Abstract
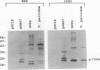
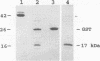
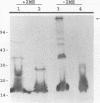
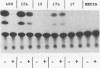
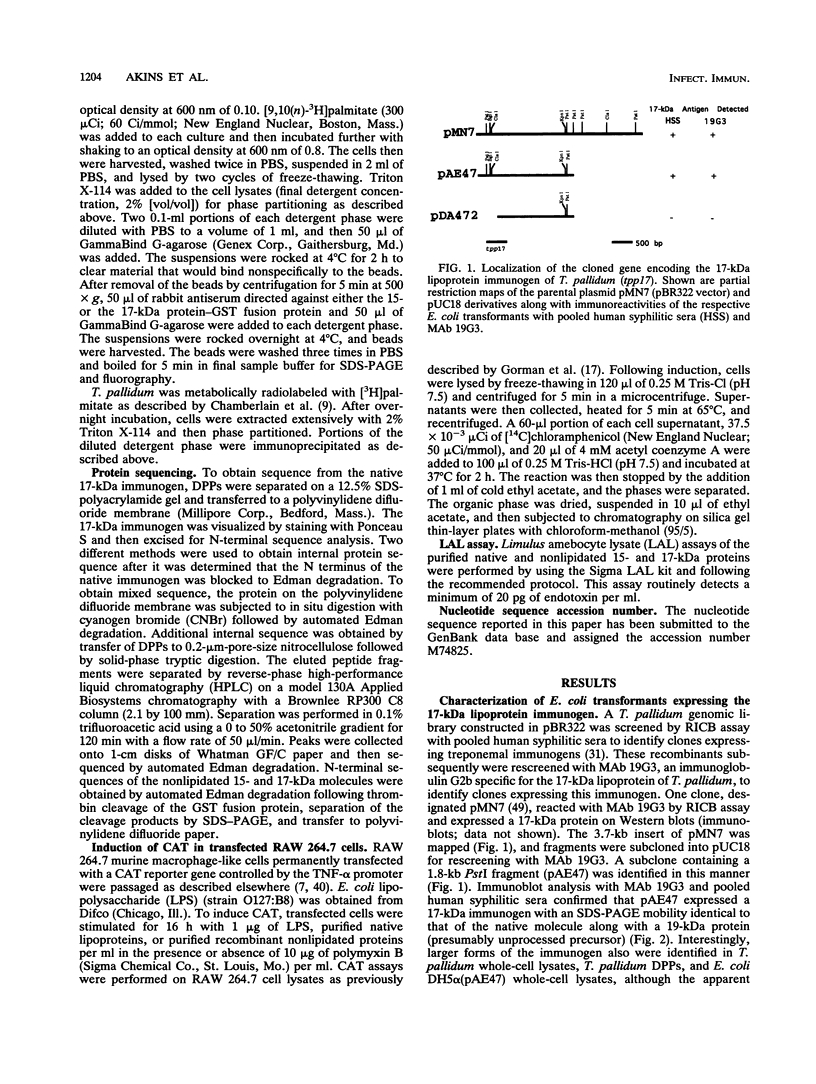
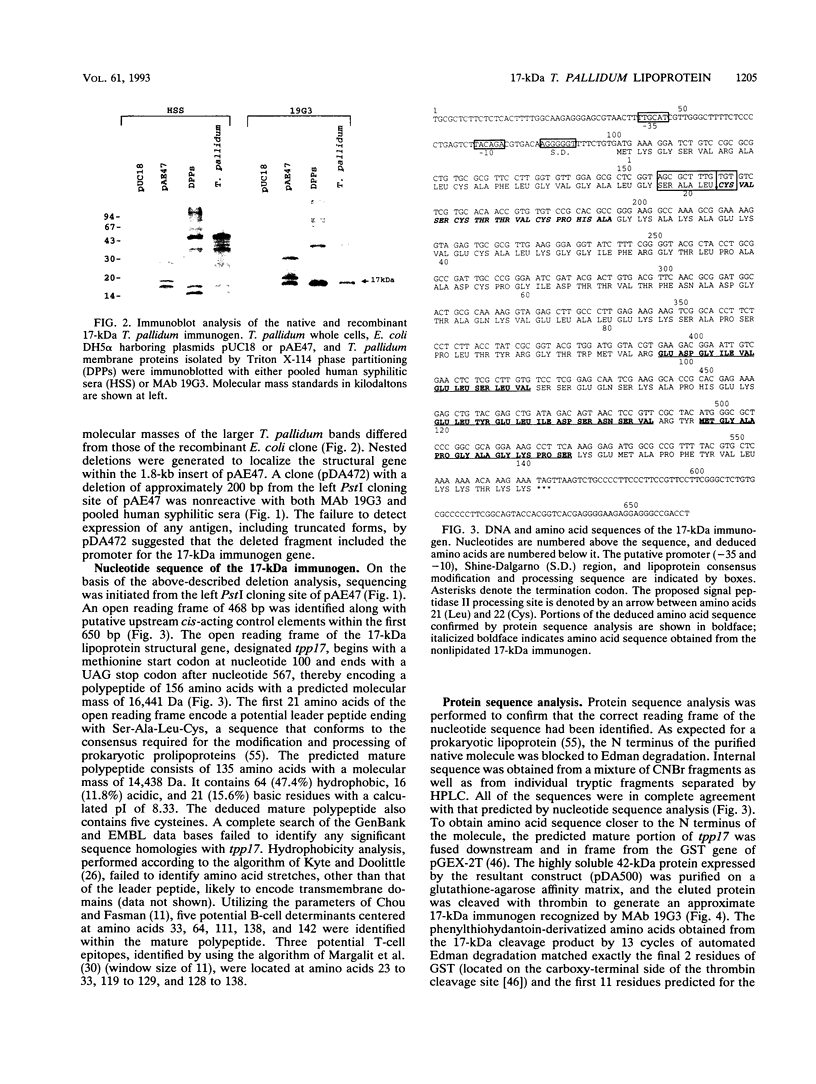
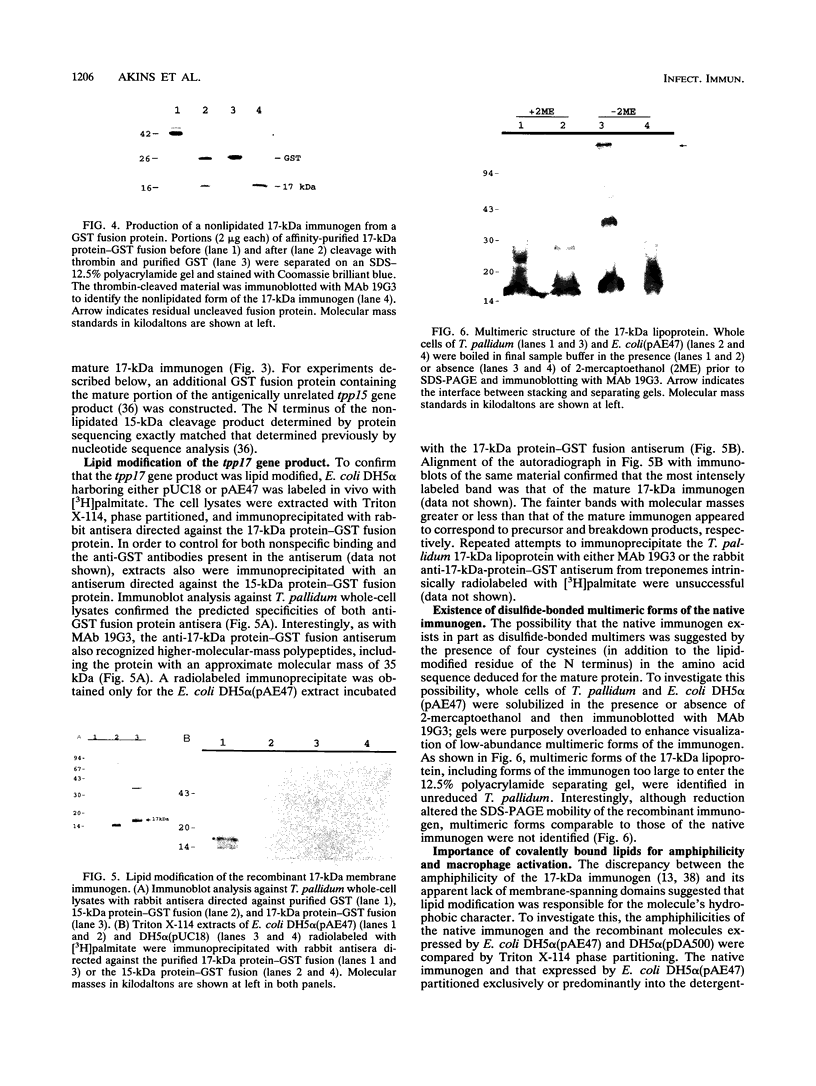
A murine monoclonal antibody specific for a 17-kDa major membrane immunogen of Treponema pallidum was used to select recombinant Escherichia coli clones expressing the molecule from a T. pallidum genomic library. Sequence analysis of the structural gene for the immunogen (designated tpp17) revealed a 468-bp open reading frame encoding a polypeptide of 156 amino acids with a calculated molecular mass of 16,441 Da. The deduced amino acid sequence included a putative leader peptide terminated by a consensus tetrapeptide for the modification and processing of prokaryotic lipoproteins. Immunoprecipitation of the cloned immunogen radiolabeled with [3H]palmitate confirmed that it was a lipoprotein. The amino acid sequence also predicted that the mature protein contains four cysteine residues in addition to the lipid-modified cysteine of the N terminus. The existence of disulfide-bonded multimeric forms of the native immunogen was demonstrated by immunoblotting T. pallidum solubilized in the presence and absence of 2-mercaptoethanol. Triton X-114 phase partitioning of a nonlipidated form of the 17-kDa immunogen cleaved from a glutathione S-transferase fusion protein demonstrated that lipid modification is responsible for the immunogen's hydrophobic character. The same nonlipidated form of the immunogen also was used to demonstrate that lipid modification is essential for the molecule's ability to stimulate production of tumor necrosis factor alpha by murine macrophages. We conclude that covalently attached fatty acids not only anchor T. pallidum lipoproteins to spirochetal membranes but also confer upon these molecules the ability to activate immune effector cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S. A., Fohn M. J., Lukehart S. A. Development of cellular immunity to individual soluble antigens of Treponema pallidum during experimental syphilis. J Immunol. 1988 Dec 15;141(12):4363–4369. [PubMed] [Google Scholar]
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S. A., Lukehart S. A. Macrophage-mediated killing of opsonized Treponema pallidum. J Infect Dis. 1992 Jan;165(1):69–74. doi: 10.1093/infdis/165.1.69. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S. A., Lukehart S. A. Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue. Infect Immun. 1983 Nov;42(2):634–638. doi: 10.1128/iai.42.2.634-638.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Brown T. A CAT reporter construct allows ultrasensitive estimation of TNF synthesis, and suggests that the TNF gene has been silenced in non-macrophage cell lines. J Clin Invest. 1991 Apr;87(4):1336–1344. doi: 10.1172/JCI115137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., DeOgny L., Slaughter C., Radolf J. D., Norgard M. V. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989 Sep;57(9):2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cox D. L., Chang P., McDowall A. W., Radolf J. D. The outer membrane, not a coat of host proteins, limits antigenicity of virulent Treponema pallidum. Infect Immun. 1992 Mar;60(3):1076–1083. doi: 10.1128/iai.60.3.1076-1083.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. M., Walker E. M., Miller J. N., Lovett M. A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988 Dec;170(12):5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Beckwith J. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J Bacteriol. 1991 Dec;173(23):7719–7722. doi: 10.1128/jb.173.23.7719-7722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Lovett M. A. Properties of an ordered ring structure formed by recombinant Treponema pallidum surface antigen 4D. J Bacteriol. 1986 Mar;165(3):732–739. doi: 10.1128/jb.165.3.732-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Bishop N. H., Miller J. N., Lovett M. A. Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J Immunol. 1983 Oct;131(4):1973–1977. [PubMed] [Google Scholar]
- Hanff P. A., Fehniger T. E., Miller J. N., Lovett M. A. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J Immunol. 1982 Sep;129(3):1287–1291. [PubMed] [Google Scholar]
- Hardy P. H., Jr, Levin J. Lack of endotoxin in Borrelia hispanica and Treponema pallidum. Proc Soc Exp Biol Med. 1983 Oct;174(1):47–52. doi: 10.3181/00379727-174-41702. [DOI] [PubMed] [Google Scholar]
- Hensel U., Wellensiek H. J., Bhakdi S. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting as a serological tool in the diagnosis of syphilitic infections. J Clin Microbiol. 1985 Jan;21(1):82–87. doi: 10.1128/jcm.21.1.82-87.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Cockayne A., Schouls L. M., van Emden J. D. Immunochemical characterization and purification of Treponema pallidum antigen TpD expressed by Escherichia coli K12. Sex Transm Dis. 1986 Oct-Dec;13(4):237–244. doi: 10.1097/00007435-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Hubbard C. L., Gherardini F. C., Bassford P. J., Jr, Stamm L. V. Molecular cloning and characterization of a 35.5-kilodalton lipoprotein of Treponema pallidum. Infect Immun. 1991 Apr;59(4):1521–1528. doi: 10.1128/iai.59.4.1521-1528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Cloning and expression of Treponema pallidum (Nichols) antigen genes in Escherichia coli. Infect Immun. 1983 Nov;42(2):435–445. doi: 10.1128/iai.42.2.435-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Rhodes J. G. Surface-associated antigens of Treponema pallidum concealed by an inert outer layer. Immunology. 1982 May;46(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Chamberlain N. R., Goldberg M. S., Andrews L. P., Robinson E. J., Norgard M. V., Radolf J. D. Molecular cloning and characterization of the 15-kilodalton major immunogen of Treponema pallidum. Infect Immun. 1989 Dec;57(12):3708–3714. doi: 10.1128/iai.57.12.3708-3714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell B. K., Swancutt M. A., Radolf J. D. Lipid modification of the 15 kiloDalton major membrane immunogen of Treponema pallidum. Mol Microbiol. 1990 Aug;4(8):1371–1379. doi: 10.1111/j.1365-2958.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Borenstein L. A., Kim J. Y., Fehniger T. E., Lovett M. A. Role of disulfide bonds in the oligomeric structure and protease resistance of recombinant and native Treponema pallidum surface antigen 4D. J Bacteriol. 1987 Apr;169(4):1365–1371. doi: 10.1128/jb.169.4.1365-1371.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Brandt M. E., Isaacs R. D., Thompson P. A., Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991 Sep 15;147(6):1968–1974. [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect Immun. 1988 Jul;56(7):1825–1828. doi: 10.1128/iai.56.7.1825-1828.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Schulz W. W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley B. S., Oppenheimer-Marks N., Hansen E. J., Radolf J. D., Norgard M. V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls L. M., Mout R., Dekker J., van Embden J. D. Characterization of lipid-modified immunogenic proteins of Treponema pallidum expressed in Escherichia coli. Microb Pathog. 1989 Sep;7(3):175–188. doi: 10.1016/0882-4010(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sommer B., Köhler M., Sprengel R., Seeburg P. H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991 Oct 4;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Radolf J. D., Norgard M. V. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990 Feb;58(2):384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Twehous D. A., Norgard M. V. Monoclonal antibody selection and analysis of a recombinant DNA-derived surface immunogen of Treponema pallidum expressed in Escherichia coli. Infect Immun. 1986 Apr;52(1):110–119. doi: 10.1128/iai.52.1.110-119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young E. J., Weingarten N. M., Baughn R. E., Duncan W. C. Studies on the pathogenesis of the Jarisch-Herxheimer reaction: development of an animal model and evidence against a role for classical endotoxin. J Infect Dis. 1982 Nov;146(5):606–615. doi: 10.1093/infdis/146.5.606. [DOI] [PubMed] [Google Scholar]