Abstract
Objective
Elderly patients with cardiovascular disease (CVD) often report cognitive difficulties including reduced cognitive processing speed and attention. On cross-sectional examination, such reports relate more closely to mood than to objective measures of cognitive performance, thus questioning the validity of subjective cognitive complaints as a marker of neurodegenerative processes. This study examined the longitudinal relationship between self-reported cognitive difficulties, depression, and performance on objective tests of global cognition in patients with CVD.
Participants and Methods
Forty-seven CVD patients (ages 55 to 85 years) completed a measure of perceived cognitive dysfunction (Cognitive Difficulties Scale), a medical history questionnaire, the Dementia Rating Scale (DRS), and the Beck Depression Inventory (BDI) at baseline and 12 months later. Baseline brain imaging was available on a small sub-sample (n = 17).
Results
Hierarchical linear regression revealed that increased report of cognitive difficulties at baseline was significantly associated with poorer DRS performance at follow-up (F(3, 43) = 4.45, p = .008, CDS partial r = −.30, p = .048), independent of age, education, baseline DRS and BDI scores. Greater perceived cognitive dysfunction at baseline also related to higher level of white matter lesions (r = .53, df = 15, p = .028).
Conclusions
Self-reported cognitive difficulties may reflect early changes in cognitive aging that are difficult to detect using global cognitive screening measures at a single time point. Yet, these perceived difficulties relate to objectively measured cognitive decline over time. Thus, they may provide important clinical information about early neurodegenerative processes that should be carefully monitored.
Keywords: Subjective Cognitive Complaints, Cognition, Cardiovascular Diseases, Dementia Ratings Scale, White Matter Hyperintensities
OBJECTIVE
Subjective cognitive complaints among the elderly have been a focus of intense debate in the research literature over the past decade. Some studies have documented a significant relationship between perceived cognitive dysfunction, current cognitive impairment, and risk for future cognitive decline (1–6). Others have demonstrated that self-reported cognitive difficulties relate more closely to current physical and emotional state (e.g., anxiety and depression), and certain personality characteristics, such as perceived locus of control (mastery), self-efficacy, and level of neuroticism (7–10). In most studies, however, the assessment of perceived cognitive dysfunction has been limited to memory, often including only a single question about perceived forgetfulness. In view of the current consensus that mild cognitive impairment (MCI) is a heterogeneous diagnostic category, including patients with non-amnestic deficits (11), self-reported cognitive difficulties and their relationship to long-term clinical outcomes deserve further investigation.
In recent years, our laboratory and others have demonstrated that patients with cardiovascular disease (CVD) show subtle deficits in attention-executive-psychomotor functions even before the onset of dementia (12–16). Consistent with these findings, patients with CVD often report experiencing cognitive difficulties in everyday life regardless of whether they have undergone cardiac surgery (17–22). Common self-reported complaints include forgetfulness, difficulties with sustained attention, verbal fluency, and short-term memory (20). Since the objectively detectable cognitive deficits in patients with CVD have been associated with mild systemic hypoperfusion (14), endothelial dysfunction (16), higher burden of atherosclerosis (13), increased microvascular lesions in the brain (23, 24), and reduced cerebral hemodynamic response to cognitive function (25), it is reasonable to assume that the self-reported cognitive difficulties reflect similar underlying neurodegenerative processes. This proposition is supported by reports of brain volume losses (26) and microvascular lesions (5, 27–29) in relation to subjective memory complaints in community samples.
However, the clinical picture is complicated by the fact that, consistent with community studies, cross-sectional and brief follow-up studies (< 6 months) with CVD patients have found that perceived cognitive difficulties relate more closely to measures of psychological distress than to objective measures of cognitive function (17–20). The long-term clinical outcomes of CVD patients with high levels of subjective cognitive complaints remain unclear. Longitudinal studies with more extensive follow-up periods (12 months or greater) have been limited to patients undergoing coronary artery bypass grafting (CABG), and have yielded discrepant results. Some have suggested that subjective memory complaints following CABG are related to depressed mood and high levels of anxiety (21), while others have noted that a high level of self-reported cognitive difficulties post-CABG cannot be fully accounted for by depression (22).
The main goal of this study was to examine the longitudinal relationships between self-reported cognitive difficulties, mood, and changes in global cognitive function in a sample of patients with a variety of CVD diagnoses. We chose a heterogeneous group of cardiac patients as our target population because it is representative of the wide range of patients treated by Outpatient Cardiology Clinics, and enrolled in Outpatient Cardiac Rehabilitation Programs. We were interested in the clinical utility of subjective cognitive complaints that may be related to a treating cardiologist during routine follow-up visits. Furthermore, in the face of evidence that even subtle changes in peripheral cardiovascular health relate to differences in cognitive functioning (13, 14, 16, 30), our goal was to increase external validity by including patients with a wide range of disease severity, systemic perfusion, endothelial function, and large vessel atherosclerosis.
Participants completed a 39-item scale of perceived cognitive dysfunction (Cognitive Difficulties Scale, CDS (31)), a medical history questionnaire, the Beck Depression Inventory (BDI (32)), and the Dementia Rating Scale (DRS (33)) at study entry and approximately one year later. Baseline neuroimaging was available on a small set of participants (n = 17). Therefore, in addition to our main goal of investigating the utility of subjective cognitive complaints in predicting objective cognitive decline over time, we were able to tentatively explore the relationship between perceived cognitive dysfunction, total brain volume (TBV), and cerebrovascular health as measured by white matter hyperintensities (WMH) on magnetic resonance imaging (MRI) in this population. In view of our previous findings that among patients with mild CVD, systemic perfusion, endothelial function, and large vessel atherosclerosis were all associated with diminished cognition (13, 14, 16), and cerebral microvascular and hemodynamic changes (23, 25), we hypothesized that subjective cognitive complaints may reflect subclinical changes in brain function that in time will manifest as objectively detectable cognitive decline. Thus, we hypothesized that higher levels of subjective cognitive complaints at study entry would be related to significantly lower TBV and higher levels of WMH in the brain at study entry, and lower global cognitive function at one-year follow-up as measured by age and education corrected scaled DRS scores.
METHODS
Study Sample
Participants between the ages of 55 and 85 were recruited from cardiology outpatient clinics, cardiac rehabilitation programs, and community fliers in the Providence, RI area for participation in a longitudinal study of cognitive function in older adults with CVD. Volunteers were screened for participation if they had a documented history of at least one of the following: a diagnosis of coronary artery disease (CAD), angina pectoris, previous myocardial infarction (MI), heart failure, cardiac surgery, arrhythmia, or hypertension. Patients were excluded if they had a history of neurological disease (i.e., large vessel stroke, seizure disorder, Parkinson’s disease, clinically significant traumatic brain injury, multiple sclerosis, brain infection/meningitis, or diagnosed dementia), major psychiatric illness (e.g. schizophrenia, bipolar disorder), substance abuse (i.e., diagnosed abuse and/or previous hospitalization for substance abuse), or if they scored below the cut off for dementia (total score < 123) on the DRS at study entry.
The current study was limited to those participants who completed a measure of subjective cognitive complaints, the CDS, at study entry, and returned for one-year follow-up. The CDS was completed by 83 participants at study entry (20). One year later, 55 of the 83 participants returned for follow-up (i.e., 66% retention rate). Participants who returned (n = 55) were on average two years more educated than those who withdrew (14.70 years of education for the returning participants vs. 12.82 for those who withdrew; t = −3.04, df = 79, p = .003). However, the two subgroups did not differ significantly for age, DRS scaled scores, CDS, or BDI scores at study entry (Table 1). Eight participants who returned for follow-up evaluation were missing data for at least one key variable, so the present analysis is based on 47 participants. MRI data were available on a subset of participants (n = 17). Descriptive statistics regarding the sample demographic and medical characteristics are presented in Table 2.
Table 1.
A Comparison of Demographics and Baseline Performances Between the Retained and Lost Participants
| Retained | Lost | t (df) | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 70.58 | 7.50 | 71.00 | 8.12 | .23 (81) | .816 |
| Education | 14.70 | 2.70 | 12.82 | 2.54 | −3.04 (79) | .003 |
| NYHAC | 1.15 | .74 | 1.29 | .69 | .72 (63) | .474 |
| DRS total (raw) | 138.05 | 4.41 | 135.18 | 7.05 | −2.28 (81) | .025 |
| DRS total (scaled)† | 10.34 | 2.56 | 9.58 | 2.87 | −1.22 (79) | .224 |
| BDI total | 5.14 | 3.73 | 7.45 | 5.69 | 1.60 (67) | .115 |
| CDS total | 35.78 | 18.54 | 40.44 | 27.19 | .19 (80) | .364 |
NYHAC = New York Heart Association Class; DRS=Dementia Rating Scale;
Raw DRS scores were converted to age- and education-corrected scaled scores using the Mayo’s Older Americans Normative Studies (MOANS) norms (36); BDI = Beck Depression Inventory; CDS = Cognitive Difficulties Scale
Table 2.
Sample Demographic and Medical Information
| Frequency % | Mean | SD | |
|---|---|---|---|
| Sex (% female) | 40 | ||
| Caucasian | 79 | ||
| African American | 2 | ||
| Did not specify | 19 | ||
| Age, years | 70.09 | 7.60 | |
| Education, years | 14.89 | 2.60 | |
| Hypertension | 77 | ||
| Hypercholesterolemia | 53 | ||
| Diabetes | 23 | ||
| Tobacco Use | 36 | ||
| Angina | 21 | ||
| Angioplasty/Stents | 23 | ||
| CAD | 30 | ||
| Heart Failure | 13 | ||
| MI | 43 | ||
| CABG | 38 | ||
| Arrhythmia | 13 | ||
| Mitral Valve Prolapse | 6 | ||
| Valve surgery | 10 | ||
| Pacemaker | 2 | ||
| New York Heart Association Class | |||
| 0 | 17 | ||
| 1 | 47 | ||
| 2 or 3 | 25 | ||
Procedures
The local Institutional Review Board approved the study and all volunteers provided written informed consent before enrollment. Participants completed a detailed medical history questionnaire with a focus on CVD, and responses were reviewed by a trained research assistant under the supervision of the study PI (RAC). Cardiovascular risk factors (hypertension, hypercholesterolemia, tobacco use, and diabetes), and cardiovascular diagnoses, events, and procedures (e.g., CAD, CABG, MI, angioplasty, angina, arrhythmia, heart failure, valve surgery) were coded as either present or absent according to participants’ self-report. Severity of functional limitation (New York Heart Association Class, NYHAC) was graded by the study cardiologist (AP) using the New York Heart Association functional classification system for patients with heart failure (34). Participants underwent cardiovascular and neuropsychological assessments at study entry and 12 months later.
Instrumentation
Subjective Cognitive Complaints
The CDS is a 39-item questionnaire of subjective difficulties in attention, memory, perception, and psychomotor abilities (31). It has been used in both healthy and patient samples, including persons with CVD (17, 19, 20, 31, 35). Participants are asked to rate themselves using a 5-point Likert scale (from “never” to “most of the time”) on several statements describing everyday inefficiencies (e.g., “I find it hard to keep my mind on a task or a job.”), lapses of memory (e.g., “I have trouble recalling frequently used phone numbers”), and related functions that people often notice about themselves (e.g., “I have to do things very slowly to be sure I’m doing them right.”). Scores range between 0 and 156. Higher scores indicate a greater number of subjective complaints.
Global Cognitive Function
The DRS is a measure of general cognitive ability for older adults with known or suspected cognitive dysfunction (33). The measure contains five modules measuring attention, verbal and motor initiation and perseveration, visuospatial construction, conceptualization, and memory, always administered in the same order. It yields a maximum total score of 144 with lower scores denoting greater impairment. This study employed the Mayo’s Older Americans Normative Studies (MOANS) (36) normative data, which is the largest and most extensive normative sample for the DRS. These norms were used to convert participants’ raw scores to age and education corrected scaled scores with a mean of 10 and a standard deviation of 3. These scaled scores served as our primary outcome variables.
Depression
The BDI assesses vegetative, affective, and cognitive symptoms of depression and has been used extensively in patient populations (32).
MRI Data Acquisition & Processing
MRI data were acquired in a single sessions on a 1.5T Siemens Symphony scanner equipped with a standard head coil. Structural imaging sequences included a fluid-attenuated inversion recovery (FLAIR) (TR = 6000 ms, TE = 105 ms, 5 mm slice thickness, 2 mm gap) sequence for WMH quantification and a high-resolution (256 × 256 matrix, FOV = 256 mm2, 1 mm slice thickness) magnetization prepared rapid gradient echo anatomical scans of the entire brain in the saggital plane.
The WMH quantification protocol used in the current study has been described elsewhere (37). Briefly, the FLAIR images obtained for each participant were used to quantify WMHs via a semi-automated threshold technique in ANALYZE® (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). The total number of voxels representing WMH from each slice was summed to calculate the total WMH for each participant. Total brain volume was calculated using the FLAIR images and threshold histogram values consistent with brain parenchyma. Total brain volume was used as a correction factor for WMH values (i.e., WMH pixel total/TBV × 100) to estimate the proportion of TBV affected by microvascular disease while correcting for potential whole brain atrophy.
Data Analyses
Variable distributions were examined using Shapiro-Wilk tests of normality. Variable transformations were performed if necessary. Differences between baseline and follow-up test scores were examined using paired t-tests. The relationship between baseline CDS score and DRS performance at follow-up was examined using hierarchical linear regression analyses where follow-up DRS scaled score was entered as the dependent variable. In the first step of the regression analysis, the relationship between baseline CDS score and scaled follow-up DRS score was adjusted for the effects of baseline cognitive functioning (DRS scaled score) and baseline depressive symptoms (BDI score). The independent contribution of CDS score to variance in global cognitive functioning at 12 months was estimated in the second step. Potential mechanisms were explored in a follow-up analysis examining the relationship between subjective cognitive complaints and severity of CVD (as indicated by NYHAC and CAD), TBV, and WMH using parametric correlations. Data were analyzed using SPSS 15.0 computer software (SPSS Inc., Chicago, IL). A two-tailed alpha level of .05 was used as the criterion for statistical significance.
RESULTS
A comparison of demographic variables and baseline test scores between the study sample and participants lost to follow-up is available in Table 1. Baseline and follow-up raw test scores for the final sample are summarized in Table 3. Shapiro-Wilk tests of normality revealed a positively skewed distribution of BDI scores (Shapiro-Wilk = .920, df = 41, p = .01) and WMH (Shapiro-Wilk = .755, df = 17, p = .001). Both variables were transformed using a square root transformation in order to fulfill assumptions of normality for inclusion in parametric analyses.
Table 3.
Study Sample Entry and Follow-up Performances
| Baseline | Follow-up | Paired t (df) | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| DRS total (raw) | 138.68 | 3.78 | 138.38 | 5.20 | .46 (46) | .651 |
| DRS total (scaled)† | 10.49 | 2.31 | 10.75 | 3.08 | −.58 (46) | .563 |
| BDI total | 5.26 | 3.77 | 4.85 | 3.51 | 1.14 (40) | .261 |
| CDS total | 36.04 | 17.71 | 32.55 | 16.95 | 1.16 (41) | .251 |
DRS = Dementia Rating Scale;
Raw DRS scores were converted to age- and education-corrected scaled scores (36); BDI = Beck Depression Inventory; CDS = Cognitive Difficulties Scale
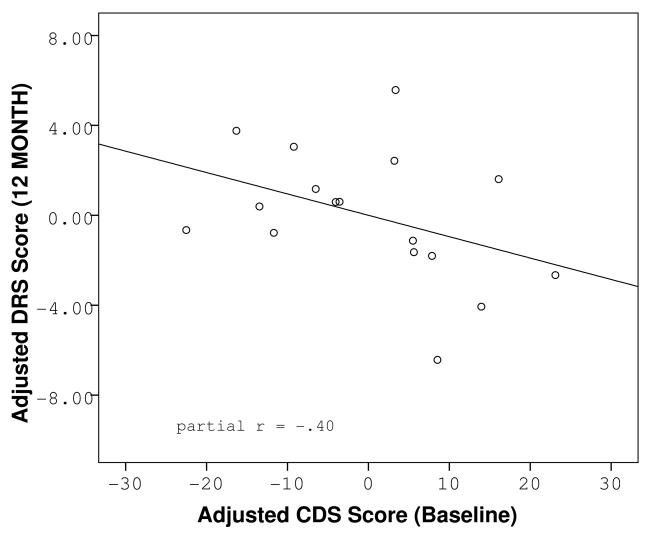
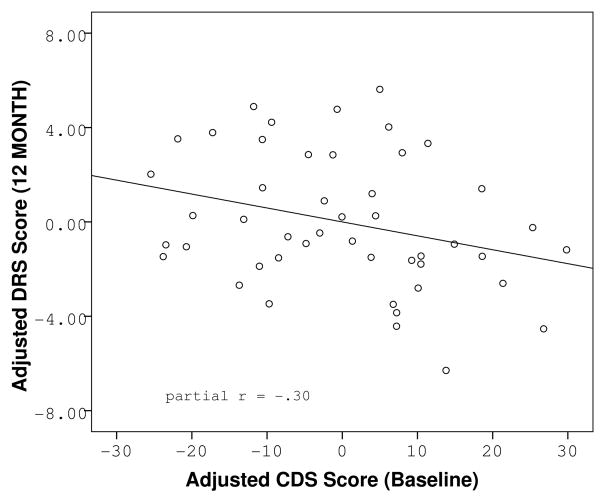
Paired t-tests revealed no overall mean differences between baseline and follow-up DRS performance (t = −.58, df = 47, p = .563), CDS score (t = 1.16, df = 42, p = .251), or BDI score (t = 1.14, df = 41, p = .261). Further investigation of individual cognitive trajectories revealed that while 42% the sample experienced no change in DRS scaled score during the follow-up period, another 42% experienced a decline, and 16% experienced an improvement. To better characterize the people with detectable cognitive decline, we examined the relationship between baseline subjective cognitive complaints and objective cognitive performance at follow-up. Higher baseline CDS scores were significantly related to lower DRS scaled scores at the 12-month follow-up (F(3, 43) = 4.45, p = .008, CDS partial r = −.30, p = .048). The effect was independent of baseline DRS scaled score, or baseline BDI score. Baseline CDS scores were not related to baseline DRS scaled scores (F(2, 44) = .31, p = .738) as previously reported (20). The relationship between baseline CDS and follow-up scaled DRS scores was maintained when the sample was limited to the patients with available neuroimaging (Figure 3), though the correlation was no longer significant due to lack of power (partial r = −.40, p = .139 in the imaging subsample vs. partial r = −.30, p = .048 in the full sample). Furthermore, when a median split was performed on baseline CDS scores, separating the sample into two categories: “low complainers” and “high complainers, ” our results revealed that 57% of the high complainers experienced a decline over the following year, while only 30% of the low complainers experienced a negative change in cognitive score. The mean DRS change scores between the two groups were significantly different (t = 2.35, df = 45, p = .023). In addition, membership in the “high complainers” group was predictive of significantly lower follow-up DRS scaled scores regardless of baseline DRS scaled score and baseline level of depressive symptoms (F(3,43) = 6.01, p = .002, partial r = −.36, p = .016) underscoring the variability in individual cognitive trajectories within the sample.
Figure 3.
Partial regression plot of the relationship between reported cognitive dysfunction at baseline and objective cognitive functioning at follow-up in the sub-sample of participants with available neuroimaging, adjusted for baseline cognitive function and baseline level of depression.
Bivariate correlations between CDS scores, DRS scaled scores, and BDI scores at study entry and follow-up are reported in Table 4. Baseline CDS scores were not related to severity of CVD as estimated by NYHAC (r = .09, df = 45, p = .574) and CAD (r = −.22, df = 45, p = .144). Neither did they relate to our overall measure of brain health, TBV, after adjusting for potential gender differences in head size (partial r = −.032, df = 14, p = .783). Baseline CDS scores, however, related to severity of microvascular disease in the brain at baseline as measured by WMH on MRI (r = .53, df = 15, p = .028). Baseline BDI scores were not significantly related to WMH (r = .31, df = 15, p = .221) at study entry in this sample.
Table 4.
Bivariate Correlations Between Study Sample Entry and Follow-up Performances
| Baseline DRS | Baseline BDI | Baseline WMH | Follow-up DRS | Follow-up BDI | |
|---|---|---|---|---|---|
| Baseline CDS | −0.05 | 0.59** | 0.53* | −0.31* | 0.54** |
| Sig. (2-tailed) | .738 | .000 | .028 | .035 | .000 |
| N | 47 | 47 | 17 | 47 | 41 |
| Baseline BDI | 0.06 | 1.00 | 0.31 | −0.10 | 0.70** |
| Sig. (2-tailed) | .712 | .221 | .506 | .000 | |
| N | 47 | 47 | 17 | 47 | 41 |
| Baseline DRS | 1.00 | 0.06 | −0.24 | 0.39** | 0.16 |
| Sig. (2-tailed) | .712 | .355 | .007 | .318 | |
| N | 47 | 47 | 17 | 47 | 41 |
CDS = Cognitive Difficulties Scale; DRS = Dementia Rating Scale; BDI = Beck Depression Inventory; WMH = White Matter Hyperintensities
Correlation is significant at the .01 level (2-tailed)
Correlation is significant at the .05 level (2-tailed)
CONCLUSIONS
The present study found that in patients with CVD, increased report of cognitive difficulties at baseline was associated with poorer global cognitive performance at 12-month follow-up. The association was independent of age, education, baseline cognitive performance, and level of depression. A relationship between self-reported cognitive difficulties and cognitive performance on objective neuropsychological measures has been inconsistently reported in past studies. While some studies have documented a significant relationship between perceived cognitive dysfunction and risk for future cognitive decline in community dwelling elderly (1–6), other studies have demonstrated that subjective assessments of cognitive abilities relates more closely to current physical and emotional state and personality (7–10). However, a systematic review of the clinical and community-based research suggests a significant interaction between the length of follow-up and the findings (38). Most cross-sectional and brief follow-up studies (< 6 months) tend to report no relationship between self-reported cognitive difficulties and objective measures of cognition (8–10, 17, 19, 20), while the majority of longer follow-ups (2 – 8 years) reveal a significant relationship between subjective memory complaints and objectively measured cognitive decline even after adjustment for depressive symptoms (1, 2, 4–6). Consistent with this review, we previously observed modest cross-sectional relationships between reported cognitive difficulties and cognition, yet depressive symptoms were moderately related to cognitive complaints (20). At one-year follow-up, however, baseline cognitive complaints in the same sample were significantly associated with poorer global cognitive performance, independent of age, education, baseline cognitive performance, or level of depressive symptoms. To further characterize the people with detectable cognitive decline, we performed a median split on baseline CDS scores, separating the sample into two categories: “low complainers” and “high complainers.” Our results revealed that while only 30% of the low complainers experienced a negative change in score over the following year, 57% of the high complainers experienced a decline leading us to the conclusion that subjective cognitive complaints may contain useful clinical information. Furthermore, membership in the “high complainers” group was predictive of significantly lower follow-up DRS scaled scores regardless of baseline DRS scaled score and baseline level of depressive symptoms underscoring the variability in individual cognitive trajectories within the sample where no mean change in cognition was observed between baseline and follow-up.
While subjective cognitive complaints were related to depressive symptoms cross-sectionally and longitudinally, they also seem to have a unique contribution in explaining differences in long-term cognitive outcome. The proportion of unique variance in long-term global cognitive function accounted for by self-reported cognitive difficulties at baseline was modest (~12%) but statistically significant. Self-reported cognitive difficulties by patients with CVD therefore may reflect early changes in brain health and cognitive aging that are difficult to detect at a single time point using standard neuropsychological screening tools.
The idea that subjective cognitive complaints may reflect early neurodegenerative changes is supported by findings of reduced gray matter density in non-depressed elderly adults with normal test performance who report cognitive difficulties relative to age-matched healthy controls without subjective cognitive complaints (26). These volume losses were found to be as severe as changes observed in individuals diagnosed with MCI (26). This finding was replicated in a large community-base study reporting a strong linear association between hippocampal volume and subjective memory complaints after adjustment for other variables, including measures of cognitive function (27). Subjective cognitive deficits have also been related to measures of microvascular disease in the brain, such as white matter lesions (5, 27–29). Although reports are somewhat mixed as to whether the association is linear, it is clear that adults with white matter lesions report cognitive failures at a rate twice as high as the rate for adults without such lesions (28), and that the association between subjective cognitive complaints and future cognitive decline is strengthened by the presence of severe white matter lesions (5).
Consistent with prior research, we found that self-reported cognitive difficulties were related significantly to increased microvascular lesions in the brain as measured by WMH on MRI in the sub-set of our participants with available brain imaging. The unique value of self-reported cognitive difficulties in estimating long-term clinical outcomes is highlighted by the fact that the complaints could not simply be explained away by the severity of clinical CVD symptoms. In our sample, self-reported cognitive difficulties at baseline showed little relationship to current CVD severity as indicated by New York Heart Association functional class or presence of clinically significant CAD, MI, or cardiac surgery. The association between cognitive complains and microvascular disease in this sample did not seem to be confounded by association between WMH and depression as one was not detected in our study. This lack of correlation between BDI and WMH was slightly surprising given prior literature documenting a significant relationship between cerebrovascular health and depression in the elderly (39). However, it is likely due to the very mild depressive symptoms in our sample (mean BDI score = 5.26, SD = 3.77) as well as the global measure of cerebrovascular health we employeed (total WMH volume).
A potential limitation of our study is the loss of approximately 33% percent of the participants to follow-up. Drop out rates on the order of 25–30% are not unusual in longitudinal studies with community participants (6, 40). Studies involving severely ill patient populations have published data losses as high as 42–74% (41, 42). Our rate, however, is a little high when compared to the reported 17–21% drop out rates in similar quasi-experimental studies of participants with cardiovascular disease (17, 22). Missing data can significantly influence the results if the loss is non-randomly related to the variables of interest. As demonstrated in Table 1, however, at baseline, our retained and lost participants did not differ from each other on any of the variables of interest (subjective cognitive complaints, objective global cognitive functioning, depressive symptoms, CVD severity). The only significant difference was that the lost participants were on average two years less educated. Lower education, older age, cerebrovascular accidents, and decreased quality of life are some drop-out reasons reported in prior longitudinal studies (43). Loss of data for any of these reasons, however, has the potential to underestimate, rather than overestimate our effect. Therefore, we believe that our results are worthy of consideration and further study.
In conclusion, some patients with CVD appear to be at high risk for cerebrovascular complications and cognitive decline. At a single time point, this risk is difficult to assess using standard neuropsychological screening tools, mood evaluations, or measures of CVD severity. However, this risk for cognitive decline seems to be captured by patients’ subjective reports of relative decrements in their cognitive function. Over time, self-reported cognitive difficulties are related to a decline in performance on objective measures of global cognitive function and may provide important clinical information about early cognitive changes possibly reflecting early neurodegenerative processes that should be carefully monitored among patients with CVD. These findings have implications for other clinical samples, including assessing subjective complaints in formulating the MCI diagnosis for aging adults.
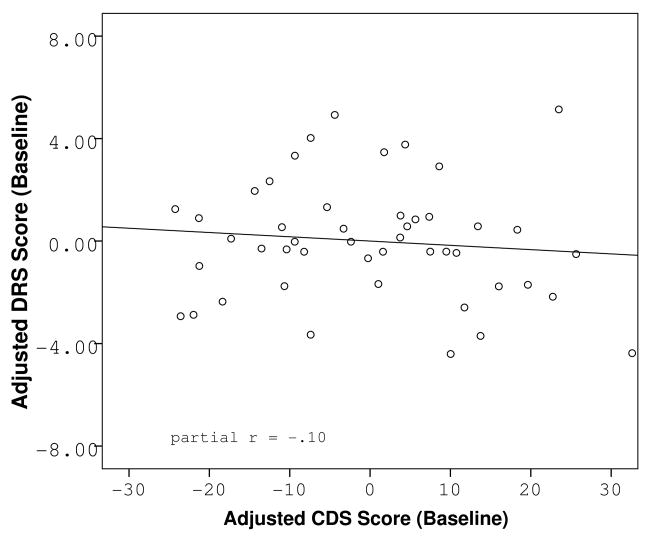
Figure 1.
Partial regression plots of the relationship between reported cognitive dysfunction at baseline and objective cognitive functioning at baseline (A) and follow-up (B), adjusted for baseline cognitive function and baseline level of depression.
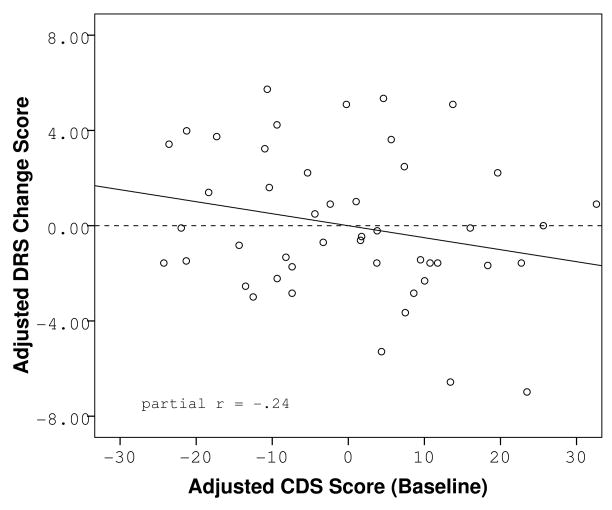
Figure 2.
Partial regression plot of the relationship between reported cognitive dysfunction at baseline and change in objective cognitive functioning over time, adjusted for baseline level of depression.
Acknowledgments
This work was supported by National Institute of Health grants AG017975 (RAC), AG020498 (APH, BAJ), AG026850 (KFH), HL074568 (JG), MH065857 and 5R01NS052470 (RHP), K23-AG030962 and P30-AG013846 (ALJ).
Footnotes
This work was presented in part at the 35th Annual Meeting of the International Neuropsychological Society, Portland, OR.
No Disclosures to Report.
References
- 1.Glodzik-Sobanska L, Reisberg B, De Santi S, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF, Christensen H, Korten AE, et al. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 3.Gallassi R, Bisulli A, Oppi F, et al. Subjective cognitive complaints, neuropsychological performance, affective and behavioural symptoms in non-demented patients. Int J Geriatr Psychiatry. 2008;23:95–101. doi: 10.1002/gps.1901. [DOI] [PubMed] [Google Scholar]
- 4.Tobiansky R, Blizard R, Livingston G, et al. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25:779–786. doi: 10.1017/s0033291700035029. [DOI] [PubMed] [Google Scholar]
- 5.Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim J-M, Stewart R, Kim S-W, et al. A prospective study of changes in subjective memory complaints and onset of dementia in South Korea. Am J Geriatr Psychiatry. 2006;14:949–956. doi: 10.1097/01.JGP.0000214857.66638.ed. [DOI] [PubMed] [Google Scholar]
- 7.Comijs HC, Deeg DJH, Dik MG, et al. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 8.Jungwirth S, Fischer P, Weissgram S, et al. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 9.Minett TSC, Da Silva RV, Ortiz KZ, et al. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry. 2008;23:49–54. doi: 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- 10.Lautenschlager NT, Flicker L, Vasikaran S, et al. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am J Geriatr Psychiatry. 2005;13:731–734. doi: 10.1176/appi.ajgp.13.8.731. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.Gunstad J, Bausserman L, Paul RH, et al. C-reactive protein, but not homocysteine, is related to cognitive dysfunction in older adults with cardiovascular disease. J Clin Neurosci. 2006;13:540–546. doi: 10.1016/j.jocn.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haley AP, Forman DE, Poppas A, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. Int J Cardiol. 2007;121:148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferson AL, Poppas A, Paul RH, et al. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser DJ, Hoth KF, Robinson RG, et al. Blood vessel function and cognition in elderly patients with atherosclerosis. Stroke. 2004;35:e369–e372. doi: 10.1161/01.STR.0000145050.35039.51. [DOI] [PubMed] [Google Scholar]
- 16.Forman DE, Cohen RA, Hoth KF, et al. Cognition and brachial endothelial responses in older adults with cardiovascular disease. Artery Research. 2(1):35–43. doi: 10.1016/j.artres.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khatri P, Babyak M, Clancy C, et al. Perception of cognitive function in older adults following coronary artery bypass surgery. Health Psychol. 1999;18:301–306. doi: 10.1037//0278-6133.18.3.301. [DOI] [PubMed] [Google Scholar]
- 18.Vingerhoets G, de Soete G, Jannes C. Subjective complaints versus neuropsychological test performance after cardiopulmonary bypass. J Psychosom Res. 1995;39:843–853. doi: 10.1016/0022-3999(95)00021-3. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys CT, Moser DJ, Hynes SM, Reese RL, Haynes WG. Predictors of subjective cognitive difficulties in older adults with atherosclerotic vascular disease. Am J Geriatr Psychiatry. 2006;15:328–334. doi: 10.1097/01.JGP.0000246868.32129.d5. [DOI] [PubMed] [Google Scholar]
- 20.Gunstad J, Cohen RA, Paul RH, Tate DF, Hoth KF, Poppas A. Understanding reported cognitive dysfunction in older adults with cardiovascular disease. Neuropsychiatric Disease and Treatment. 2006;2(2):213–218. doi: 10.2147/nedt.2006.2.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman S, Klinger L, Venn G, et al. Subjective reports of cognition in relation to assessed cognitive performance following coronary artery bypass surgery. J Psychosom Res. 1989;33:227–233. doi: 10.1016/0022-3999(89)90050-0. [DOI] [PubMed] [Google Scholar]
- 22.Selnes OA, Grega MA, Borowicz LM, et al. Self-reported memory symptoms with coronary artery disease: a prospective study of CABG patients and nonsurgical controls. Cogn Behav Neurol. 2004;17:148–156. [PubMed] [Google Scholar]
- 23.Hoth KF, Tate DF, Poppas A, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke; a journal of cerebral circulation. 2007;38:308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jefferson AL, Tate DF, Poppas A, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haley AP, Sweet LH, Gunstad J, et al. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. J Neuroimaging. 2007;17:227–233. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- 26.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart R, Dufouil C, Godin O, et al. Neuroimaging correlates of subjective memory deficits in a community population. Neurology. 2008;70:1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54. [DOI] [PubMed] [Google Scholar]
- 28.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 29.Minett TSC, Dean JL, Firbank M, et al. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry. 2005;13:665–671. doi: 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- 30.Cohen RA, Poppas A, Forman DE, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clin Exp Neuropsychol. 2009;31:96–110. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNair D, Kahn RJ. In: Self-assessment of cognitive deficits, in Assessment in Geriatric Psychopharmacology. Crook T, Ferris S, Bartus R, editors. New Canaan, CT: Mark Powley Associates Inc; 1983. pp. 137–143. [Google Scholar]
- 32.Beck A, Steer R. Manual for the Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 33.Mattis S. Dementia Rating Scale (DRS) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 34.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 35.Derouesne C, Lacomblez L, Thibault S, et al. Memory complaints in young and elderly subjects. Int J Geriatr Psychiatry. 1999;14:291–301. [PubMed] [Google Scholar]
- 36.Lucas J, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20:536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- 37.Gunstad J, Cohen RA, Tate DF, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Press. 2005;14:353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Vaishnavi S, Taylor WD. Neuroimaging in late-life depression. Int Rev Psychiatry. 2006;18:443–451. doi: 10.1080/09540260600935454. [DOI] [PubMed] [Google Scholar]
- 40.Lee AJ, Garraway WM, Simpson RJ, et al. The natural history of untreated lower urinary tract symptoms in middle-aged and elderly men over a period of five years. Eur Urol. 1998;34:325–332. doi: 10.1159/000019749. [DOI] [PubMed] [Google Scholar]
- 41.Wessells H, Roy J, Bannow J, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo-treated men with benign prostatic hyperplasia. Urology. 2003;61:579–584. doi: 10.1016/s0090-4295(02)02401-9. [DOI] [PubMed] [Google Scholar]
- 42.Barnett JH, Croudace TJ, Jaycock S, et al. Improvement and decline of cognitive function in schizophrenia over one year: a longitudinal investigation using latent growth modelling. BMC Psychiatry. 2007;7:16. doi: 10.1186/1471-244X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gades NM, Jacobson DJ, McGree ME, et al. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC Med Res Methodol. 2006;6:58. doi: 10.1186/1471-2288-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]