |
| Age of Onset | 49.7 (15) | 44.6 (19.7) | −0.7;0.5 |
| Education (years) | 16.0 (3.7) | 14.8 (1.9) | −1.6;0.1 |
| Duration of the current episode (months | 31.6 (24.4) | 22.3 (16.6) | −1.4;0.2 |
| Resilience | 60.2 (16.7) | 66.6 (17.0) | 1.2;0.2 |
| Burden | 50.6 (14.6) | 47.3 (18.4) | −0.6;0.5 |
| RMBP-care-recipient’s behaviors | 46.9 (14.8) | 44.6 (18.4) | 1.5;0.1 |
| RMBP-distress | 32.0 (7.5) | 35.3 (22.2) | 0.5;0.6 |
| HDRS baseline | 15.1 (4.6) | 15.7 (5.8) | 1.2;0.2 |
| HAM-A | 10.1 (4.8) | 8.9 (4.5) | −0.8;0,4 |
| CIRS | 2.6 (2.1) | 3.9 (3.4) | 1.9;0.06 |
| CVRF | 6.1 (3.8) | 8.7 (6.3) | 1.6;0.1 |
| MMSE baseline | 29.4 (1.0) | 29.3 (1.0) | −0.3;0.8 |
chi-square statistics (df=1) and p-values are reported for gender ratio, number of depressive episodes, frequency of chronic and major depression; t-statistics (df=38) and p-values are reported for all other variables
P (tapproxim) ≤0.05; two-sample test t approximation, two-sided P reported for all variables
RMBP-care-recipient’s behaviors and distress scales
MMSE–Mini-Mental State Examination scale at baseline
HDRS-Hamilton Depression Rating Scale at baseline
HAM-A-Hamilton Anxiety Rating Scale
CIRS-Cumulative Illness Rating Scale
CVRF-Cerebrovascualr Risk Factors scale
Figure 1. Comparison of the change scores in clinical outcomes in the completers in the two treatment groups.
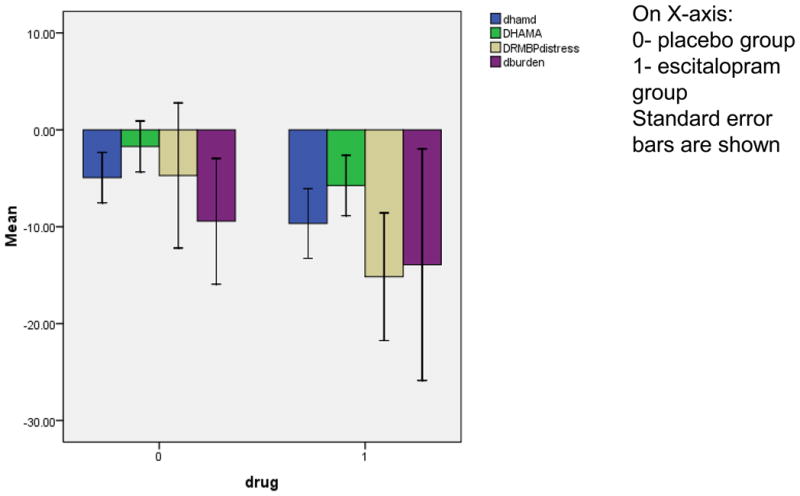
Change in Hamilton Depression Rating Scale (HDRS), Hamilton Anxiety scale (HAM-A), Resilience, Distress and Burden scores are reported as the differences between scores at week 12 and baseline in the placebo (0) and the escitalopram (1) groups.
Twelve subjects in the escitalopram group and six subjects in the placebo group achieved remission (Chi square= 4.5; df=1; p<.04), which amounts to 80% vs. 40% remission rates in the completers, or 60% vs. 30% remission rates in the entire sample. The severity of depression improved with the drug compared to placebo in the mixed random regression model in the full sample (interaction term between group and time: F=4.1; df=1;135; p≤.05). The results did not change after controlling for age, caregiver status, and the baseline HDRS scores, the CIRS or CVRF scores, or the diagnosis of major or minor depression. Participants who took escitalopram also showed improvement measures of resilience, anxiety, and perceived burden and distress despite the lack of changes in the care-recipient behaviors (Table 2). Improvement in depression severity correlated with improvement in anxiety (r=0.67; p<0.01) and perceived burden (r=0.39; p<0.05). Interestingly, the narrative subjective comments about improvement in depression, the level of distress, and the ability to cope were fairly uniform among caregivers taking escitalopram who described their improved ability to cope with the tasks of caregiving. Remitters in the escitalopram group continued taking the drug after the end of the trial. Only two non-remitters in the escitalopram group were offered other antidepressant trials upon the completion of the trial, while three of eight non-remitters in the placebo group were offered antidepressants, and three were referred to psychotherapy according to their choice.
Discussion
Our study is the first to use a placebo-controlled randomized design of the antidepressant treatment of caregiver depression. We report improvement in depression, anxiety, resilience, and subjective distress in caregivers receiving escitalopram compared to those on a placebo despite the fact that caregivers had higher levels of distress compared to other studies of caregiver stress (total scores with mean of 32–34 in our sample compared to 11–17 in other samples) (28, 33, 34). Although some prior studies used pharmacological approaches in a subgroup of patients, no prior reports used a placebo-controlled trial (4, 28, 35). Based on our findings, we concluded that antidepressant use in depressed family dementia caregivers with either major or minor depression can be used to improve symptoms of depression, boost resilience to stress, and improve coping with caregiver stress.
The accumulating evidence on the personal, social, and health impacts of caregiving has generated multiple psychosocial intervention studies aimed at decreasing the burden and stress of caregiving. Although the empirical evidence has shown that psychosocial and educational interventions can lower caregivers’ depressive symptoms and enhance mental health (36–38), the effects of interventions vary greatly in relation to the contents of programs, characteristics of caregivers and patients, and other contextual factors (39). The existing psychosocial interventions are minimally-to-modestly effective and rarely include biological measures of stress-response or document improvements in distress or resilience in caregivers as the result of these interventions (40, 41).
Recent research focusing on more rigorous randomized controlled-trial designs evaluated a broader range of intervention programs involving individual or family counseling, case management, skills training, environment modification, behavior-management strategies, anger and depression management, and combination thereof (38, 42–45). The recent multi-site study referred to as REACH I and II (Resources for Enhancing Alzheimer’s Caregiver Health) (1, 4, 44, 45) is a unique, multi-site research program sponsored by the National Institute on Aging and the National Institute on Nursing Research, studied a total of 1,222 caregivers and care-recipients with various psycho-educational interventions, home-based interventions, support groups; counseling, behavioral skills training, and medications for care-recipient and caregiver with moderate effects on caregiver depression and stress (1, 4, 43–46). Evidence from these supportive and behavioral interventions indicates that combined interventions targeting multiple levels of the stress/health model and multiple individuals simultaneously (i.e., caregiver and patient) produce a significant improvement in caregiver depression/burden, subjective well-being, perceived caregiver satisfaction, ability/knowledge, and, sometimes care-recipient symptoms (39). In the recent meta-analysis, Pinquart and Sorensen (2006) integrated the results of 127 intervention studies with dementia caregivers published or presented between 1982 and 2005 (47). Interventions had, on average, significant but small effects on burden, depression, subjective well-being, ability/knowledge and symptoms of care recipient. Only multicomponent interventions reduced the risk for institutionalization. Psychoeducational interventions that require active participation of caregivers had the broadest effects. Effects of cognitive-behavioral therapy, support, counseling, daycare, training of care recipient, and multicomponent interventions were domain specific. The authors concluded that because most interventions had domain-specific outcomes, clinicians must tailor interventions according to the specific needs of the individual caregivers. Given the magnitude of the caregiver burden and the great variety of caregiver intervention studies funded by the federal and private funding, it is surprising that a number of intervention studies have consistently failed to document positive outcomes, and there has been very little translation of the interventions into clinical practice.
On the other hand, our results are fairly striking compared to the placebo-controlled antidepressant studies of geriatric or mixed-age depression (48–51). While a relatively modest remission rate of 30–35% and high placebo response rate nearing 30–40% has been well documented in geriatric and mixed–age samples including a large cohort of the recently completed Sequenced Treatment Alternatives to Relieve Depression (STAR-D) study (48, 49, 51), 86% of caregivers in our sample on escitalopram were able to achieve remission compared to 44% in those on placebo. In fact, the commonly believed and expected effect of antidepressants to help depressed patients achieve remission or improvement only happens in 30–50% of patients with geriatric depression in clinical trials. Nearly half of all placebo-controlled clinical trials fail to find drug-placebo differences in geriatric patients. Fluoxetine performed equal or worse than placebo in improving depressive symptoms in several trials of geriatric depression, similarly to other SSRI antidepressants (18, 19, 48, 50, 51). We believe that heterogeneity of depression is one of the factors responsible for overall disappointing results in geriatric depression trials. We are impressed with the observed benefit of escitalopram and the speed of response between weeks two and four in the remitters and the improved burden and coping that are important in reducing suffering in caregivers. In the narrative subjective reports, most caregivers uniformly stated in the first two to four weeks that they felt as if they were removed from the immediate harmful effect of stress that gave them an opportunity to be more objective and less reactive to the difficulties of caregiving tasks. None of psychosocial interventions that dominate caregiver depression field provided such fast and reliable relief. There have been no placebo-controlled studies of antidepressants in caregivers, ours is the first to demonstrate such effects.
In conclusion, our study provides new evidence of antidepressant efficacy in caregiver depression that also improves resilience and coping with caregiver stress. One might consider the characteristics of our samples as the limitations of the study that includes mixed and relatively small sample of adult children and spousal caregivers who suffered from either major or minor depression. However, this sample is representative of community caregivers and these features also allowed us to examine the effects of age and severity of depression, as well as comorbid conditions associated with aging such as medical and vascular burden. We did not find any significant effects of the above variables in our outcomes. We concluded that the use of antidepressants is a viable option in helping caregivers cope with their depression and the stress of caregiving. We attribute the greater rate of remission in this cohort compared to the average study of geriatric depression to the nature of depression caused by the similar life stressors compared to the heterogeneous groups of elderly depressed patients selected by the non-discriminating-by-cause descriptive DSM diagnostic categories. Although some heterogeneity in individual life circumstances exists, for the most part, caregivers are depressed because of severe chronic stress of caregiving. They may also have lesser genetic loading, medical burden, and cognitive impairment than subjects in the studies of geriatric depression that may explain better antidepressant response in our trial. However, this study needs to be replicated in a larger study. Other non-pharmacological mind-body approaches to stress reduction such as yoga and meditation should also be tested in this difficult-to-manage population.
Acknowledgments
This work was supported by the Forest Research institute IIT LXP-103; and the NIH grants R01 MH077650 and R-21 AT003480 to Dr Lavretsky, and NIH grants T32-MH19925, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, General Clinical Research Centers Program, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core to Dr. Irwin
References
- 1.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 2.Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12:240–249. [PubMed] [Google Scholar]
- 3.Ory MG, Hoffman RR, 3rd, Yee JL, et al. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 4.Schulz R, O’Brien AT, Czaja S, et al. Dementia caregiver intervention research: in search of clinical significance. Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DL. Comparative analysis of caregiver data for caregivers to the elderly 1987 and 1997. Bethesda, MD: 1997. [Google Scholar]
- 7.Lavretsky H. Stress and depression in informal dementia caregivers. Health and Aging. 2005;1:117–133. [Google Scholar]
- 8.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 10.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavretsky H, Ballmaier M, Pham D, et al. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. Am J Geriatr Psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavretsky H, Roybal DJ, Ballmaier M, et al. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–967. doi: 10.4088/jcp.v66n0801. [DOI] [PubMed] [Google Scholar]
- 14.Mills PJ, Adler KA, Dimsdale JE, et al. Vulnerable caregivers of Alzheimer disease patients have a deficit in beta 2-adrenergic receptor sensitivity and density. Am J Geriatr Psychiatry. 2004;12:281–286. [PubMed] [Google Scholar]
- 15.Mills PJ, Yu H, Ziegler MG, et al. Vulnerable caregivers of patients with Alzheimer’s disease have a deficit in circulating CD62L- T lymphocytes. Psychosom Med. 1999;61:168–174. doi: 10.1097/00006842-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Zarit SH. Diagnosis and management of caregiver burden in dementia. Handb Clin Neurol. 2008;89:101–106. doi: 10.1016/S0072-9752(07)01209-2. [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 18.Cipriani A, La Ferla T, Furukawa TA, et al. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009:CD006117. doi: 10.1002/14651858.CD006117.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Cipriani A, Santilli C, Furukawa TA, et al. Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009:CD006532. doi: 10.1002/14651858.CD006532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharmacol. 2005;20:131–137. doi: 10.1097/00004850-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Rampello L, Alvano A, Raffaele R, et al. New possibilities of treatment for panic attacks in elderly patients: escitalopram versus citalopram. J Clin Psychopharmacol. 2006;26:67–70. doi: 10.1097/01.jcp.0000195383.96383.25. [DOI] [PubMed] [Google Scholar]
- 22.Lader M, Andersen HF, Baekdal T. The effect of escitalopram on sleep problems in depressed patients. Hum Psychopharmacol. 2005;20:349–354. doi: 10.1002/hup.694. [DOI] [PubMed] [Google Scholar]
- 23.Stein DJ, Andersen HF, Goodman WK. Escitalopram for the treatment of GAD: efficacy across different subgroups and outcomes. Ann Clin Psychiatry. 2005;17:71–75. doi: 10.1080/10401230590932335. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Gibbon M, Spitzer RL, et al. User’s guide for the structured clinical interview for DSM-IV Axis I disorders-Research version-(SCID-I), Version 2.0. 1996 February; [Google Scholar]
- 25.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 26.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 27.Lamond AJ, Depp CA, Allison M, et al. Measurement and predictors of resilience among community-dwelling older women. J Psychiatr Res. 2008;43:148–154. doi: 10.1016/j.jpsychires.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 29.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 30.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 31.American Heart Association. Stroke Risk Factor Prediction Chart. Dallas, TX: 1990. [Google Scholar]
- 32.Miller MD, Paradis CF, Houck PR, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 33.Fuh JL, Liu CY, Wang SJ, et al. Revised memory and behavior problems checklist in Taiwanese patients with Alzheimer’s disease. Int Psychogeriatr. 1999;11:181–189. doi: 10.1017/s1041610299005736. [DOI] [PubMed] [Google Scholar]
- 34.Roth DL, Burgio LD, Gitlin LN, et al. Psychometric analysis of the Revised Memory and Behavior Problems Checklist: factor structure of occurrence and reaction ratings. Psychol Aging. 2003;18:906–915. doi: 10.1037/0882-7974.18.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz R, Martire LM, Klinger JN. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatr Clin North Am. 2005;28:1007–1038. x. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Marriott A, Donaldson C, Tarrier N, et al. Effectiveness of cognitive-behavioural family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry. 2000;176:557–562. doi: 10.1192/bjp.176.6.557. [DOI] [PubMed] [Google Scholar]
- 37.Mittelman MS, Ferris SH, Shulman E, et al. A comprehensive support program: effect on depression in spouse-caregivers of AD patients. Gerontologist. 1995;35:792–802. doi: 10.1093/geront/35.6.792. [DOI] [PubMed] [Google Scholar]
- 38.Mittelman MS, Roth DL, Coon DW, et al. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. Am J Psychiatry. 2004;161:850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 40.Hosaka T, Sugiyama Y. Structured intervention in family caregivers of the demented elderly and changes in their immune function. Psychiatry Clin Neurosci. 2003;57:147–151. doi: 10.1046/j.1440-1819.2003.01094.x. [DOI] [PubMed] [Google Scholar]
- 41.Vedhara K, Miles J, Bennett P, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol Psychol. 2003;62:89–96. doi: 10.1016/s0301-0511(02)00128-x. [DOI] [PubMed] [Google Scholar]
- 42.Coon DW, Thompson L, Steffen A, et al. Anger and depression management: psychoeducational skill training interventions for women caregivers of a relative with dementia. Gerontologist. 2003;43:678–689. doi: 10.1093/geront/43.5.678. [DOI] [PubMed] [Google Scholar]
- 43.Eisdorfer C, Czaja SJ, Loewenstein DA, et al. The effect of a family therapy and technology-based intervention on caregiver depression. Gerontologist. 2003;43:521–531. doi: 10.1093/geront/43.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gitlin LN, Belle SH, Burgio LD, et al. Effect of multicomponent interventions on caregiver burden and depression: the REACH multisite initiative at 6-month follow-up. Psychol Aging. 2003;18:361–374. doi: 10.1037/0882-7974.18.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisniewski SR, Belle SH, Coon DW, et al. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): project design and baseline characteristics. Psychol Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levesque L, Gendron C, Vezina J, et al. The process of a group intervention for caregivers of demented persons living at home: conceptual framework, components, and characteristics. Aging Ment Health. 2002;6:239–247. doi: 10.1080/13607860220142468. [DOI] [PubMed] [Google Scholar]
- 47.Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18:577–595. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]
- 48.Kozel FA, Trivedi MH, Wisniewski SR, et al. Treatment outcomes for older depressed patients with earlier versus late onset of first depressive episode: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2008;16:58–64. doi: 10.1097/JGP.0b013e31815a43d7. [DOI] [PubMed] [Google Scholar]
- 49.Roose SP, Sackeim HA, Krishnan KR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004;161:2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 50.Roose SP, Schatzberg AF. The efficacy of antidepressants in the treatment of late-life depression. J Clin Psychopharmacol. 2005;25:S1–7. doi: 10.1097/01.jcp.0000162807.84570.6b. [DOI] [PubMed] [Google Scholar]
- 51.Schatzberg A, Roose S. A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. Am J Geriatr Psychiatry. 2006;14:361–370. doi: 10.1097/01.JGP.0000194645.70869.3b. [DOI] [PubMed] [Google Scholar]


