Abstract
Chronic demyelination is a pathophysiologic component of compressive spinal cord injury (SCI) and a characteristic finding in demyelinating diseases including multiple sclerosis (MS). A better characterization of endogenous cells responsible for successful remyelination is essential for designing therapeutic strategies aimed at restoring functional myelin. The present study examined the spatiotemporal response of endogenous oligodendrocyte precursor cells (OPCs) following ethidium bromide (EB)-induced demyelination of the adult rat spinal cord. Beginning at 2 days post-EB injection (dpi), a robust mobilization of highly proliferative NG2+ cells within the lesion was observed, none of which expressed the oligodendrocyte lineage-associated transcription factor Nkx2.2. At 7 dpi, a significant up-regulation of Nkx2.2 by OPCs within the lesion was observed, 90% of which coexpressed NG2 and virtually all of which coexpressed the bHLH transcription factor Olig2. Despite successful recruitment of Nkx2.2+/Olig2+ OPCs within the lesion, demyelinated axons were not remyelinated by these OPCs in regions lacking astrocytes. Rather, Schwann cell remyelination predominated throughout the central core of the lesion, particularly around blood vessels. Oligodendrocyte remyelination was observed in the astrogliotic perimeter, suggesting a necessary role for astrocytes in oligodendrocyte maturation. In addition, reexpression of the radial glial antigen, RC-1, by reactive astrocytes and ependymal cells was observed following injury. However, these cells did not express the neural stem cell (NSC)-associated transcription factors Sox1 or Sox2, suggesting that the endogenous response is primarily mediated by glial progenitors. In vivo electrophysiology demonstrated a limited and unsustained functional recovery concurrent with endogenous remyelination following EB-induced lesions.
Keywords: Demyelination, Remyelination, Nkx2.2, NG2, Oligodendrocyte precursor cell, Radial glia
Introduction
A comprehensive understanding of the cells responsible for mediating oligodendrocyte remyelination in the central nervous system (CNS) is essential to the development of therapies aimed at promoting remyelination in demyelinating diseases such as multiple sclerosis (MS). Gensert and Goldman (1997) were the first to convincingly demonstrate that the adult CNS harbors immature cycling cells with the potential to differentiate into mature myelinating oligodendrocytes following a demyelinating insult. Several subsequent studies have confirmed the capacity for remyelination by endogenous progenitors, with particular emphasis on the proliferative response to demyelination by oligodendrocyte precursor cells (OPCs) (Carroll et al., 1998; Di Bello et al., 1999; Keirstead et al., 1998; Levine and Reynolds, 1999; Redwine and Armstrong, 1998; Reynolds et al., 2002; Watanabe et al., 2002). Although its specificity is controversial, NG2 has been extensively utilized as a marker for adult OPCs in many of these studies. Recently, Nishiyama et al. (2002) proposed the term polydendrocyte to define parenchymal NG2+ cells endogenous to the CNS. Based largely on morphological criteria and CD9 expression, Berry et al. (2002) have suggested that only a small minority (approximately 1%) of polydendrocytes in the adult are truly OPCs. Therefore, the identification of markers more specific for the oligodendrocyte lineage is needed to accurately characterize the OPC response to demyelination.
Recent developmental studies have identified several transcription factors important for oligodendrocyte differentiation in the embryo. It is likely that these same factors operate in regulating differentiation of OPCs during remyelination in the adult (Franklin and Hinks, 1999). In support of this hypothesis, Sim et al. (2000) demonstrated transient up-regulation of Nkx6.2 before remyelination in the adult rat brain. Other recently identified transcription factors important for oligodendrocyte development include the bHLH protein Olig2 (Lu et al., 2000; Zhou et al., 2000) and the transiently expressed homeodomain protein Nkx2.2 (Fu et al., 2002; Qi et al., 2001; Soula et al., 2001; Sun et al., 2001; Xu et al., 2000; Zhou et al., 2001). In addition to providing specific markers for OPCs, the expression of these proteins by endogenous progenitors in the adult may identify specific stages of oligodendrocyte differentiation at which factors present or absent in the lesioned adult spinal cord interrupt the recapitulation of developmental myelination.
In addition to OPCs, there is some evidence that the adult mammalian spinal cord harbors pools of neural stem cells (NSCs), which might be activated following injury and contribute to repair (Brundin et al., 2003; Calza et al., 2002; Oumesmar et al., 1995). Several laboratories have isolated cells from the adult spinal cord that demonstrate properties of NSCs in vitro (Johansson et al., 1999; Shihabuddin, 2002; Shihabuddin et al., 1997; Yamamoto et al., 2001); however, the role these cells play in vivo during remyelination remains to be elucidated.
In the present study, we chose the ethidium bromide (EB) lesion (Blakemore, 1982) as a model to examine the temporal response and transcription factor expression of endogenous OPCs following demyelination. Moreover, we addressed the question of whether NSCs are mobilized following EB-induced demyelination. Finally, we analyzed the extent to which in vivo electrophysiology measures recover within ventrolateral funiculus (VLF) motor path-ways concurrent with endogenous remyelination.
Materials and methods
Animal surgery
A total of 50 female Fischer 344 rats (160–200 g) were used throughout the study. Animals were anesthetized with sodium pentobarbital (50 mg/kg ip). Following induction of anesthesia, dorsal laminectomies were performed at the T9/T10 vertebral level and the dura was opened transversely at two sites 2 mm apart. To assess the endogenous precursor response to demyelination, unilateral injections (0.75 µl/injection, n = 20) of 0.1 mg/ml EB were delivered at stereotactic coordinates for the VLF (0.7 mm lateral to midline and depths of 1.3 and 1.6 mm) of the thoracic spinal cord using custom-pulled and -beveled micropipettes attached to a Parker picospritzer (Loy et al., 2002a). For transcranial magnetic motor-evoked potential (tcMMEP) studies (described below), bilateral VLF lesions were created (n = 20). Surgical interventions and perioperative care were provided in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Tissue processing
To evaluate the response of endogenous precursors following demyelination, animals were sacrificed at 2 (n = 4), 7 (n = 4), 14 (n = 4), and 28 (n = 8) days post EB injection (dpi). Uninjured, age-matched animals were used as controls (n = 3). Animals were deeply anesthetized with sodium pentobarbitol (60 mg/kg) and transcardially per-fused with 100 ml of 0.1 M PBS followed by 300 ml of 4% paraformaldehyde in 0.1 M PBS. Spinal cords were dissected and postfixed overnight at 4°C, then transferred to 30% sucrose at 4°C for cryoprotection (48 h). Specimens were cut transversely at the epicenter of the lesion and mounted with the cut surfaces facing down in TBS tissue freezing medium (Triangle Biomedical Sciences, Durham, NC).
Immunohistochemistry and fluorescence microscopy
After tissue embedding, 20-µm-thick transverse sections were cut on a Leica CM3050 cryostat and mounted on microscope slides. Before incubation with primary antibodies, sections were permeabilized and blocked with 0.3% Triton X-100/10% normal donkey serum (NDS) in 0.1 M TBS (7.4) for 30 min. Primary antibodies were then applied for 48 h at 4°C. The following primary antibodies were used: Sox1 for NSC (1:2000; kind gift of Dr. L. Pevny), Sox2 for NSC (1:3000; kind gift of Dr. L. Pevny), Nestin for NSC/radial glia (1:20; Developmental Studies Hybridoma Bank-DSHB), RC1 for radial glia (1:20; DSHB), NG2 for polydendrocytes (1:200; Chemicon), Nkx2.2 for oligodendrocyte lineage cells (1:5; DSHB), Olig2 for oligodendrocyte lineage cells (1:8000; kind gift of Dr. D. Rowitch), BrdU for dividing cells (1:200; Biodesign), glial fibrillary acidic protein (GFAP) for astrocytes (1:200; Dako), adenomatous polyposis coli (APC) for mature oligodendrocytes (1:100; Oncogene), P0 for Schwann cells (1:800; Dr. J.J. Archelos), and ED-1 for activated microglia/macrophages (1:500; Chemicon). Appropriate secondary antibodies were applied for 90 min at room temperature. The following species-specific secondary antibodies were used: donkey anti-sheep 7-amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated IgG (1:100), donkey anti-mouse fluoroisothiocyanate (FITC)-conjugated (1:100) and rhodamine red-conjugated IgG (1:200), donkey anti-rabbit rhodamine red (1:200), and FITC-conjugated Fab’ fragments (1:100). All secondary antibodies were supplied by Jackson Immunoresearch Lab (Baltimore, MD). Sections were then rinsed in TBS and coverslipped with antifade mounting media (Molecular Probes, Eugene, OR). Staining was visualized with a Nikon Eclipse TE300 microscope and photographed with a Spot RT Color CCD camera. Figures were assembled using Adobe Photoshop and Illustrator software.
BrdU pulse labeling and immunohistochemistry
BrdU (Sigma, St. Louis, MO) solutions (20 mg/ml) in normal saline were prepared and syringe filtered. Animals received a total of three intraperitoneal injections of 50 mg/kg BrdU delivered every 8 h over a 24-h pulse period. Table 1 describes the timing between BrdU pulse labeling and animal sacrifice for experimental groups 1–5. Immunohistochemistry for BrdU was performed as above, except that sections were incubated in 2 N HCl (30 min) and 0.1 M borate buffer (10 min) before blocking steps.
Table 1.
Experimental groups for assessing endogenous remyelination
| Group no. | N | 24-h BrdU pulse | Sacrifice (dpi) |
|---|---|---|---|
| 1 | 4 | 24–48 h | 2 |
| 2 | 4 | Day 6 | 7 |
| 3 | 4 | Day 13 | 14 |
| 4 | 4 | Day 27 | 28 |
| 5 | 4 | Day 2 | 28 |
Histological analysis of lesion
Schwann cell and oligodendrocyte remyelinations were examined in 1 µm-thick plastic toluidine blue-stained sections from animals sacrificed at 74 dpi. For luxol fast blue (LFB) staining, cryostat sections were dehydrated in graded alcohol and placed in 0.1% LFB at 37°C overnight. Sections were then differentiated in 0.05% LiCO3 for 4 min, dehydrated in graded alcohol and xylene, and coverslipped.
Electron microscopy
Following anesthetization with 100 mg/kg IP sodium pentobarbital, animals were transcardially perfused at 7 (n = 4) and 28 dpi (n = 3) with 100 ml 0.1 M phosphate buffer (PB; pH 7.4) then with 300 ml of 4% paraformaldehyde (PFA) and 2% glutaraldehyde in 0.1 M PB. One millimeter tissue slices was cut from the lesion epicenter and immersed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h. Tissues were rinsed in buffer, dehydrated in graded ethanol and propylene oxide, and embedded in Embed812 epoxy resin (Electron Microscopy Sciences, Fort Washington, PA). Ultrathin sections were prepared and stained with uranyl acetate and lead citrate and examined in a Philips CM10 transmission electron microscope.
Transcranial magnetic motor-evoked potential (tcMMEP) responses
tcMMEP responses, which are carried in the VLF (Loy et al., 2002a,b), were recorded from stimulation of awake, nonanesthetized, restrained rats, as described previously (Linden et al., 1999; Magnuson et al., 1999). Briefly, rats were placed in a prone position on a wooden board and wrapped in a cloth stockingette tacked to the board surface. The hindlimbs were pulled through the cloth and left exposed in order to allow the insertion of needle electrodes into the gastrocnemius muscle of each hindlimb, with the active electrode placed into the gastrocnemius muscle belly and the reference electrode placed near the distal tendon. The ground electrode was placed subcutaneously between the coil and recording electrodes. tcMMEP responses were elicited by activation of subcortical structures with a Cadwell MES10 (Kennewick, WA) electromagnetic coil placed over the cranium. A single magnetic pulse with a stimulus intensity of 60 mV and a gain of 5000 was used. The onset latency and amplitude were recorded with a Cadwell Sierra II console. All animals were recorded biweekly for up to 74 days postinjury. Left and right tcMMEP responses were analyzed independently; thus, two tcMMEP responses were counted for each animal. Animals were sacrificed at 7 (n = 5 animals), 14 (n = 5 animals), 28 (n = 5 animals), and 74 days (n = 5 animals) following bilateral EB injections. For statistical analysis, amplitude and latency values for all recovered tcMMEP signals (those observed after 14 dpi) were collapsed. Paired t tests were performed to compare amplitude and latency values between baseline and recovered tcMMEP signals.
Cell quantitation
For quantification, four cryostat sections from the epicenter of lesions from at least three different animals were analyzed. Digitized images of the lesion were obtained with a Spot RT Color CCD camera (Sterling Heights, MI) at 40× magnification. Cell proliferation within the lesion was assessed by manually counting the total number of BrdU+ nuclei in a 190 × 150 µm rectangle drawn within the lesion borders using SPOT RT Software v3.1 (Sterling Heights). The density of NG2+ cells within lesions was assessed at 2, 7, and 28 days following EB injection as well as in the VLF of sections from uninjured animals. Identification of NG2+ cells for quantification was performed as previously described (McTigue et al., 2001). Briefly, NG2+ cells were only counted if a clearly defined cell body was observed. Double-label quantification for NG2 and BrdU (2 dpi) or NG2 and Nkx2.2 (0, 7 dpi) was also performed. Cells were only counted as double labeled if the BrdU+ or Nkx2.2+ nuclei were completely surrounded by NG2 immunoreactivity. Data are expressed as cells/mm2. The VLF of uninjured animals served as controls for comparison with experimental groups. To assess NG2 and Nkx2.2 up-regulation following injury, one-way ANOVA was used to compare control values with experimental groups followed by a Tukey’s post hoc comparison test. Independent t tests were performed to analyze the proportion of cells coexpressing NG2 and Nkx2.2 in control and injured tissue.
Results
EB-induced demyelination in the VLF
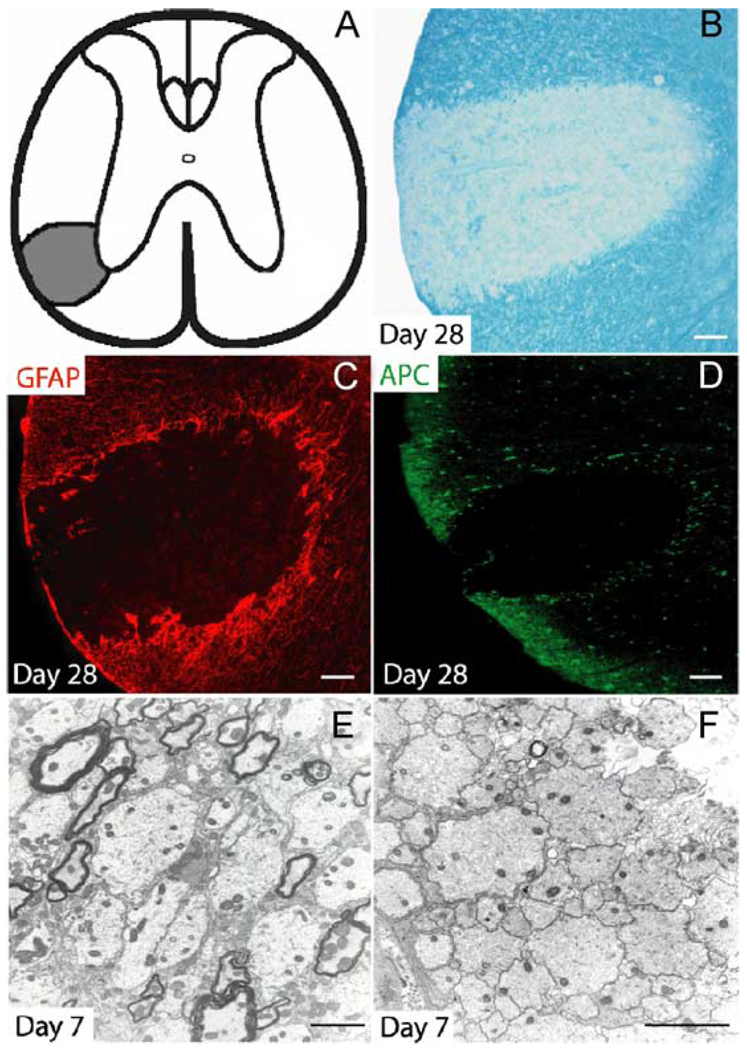
Injection of EB into the VLF of the adult rat spinal cord consistently resulted in a characteristic demyelinating plaque as evidenced by LFB staining of frozen sections from the lesion epicenter 28 days after injection (Figs. 1A and B). Well-defined demyelination consistently followed a linear diffusion pattern extending away from the injection point medially toward the ventral gray matter and peripherally toward the pia mater. After 28 days, the lesion was largely devoid of both astrocytes and oligodendrocytes as evidenced by a lack of GFAP and APC immunoreactivity (Figs. 1C and D), although a rim of oligodendrocytes and reactive astrocytes was present within the perimeter of the lesion and delineated the lesion from adjacent uninjured white matter. Ultrastructural analysis revealed that many viable demyelinated axons in both the peripheral (Fig. 1E) and central (Fig. 1F) regions of the lesion survived EB treatment.
Fig. 1. EB-induced demyelination in the VLF.
(A) Stereotactic injection of EB into the VLF consistently resulted in well-defined lesions as schematically illustrated. (B) LFB staining reveals a loss of myelin throughout most of the lesion after 28 days. EB is toxic to both GFAP+ astrocytes (C) and APC+ oligodendrocytes (D). Ultrastructural analysis revealed that after 7 days, many viable demyelinated axons survive EB treatment in both the peripheral (E) and central (F) portions of the lesion. Scale bar = 100 µm in (B–D); 2 µm in (E and F).
NG2+ polydendrocyte response to EB injection
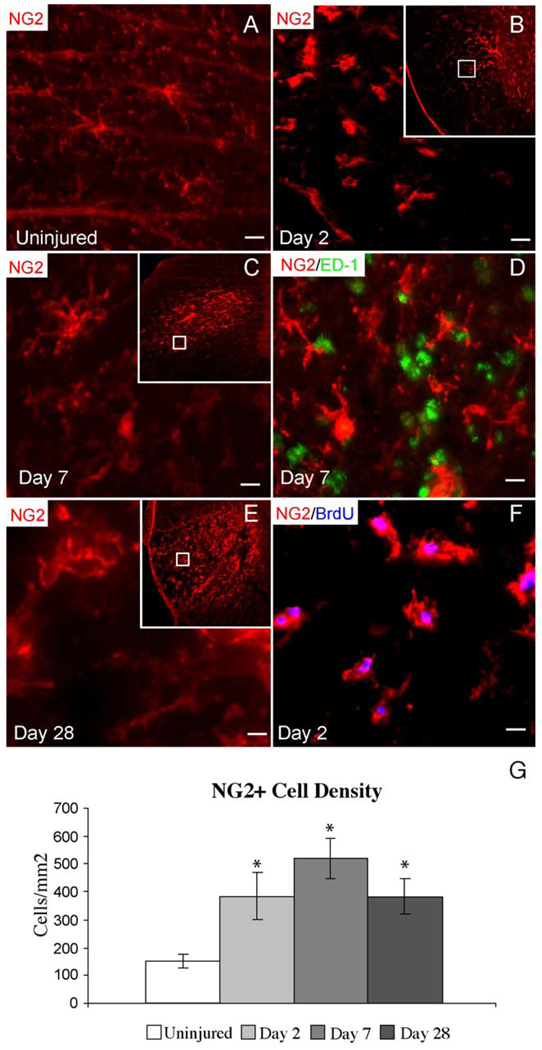
NG2 immunohistochemistry revealed an evenly spaced distribution of polydendrocytes throughout the white matter in uninjured adult spinal cord. They were bipolar and multipolar cells with irregularly shaped cell bodies (Fig. 2A). In the uninjured VLF, polydendrocyte density was 153 ± 28 cells/mm2. Beginning at 2 dpi, reactive polydendrocytes within the EB injection site demonstrated increased staining for NG2 and extended only short processes such that they could be easily distinguished morphologically from NG2+ cells in unaffected white matter (Fig. 2B). There were no ED-1+ macrophages in Day 2 lesions suggesting that NG2+ cells were not invading blood macrophages at this time point (data not shown). The density of polydendrocytes within the lesion at 2 dpi was significantly increased compared with controls (394 ± 85 cells/mm2). At 7 dpi, demyelination was nearly complete and polydendrocyte density remained increased within the lesion relative to controls (521 ± 73 cells/mm2). At this stage, many polydendrocytes exhibited a complex morphology with numerous branching processes (Fig. 2C). By 7 dpi, many ED-1+ macrophages were also present within the lesion; however, coexpression with NG2 was not observed (Fig. 2D). Increased NG2+ cell density within the lesion was maintained through 28 dpi (381 ± 64 cells/mm2).
Fig. 2. NG2+ polydendrocyte response to EB injection.
(A) NG2+ cells are evenly spaced in the uninjured VLF and have irregularly shaped cell bodies. (B) At 2 dpi, NG2+ polydendrocytes demonstrate increased NG2+ staining and short processes. In (B, C, and E), the larger inset box shows the entire lesioned VLF while the inner box shows the region shown at higher magnification. (C) By 7 dpi, NG2+ polydendrocytes exhibited complex morphologies with many branching processes. (D) NG2 did not label activated macrophages at 7 dpi as evidenced by a lack of NG2 and ED-1 coexpression. (E) Increased NG2+ immunoreactivity is observed in EB lesions up to 28 dpi. (F) In animals sacrificed at 2 dpi, 85% of the polydendrocytes within the lesion express BrdU following a 24-h BrdU pulse on Day 2, suggesting that the increase in polydendrocyte density within EB lesions is at least partially due to local proliferation. (G) Polydendrocyte density within EB lesions is significantly increased at 2, 7, and 28 dpi compared to uninjured controls. Data are expressed as the mean ± SD (uninjured; n = 10, Day 2; n = 9, Day 7; n = 8, Day 28; n = 8). One-way ANOVA (F = 50.5, df = 3.31, P < 0.001). Tukey post hoc (all comparisons, *P < 0.001). Scale bar = 5 µm in (A, D, and F); 8 µm in (B); 3 µm in (C and E).
To evaluate the proliferative response of polydendrocytes, lesioned animals were subjected to 24 h BrdU pulse labeling at time points ranging from 2 to 28 dpi. The early increase in NG2+ cell number after EB injection was at least in part due to cell division as evidenced by incorporation of BrdU in the nuclei of polydendrocytes within the lesion following 24 h BrdU pulse labeling beginning 24 h after injury (Fig. 2F). Polydendrocytes that incorporated BrdU during this pulse were primarily located within the EB injection site. Eighty-five percent of the polydendrocytes within the lesion were BrdU+ and they accounted for 83% of all BrdU+ cells within the lesion. In addition to the lesion area, NG2+/BrdU+ cells were also noted in the pia and adjacent subpial region of the dorsal spinal cord in response to trauma associated with laminectomy, durotomy, and micropipette injection. However, these cells were not quantified. At all BrdU pulse time points examined, NG2+/BrdU+ cells were not observed in the contralateral VLF, indicating that the NG2+ cell reaction in the injured VLF was specific to EB injection and not a response to the laminectomy and/or durotomy (data not shown).
Nkx2.2 and Olig2 expression within the lesion
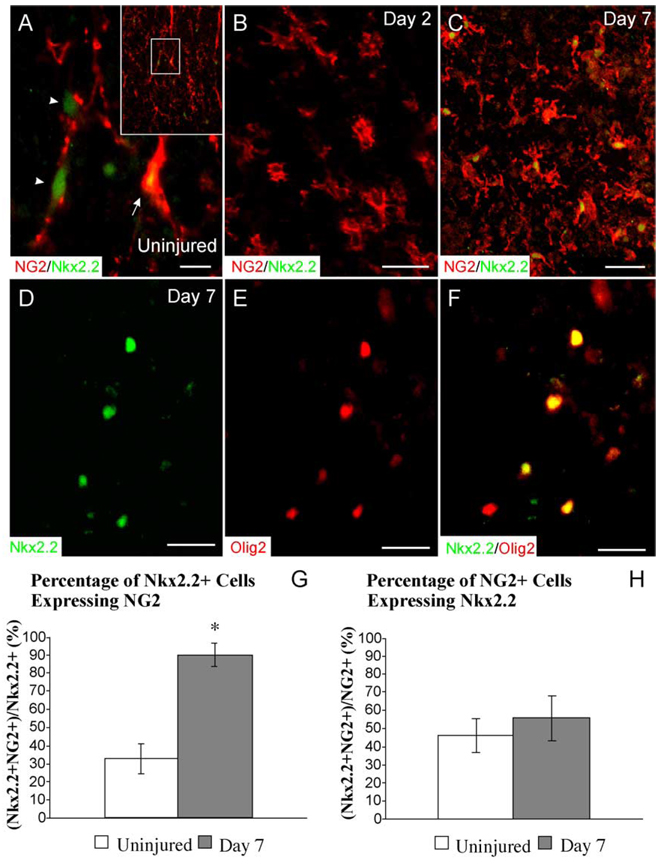
The expression of two transcription factors, Nkx2.2 and Olig2, by OPCs following EB injection was examined to better understand the extent to which these factors potentially modulate adult OPC-mediated remyelination. In uninjured white matter, faint Nkx2.2 expression was observed in the nuclei of many cells (212 ± 35 cells/mm2; Fig. 3A). Approximately one third of all Nkx2.2+ nuclei in normal white matter demonstrated clear coexpression with NG2 (33% ± 8%). Many Nkx2.2+ nuclei appeared to be associated with faint NG2+ processes; however, they did not meet criteria for double labeling. At 2 dpi, Nkx2.2 expression within the lesion was absent (Fig. 3B). At 7 dpi, Nkx2.2 expression within the lesion was up-regulated to levels significantly greater than uninjured white matter (327 ± 99 cells/mm2, P < 0.05, t = 3.1, df = 8.4) and 90% ± 7% of the Nkx2.2 cells within the lesion coexpressed NG2, representing a significant increase compared to the proportion of Nkx2.2+ cells coexpressing NG2 in normal white matter (Fig. 3C). While the proportion of Nkx2.2+ cells within the lesion coexpressing NG2 was significantly increased compared to controls at 7 dpi, the proportion of NG2+ cells expressing Nkx2.2 was not significantly different from uninjured controls (Figs. 3G and H).
Fig. 3. Nkx2.2 and Olig2 expression within EB lesions.
(A) In the uninjured VLF, faint Nkx2.2 expression is observed in many cells, approximately one third of which are NG2+ (arrow). Many Nkx2.2+ nuclei are associated with NG2 processes; however, they do not meet criteria for NG2 coexpression (arrowheads). The inset shows the entire region of the VLF and the inner box shows the area presented at higher magnification. (B) At 2 dpi, Nkx2.2 is not expressed within the lesion despite the increased density of NG2+ polydendrocytes. (C) At 7 dpi, 90% of Nkx2.2+ nuclei coexpress NG2. (D–F) All Nkx2.2+ nuclei coexpress Olig2 at 7 dpi. The proportion of Nkx2.2+ cells coexpressing NG2 is significantly increased in Day 7 lesions compared to uninjured controls (G), whereas the proportion of NG2+ cells coexpressing Nkx2.2 is not (H). Data are expressed as the mean ± SD. *P < 0.05, t = 3.1, df = 8.4. Scale bar = 6 µm in (A); 15 µm in (B and D– F); 24 µm in (C).
During development, Nkx2.2 and Olig2 are expressed by OPCs in the spinal cord (Liu et al., 2002). Double label immunohistochemistry for Nkx2.2 and Olig2 revealed that virtually all Nkx2.2+ cells within the lesion also coexpressed Olig2 at 7 dpi (Figs. 3D–F). Since nearly all Nkx2.2+ cells also express NG2 at 7 dpi, this suggests that the predominant population to respond after injury is an NG2+/Nkx2.2+/Olig2+ precursor cell.
Radial glia antigen expression by reactive astrocytes and ependymal cells
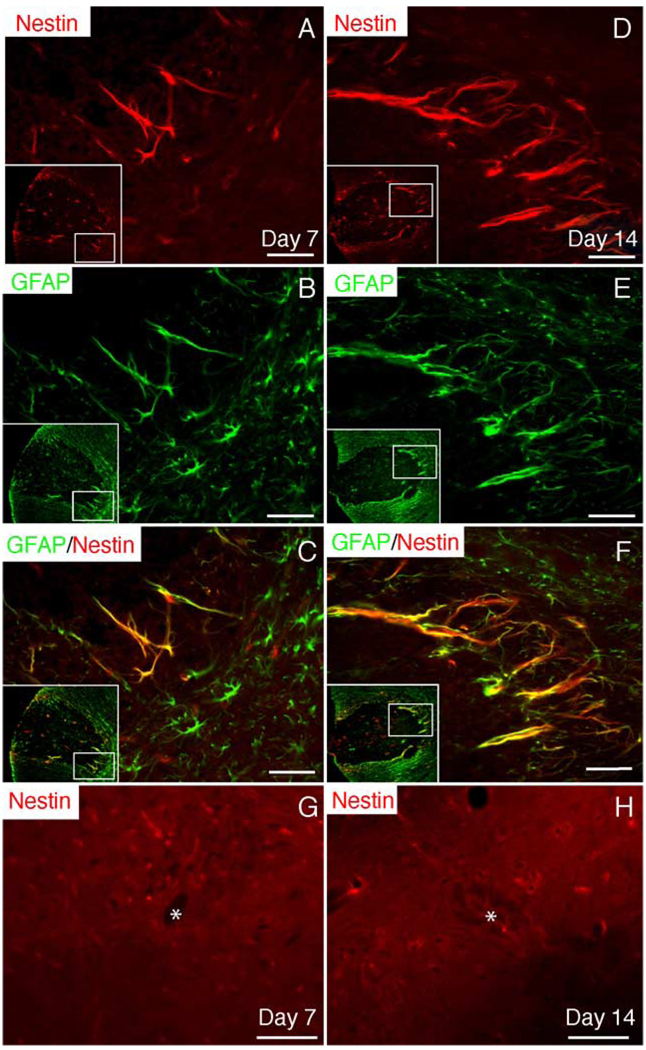
Immunohistochemistry for RC-1, nestin, and GFAP was performed sequentially at 0, 2, 7, 14, and 28 dpi. Beginning at 7 dpi, long arborized processes positive for nestin immunoreactivity were observed around the perimeter of the lesion (Fig. 4A). Maximal nestin expression in the VLF was observed at 14 dpi and was still confined to the perimeter of the lesion (Fig. 4D). By 28 dpi, nestin immunoreactivity had decreased but could still be detected in arborized processes in the perimeter of the lesion (data not shown). Increased nestin expression was never observed in ependymal cells or in the subependymal region (Figs. 4G and H).
Fig. 4. Nestin up-regulation by reactive astrocytes.
(A) Beginning at 7 dpi, nestin is up-regulated by cells surrounding the lesion. In (A–F), the insets show the entire region of the VLF and the inner box designates the area shown at higher magnification. (B and C) GFAP double labeling reveals that nestin+ cells are GFAP+-reactive astrocytes. (D–F) Nestin up-regulation by reactive astrocytes around the lesion is maintained at 14 dpi. Nestin up-regulation by ependymal and/or subependymal cells was not observed at 7 (G) or 14 (H) dpi. Asterisks (*) identify the central canal. Scale bar = 30 µm.
Because up-regulation of nestin by reactive astrocytes has been demonstrated in other injury models (Lin et al., 1995; Shibuya et al., 2002) and the pattern of nestin expression overlapped with the region of reactive astrocytosis, double label immunohistochemistry for nestin and GFAP was performed. Coexpression of nestin and GFAP at 7 and 14 dpi confirmed that virtually all nestin+ cells bordering the lesion were indeed GFAP+ reactive astrocytes (Figs. 4C and F). Twenty-four-hour BrdU pulse labeling at 7, 14, or 28 dpi revealed that, despite extensive proliferation at these times, nestin+ cells did not undergo cell division as BrdU+/nestin+ cells were not observed (data not shown). Therefore, nestin+ astrocytes do not proliferate and give rise to NG2+ OPCs.
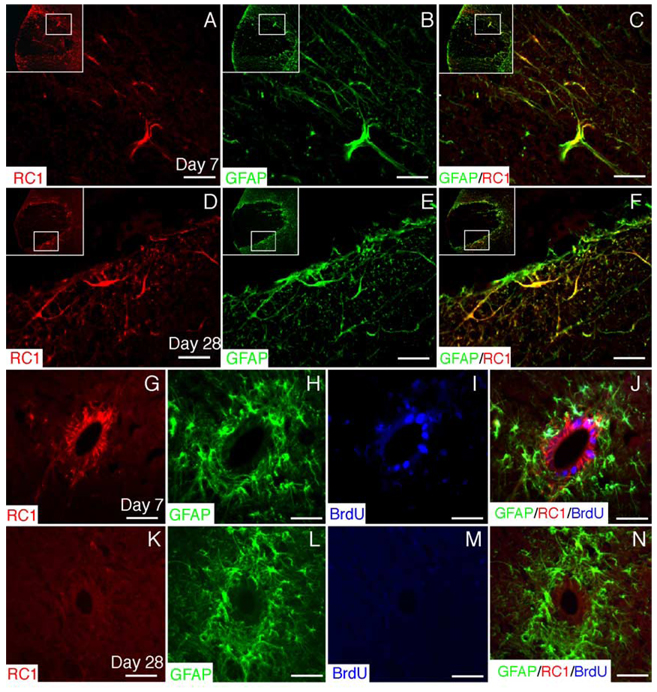
Beginning at 7 dpi, a marked increase in the expression of RC-1 was observed surrounding the lesion in a pattern very similar to that observed for nestin (Fig. 5A). As with nestin, complete coexpression of RC-1 with GFAP was observed by reactive astrocytes in the perimeter of the lesion at both 7 and 28 dpi (Figs. 5C and F). RC-1+/GFAP+ astrocytes surrounding the lesion did not incorporate BrdU during 24 h pulse labeling at 7, 14, or 28 dpi (data not shown). In contrast to nestin, RC-1 was expressed by ependymal cells beginning at 7 dpi and these cells did not coexpress GFAP (Figs. 5G and H). BrdU pulse labeling at 7 dpi revealed that many RC-1+/GFAP− ependymal cells were dividing (Figs. 5I and J). Ependymal cell up-regulation of RC-1 was transient and by 28 dpi, RC-1 expression was limited to reactive astrocytes in the perimeter of the lesion (Figs. 5K–N).
Fig. 5. RC-1 up-regulation by reactive astrocytes and ependymal cells.
(A) RC-1, a marker for radial glia, is up-regulated by cells surrounding EB lesions. In (A–F), the inset shows the entire region of the VLF and the inner box illustrates the areas shown at higher magnification. (B and C) GFAP double labeling reveals that RC-1+ cells are GFAP+-reactive astrocytes. (D– F) RC-1 up-regulation by reactive astrocytes around the lesion is maintained at 28 dpi. (G) RC-1 is also up-regulated by ependymal cells surrounding the central canal at 7 dpi. (H) GFAP is expressed by subependymal astrocytes, but not ependymal cells. (I) Twenty-four-hour BrdU pulse labeling at 7 dpi reveals that many ependymal cells are dividing at this time. (J) Merged image of (G– I). (K) At 28 dpi, ependymal cells no longer express RC-1. (L) GFAP expression by subependymal astrocytes at 28 dpi. (M) Twenty-four-hour BrdU pulse labeling at 28 dpi shows that ependymal cells are no longer dividing at this time. (N) Merged image (K– M). Scale bar = 30 µm in (A– F); 25 µm in (G–N).
Because the up-regulation of the NSC/radial glial cell antigens nestin and RC1 after injury may signify a stem cell response, the NSC identity of these cells was investigated by examining the expression of Sox1 and Sox2, transcription factors that function to maintain neural progenitor identity (Aubert et al., 2003; Cai et al., 2002; Graham et al., 2003). Neither Sox1 nor Sox2 expression was observed (data not shown). The specificity of the antibodies was tested by examining hippocampal sections where Sox-2+ and Sox-1+ cells are present in the adult (Ellis et al., 2004; and data not shown).
Remyelinating oligodendrocytes are restricted to the perimeter of the lesion
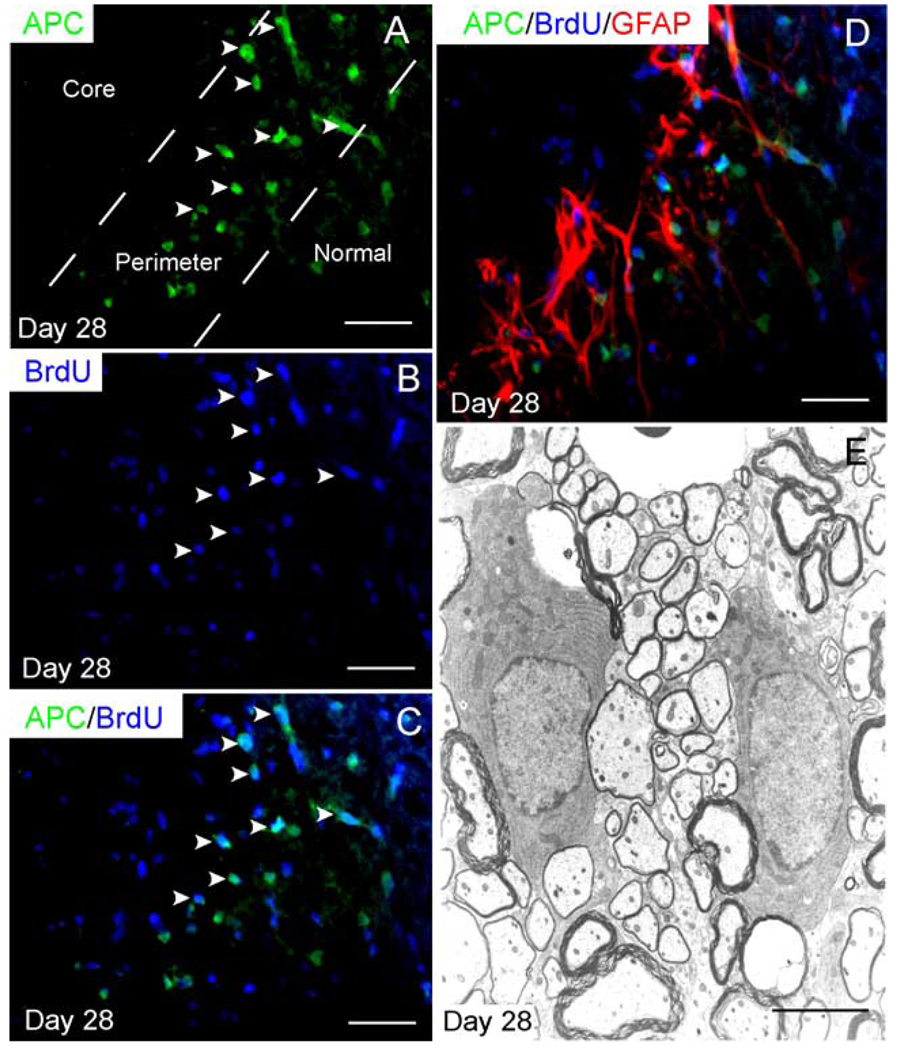
Both immunohistochemistry and electron microscopy were utilized to determine the extent of oligodendrocyte remyelination 28 dpi. Immunohistochemistry for APC, a marker for mature oligodendrocytes, revealed that the central, astrocyte-free portions of the lesion were devoid of mature oligodendrocytes after 28 days. APC+ oligodendrocytes were observed in the perimeter of the lesion (excluding the pia border) and appeared to be more densely distributed than in adjacent normal white matter (Fig. 6A). This result is consistent with previous studies demonstrating increased oligodendrocyte densities in remyelinating regions (Blakemore and Keirstead, 1999). To determine if the APC+ oligodendrocytes observed in the perimeter of the lesion were derived from cells dividing on Day 2, we pulsed animals with BrdU for a 24-h interval at 2 dpi and then allowed the animals to survive to 28 dpi. Many APC+ oligodendrocytes within the perimeter of the lesion coexpressed BrdU in these animals. Because APC+ oligodendrocytes do not incorporate BrdU when pulsed on Day 2 and sacrificed immediately thereafter (data not shown), BrdU+ oligodendrocytes observed in animals pulsed on Day 2 and sacrificed on Day 28 must be derived from undifferentiated precursor cells proliferating on Day 2 (Figs. 6A–C). Moreover, remyelinating oligodendrocytes were closely associated with reactive astrocytes in the perimeter of the lesion (Fig. 6D). Ultrastructural analysis confirmed that the astrocyte-free region of the lesion was devoid of CNS-type myelin. CNS-type remyelination was confined to the perimeter of the lesion, confirming that APC+-positive oligodendrocytes observed in this region were indeed synthesizing new myelin (Fig. 6F).
Fig. 6. Remyelinating oligodendrocytes are restricted to the perimeter of the lesion.
(A) APC+ oligodendrocytes are present in the perimeter of the lesion after 28 days, but not in the central region. Twenty-four-hour pulse labeling with BrdU at 2 dpi in animals, which were sacrificed at 28 dpi, reveals that (A–C) many APC+ oligodendrocytes in the perimeter of the lesion are BrdU+. (D) Triple label immunohistochemistry reveals that new oligodendrocytes are closely associated with the band of reactive astrocytes in the perimeter of the lesion. (E) Ultrastructural analysis reveals that oligodendrocytes in the perimeter of the lesion produce thin myelin, characteristic for CNS-type remyelination; these may be distinguished from the thicker, spared myelin. Scale bar = 25 µm in (A– C); 28 µm in (D); and 6 µm in (E).
Schwann cell remyelination predominates in astrocyte-free regions
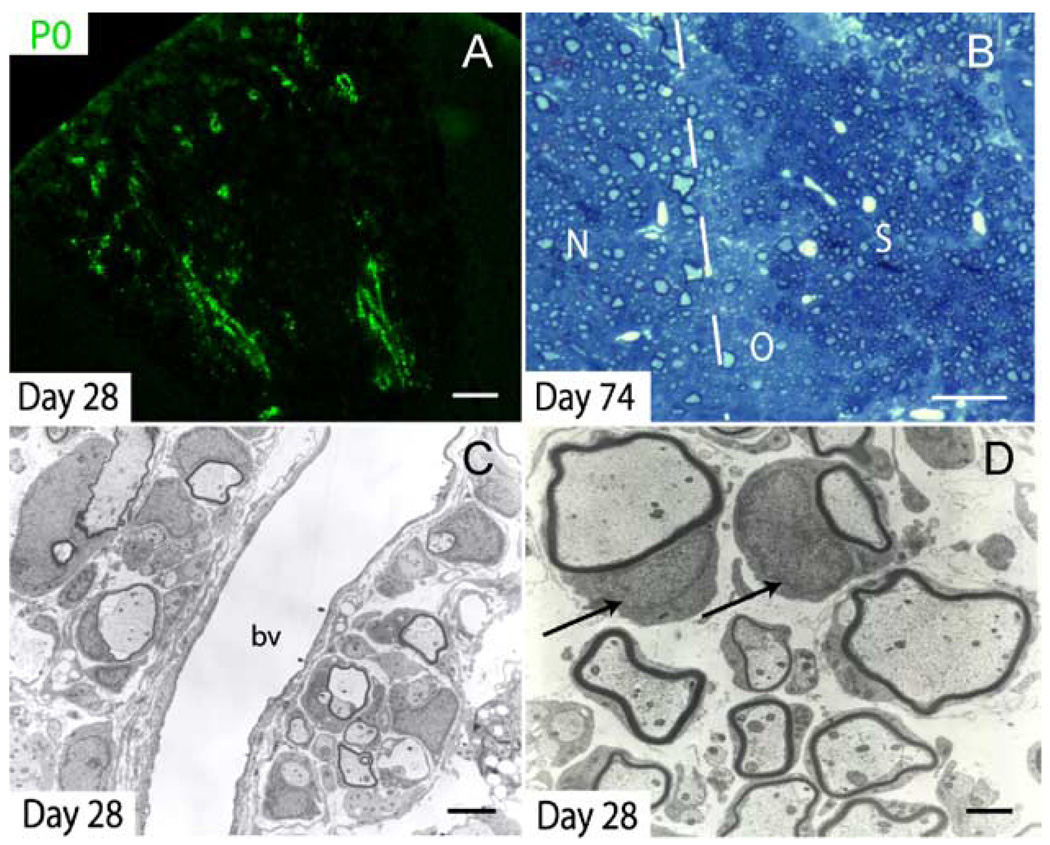
P0 immunohistochemistry revealed that by 28 dpi, a large number of axons within the lesion were remyelinated by Schwann cells in a distinct perivascular pattern (Fig. 7A). Ultrastructural analysis confirmed the presence of Schwann cell myelin, especially around perivascular axons (Figs. 7C and D). In more chronic lesions (74 days), toluidine blue-stained plastic sections demonstrated extensive Schwann cell remyelination in the central portions of the lesion (Fig. 7B). Schwann cell myelin sheaths are identified by the presence of a nucleus adjacent to the myelin (Fig. 7D).
Fig. 7. Schwann cell remyelination in the lesion core.
(A) P0 immunohistochemistry demonstrates considerable Schwann cell-mediated remyelination as early as 28 dpi. Schwann cell remyelination begins in a distinct perivascular pattern. (B) After 74 days, Schwann cell (S) remyelination is extensive in the central portion of the lesion while oligodendrocyte (O) remyelination is limited to the perimeter of the lesion, adjacent to normal (N), uninjured white matter. In toluidine blue-stained plastic sections, Schwann cell myelin appears darker than CNS myelin. (C) Ultrastructural analysis confirmed the presence of characteristic Schwann cell remyelination in the central core of the VLF at 28 dpi; bv = blood vessel. (D) Higher magnification of remyelinating Schwann cells in the center of Day 28 lesions. Schwann cell nuclei are indicated by arrows. Scale bar = 90 µm in (A); 30 µm in (B); 3 µm in (C); and 1 µm in (D).
Endogenous remyelination fails to restore sustained electrophysiologic function
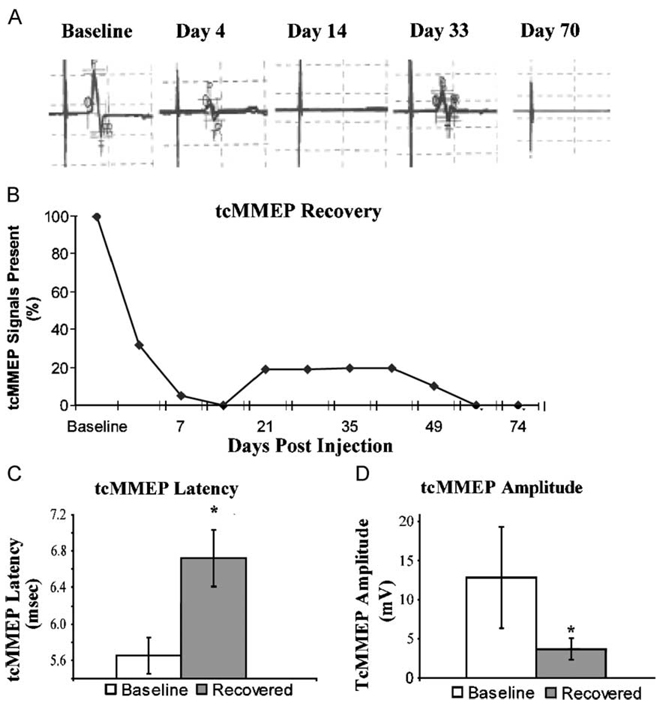
To assess recovery following demyelination, tcMMEP responses were utilized as an in vivo functional measurement of descending axonal conduction in the VLF (Loy et al., 2002a,b). Left and right tcMMEPs were analyzed independently; thus, two signals were counted for each animal. Bilateral baseline tcMMEPs were consistently observed in all animals with an average latency of 5.8 ± 0.1 ms (Fig. 8C) and an average amplitude of 12.6 ± 3.6 mV (Fig. 8D). The functional consequence of EB injection was apparent at 7 dpi at which point 95% of the initial tcMMEP signals analyzed were abolished. Complete loss of all tcMMEP signals at 14 dpi indicated that functional demyelination was complete at this time point. In 3/10 of the lesions analyzed from five animals surviving to Day 74, functional recovery was observed as evidenced by a return of tcMMEP signals. Partial tcMMEP recovery is first observed at 21 dpi, which correlates in time with the appearance of significant endogenous Schwann cell remyelination in the central portions of the lesion (data not shown). However, the recovery was less than optimal. Further analysis of recovered tcMMEP signals revealed that, on average, they were significantly lower in amplitude (3.9 ± 1.4 mV; Fig. 8C) and had significantly increased latencies (6.6 ± 0.2 ms; Fig. 8D) compared to baseline controls. This limited functional recovery did not improve with time and indeed, by Day 56, no tcMMEP signals were detected.
Fig. 8. Endogenous remyelination fails to restore sustained electrophysiologic function.
(A) A representative sample of tcMMEP responses from an animal demonstrating transient electrophysiologic functional recovery. (B) By 14 dpi, 100% of tcMMEP signals were abolished (n = 22). Electrophysiologic function returned in some lesions as evidenced by a return of tcMMEP signals. (C) Recovered tcMMEP signals demonstrated significantly increased latencies (P < 0.001, t = 10.7, df = 6) and significantly decreased amplitudes (P < 0.01, t = 4.1, df = 6) compared to baseline controls.
Discussion
Nkx2.2+/Olig2+ OPC are mobilized within EB lesions
Recruitment of OPCs is a critical first step in remyelination. Here, robust mobilization of polydendrocytes within EB lesions was observed. These results are consistent with previous studies that have similarly demonstrated survival and proliferation of polydendrocytes within EB lesions in the brainstem (Levine and Reynolds, 1999; Penderis et al., 2003a). Despite local proliferation of polydendrocytes, present results do not exclude the possible contribution of migration from surrounding regions. Nevertheless, increased polydendrocyte density within EB lesions up to 28 dpi suggests that recruitment of polydendrocytes within EB lesions is not impaired.
Although the importance of Nkx2.2 and Olig2 for regulating oligodendrocyte specification and maturation in the embryonic spinal cord has been demonstrated (Fu et al., 2002; Qi et al., 2001; Sun et al., 2001; Zhou et al., 2001), their coexpression during remyelination in the adult was unknown. Watanabe et al. (2004) recently showed that Nkx2.2 is up-regulated by differentiating OPCs following lysolecithin-induced demyelination, suggesting a recapitulatory role for this protein in the adult. In the uninjured adult spinal cord, Nkx2.2 recognizes a heterogeneous population of cells, approximately 1/3 of which are NG2+ polydendrocytes (Fig. 3G). At 2 dpi, Nkx2.2 expression was absent in regions of EB injection despite a significant increase in polydendrocyte density. The lack of Nkx2.2 expression by reactive polydendrocytes within the EB injection site may suggest that Nkx2.2+/NG2+ polydendrocytes are susceptible to EB toxicity. Alternatively, the lack of Nkx2.2+ polydendrocytes within EB lesions at 2 dpi could reflect Nkx2.2 down-regulation among NG2+/Nkx2.2+ polydendrocytes concomitant with ‘dedifferentiation’ of resident OPCs in response to local signals. This possibility is consistent with the highly proliferative capacity and less complex morphology of polydendrocytes within the lesion at 2 dpi (Figs. 2B and F). In vitro studies have demonstrated a similar reversion by OPCs in response to specific growth factors (Shi et al., 1998; Wolswijk and Noble, 1992). Therefore, in the adult, NG2+/Nkx2.2+ cells may represent a transitional phenotype between immature NG2+/Nkx2.2− OPCs and mature oligodendrocytes. Consistent with this possibility, Liu et al. (2002) noted that NG2 expression is coincident with PDGFRα expression and precedes the acquisition of Nkx2.2 expression. Importantly, Nkx2.2+ cell density within the lesion is above control levels by 7 dpi. However, this increase did not reproduce the pattern of Nkx2.2 expression observed in normal white matter in regards to coexpression with NG2. Whereas 33% of Nkx2.2+ cells coexpress NG2 in normal white matter, 90% of Nkx2.2+ cells within the lesion coexpress NG2 at 7 dpi (Fig. 3G). Thus, among the Nkx2.2+ cell population, there is a selective increase in the NG2+ subpopulation at 7 dpi. Watanabe et al. (2004) similarly observed a transient up-regulation of Nkx2.2 in NG2+ cells as they differentiated in a demyelinating/remyelinating lesion in the rat spinal cord and demonstrated Nkx2.2 expression in a subpopulation of both OPCs and mature oligodendrocytes. Collectively, these data are consistent with the hypothesis that NG2+/Nkx2.2+ cells represent a transitional phenotype between NG2+/Nkx2.2− OPCs and mature oligodendrocytes, as this phenotype is significantly up-regulated when OPCs begin differentiating.
Among the polydendrocyte population at 7 dpi, at least half are OPCs as evidenced by Nkx2.2 expression. The identity of the remaining NG2+ cells remains unknown. Recent studies demonstrated NG2 expression by blood macrophages in contusion (Jones and Tuszynski, 2002; McTigue et al., 2001) and kainic acid-induced (Bu et al., 2001) lesions. However, consistent with previous studies examining NG2 up-regulation in demyelinating lesions (Di Bello et al., 1999; Levine and Reynolds, 1999; Nishiyama et al., 1997), no overlap between NG2+ cells and activated macrophages was observed. Thus, remaining NG2+/Nkx2.2− cells are likely Nkx2.2− OPCs, synantocytes (Butt et al., 2002), or nonmyelinating Schwann cells invading the lesion (Jones et al., 2003).
Further, supporting the oligodendrocyte lineage of NG2+/Nkx2.2+ polydendrocytes was the complete coexpression of Nkx2.2 with Olig2. Embryonic coexpression of Nkx2.2 and Olig2 in the CNS is specific for OPCs. Since Nkx2.2 expression is up-regulated in differentiating OPCs (Fu et al., 2002), the up-regulation of Nkx2.2 in Olig2+ OPCs present throughout the lesion demonstrates that these cells have initiated differentiation towards mature myelinating oligodendrocytes.
OPC remyelination failure in the absence of astrocytes
A failure in OPC recruitment is not responsible for the limited oligodendrocyte-mediated remyelination observed. Whereas previous studies have similarly demonstrated poly-dendrocyte recruitment following EB lesions in the brainstem (Levine and Reynolds, 1999; Penderis et al., 2003b), we further show that a significant population of Nkx2.2+/Olig2+ OPCs are mobilized within astrocyte-free regions, which ultimately remain demyelinated or are remyelinated by Schwann cells after 1 month. This is surprising as coexpression of Nkx2.2 and Olig2 by OPCs in the embryo is tightly associated with oligodendrocyte differentiation (Qi et al., 2001; Zhou et al., 2001). Ultrastructural evidence of spared, demyelinated axons coupled with eventual Schwann cell remyelination in astrocyte-free regions indicates that a lack of viable axons cannot explain the observed OPC remyelination failure. It is unlikely that Schwann cells outcompete OPCs for axons to remyelinate, as Schwann cell remyelination occurs over several months, providing ample opportunity for OPC-mediated remyelination. Together, these results suggest that although Olig2+ OPCs initiate differentiation by up-regulating Nkx2.2, these two transcription factors are not sufficient to induce terminal differentiation and remyelination in astrocyte-free regions.
There are two possible explanations for oligodendrocyte remyelination failure. The astrocyte-free regions of the lesion (i) contain inhibitory signals that prevent terminal differentiation of OPCs or (ii) lack appropriate signals necessary for OPCs to undergo terminal differentiation. The relationship between astrocytes, Schwann cells, and oligodendrocytes within demyelinating lesions has been extensively examined by Blakemore and Colleagues (Blakemore, 1992; Blakemore and Crang, 1988, 1989; Blakemore et al., 1977; Franklin et al., 1992; Shields et al., 2000). Their studies suggest that astrocytes inhibit Schwann cell infiltration and possibly promote oligodendrocyte remyelination. Why Nkx2.2+/Olig2+ OPCs fail to remyelinate viable, denuded axons in the absence of astrocytes remains unresolved. The localization of remyelinating oligodendrocytes to the zone of reactive astrocytes is consistent with previous studies in support of a promyelinating role for reactive astrocytes (Blakemore and Crang, 1989; Franklin et al., 1991; Jasmin and Ohara, 2002). However, Blakemore et al. (2003) recently demonstrated that the transplantation of nonmitotic, resting astrocytes into EB lesions inhibits oligodendrocyte remyelination by transplanted OPCs, thereby emphasizing that the activation state of astrocytes likely determines their permissive or inhibitory influence. Activated astrocytes express growth factors known to promote oligodendrocyte proliferation and differentiation including PDGF, IGF-1, neuregulin, and CNTF. Woodruff et al. (2004) recently demonstrated that overexpression of PDGF, an astrocyte-derived growth factor known to be mitogenic for OPCs, enhances OPC recruitment but does not increase the extent or efficiency of remyelination following lysolecithin-induced demyelination in the mouse. Therefore, because OPCs are successfully recruited, a shortage of PDGF within the central portions of EB lesions does not explain oligodendrocyte remyelination failure observed in the present study. Mason et al. (2001) showed that microglia- or macrophage-derived IL-1β induces astrocytes to secrete IGF-1, which is crucial for successful remyelination of cuprizone-induced demyelinating lesions by endogenous progenitors in mice. Thus, macrophages, which are abundant throughout EB lesions, in coordination with reactive astrocytes, possibly contribute to promoting OPC differentiation in the perimeter of EB lesions. Further studies, however, are needed to confirm this conclusion. Alternatively, inhibitory signals within the lesion may impair oligodendrocyte differentiation. The intense NG2 expression within EB lesions up to 28 dpi coupled with recent data demonstrating that NG2 impairs oligodendrocyte differentiation (Larsen et al., 2003) points to NG2 as a candidate inhibitory signal.
Radial glia antigen expression by reactive astrocytes and ependymal cells
The close relationship between radial glia and NSCs coupled with the NSC potential of late embryonic radial glia has led investigators to propose that adult NSCs may indeed be radial glia (Tramontin et al., 2003;Weissman et al., 2003). Recent data demonstrated that a subpopulation of astrocytes reexpress the radial glial antigen 3CB2 following spinal cord injury (SCI) (Shibuya et al., 2003). These authors hypothesized that such cells may play an active role in repair. We likewise observed a radial glia response, as evidenced by the up-regulation of RC-1 in reactive astrocytes surrounding EB lesions. Although RC-1 and nestin double labeling were not performed because the antibodies are of identical species and isotype, examination of adjacent sections reveals that both markers appear to identify the same population of reactive astrocytes surrounding the lesion. The reexpression of embryonic antigens by reactive astrocytes occurs in numerous injury models and may represent an attempt at astrocytic dedifferentiation (Lin et al., 1995; Shibuya et al., 2002). A lack of cell division by RC-1-positive astrocytes around lesions at 7, 14, and 28 dpi in the present study, however, indicates that they do not reenter the cell cycle. Thus, dedifferentiation appears to be incomplete.
RC-1 was up-regulated by ependymal cells, many of which were dividing at 7 dpi. Previous studies have suggested that ependymal cells in the adult spinal cord are NSCs that may be mobilized following injury (Calza et al., 2002; Oumesmar et al., 1995). Although ependymal cell migration was not addressed in the present study, the simultaneous up-regulation of RC-1 in ependymal cells and reactive perilesional astrocytes suggests that RC-1+ ependymal cells do not give rise to all perilesional RC-1+ astrocytes. Evidence that both ependymal and subependymal cells contribute to the recruitment of reactive astrocytes around clip compression (Namiki and Tator, 1999) and laceration (Frisen et al., 1995) SCI exists; therefore, a portion of perilesional RC-1+ astrocytes observed in the present study may be derived from ependymal cells. The lack of Sox1 and Sox2 expression by endogenous cells, however, further indicates that RC-1 does not recognize a population of NSCs and that NSC mobilization following EB-induced demyelination does not occur. Similarly, Han et al. (2004) demonstrated up-regulation of glial precursor antigens with no equivalent increase in neuronal precursor markers following a laceration SCI, suggesting that the endogenous precursor response to multiple injury types is predominated by glial-restricted progenitors.
Endogenous remyelination elicits limited and unsustained functional recovery
tcMMEP responses define the conduction capacity of descending myelinated fibers in the VLF (Loy et al., 2002a,b). Present data are the first demonstration of in vivo electrophysiological recovery. Jeffery et al. (1999) similarly demonstrated recovery in locomotor ability following spontaneous remyelination of EB lesions in the rat dorsal funiculus. Importantly, neither locomotor or electrophysiological recovery occurs when doses of irradiation sufficient to prevent spontaneous remyelination are delivered to thoracic spinal cord (Jeffery et al., 1999; Loy et al., 2002b). These data suggest that the observed recovery results from remyelination rather than reorganization of propriospinal pathways caudal to the lesion. Prolonged latencies, diminished amplitudes, and the transient nature of these responses observed over time in the present study, however, indicates a more restricted capacity for long-term functional recovery following endogenous remyelination attempts in the VLF compared to the dorsal funiculus (Jeffery and Blakemore, 1997).
Development of remyelinating therapies will require identification of cells capable of differentiating into mature, myelinating oligodendrocytes in the restrictive environment of the lesioned adult CNS. Importantly, the present study demonstrates that Nkx2.2+/Olig2+ OPCs are unable to remyelinate the VLF in the absence of astrocytes. Identification of putative factors and/or cell interactions responsible for remyelination success or failure is critical to facilitating both endogenous remyelination and potential myelinating cell engraftment approaches for spinal cord repair.
Acknowledgments
This research was supported by NS38665 (SRW and MBB), RR15576, the Kentucky Spinal Cord and Head Injury Research Trust, the Commonwealth of Kentucky Research Challenge Trust Fund (SRW), and the Buoniconti Fund (MBB). We thank Margaret L. Bates for preparation of plastic embedded tissue and the results obtained from studying this tissue. We also thank Cathie Caple for her excellent technical assistance with electron microscopy and Darlene Burke for her assistance with statistical analyses. The hybridomas for nestin developed by S. Hockfield, RC1 developed by A. Alvarez-Buylla, and Nkx2.2 developed by T. Jessell were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Abbreviations
- OPC
oligodendrocyte precursor cell
- NSC
neural stem cell
- tcMMEP
transcranial magnetic motor-evoked potential
- APC
adenomatous polyposis coli (mature oligodendrocyte marker)
- EB
ethidium bromide
- LFB
luxol fast blue
References
- Aubert J, Stavridis MP, Tweedie S, O’Reilly M, Vierlinger K, Li M, Ghazal P, Pratt T, Mason JO, Roy D, Smith A. Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. U. S. A. 2003;100 Suppl. 1:11836–11841. doi: 10.1073/pnas.1734197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J. Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol. Appl. Neurobiol. 1982;8:365–375. doi: 10.1111/j.1365-2990.1982.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Transplanted cultured type-1 astrocytes can be used to reconstitute the glia limitans of the CNS: the structure which prevents Schwann cells from myelinating CNS axons. Neuropathol. Appl. Neurobiol. 1992;18:460–466. doi: 10.1111/j.1365-2990.1992.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. Extensive oligodendrocyte remyelination following injection of cultured central nervous system cells into demyelinating lesions in adult central nervous system. Dev. Neurosci. 1988;10:1–11. doi: 10.1159/000111949. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. The relationship between type-1 astrocytes, Schwann cells and oligodendrocytes following transplantation of glial cell cultures into demyelinating lesions in the adult rat spinal cord. J. Neurocytol. 1989;18:519–528. doi: 10.1007/BF01474547. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Keirstead H. The origin of remyelinating cells in the central nervous system. J. Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Eames RA, Smith KJ, McDonald WI. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J. Neurol. Sci. 1977;33:31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Gilson JM, Crang JA. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Exp. Neurol. 2003;184:955–963. doi: 10.1016/S0014-4886(03)00347-9. [DOI] [PubMed] [Google Scholar]
- Brundin L, Brismar H, Danilov AI, Olsson T, Johansson CB. Neural stem cells: a potential source for remyelination in neuroinflammatory disease. Brain Pathol. 2003;13:322–328. doi: 10.1111/j.1750-3639.2003.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J, Akhtar N, Nishiyama A. Transient expression of the NG2 proteoglycan by a subpopulation of activated macrophages in an excitotoxic hippocampal lesion. Glia. 2001;34:296–310. doi: 10.1002/glia.1063. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J. Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Cai J, Wu Y, Mirua T, Pierce JL, Lucero MT, Albertine KH, Spangrude GJ, Rao MS. Properties of a fetal multipotent neural stem cell (NEP cell) Dev. Biol. 2002;251:221–240. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3258–3263. doi: 10.1073/pnas.052704499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR, Ironside LJ. Identification of the adult resting progenitor cell by autoradiographic tracking of oligodendrocyte precursors in experimental CNS demyelination. Brain. 1998;121(Pt 2):293–302. doi: 10.1093/brain/121.2.293. [DOI] [PubMed] [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J. Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Ellis P, Hayashi S, McMahon A, Anton E, Rao M, Pevny L. SOX-2, a persistent marker for neural stem cells derived from ES cells, the embryo or the adult. Dev. Neurosci. 2004 doi: 10.1159/000082134. (in press) [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Hinks GL. Understanding CNS remyelination: clues from developmental and regeneration biology. J. Neurosci. Res. 1999;58:207–213. [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J. Neurocytol. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Type 1 astrocytes fail to inhibit Schwann cell remyelination of CNS axons in the absence of cells of the O-2A lineage. Dev. Neurosci. 1992;14:85–92. doi: 10.1159/000111651. [DOI] [PubMed] [Google Scholar]
- Frisen J, Johansson CB, Torok C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J. Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W, Qiu M. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 2002;129:681–693. doi: 10.1242/dev.129.3.681. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Ohara PT. Remyelination within the CNS: do Schwann cells pave the way for oligodendrocytes? Neuroscientist. 2002;8:198–203. doi: 10.1177/1073858402008003005. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120(Pt 1):27–37. doi: 10.1093/brain/120.1.27. [DOI] [PubMed] [Google Scholar]
- Jeffery ND, Crang AJ, O’leary MT, Hodge SJ, Blakemore WF. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur. J. Neurosci. 1999;11:1508–1514. doi: 10.1046/j.1460-9568.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J. Neurosci. 2002;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J. Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Lin RC, Matesic DF, Marvin M, McKay RD, Brustle O. Reexpression of the intermediate filament nestin in reactive astrocytes. Neurobiol. Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- Linden RD, Zhang YP, Burke DA, Hunt MA, Harpring JE, Shields CB. Magnetic motor evoked potential monitoring in the rat. J. Neurosurg. 1999;91:205–210. doi: 10.3171/spi.1999.91.2.0205. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z, Rao MS. Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia. 2002;40:25–43. doi: 10.1002/glia.10111. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 2002a;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DS, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol. 2002b;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB. Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 1999;156:191–204. doi: 10.1006/exnr.1999.7016. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1β promotes repair of the CNS. J. Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J. Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki J, Tator CH. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J. Neuropath. Exp. Neurol. 1999;58:489–498. doi: 10.1097/00005072-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yu M, Drazba JA, Tuohy VK. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J. Neurosci. Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J. Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Oumesmar BN, Vignais L, Duhamel-Clerin E, Avellana-Adalid V, Rougon G, Baron-Van Evercooren A. Expression of the highly polysialylated neural cell adhesion molecule during postnatal myelination and following chemically induced demyelination of the adult mouse spinal cord. Eur. J. Neurosci. 1995;7:480–491. doi: 10.1111/j.1460-9568.1995.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Penderis J, Shields SA, Franklin RJ. Impaired remyelination and depletion of oligodendrocyte progenitors does not occur following repeated episodes of focal demyelination in the rat central nervous system. Brain. 2003a;126:1382–1391. doi: 10.1093/brain/awg126. [DOI] [PubMed] [Google Scholar]
- Penderis J, Woodruff RH, Lakatos A, Li WW, Dunning MD, Zhao C, Marchionni M, Franklin RJ. Increasing local levels of neuregulin (glial growth factor-2) by direct infusion into areas of demyelination does not alter remyelination in the rat CNS. Eur. J. Neurosci. 2003b;18:2253–2264. doi: 10.1046/j.1460-9568.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J. Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J. Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J. Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 2002;114:905–916. doi: 10.1016/s0306-4522(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Shibuya S, Miyamoto O, Itano T, Mori S, Norimatsu H. Temporal progressive antigen expression in radial glia after contusive spinal cord injury in adult rats. Glia. 2003;42:172–183. doi: 10.1002/glia.10203. [DOI] [PubMed] [Google Scholar]
- Shields SA, Blakemore WF, Franklin RJ. Schwann cell remyelination is restricted to astrocyte-deficient areas after transplantation into demyelinated adult rat brain. J. Neurosci. Res. 2000;60:571–578. doi: 10.1002/(SICI)1097-4547(20000601)60:5<571::AID-JNR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS. Adult rodent spinal cord derived neural stem cells. Isolation and characterization. Methods Mol. Biol. 2002;198:67–77. doi: 10.1385/1-59259-186-8:067. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp. Neurol. 1997;148:577–586. doi: 10.1006/exnr.1997.6697. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Hinks GL, Franklin RJ. The re-expression of the homeodomain transcription factor Gtx during remyelination of experimentally induced demyelinating lesions in young and old rat brain. Neuroscience. 2000;100:131–139. doi: 10.1016/s0306-4522(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Soula C, Danesin C, Kan P, Grob M, Poncet C, Cochard P. Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development. 2001;128:1369–1379. doi: 10.1242/dev.128.8.1369. [DOI] [PubMed] [Google Scholar]
- Sun T, Echelard Y, Lu R, Yuk DI, Kaing S, Stiles CD, Rowitch DH. Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr. Biol. 2001;11:1413–1420. doi: 10.1016/s0960-9822(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb. Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hadzic T, Nishiyama A. Transient up-regulation of Nkx2.2 expression in oligodendrocyte lineage cells during remyelination. Glia. 2004;46:311–322. doi: 10.1002/glia.20006. [DOI] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb. Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Noble M. Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J. Cell Biol. 1992;118:889–900. doi: 10.1083/jcb.118.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Xu X, Cai J, Fu H, Wu R, Qi Y, Modderman G, Liu R, Qiu M. Selective expression of Nkx-2.2 transcription factor in chicken oligodendrocyte progenitors and implications for the embryonic origin of oligodendrocytes. Mol. Cell. Neurosci. 2000;16:740–753. doi: 10.1006/mcne.2000.0916. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp. Neurol. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]