Abstract
Advances in spinal cord injury (SCI) research are dependent on quality animal models, which in turn rely on sensitive outcome measures able to detect functional differences in animals following injury. To date, most measurements of dysfunction following SCI rely either on the subjective rating of observers or the slow throughput of manual gait assessment. The present study compares the gait of normal and contusion-injured mice using the TreadScan® system. TreadScan utilizes a transparent treadmill belt and a high-speed camera to capture the footprints of animals and automatically analyze gait characteristics. Adult female C57Bl/6 mice were introduced to the treadmill prior to receiving either a standardized mild, moderate, or sham contusion spinal cord injury. TreadScan gait analyses were performed weekly for 10 weeks and compared with scores on the Basso Mouse Scale (BMS). Results indicate that this software successfully differentiates sham animals from injured animals on a number of gait characteristics, including hindlimb swing time, stride length, toe spread, and track width. Differences were found between mild and moderate contusion injuries, indicating a high degree of sensitivity within the system. Rear track width, a measure of the animal's hindlimb base of support, correlated strongly both with spared white matter percentage and with terminal BMS. TreadScan allows for an objective and rapid behavioral assessment of locomotor function following mild-moderate contusive SCI, where the majority of mice still exhibit hindlimb weight support and plantar paw placement during stepping.
Key words: behavioral assessments, locomotor function, spinal cord injury (SCI)
Introduction
The successful treatment of spinal cord injury (SCI) in humans will ultimately be dependent on research using reliable animal models. Until recently, rats had been considered the most advantageous model of SCI due to the pathophysiology consistent with human injury and well-established protocols for producing consistent and graded injuries (Kuhn and Wrathall, 1998). However, a lack of consistency and reliability in the outcome measures utilized has been problematic, especially across independent laboratories (Basso, 2004). The advent of the transgenic mouse has advanced the murine model of SCI research tremendously in recent years. These mutations, both naturally occurring and genetically engineered, could offer invaluable insight into the mechanisms of SCI and possible therapeutic agents (Basso et al., 2006; Farooque, 2000; Guertin, 2005; Kuhn and Wrathall, 1998).
As more studies begin to utilize mouse models of SCI, the search for a reliable, consistent, and objective behavioral assessment tool becomes more vital than ever. Many assessment measures exist, each with inherent advantages and disadvantages (Basso, 2004). Open-field assessments offer valuable insight into the animal's overground locomotor behavior. Until recently, open-field assessment measures used for the mouse were variations of assessment tools designed for the rat. For example, several studies (Farooque, 2000; Hsu et al., 2006; Mikami et al., 2002; Stieltjes et al., 2006) utilized the Basso, Beattie, and Bresnahan (BBB) (Basso et al., 1995) scale to assess mouse behavior following SCI, despite the fact that this assessment tool was created specifically for scoring motor deficits in the rat. Other laboratories have attempted to modify the BBB specifically for use in mouse studies (Apostolova et al., 2006; Joshi and Fehlings, 2002a, 2002b; Li et al., 2006; Ma et al., 2001). While these adaptations of rat assessment tools provided useful information of locomotor function in the mouse following SCI, it was clear that a more specific mouse scale was necessary to provide more accurate data.
Basso et al. (2006) created the Basso Mouse Scale (BMS), a 10 point assessment scale designed specifically for studying mouse behavior. In addition to high inter-rater reliability, the BMS has significant face validity (sensitive to locomotor recovery across lesion severity and time) and predictive validity (correlates significantly with spared white matter). Overall the BMS has proved reliable for assessing the locomotor activity of several strains of mouse following SCI (Basso et al., 2006). The BMS is not free of criticism, however, due to its inherent subjectivity and ordinal nature, as recognized by the assessment's creators. The BMS relies on the visual observation of two raters, and the small, rapid movements of the mouse are difficult to assess. The most significant example of this is coordination. The mouse often moves in quick bursts throughout the open field, making observation of a one-to-one relationship between forelimbs and hindlimbs very difficult to discern. In addition, the scale is ordinal and non-linear, leading to potentially confusing results following treatment. For example, if a therapeutic agent increases the final BMS score from a 3 to a 4, this seemingly small increase has profound effects on the animal's locomotor abilities, as the animal has improved from not stepping to stepping. However, a treatment that increases the final BMS score from a 1 to a 2 (slight ankle movement to extensive ankle movement) has less biological relevance (Basso et al., 2006).
In contrast to subjective open-field assessments, gait analysis is especially useful for objectively quantifying locomotor behavior following SCI. Computer-assisted footprint analysis such as the CatWalk system allows the experimenter to calculate gait characteristics such as velocity, stance time, swing time, and stride length as the animal traverses a clear stationary walkway (Apostolova, 2006; Clarke and Still, 1999; Hamers et al., 2001, 2006). The drawback of this system, however, is the slow throughput associated with manual identification of each step cycle. Additionally, the experimenter is unable to control for the speed of individual animals using the CatWalk system. A clear belt-driven treadmill device offers the same ability to manually identify step cycles and calculate gait characteristics, but with the added advantage of speed control (Amende et al., 2005; Guertin, 2004; Herbin et al., 2004; Kale et al., 2004; Leblond et al., 2003). Until recently, the drawback of the treadmill system was the time-consuming nature of the video analysis. Individual footprints were identified manually, and many of the gait characteristics such as stance time, swing time, stride length, paw rotation, hindlimb base of support, were measured and/or calculated by the experimenter.
The purpose of the present study is to characterize a new motor-driven treadmill device in conjunction with the TreadScan® software system (CleverSys, Inc., Reston, VA) for gait analysis. Similar to the computer-assisted footprint analysis method, animals are recorded from underneath by a high-speed digital camera as they walk across a clear surface. The treadmill allows the experimenter to control for speed, and with minimal training, the TreadScan software automatically detects the individual footprints of the animal. The present study evaluated training effects, optimal training protocols, discrimination between injury severities, and correlation with BMS evaluation. Data show that the TreadScan system is a highly sensitive and objective measure for assessing deficits in mild-to-moderate SCI, and the automated data acquisition of TreadScan allows for fast and accurate throughput gait analysis.
Methods
Treadmill training
Adult female C57Bl/6 mice were used to obtain normal baseline levels for mouse treadmill locomotion. Prior to treadmill introduction, mice were gentled in the BMS field for four sessions over a period of 1 week. Initial BMS scores were obtained before treadmill training to ensure that all animals performed at the highest behavioral level (BMS = 9). An initial group of mice (n = 15) was trained to walk on the motor-driven treadmill belt at a constant speed of 15 cm/s for 20 s periods. This group (high training) received 34 training sessions over a period of 7 weeks. A second (n = 20) and third group (n = 20) were trained on the treadmill at 15 cm/s for 20 s periods for 12 training sessions over a 3 week period (medium training) or five training sessions over a 10 day period (low training), respectively. The final three training sessions were recorded for each group to serve as the baseline for normal locomotion. To assess which training group performed most frequently on the treadmill, the response rates of each animal were recorded throughout the three recording sessions. Heglund and Taylor (1988) suggest that the mouse changes its locomotor pattern from a walk to a trot at 19 cm/s. Since our interest was in the walking pattern of the mouse, we chose a baseline treadmill speed of 15 cm/s, a speed also favored by Leblond et al. (2003). Although baseline animals were capable of performing consistently at this speed, injured animals were incapable of achieving this walking speed. Thus, injured animals were walked at the slower speeds of 7 cm/s and 10 cm/s.
Surgical procedures and postoperative care
All animal care and surgical interventions were undertaken in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996), and with the approval of the University of Louisville Institutional Animal Care and Use Committee.
A total of 75 adult female C57BL/6 mice (20 g) were purchased from Jackson Laboratory (Bar Harbor, ME). Of these, mice in the initial medium and high training groups were excluded from future study due to treadmill training effects. The remaining 40 mice received a low training regimen and were divided randomly into three injury groups. A first group (n = 8) received mild contusion injuries. A second group (n = 15) received moderate contusion injuries. The final group (n = 13) were sham controls. The remaining four mice were excluded from the study because they did not regain plantar placement of the hindpaws. The mice were anesthetized using 0.1 mL ketamine/xylazine administered by intraperitoneal injection. A dorsal laminectomy was performed at the ninth thoracic vertebral (T9) level to expose the spinal cord. Mice were placed in a custom stabilizer device, which holds the spinal cord level and steady in the Louisville Injury System Apparatus (LISA) (Hill et al., 2009; Zhang et al., 2007). Briefly, the LISA utilizes a laser sensor to measure the velocity and displacement of an injury obtained via a pneumatically driven impactor. Mice in the mild injury group received a 0.25 mm displacement contusion at a velocity of 1.0 m/s. Mice in the moderate injury group received a 0.40 mm displacement contusion at a velocity of 1.0 m/s. Sham control mice remained uncontused and received a T9 laminectomy only. After surgery, animals were given 1 cc of sterile saline subcutaneously, 0.1 cc of gentamycin intramuscularly on the day of surgery and days 3 and 5 post-surgery, and 0.1 ml bupronorphine subcutaneously on the day of surgery and days 1 and 2 post-surgery. Animals were placed on a heating pad until full recovery from anesthesia. Postoperative care included the manual expression of bladders twice a day for 7–10 days or until spontaneous voiding returned. The animals were sacrificed 10 weeks post-injury.
Recording and analysis procedures
The treadmill device was purchased from Columbus Instruments (Columbus, OH). Briefly, the treadmill consisted of a motor-driven transparent treadmill belt with an angled mirror mounted below. A high-speed digital video camera was mounted to record the ventral view of the treadmill belt reflected off the mirror; digital video images of the underside of the mouse were recorded at 100 frames/s. An adjustable compartment measuring 17 cm × 5 cm was mounted over the treadmill belt, ensuring that the mouse would remain in the view of the camera at all times. TreadScan software (CleverSys) identified each individual paw of the mouse in each frame as it walked on the treadmill. With minimal training, this software was used to correctly identify initial foot contact, stance duration, stride duration, foot liftoff, swing duration, stride length, track width, and toe spread data for each foot (Fig. 1). A single training session video was chosen to train the software based on its representation of normal mouse locomotion. The outline of each paw was drawn on the computer screen using the software's built-in tracing system. Extreme caution was taken to ensure that only the paws were selected, as the software uses color and pixel differentiation to identify paw placement. A sampling of 10 to 12 outlines for each paw was sufficient to train the software to correctly identify paw placement and liftoff. Software training and optimization can be completed in a matter of hours with proper training, lighting, and supervision. Mice walked on the treadmill for 20 s sessions resulting in 2000 captured frames. The first video recorded each day was previewed to insure that the software was optimally calibrated. For each 20 s session, the video was previewed to determine a minimum of four to six consecutive step cycles of consistent walking for video analysis. Fewer strides have been shown to increase variability, while additional strides do not tend to reduce variability (Wooley et al., 2005). If the animal walked consistently and provided more than six consecutive step cycles, these additional steps were included for analysis. Care was taken to insure that selected step cycles were representative of consistent walking with constant speed. Step cycles in which animals ran to the front, drifted backward on the belt, or explored the chamber were excluded from analysis. Toe spread was manually identified for each hindlimb using the software's built-in toe spread tool. For each hindlimb step cycle analyzed, each of the five toes and the base of the foot were identified at initial contact to reduce variability. Previewing the video to choose the most representative step cycles, toe spread identification, and software analysis took approximately 8–10 min for each video once the software was optimized and the experimenter familiarized with the system.
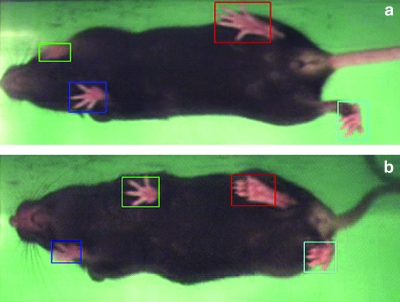
FIG. 1.
(a) A sham animal (BMS = 9) walking at 10 cm/s on the treadmill 4 weeks post-injury. In TreadScan, each paw is automatically color-coded and measured over 20 s of locomotion, and gait parameters are automatically calculated. (b) Note the considerably narrower rear track width, decreased hindlimb toe spread, and decreased hindlimb stride length in a moderately contused mouse (BMS = 5) walking at 10 cm/s 4 weeks post-injury.
Beginning 1 week post-injury and continuing once weekly for 10 weeks, BMS scores were obtained for all mice by observers who had completed BMS training at Ohio State University under the direction of Dr. Basso and other faculty who designed the scale (Basso, 2006). BMS observers were blinded to the injury group. On the same day as BMS testing, mice from the mild, moderate, and sham groups were run on the motor-driven treadmill at constant speeds. Each animal was walked for 20 s at 7 cm/s and 20 s at 10 cm/s for each week, as injured animals were incapable of matching the baseline treadmill speed of 15 cm/s.
Histochemistry and analysis
Following final behavioral and physiological assessments, the mice were sacrificed at 10 weeks post-injury. Animals were anesthetized with a solution of 60% ketamine−40% xylazine (0.25 ml/20 g) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA), consecutively. The spinal cords were dissected, submerged in PFA for 1 h, and stored in a 30% sucrose solution overnight at 4°C. The spinal cords were cut into 1 cm segments centered on the epicenter, embedded in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC), flash frozen on dry ice, and stored at −80°C. Serial 30 μm thick sections spanning the injury site were cut coronally and stored at −80°C. After thawing, iron eriochrome cyanine (EC) staining was done to delineate spared myelin (Rabchevsky et al., 2003). Slides were cover-slipped with Cytoseal (Fisher, Atlanta, GA) and dried in a ventilated hood overnight. The total cross-sectional area of the spinal cord and the lesion boundary were captured with an Olympus BX60 microscope and measured and analyzed using Neurolucida (Microbrightfield, Colchester, VT). The epicenter of each injury was determined based on the section with the least amount of spared white matter. A code was used to randomize the epicenter sections, allowing for an unbiased quantification. The data were normalized to find an estimated area at the epicenter of the injury. This was confirmed by evaluation of uninjured animals. The spared white matter area at the epicenter was compared to normal average white matter area at the same location. The percentage of spared white matter at the epicenter was calculated for each animal. At this point, the code was broken and the subjects were divided into mild, moderate, and sham groups. Mean values of percent spared white matter area were compared statistically using one-way ANOVA followed by Tukey HSD post hoc t tests.
Statistical analyses
To assess the optimal number of training sessions for non-injured animals, chi-square analyses were run on baseline response rates for each training group. Repeated measures analyses of variance (ANOVA) were run on baseline data to determine any gait parameter differences between recording sessions. When no significant differences were found, baseline data from the three recording sessions were averaged for each group, resulting in an average baseline performance for each training group. Repeated measures ANOVA with Tukey HSD post hoc t tests were run on the new averages to assess whether any significant differences existed in the gait characteristics of the training groups.
To assess whether hindlimb gait characteristics changed over time, as well as to determine whether any differences existed between injury groups, mixed model ANOVA with Bonferroni post hoc t tests were run on gait parameter data over the entire 10 week testing period.
To determine the sample sizes necessary to uncover differences between sham and mild-injured animals if moderate-injured animals had been excluded from analysis, power analyses were run on BMS and two of the gait characteristics measured by TreadScan. Power analyses were run using a two-tailed t test on terminal BMS scores and rear track width and hindlimb swing times at 7 cm/s (Lenth, 2006). Identical power analyses were run on these gait parameters to determine the sample sizes needed to differentiate the mild group from the moderate group.
To reveal whether any differences existed in spared white matter between injury groups, a one-way ANOVA was run on the spared white matter percentage for each injury group. In addition, Pearson two-tailed correlations were run on several of the hindlimb gait parameters and spared white matter percentage, BMS and spared white matter percentage, and hindlimb gait parameters and BMS. All data are reported as mean ± SD.
Results
Treadmill training
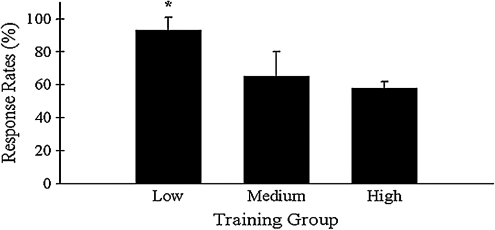
Animals that received the most training prior to recording sessions performed less consistently than animals that received less training. Response rate is defined as the number of sessions in which animals successfully walked on the treadmill divided by the total number of sessions. For the three baseline recording sessions (treadmill speed, 15 cm/s), the response rate for mice receiving only five training sessions (low) was 93.3%. In contrast, animals that received 12 training sessions (medium) had a response rate of 65.0%, while animals with 34 training sessions (high) exhibited a response rate of only 57.8%. Chi-square analysis of this data indicated that the low group had a significantly higher response rate than both the medium and the high groups (Fig. 2).
FIG. 2.
Animals in the low training group had a higher response rate to the treadmill task than animals in both the medium training and high training groups (χ2 = 7.1, df = 2, *p < 0.05). Left hindlimb stance time was longer in the medium group than the low group (F = 6.65, df = 2, 47, p < 0.005; t = 24.6, p < 0.05), while right hindlimb stance time was longer in the high group than in the low group (t = 22.6, p < 0.05). Left hindlimb stride length was longer in both the medium (F = 18.6, df = 2, 47, p < 0.001; t = 8.2, p < 0.01) and high (t = 8.7, p < 0.01) groups than the low group. Medium (t = 7.9, p < 0.01) and high (t = 8.8, p < 0.01) groups also had longer right hindlimb stride lengths than the low training group.
Due to the injury model chosen (T9 contusion), we focused on differences in hindlimb gait parameters. Thus, forelimb data were ignored in all statistical analyses. Animals in the low training group exhibited significant hindlimb differences in gait characteristics compared to more extensively trained animals. Left hindlimb stance times (p < 0.05) and left and right hindlimb stride lengths (p < 0.01) were significantly longer and had greater variability in the medium and high trained groups than in the low training group (data not shown). The higher response rates and lower standard deviations of the low training group support the conclusion that this group's data is the most representative of baseline locomotion in the intact mouse.
Open-field assessment
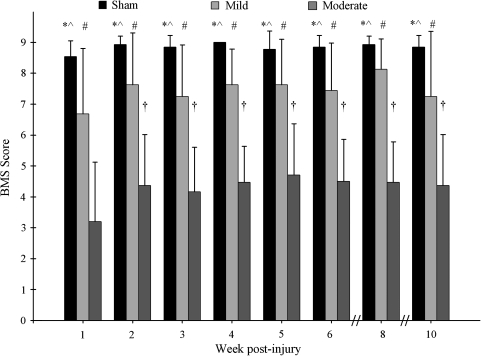
Due to the treadmill training effect and lower response rates of the higher trained groups, animals for the remainder of this study received the low training paradigm. Following training, animals were divided randomly into mild SCI (n = 8), moderate SCI (n = 15), and sham (n = 13). A significant difference in BMS scores was found between injury groups. Sham animals exhibited significantly higher BMS scores than both the mild and moderate injury groups. Mild injury animals showed higher BMS scores than moderate injury animals at all weeks. In addition, moderate animals exhibited a change in BMS over time; in these animals, week 1 BMS scores were significantly lower than those at all other time points. Terminal BMS scores were 8.85 ± 0.38 for shams, 7.25 ± 2.10 for the mild group, and 4.37 ± 1.65 for the moderate group (Fig. 3). These data are consistent with those reported previously (Basso et al., 2006).
FIG. 3.
BMS scores for sham, mild, and moderate groups. The BMS successfully distinguished between all three groups at all time points (F = 58.1, df = 2, 33, p < 0.001). *Significant difference between sham and mild (p < 0.01 at all weeks except week 8, p < 0.05); ^significant difference between sham and moderate (p < 0.01 at all weeks); #difference between mild and moderate (p < 0.01 at all weeks). The moderately injured animals exhibited a significant increase in BMS over time (F = 7.49, df = 3.9, 55.2, p < 0.001); week 2 and all subsequent weeks were significantly higher than the initial BMS score at week 1. †p < 0.01 at all weeks except week 3, p < 0.05. BMS data are mean left and right sides ± SD (sham, n = 13; mild, n = 8; moderate, n = 15).
Treadmill assessment
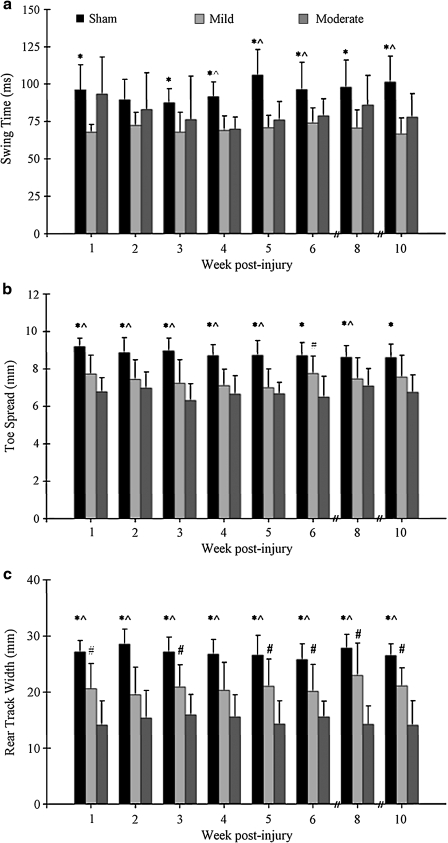
TreadScan differentiated injured from non-injured animals. At 7 cm/s, the left hindlimb swing times were longer in the sham group than in the mild group at nearly all time points (Fig. 4a). Left hindlimb swing times were also longer in shams than in moderate animals at weeks 4, 5, 6, and 10. Similarly, the right hindlimb swing times were longer in the sham group than in the mild group at nearly all time points, and longer in the sham group than in the moderate group at weeks 2, 4, 5, and 6. Left hindlimb stride length was longer in the shams than in the mild group at weeks 4 and 10, and longer in shams than in the moderate group at weeks 4, 5, 6, and 10 (data not shown). Similarly, right hindlimb stride length was longer in the sham group than in the mild group at weeks 4, 6, and 10, and longer in shams than in moderates at weeks 2, 4, 5, 6, and 10 (data not shown). Mild injured animals had a longer right hindlimb stride length than the moderates at week 2. Left hindlimb toe spread was wider in the sham group than in both the mild group and the moderate group at nearly all time points (Fig. 4b). Additionally, left hindlimb toe spread was wider in the mild group than in the moderate group at week 6 (p < 0.05). Right hindlimb toe spread was wider in shams than in mild-injured animals at weeks 1, 2, and 5, and wider than moderate-injured animals at all weeks. Mild animals exhibited wider right hindlimb toe spread than moderate animals at weeks 1, 3, 6, and 10 (data not shown). Finally, rear track width was significantly wider in the sham group than in both the mild group and the moderate group at all time points, and wider in the mild group than the moderate group at nearly all time points (Fig. 4c).
FIG. 4.
Gait parameters measured by TreadScan consistently differentiated sham animals from injured animals. Left hindlimb swing time was significantly reduced in injured animals (a). Mild-injured animals differed from shams at weeks 1, 4, 5, 6, 8, and 10 (p < 0.01) and week 3 (p < 0.05). Moderate-injured animals differed from shams at weeks 4 and 5 (p < 0.001), week 6 (p < 0.05), and week 10 (p < 0.01). Left hindlimb toe spread is narrower following SCI (b). Mild animals had a narrower toe spread than shams at weeks 1, 3, 4, and 5 (p = 0.001) and weeks 2 and 8 (p < 0.05); moderates had narrower toe spread than shams at all weeks: 1, 3, 4, 5, 6, and 10 (p < 0.001); 2 and 8 (p = 0.001). Hindlimb toe spread was narrower in moderate animals than in mild ones at week 6 (p < 0.05). Rear track width, a measure of the animal's hindlimb base of support, was narrower in injured animals than in shams (c). Mild animals exhibited a narrower rear track width than shams (p < 0.01 at weeks 1, 2, 3, 4, 6, and 10; p < 0.05 at weeks 5 and 8), as did moderate animals (p < 0.001 at all weeks). Moderate animals exhibited a narrower rear track width than milds (p < 0.01 at weeks 5, 8, and 10; p < 0.05 at weeks 1, 3, and 6). These gait parameters do not show improvement over time, and thus may be markers of the efficacy of therapeutic agents in future studies. *Significant differences between sham and mild; ^significant differences between sham and moderate; #significant differences between mild and moderate. Data are mean ± SD (sham, n = 13; mild, n = 8; moderate, n = 15).
At 10 cm/s, sham animals had longer left hindlimb swing times than mild animals at all weeks. Similarly, right hindlimb swing times were longer in shams than mild animals at all weeks (data not shown). Left hindlimb toe spread was wider in shams than in mild animals at nearly all time points, although right hindlimb toe spread was only wider in shams than in mild animals at weeks 1, 6, and 10. Rear track width was significantly wider in sham animals than in the mild group at all time points (data not shown).
The only gait characteristic difference over time was found in right hindlimb stride length at 7 cm/s and at 10 cm/s. Specifically, post hoc analyses revealed that at week 6, sham animals exhibited a longer right hindlimb stride length at the 7 cm/s speed than at other weeks (data not shown). Post hoc analyses did not reveal any significant differences over time for the 10 cm/s condition.
There was a significant week by group interaction in the right hindlimb stride length at 7 cm/s. This interaction revealed a general lengthening over time of the right hindlimb stride length in the sham group up to week 6. The mild- and moderate-injured groups, on the other hand, exhibited a relatively stable right hindlimb stride length over time (data not shown).
Histological analyses
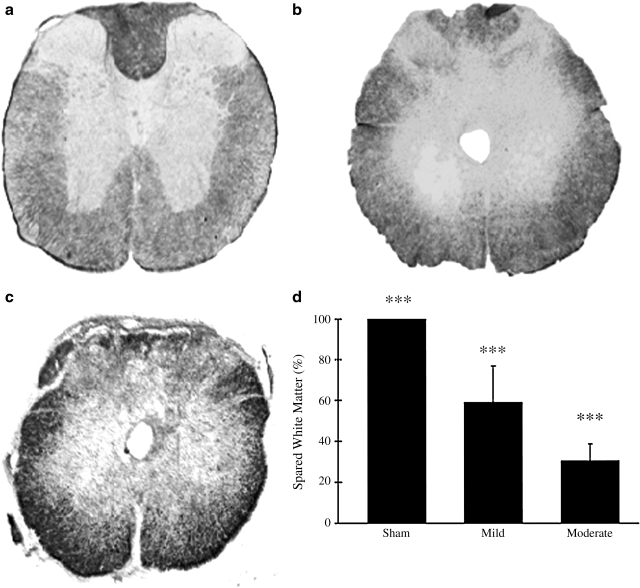
Ten weeks post-injury, sham animals had 100% spared white matter, compared with 59.25 ± 17.53% in mild- and 30.53 ± 8.34% in moderate-injured animals (Fig. 5). Sham animals had a significantly higher spared white matter percentage than both mild- and moderate-injured animals. In addition, mild animals had a significantly higher spared white matter percentage than moderate animals.
FIG. 5.
(a) Sham mice show normal white matter staining at 10 weeks post-injury. (b) In mildly injured animals (0.25 mm displacement injury), a small loss of white matter is evident 10 weeks post-injury. (c) Moderately injured animals (0.40 mm displacement) show significant loss of white matter 10 weeks post-injury. (d) All groups differed significantly from one another (***p < 0.001). Data are mean ± SD (sham, n = 13; mild, n = 8; moderate, n = 15).
Power analyses
Due to the relatively large variability seen in the BMS scores of the mild group, we performed a power analysis using a two-tailed t test to determine the sample size needed to detect a significant difference between the groups at the 0.05 level (Lenth, 2006). Although the BMS successfully distinguished between sham and mild animals at all time points, the mild group exhibited an average standard deviation in BMS scores of 1.6 across the 10 weeks of the study. The power analysis indicated that, assuming equal group sizes and variability, a sample size of 18 for each group would be necessary to distinguish between shams and mild-injured animals with a probability of 95%.
Conversely, the TreadScan system revealed much smaller relative variability within the mild group. Power analyses revealed that, using the 7 cm/s week 10 terminal rear track width data and assuming equal group sizes and variability, a sample size of eight animals per group would successfully distinguish sham from mild animals. Similarly, 7 cm/s week 10 left hindlimb swing data would require a sample size of only six animals per group to separate shams from mild-injured animals with a power of 95%. Additionally, power analyses revealed that a sample size of 10 animals per group would be necessary to successfully distinguish mild from moderate animals on the BMS. On the TreadScan, rear track width would require sample sizes of nine animals per group to separate mild from moderate injuries.
Correlations
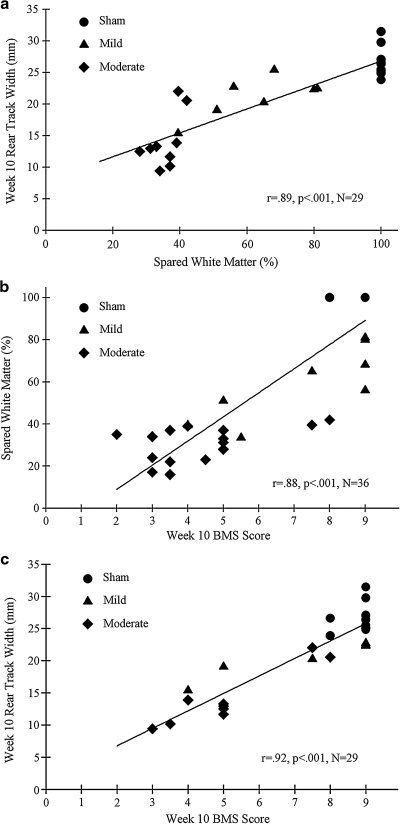
To define how well performance on the TreadScan system corresponds to spared white matter, several two-tailed Pearson correlations were run. The best predictor of spared white matter on the TreadScan was rear track width (Fig. 6a). As expected, the percentage of spared white matter correlated strongly with the final BMS score (Fig. 6b). Terminal rear track width and BMS scores also exhibited a strong correlation (Fig. 6c). While terminal stride length (r2 = 0.55, p = 0.001), swing time (r2 = 0.31, p < 0.005), and toe spread (r2 = 0.42, p < 0.001) also correlated significantly with spared white matter percentage, the low r2 values associated with these correlations indicate that they are not as strong as the rear track width and BMS correlations.
FIG. 6.
(a) At 7 cm/s, terminal rear track width, a measure of an animal's hindlimb base of support, correlated strongly with the percentage of spared white matter. (b) Terminal BMS scores also correlated well with percentage of spared white matter. (c) Terminal 7 cm/s rear track widths and BMS scores also exhibited a strong correlation. BMS data are mean left and right sides.
Discussion
We describe the utility of the TreadScan software system in conjunction with a motor-driven treadmill device in assessing locomotor function in mice (Fig. 1). In naïve animals, we found that increased exposure to the treadmill prior to injury led to undesired training effects. Animals receiving more than minimal exposure to the treadmill prior to injury were much less likely to perform the treadmill walking task (Fig. 2), and had more variability in gait characteristics than animals in the lowest training group. We attribute this effect to the initial novelty of the treadmill. When first placed on the treadmill, the animal is compelled to respond to the moving belt or it will strike the rear bumper of the enclosure. With increased exposure to the treadmill, animals learn that the bumper is not an aversive stimulus, and thus tend to ride to the back of the treadmill rather than perform the walking task consistently.
Studies using several species of animals have found similar adverse acclimatization effects in response to treadmill training. For example, Barrey et al. (1993) found that horses exhibit a significantly longer stride length on a treadmill than during overground locomotion; Blaszczyk and Loeb (1993) described similar findings in cats. Interestingly, Herbin et al. (2007) found that SWISS-OF1 mice exhibited shorter stride lengths on the treadmill than overground, although the speeds chosen (20–101 cm/s) are more indicative of trotting or galloping than walking.
The present data suggest that the animals in the low training group did not acquire the tendency to alter their gait in response to the treadmill, and thus exhibited gait characteristics that are more relevant to overground locomotion. To our knowledge, this is the first study to look at changes accompanying acclimatization in mouse treadmill locomotion. These data strongly suggest that caution should be exercised when designing experiments utilizing a treadmill system, and that novelty can actually be an advantage when assessments use tasks that do not have to be learned.
Previous studies into mouse behavior following SCI have found that injured animals exhibit a period of initial recovery that tends to plateau after 2–3 weeks (Basso et al., 2006; Kuhn and Wrathall, 1998; Ma et al., 2001). Consistent with these findings, the moderate group showed an initial improvement on the BMS during the first 2 weeks with no additional improvements for the remainder of the study (Fig. 3). These data substantiate previous findings indicating that the BMS is sensitive to early improvements in the repertoire of behaviors exhibited by injured animals (Basso et al., 2006). The BMS did not find significant improvement over time in the mild-injured group, although this is not surprising given the small displacement of this injury. Additionally, our first injured BMS measurement was taken 1 week post-injury, while animals with mild lesion severity have been shown to recover the most functioning within 5 days post-injury (Basso et al., 2006; Ma et al., 2001). Interestingly, TreadScan did not find any changes over time in either of the injury groups, indicating that perhaps the BMS is a better behavioral assessment for moderately injured animals than a treadmill-based system. This is intuitive, as many moderately injured animals scored a 4 on the BMS, a level associated with occasional plantar stepping. Occasional plantar stepping is scored when the mouse plantar steps less than half of the time that it is moving forward (Basso et al., 2006). Thus, these animals had difficulty performing the treadmill task and many were simply unable to perform the requisite four to six consecutive step cycles.
Following spinal cord contusion, the BMS consistently distinguished between sham animals and injured animals. In the mild group, however, the injury was quite subtle, and half of our mild animals were able to achieve a BMS score of 9 as early as 2 weeks following this injury. While the BMS was not sensitive to the injury in some mildly injured animals, the TreadScan system consistently distinguished between shams and mild-injured animals. The significantly shorter hindlimb swing times (Fig. 4a), narrower toe spread (Fig. 4b), and narrower rear track width (Fig. 4c) exhibited by the mild injury group appear to stem from a balance deficit and/or trunk instability that the BMS cannot always discern. Power analyses indicated that sample sizes as small as 6 could distinguish sham animals from mild-injured animals at a power of 95%, whereas a sample size of 18 would be required to do the same on the BMS. This sensitivity may also prove useful in analyzing subtle differences in transgenic knockout mice. Uninjured mice null for the endoplasmic reticulum stress gene CHOP exhibit BMS scores of 9, while TreadScan analysis revealed unique baseline locomotor patterns (unpublished observations).
The rear track width difference previously discussed is consistent with data from both Ma et al. (2001) and Hill et al. (2009), who reported decreased hindlimb base of support in C57Bl/6 mice following SCI. According to Thota et al. (2005), thoracic SCI can lead to deleterious effects in rhythmic locomotor activity, balance, and posture. In the present study, both balance and posture were negatively affected. The injured animals seemed to rush through the hindlimb swing phase and take shorter steps (observed as shorter stride lengths) due to a lack of balance, and their narrower hindlimb base of support suggests postural deficits (Fig. 1). It is important to note that these deficits remained consistent over the entire 10 week course of this study, suggesting that these gait parameters may provide a useful analysis for locomotor improvement following therapeutic intervention.
Toe spread differences, while variable, could provide useful insight into recovery following SCI. Blakeman et al. (2003) and Hygge-Blakeman et al. (2004) described a narrowing of hindlimb toe spread following sciatic nerve crush in mice. It appears that narrowed toe spread may be indicative of deficits in hindlimb sensory motor function. Interestingly, there are no current SCI studies that discuss the relevance of toe spread on locomotion in mice. Future studies should be conducted to ascertain the anatomical and clinical relevance of toe spread following SCI.
TreadScan did not uncover any differences over time in any of the gait parameters measured for either of the injury groups. It is important to note, however, that the first treadmill session following SCI was conducted at 7 days post-injury, and most of the functional recovery following SCI occurs within the first week (Ma et al., 2001; Basso et al., 2006). Additionally, the mild injury was in fact quite mild, as evidenced by the lack of recovery on the BMS. The moderate injury, due to its increased lesion severity, often prevented animals from achieving the requisite four-to-six step cycles necessary for analysis. Thus, the seeming lack of recovery over time can be addressed by adding an earlier time point and by allowing the animals to walk at their own best speed so that more animals may be included in analyses.
Furthermore, it is important to recognize that TreadScan analysis forces animals to walk, as opposed to self-initiated locomotion as in the open field. Animals able to perform the treadmill task experience a reflex initiation of locomotion soon after injury, which may enhance initial performance and thus explain the lack of improvement on TreadScan over the course of our 10 week study.
The fact that injured animals exhibited the same gait parameter deficits at week 1 as those at week 10 suggests that future studies can safely minimize the number of treadmill sessions to only one or two time points. Rather than following animals weekly throughout the course of a long study, experimenters utilizing the TreadScan system could instead employ an experimental design using one acute treadmill session for insight into initial injury locomotor deficits, and one terminal treadmill session to ascertain the effects of any experimental treatments. This would greatly reduce the amount of data collection and analyses required to obtain significant group differences.
It is interesting to note that the sham group exhibited the only significant change in any gait parameter over time. This seems counterintuitive, as one would expect the non-injured animals to perform consistently from week to week. However, the difference over time occurred only at the slowest treadmill setting, 7 cm/s. Non-injured animals appeared not to prefer walking at such a slow speed, as evidenced by the fact that baseline animals performed very inconsistently at speeds lower than 12 cm/s (unpublished observations). This finding, coupled with the fact that injured animals had difficulty keeping up with higher treadmill speeds, suggests that optimal treadmill results may be obtained by allowing each animal to walk at its own preferred speed. Consistent with previous studies (Clarke and Still, 1999; Herbin et al., 2004; Leblond et al., 2003), we found that stance time and stride length are the two gait parameters affected by speed. Specifically, stance times decrease and stride lengths increase in response to increasing speeds (data not shown). Interestingly, our results found no differences across speeds for any of the balance- and posture-associated gait parameters (hindlimb swing, track width, and toe spread). Leblond et al. (2003) described similar hindlimb swing time consistency regardless of changes in speed. Because these SCI-related gait characteristics are unaffected by the speed of the treadmill, allowing the animals to walk at whatever speed best suits their ability should lead to more consistent treadmill walking without compromising data integrity, provided the experimenter is interested in only the speed-independent measures previously described. If speed-dependent measures such as stance time and/or stride length are important to the study, then careful speed control must be taken into account.
Spared white matter correlates well with various open-field assessment measures in rodents (Cao et al., 2005; Li et al., 2006; Kuhn and Wrathall, 1998; Ma et al., 2001). Consistent with these findings, we found a strong correlation between BMS and spared white matter (Fig. 6b). Interestingly, we found a similarly strong correlation between rear track width and spared white matter (Fig. 6a). Hill et al. (2009) suggested that hindlimb base of support may be adversely affected by damage to the propriospinal tracts in the dorsolateral white matter. This may indeed be the case, as our dorsal contusion model would inherently affect dorsolateral tracts in the spinal cord. Further study is needed to elucidate the anatomical basis for this functional deficit.
BMS and rear track width were also strongly correlated (Fig. 6c). This is not surprising, as the latter measure reflects the animals' balance and postural deficits, which are reflected in the higher range of BMS scores. Present data suggest that TreadScan is a very sensitive measure of behavioral dysfunction following mild-to-moderate SCI. Additionally the software system removes the subjectivity from behavioral assessment, maximizing unbiased comparisons between animals.
TreadScan has a very fast throughput since the foot positions are detected automatically and all gait parameters are calculated directly by the software. Moreover, this software provides objective ratio data on numerous gait parameters relating to the injury. Importantly, velocity-dependent data also can be collected and analyzed. The limitation of the TreadScan is that injured animals must be able to sustain hindlimb weight-bearing locomotion. However, the low variability in TreadScan analyses is of substantive importance in evaluating mild-to-moderate SCI in both mice (Figs. 3, 4, and 6) and rats (unpublished data). The BMS is an excellent outcome measure for distinguishing animals with moderate-to-severe SCI that cannot reliably and consistently walk on the treadmill. Optimally, TreadScan can be used in conjunction with the BMS and/or other locomotor analyses to provide sensitive and objective insight into the locomotor abilities of mice following SCI.
Acknowledgments
We would like to acknowledge that one of the authors (JEB) was supported by a University of Louisville Fellowship in the Integrated Program in Biomedical Sciences (IPIBS). Additional funding was provided by RR15576, NS054708 (SRW, DSKM), Norton Healthcare, the Commonwealth of Kentucky Challenge for Excellence, and the Kentucky Spinal Cord and Head Injury Research Trust (SRW). Special thanks go to Dr. Yi Ping Zhang and Dr. Jason F. Talbott for surgical support, Aaron Puckett and the KSCIRC animal care staff for post-operative animal care, and Melissa Maddie and Conner Means for BMS scoring.
Author Disclosure Statement
No competing financial interests exist.
References
- Amende I. Kale A. McCue S. Glazier S. Morgan J.P. Hampton T.G. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J. Neuroengineering Rehab. 2005;2:20. doi: 10.1186/1743-0003-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova I. Irintchev A. Schachner M. Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice. J. Neurosci. 2006;26:7849–7859. doi: 10.1523/JNEUROSCI.1526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrey E. Galloux P. Valette J.P. Auvient B. Wolter R. Stride characteristics of overground versus treadmill locomotion in the saddle horse. Acta Anat. 1993;146:90–94. doi: 10.1159/000147427. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Fisher L.C. Anderson A.J. Jakeman L.B. McTigue D.M. Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Blakeman K.H. Hao J.X. Xu X.J. Jacoby A.S. Shine J. Crawley J.N. Iismaa T. Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Blaszczyk J. Loeb G.E. Why cats pace on the treadmill. Physiol. Behav. 1993;53:501–507. doi: 10.1016/0031-9384(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Cao Q. Zhang Y.P. Iannotti C. DeVries W.H. Xu X.M. Shields C.B. Whittemore S.R. Functional and electrophysiological changes after graded traumatic spinal cord injury in the adult rat. Exp. Neurol. 2005;191:S3–S16. doi: 10.1016/j.expneurol.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Clarke K.A. Still J. Gait analysis in the mouse. Physiol. Behav. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Farooque M. Spinal cord compression injury in the mouse: presentation of a model including assessment of motor dysfunction. Acta Neuropathol. (Berl) 2000;100:13–22. doi: 10.1007/s004010051187. [DOI] [PubMed] [Google Scholar]
- Guertin P.A. Synergistic activation of the central pattern generator for locomotion by l-beta-3,4-dihydroxyphenylalanine and quipazine in adult paraplegic mice. Neurosci. Lett. 2004;358:71–74. doi: 10.1016/j.neulet.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Guertin P.A. Paraplegic mice are leading to new advances in spinal cord injury research. Spinal Cord. 2005;43:459–461. doi: 10.1038/sj.sc.3101754. [DOI] [PubMed] [Google Scholar]
- Hamers F.P. Lankhorst A.J. van Laar T.J. Veldhuis W.B. Gispen W.H. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma. 2001;18:187–201. doi: 10.1089/08977150150502613. [DOI] [PubMed] [Google Scholar]
- Hamers F.P. Koopmans G.C. Joosten E.A. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma. 2006;23:537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Heglund N.C. Taylor C.R. Speed, stride frequency and energy cost per stride: how do they change with body size and gait? J. Exp. Biol. 1988;138:301–318. doi: 10.1242/jeb.138.1.301. [DOI] [PubMed] [Google Scholar]
- Herbin M. Gasc J.P. Renous S. Symmetrical and asymmetrical gaits in the mouse: patterns to increase velocity. J. Comp. Physiol. A. 2004;190:895–906. doi: 10.1007/s00359-004-0545-0. [DOI] [PubMed] [Google Scholar]
- Herbin M. Hackert R. Gasc J.P. Renous S. Gait parameters of treadmill versus overground locomotion in mouse. Behav. Brain Res. 2007;181:173–179. doi: 10.1016/j.bbr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Hill R.L. Zhang Y.P. Zhang Y. Burke D.A. DeVries W.H. Magnuson D.S.K. Whittemore S.R. Shields C.B. Anatomical and functional outcomes following a precise, graded dorsal laceration spinal cord injury in C57Bl/6 mice. J. Neurotrauma. 2009;26:1–15. doi: 10.1089/neu.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J.Y. McKeon R. Goussev S. Werb Z. Lee J.U. Trvedi A. Noble- Haeusslein L.J. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J. Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygge-Blakeman K. Brumovsky P. Hao J.X. Xu X.J. Hokfelt T. Crawley J.N. Wiesenfeld-Hallin Z. Galanin over-expression decreases the development of neuropathic pain-like behaviors in mice after partial sciatic nerve injury. Brain Res. 2004;1025:152–158. doi: 10.1016/j.brainres.2004.07.078. [DOI] [PubMed] [Google Scholar]
- Joshi M. Fehlings M.G. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J. Neurotrauma. 2002a;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- Joshi M. Fehlings M.G. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J. Neurotrauma. 2002b;19:191–203. doi: 10.1089/08977150252806956. [DOI] [PubMed] [Google Scholar]
- Kale A. Amende I. Meyer G.P. Crabbe J.C. Hampton T.G. Ethanol's effects on gait dynamics in mice investigated by ventral plane videography. Alcohol Clin. Exp. Res. 2004;28:1839–1848. doi: 10.1097/01.alc.0000148103.09378.81. [DOI] [PubMed] [Google Scholar]
- Kuhn P.L. Wrathall J.R. A mouse model of graded contusive spinal cord injury. J. Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- Leblond H. L'Esperance M. Orsal D. Rossignol S. Treadmill locomotion in the intact and spinal mouse. J. Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R.V. Java Applets for Power and Sample Size [computer software] 2006. [Jul 3;2008 ]. http://www.stat.uiowa.edu/∼rlenth/Power http://www.stat.uiowa.edu/∼rlenth/Power
- Li Y. Oskouian R.J. Day Y.J. Kern J.A. Linden J. Optimization of a mouse locomotor rating system to evaluate compression-induced spinal cord injury: correlation of locomotor and morphological injury indices. J. Neurosurg. (Spine) 2006;4:165–173. doi: 10.3171/spi.2006.4.2.165. [DOI] [PubMed] [Google Scholar]
- Ma M. Basso D.M. Walters P. Stokes B.T. Jakeman L.B. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Mikami Y. Toda M. Watanabe M. Nakamura M. Toyama Y. Kawakami Y. A simple and reliable behavioral analysis of locomotor function after spinal cord injury in mice. J. Neurosurg. (Spine 1) 2002;97:142–147. doi: 10.3171/spi.2002.97.1.0142. [DOI] [PubMed] [Google Scholar]
- Rabchevsky A.G. Sullivan P.G. Fugaccia I. Scheff S.W. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats. J. Neurotrauma. 2003;20:659–669. doi: 10.1089/089771503322144572. [DOI] [PubMed] [Google Scholar]
- Stieltjes B. Klussmann S. Bock M. Umathum R. Mangalathu J. Letellier E. Rittgen W. Edler L. Krammer P.H. Kauczor H.U. Martin-Villalba A. Essig M. Manganese-enhanced magnetic resonance imaging for in vivo assessment of damage and functional improvement following spinal cord injury in mice. Magn. Reson. Med. 2006;55:1124–1131. doi: 10.1002/mrm.20888. [DOI] [PubMed] [Google Scholar]
- Thota A.K. Watson S.C. Knapp E. Thompson B. Jung R. Neuromechanical control of locomotion in the rat. J. Neurotrauma. 2005;22:442–465. doi: 10.1089/neu.2005.22.442. [DOI] [PubMed] [Google Scholar]
- Wooley C.M. Sher R.B. Kale A. Frankel W.N. Cox G.A. Seburn K.L. Gait analysis detects early changes in transgenic SOD1 (G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.P. Burke D.A. Shields L.B. Chekmenev S.Y. Dincman T. Zhang Y. Zheng Y. Smith R.R. Benton R.L. DeVries W.H. Hu X. Magnuson D.S. Whittemore S.R. Shields C.B. Spinal cord contusion based on precise vertebral stabilization and tissue displacement measured by combined assessment to discriminate small functional differences. J. Neurotrauma. 2007;25:1227–1240. doi: 10.1089/neu.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]