Abstract
The development of remyelinating strategies designed to enhance recruitment and differentiation of endogenous precursor cells available to a site of demyelination in the adult spinal cord will require a fundamental understanding of the potential for adult spinal cord precursor cells to remyelinate as well as an insight into epigenetic cues that regulate their mobilization and differentiation. The ability of embryonic and postnatal neural precursor cell transplants to remyelinate the adult central nervous system is well documented, while no transplantation studies to date have examined the remyelinating potential of adult spinal-cord-derived oligodendrocyte precursor cells (adult OPCs). In the present study, we demonstrate that, when transplanted subacutely into spinal ethidium bromide/X-irradiated (EB-X) lesions, adult OPCs display a limited capacity for oligodendrocyte remyelination. Interestingly, the glia-free environment of EB lesions promotes engrafted adult OPCs to differentiate primarily into cells with immunophenotypic and ultrastructural characteristics of myelinating Schwann cells (SCs). Astrocytes modulate this potential, as evidenced by the demonstration that SC-like differentiation is blocked when adult OPCs are co-transplanted with astrocytes. We further show that inhibition of bone morphogenetic protein (BMP) signaling through noggin overexpression by engrafted adult OPCs is sufficient to block SC-like differentiation within EB-X lesions. Present data suggest that the macroglial-free environment of acute EB lesions in the ventrolateral funiculus is inhibitory to adult spinal cord-derived OPC differentiation into remyelinating oligodendrocytes, while the presence of BMPs and absence of noggin promotes SC-like differentiation, thereby unmasking a surprising lineage fate for these cells.
Keywords: demyelination, remyelination, noggin, adult oligodendrocyte precursor cell, astrocyte, spinal cord
INTRODUCTION
The source of remyelinating cells in the adult central nervous system (CNS) has been a subject of considerable interest over the past two decades (Blakemore and Keirstead, 1999; Franklin and Hinks, 1999; Imitola et al., 2003). Characterization of the cell type(s) responsible for remyelination in the adult will potentially lead to therapeutic strategies aimed at enhancing remyelination when endogenous attempts fail. Numerous studies using a variety of experimental injury paradigms have identified a wide-spread population of endogenous oligodendrocyte precursor cells (OPCs) as a likely source for new oligodendrocyte myelin in the adult (Carroll et al., 1998; Gensert and Goldman, 1997; Keirstead et al., 1998; Levine and Reynolds, 1999; McTigue et al., 2001; Reynolds et al., 2002; Watanabe et al., 2002; Zhang et al., 1999). Several reports suggest that OPCs endogenous to the CNS remyelinate experimental demyelinating lesions in which astrocytes are spared (Gensert and Goldman, 1997; Watanabe et al., 2002; Woodruff and Franklin, 1999). However, demyelinating injury models of the spinal cord in which astrocytes are also lost are primarily remyelinated by Schwann cells (SCs) and exhibit relatively little oligodendrocyte remyelination, which is usually confined to the perimeter of such lesions (Blakemore, 1975; Talbott et al., 2005; Woodruff and Franklin, 1999). Talbott et al. (2005) recently demonstrated that oligodendrocyte remyelination failure in astrocyte-depleted ethidium bromide (EB) lesions does not result from a failure in OPC recruitment. Indeed, OPCs are recruited to, and partially differentiate within, EB lesions. Nevertheless, SC remyelination eventually predominates. The ultimate fate of recruited OPCs and the source of remyelinating SCs in such lesions remain a matter of speculation.
The source of remyelinating SCs in astrocyte-ablated CNS lesions has been attributed to invasion from peripheral nerve roots and peripherally innervated spinal vasculature concomitant with a breakdown in the glial-limitans (Duncan and Hoffman, 1997; Franklin et al., 1993a; Sims et al., 1998). An alternative hypothesis is that a precursor cell resident of the CNS parenchyma may maintain the potential to give rise to remyelinating SCs. There is now a growing body of literature that suggests that a variety of CNS-derived precursors have the potential to differentiate into SCs under appropriate conditions both in vitro and in vivo (Akiyama et al., 2001; Blakemore et al., 2003; Chandran et al., 2004; Crang et al., 2004; Keirstead et al., 1999; Mujtaba et al., 1998). Thus, in addition to migrating SCs from the periphery, progenitor/stem cells endogenous to the CNS may provide another source for remyelinating SCs (Blakemore, 2005). The presence of increased numbers of OPCs in regions eventually remyelinated by SCs suggests that OPCs may represent a source of remyelinating SCs. To determine the fate of OPCs within astrocyte-free EB lesions in the rat spinal cord, A2B5+/NG2+ spinal OPCs isolated from adult rats genetically engineered to express the human placental alkaline phosphatase protein were transplanted subacutely into EB-induced demyelinating lesions. Present data suggest that the astrocyte-free environment of subacute EB lesions promotes SC-like differentiation of adult OPCs and define a modulatory role for BMPs in mediating this lineage plasticity.
MATERIALS AND METHODS
Isolation and Culture of Cells
Adult OPCs were immunopanned with an A2B5 antibody from the adult spinal cord of Fischer rats expressing human placental alkaline phosphatase (hPAP) (Kisseberth et al., 1999), using a protocol modified from that of Shi et al. (1998). Briefly, the dissected spinal cords were minced into 1-mm3 pieces and incubated in HBSS containing 0.1% papain, 0.1% neutral protease, and 0.01% DNAse for 30 min at 37°C. The digestion was stopped by the addition of an equal volume of DMEM containing 10% fetal bovine serum. Tissues were dissociated by repeated trituration with fire-polished Pasteur pipettes and were filtered through a 70-μm nylon mesh. The cells were then incubated on an anti-RAN-2 antibody-coated dish for 30 min to deplete Type-1 astrocytes and meningeal cells and then transferred to an A2B5-coated dish for 45 min to select for adult OPCs. The purified adult OPCs on the dish were removed with trypsin and cultured in DMEM/F12 medium containing N2 and B27 supplements, FGF2 (20 ng/mL), PDGFaa (10 ng/mL), insulin (5 μg/mL), and BSA (0.1%). In all cases, an aliquot of cells was analyzed the next day to determine the efficiency of the immunopanning. Only those cell preparations in which >95% of the bound cells expressed A2B5 were used in the experiments. The results were confirmed by fluorescence-activated cell sorting (FACS) analysis.
Embryonic A2B5+ glial-restricted precursor (GRP) cells were isolated from hPAP-expressing rats as previously described (Mujtaba et al., 2002; Rao et al., 1998). Briefly, cell suspensions from E13.5 spinal cords were generated, and cells expressing the embryonic neural cell adhesion molecule (E-NCAM) were removed by negative immunopanning with an anti-E-NCAM antibody. GRPs were purified from the remaining suspension by positive selection on immunopanning plates coated with the A2B5 antibody. Adherent cells were removed by scraping and plated on fibronectin/laminin-coated tissue culture plates.
Primary astrocyte cultures were obtained from P2 rat cortices using previously described methods (McCarthy and De Vellis, 1980). Briefly, cortices were dissected from P2 rats and digested with trypsin and DNAse I. Cells were then suspended in 75-cm2 culture flasks and incubated at 37°C in a moist 5% CO2, 95% air atmosphere for 2 days prior to medium change. After 7 days in culture, cells were placed on an orbital shaker at 37°C for 18 h at 250 rpm Flasks were then vigorously shaken by hand and the attached astrocytes were resuspended in fresh medium.
SC cultures were generously provided by Dr. Xiao-Ming Xu and isolated from the sciatic nerve as previously described (Xu et al., 1995).
In Vitro Differentiation Experiments
Adult rat spinal cord-derived OPCs were differentiated for 3 days in serum-free conditions or 10% FBS ± the indicated concentrations of noggin (Table 2). Data are mean ± SD of the percentage of cells that immunostained for O1 or GFAP (glial fibrillary acidic protein) from 3 to 7 independent experiments using adult OPCs at P5, P7, and P9. Data were analyzed by repeated measures ANOVA (for O1, df = 4,29; F = 22.3; P < 0.001 and for GFAP, df = 2,4; F = 26.3; P = 0.005) and Tukey HSD post-hoc t-tests.
TABLE 2. Effects of Noggin on Serum-Induced Differentiation of Adult OPCs.
| Culture conditions | Serum free | 10% FBS | FBS + 50 ng noggin/ml | FBS + 100 ng noggin/ml | FBS + 200 ng noggin/ml |
|---|---|---|---|---|---|
| % O1+ | 56.8 ± 8.9 | 19.9 ± 7.1*** | 25.1 ± 4.6 | 36.3 ± 11.6## | 38.5 ± 4.4@@ |
| % GFAP+ | 0 | 24.6 ± 6.3*** | 7.4 ± 0.4* | 3.7 ± 0.6* | 3.5 ± 1.0* |
Adult rat spinal cord derived OPCs were differentiated for 3 days in serum-free conditions or the 10% FBS ± the indicated concentrations of noggin. Data are mean ± SD of the percentage of cells that immunostained for O1 or GFAP from 3–7 independent experiments using adult OPCs at P5, P7, and P9. Data were analyzed by repeated measures ANOVA (for O1, df = 4,29; F = 22.3; P < 0.001. For GFAP, df = 2,4; F = 26.3; P = 0.005) and Tukey HSD post-hoc t-tests. Significant differences are as follows:
P < 0.001 when compared with serum-free
P < 0.01 when compared with FBS alone
P < 0.005 when compared with FBS alone
P < 0.05 when compared with FBS alone.
Animal Surgery, X-Irradiation, and Cell Transplantation
A total of 39 female Fischer 344 rats (160–200 g) were used throughout the study. Animals were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal, i.p.). Surgical interventions and perioperative care were provided in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Louisville Institutional Animal Care and Use Committee.
For ethidium bromide (EB) lesions, following induction of anesthesia, dorsal laminectomies were performed at the T9/T10 vertebral level and the dura was opened transversely at two sites 2 mm apart. Unilateral injections (0.75 μL/injection) of EB (0.1 mg/mL) were delivered at stereotactic coordinates for the ventrolateral funiculus (VLF) (0.7 mm lateral to midline and depths of 1.3 and 1.6 mm) of the thoracic spinal cord using custom pulled and beveled micropipettes attached to a Parker picospritzer (Loy et al., 2002).
In some animals receiving EB injections, X-irradiation was applied 3 days after induction of EB lesions (EB-X) to block endogenous remyelination as previously described (Loy et al., 2002). Briefly, 40 Gy photon irradiation was delivered to the spinal cord using two Elekta SL-25 linear accelerators delivering 6 MV lateral opposed photon fields at a 100 cm source to axis distance. Radiation exposure was planned as previously described (Loy et al., 2002). Briefly, irradiation was focused along the thoracic spinal cord using collimators designed to expose the entire thoracic spinal cord while protecting internal organs, thus significantly reducing morbidity and mortality. The linear accelerators are housed at the James Graham Brown Cancer Center (University of Louisville). After irradiation, animals were given gentamycin (0.03 mg/kg, i.m.) and a 10 cc subcutaneous bolus of lactated Ringer’s solution, and their cages were placed on a 37°C heating pad overnight.
All cell transplants were performed 4 days after EB lesion induction. Following induction of anesthesia, T9/T10 laminectomy sites were re-exposed. For all single cell type transplants, a total of 4 μL (5 × 104 cells/μL) of cells was injected into the stereotactic coordinates initially used for creation of EB lesions (see earlier). In some animals, prior to engraftment, adult OPCs were infected with the LZRS retrovirus expressing both noggin (generous gift of Dr. R.M. Harland, University of California, Berkeley) and enhanced green fluorescent protein (Cao et al., 2002). For astrocyte/adult OPC co-transplants, a total concentration of 1 × 105 cells/μL (5 × 104 astrocytes + 5 × 104 adult OPCs) was used to ensure that the number of engrafted adult OPCs was comparable to other groups. As shown in Table 1, a total of seven groups received cell grafts as follows: Group 1, EB + adult OPCs (n = 3); Group 2, EB-X + adult OPCs (n = 9, 4 for immunohistochemistry, 3 for ultrastructure, and 2 for immunoelectron microscopy); Group 3, EB-X + adult OPCs + astrocytes (n = 5); Group 4, EB + embryonic GRPs (n = 4); Group 5, EB-X + embryonic GRPs (n = 4); Group 6, EB + noggin-adult OPCs (n = 2); Group 7, EB-X + noggin-adult OPCs (n = 4). In addition, one EB-X group (n = 4) and one EB group (n = 4) did not receive grafts and served as nontransplanted controls. Adult OPCs were transplanted after ~6–8 weeks in culture, thereby providing a sufficient amount of time to expand the cells for transplantation.
TABLE 1. Summary of Results of Transplantation Experimentsa.
| Remyelination phenotype |
|||||
|---|---|---|---|---|---|
| Graft | Lesion | Schwann cell | Oligodendrocyte | ||
| Center | Edge | Center | Edge | ||
| Adult OPCs | EB | +++ | − | − | + |
| EB-X | +++ | − | − | + | |
| Adult OPCs | EB-X | − | − | − | + |
| + astrocytes | |||||
| Embryonic | EB | − | − | − | − |
| glial-restricted | EB-X | − | − | − | − |
| precursors | |||||
| Noggin-transduced | EB | − | − | − | + |
| adult OPCs | EB-X | − | − | − | + |
For each experimental group.
indicates extensive
occasional
not observed.
Tissue Preparation
Animals from all groups were processed similarly. Four weeks after cell transplantation, animals were deeply anesthetized with sodium pentobarbitol (100 mg/kg) and transcardially perfused with 100 mL of 0.1 M PBS, followed by 300 mL of 4% paraformaldehyde in 0.1 M PBS. Spinal cords were dissected and postfixed overnight at 4°C, then transferred to 30% sucrose at 4°C for cryoprotection (48 h). Specimens were cut transversely at the epicenter of the lesion and mounted with the cut surfaces facing down in TBS tissue freezing medium (Triangle Biomedical Sciences, Durham, NC).
Immunohistochemistry of Cells and Tissue Sections
After tissue embedding, 20-μm-thick transverse sections were cut on a Leica CM3050 cryostat and mounted on microscope slides. Prior to incubation with primary antibodies, sections were permeabilized and blocked with 0.3% Triton X-100/10% normal donkey serum in 0.1 M TBS (7.4) for 30 min. Primary antibodies were then applied for 48 h at 4°C. The following primary antibodies were used: rabbit (1:200; Accurate, Westbury, NY) and mouse (1:200, Sigma) anti-hPAP for engrafted cells, mouse anti-A2B5 for glial precursors (1:2; ATCC), rabbit anti-NG2 for OPCs (1:200; Chemicon, Temecula, CA), mouse anti-neurofilament M (NF-M) for axons (1:200; Chemicon), mouse anti-p75NTR for SCs and their precursors (1:200; Chemicon), rabbit anti-GFAP for astrocytes (1:200; Dako), mouse anti-adenomatous polyposis coli (APC) for mature oligodendrocytes (1:100; Oncogene), and mouse anti-P0 for SCs (1:800; Dr. J.J. Archelos). Appropriate secondary antibodies were applied for 90 min at room temperature. The following species-specific secondary antibodies were used: donkey anti-mouse fluoroisothiocyanate (FITC)-conjugated (1:100) and rhodamine-red-conjugated IgG (1:200), donkey anti-rabbit rhodamine-red (1:200)- and FITC-conjugated Fab’ fragments (1:100). All secondary antibodies were supplied by Jackson Immunoresearch Lab (Baltimore, MD). Sections were then rinsed in TBS and cover-slipped with antifade mounting media (Molecular Probes, Eugene, OR). Staining was visualized with a Nikon Eclipse TE300 microscope and photographed with a Spot RT Color CCD camera. Figures were assembled using Adobe Photoshop® and Illustrator® software.
Ultrastructural Analysis
Following anesthetization with sodium pentobarbital (100 mg/kg, i.p.), animals were transcardially perfused, 28 days after EB injection, with 100 mL of 0.1 M phosphate buffer (PB; pH = 7.4), then with 300 mL of 2% paraformaldehyde (PFA) and 2% glutaraldehyde in 0.1 M PB. Tissue slices (1 mm) were cut from the lesion epicenter and immersed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h. Tissues were rinsed in buffer, dehydrated in graded ethanol and propylene oxide, and embedded in Embed812 epoxy resin (Electron Microscopy Sciences (EMS), Fort Washington, PA). Ultrathin sections were prepared and stained with uranyl acetate and lead citrate and examined under a Philips CM10 transmission electron microscope.
For immuno-electron microscopy, animals were perfused with 4% PFA as described earlier. A 1-mm section of tissue was cut from the epicenter and 30-μm sections were cut on a vibratone. Sections were incubated overnight with an antibody to hPAP (1:200, Sigma). A Vectastain Elite ABC antibody kit was used for detection with 0.05% 3,3-diaminobenzidine. Tissue was postfixed in osmium tetroxide, dehydrated, and embedded in EMbed8-12 (EMS). Sections were photographed on a Philip CM10 transmission electron microscope.
Flow Cytometry Analysis
Both adult rat OPCs and SCs of different passages were analyzed on a FACS Calibur (Becton Dickinson, Franklin Lakes, NJ). Briefly, cells were stained live with either directly conjugated or unconjugated titrated antibodies against the surface markers A2B5 (ATCC, Manassas, VA), NG2 (Immunotech, Marseille, France; Chemicon), and p75NTR (Advanced Targeting System, San Diego, CA), followed by sandwich reagents as required, and fixed with 1% PFA. Appropriate isotype controls were performed parallel to determine any unspecific binding.
RT-PCR
For determination of bmp-2, bmp-4, and noggin mRNA expression in EB lesions, four animals were transcardially perfused with cooled (4°C) Svenka’s PBS 5 days after EB injection into the VLF. A 1-cm section of spinal cord centered around the EB injection sites was quickly removed and immediately frozen on dry ice and placed at −80°C. For isolation of RNA, three sections of cord (1 mm thick each) were transversely cut from the epicenter of frozen EB-lesioned specimens using a sterile scalpel blade. Demyelinated lesions were easily visualized in the VLF of 1-mm-thick sections with 10× magnification and a scalpel was used to carefully dissect out the demyelinated region of the VLF. For control tissue, equal-sized sections of contralateral VLF were identically dissected. For dissected VLF tissue, adult OPCs, noggin-transduced adult OPCs, and astrocytes, tissues/cells were homogenized in buffer containing 4 M guanidinium isothiocyanate, 142 mM β-mercaptoethanol, and 5% N-lauroyl-sarcosine. Homogenates were centrifuged for 18 h at 36,000 rpm on a 5.7 M CsCl cushion. Pelleted total RNA was washed and resuspended in RNAse-free TE buffer. Total RNA concentrations were determined spectrophotometrically. One microgram of total RNA was reverse-transcribed into cDNA in reactions containing M-MLV RT (100 U; Promega, Madison, WI), DTT (5 mM), dNTPs (1 mM each), random hexamers (4 mg), and RNAse inhibitor (20 U; Boehringer Mannheim, Mannheim, Germany). PCRs were performed using specific primers for BMPs, noggin, BMP-Rs, and housekeeping genes designed using freeware Oligos-U-Like and purchased from Integrated DNA Technologies (Coralville, IA). Optimum annealing temperatures, cycle numbers, and RT input were empirically determined by amplification of a single PCR product at the appropriate molecular weight for each target cDNA. All RT and PCR reactions were performed using a PTC-200 gradient thermocycler (MJ Research, Watertown, MA) in 10 μL reaction volumes. PCR products were analyzed by native polyacrylamide gel electrophoresis, followed by resolution with SYBR Gold (Molecular Probes, Eugene, OR). The following primers were used at the indicated number of cycles: BMP-2 (27 cycles, sense (S) 5′-CGACGGTAAAGGACATCCAC–3′, antisense (A) 5′-TCCACATACAAAGGGTGCCT–3′), BMP-4 (27 cycles, S 5′-TCAGGGCAGAGCCATGAG–3′, A 5′-TCTGGGATGCTGCTGAGG–3′), noggin (27 cycles, L S′- TGTGGTCACAGACCTTCTGC–3′, A 5′-GTGAGGTGCACAGACTTGGA–3′), BMPR-1A (25 cycles, S 5′-TGACTTTAGCACCAGAGGATA-CCT–3′, A 5′-ACCCCTGCTTGAGATACTCTTACAA–3′), BMPR-1B (25 cycles, S 5′-TTGTCACCTCTGGATGTC-TAG GACT–3′, A 5′-CATAGCGGCCTTTTCCAATCT–3′), BMPR-2 (25 cycles, S 5′-GAATGGTGTGGCAGGTAG-AA–3′, A 5′-CCTGATTTGCCATCTTGTGT–3′), Cyclophillin A (S 5′-GCACTGGGGAGAAAGGATTT–3′, A 5′-ATGCCTTCTTTCACCTTCCC–3′).
RESULTS
Adult OPC Cultures are not Contaminated with SCs
Isolation of adult OPCs from dissociated adult hPAP-expressing rat spinal cords for transplantation was accomplished by immunopanning with the A2B5 antibody for cell capture. In vitro, virtually all immunopurified cells displayed a characteristic OPC morphology with several small processes emanating from a round cell body (Fig. 1A). Cells morphologically resembling SCs were never observed within adult OPC cultures. Immunohistochemical analysis revealed uniform expression of A2B5 by nearly all cells within adult OPC cultures (Fig. 1B). FACS analysis of the immunopanned cells demonstrated homogenous hPAP expression (data not shown) and confirmed their OPC identity with greater than 98% expressing A2B5 and 95% expressing the OPC marker NG2 (Figs. 1C,D). Approximately 90% of immuno-isolated cells also expressed the more mature OPC marker 04 (data not shown). FACS analysis of SC cultures showed that they do not express the OPC markers A2B5 or NG2 (Figs. 1F,G). Both FACS and immunohistochemistry confirmed that isolated adult OPCs (Figs. 1E,I), in contrast to SCs (Figs. 1H,J), do not express the low affinity neurotrophin receptor p75. These data suggest that adult OPC cultures represent a homogenous population that lack detectable SC contamination. Characteristic of the O2-A lineage, A2B5+ adult OPCs do not express GFAP but can be induced to differentiate into astrocytes following exposure to 10% FBS (Table 2). No expression of the early neuronal marker βIII-tubulin was observed by adult OPCs in vitro (data not show).
Fig. 1.
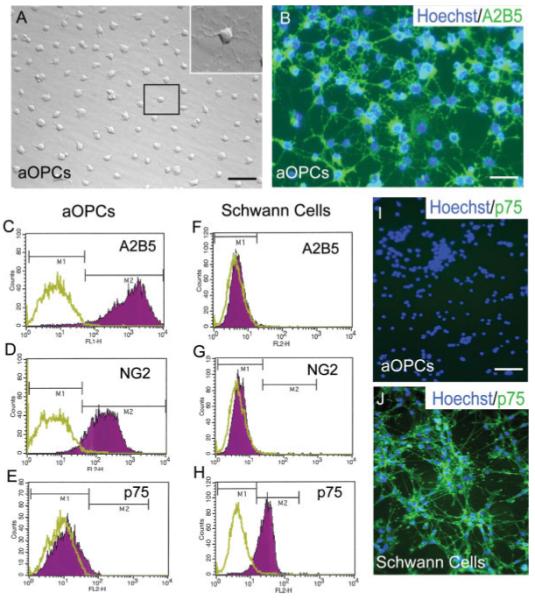
Adult oligodendrocyte precursor cell (adult OPC) cultures are not contaminated with SCs. (A) Phase contrast microscopy shows that adult OPCs exhibit several processes emanating from a small round cell body (inset shows higher magnification view of boxed cell). (B) Nearly all cells express A2B5, as demonstrated by immunohistochemistry. FACS analysis shows uniform expression of the glial precursor markers A2B5 (98%) and NG2 (93%) and an absence of p75 expression by adult OPC cultures (C–E). In contrast to adult OPCs, SCs do not express A2B5 or NG2 but greater than 95% express p75 (F–H). (I) Immunohistochemistry confirms a lack of p75 expression by adult OPCs while SCs demonstrate uniform expression (J). (C–H) The green plot represents unlabeled control cells while the purple plot represents the population of cells labeled with fluorescent antibody. (C–H) Data are representative of three independent experiments. Scale bar = 24 μm (A); 18 μm(B); 28 μm (I, J).
Adult OPCs Transplanted into EB Lesions do not Differentiate Significantly into Astrocytes or Oligodendrocytes
Adult OPCs were subacutely (4 days post injury) transplanted into both EB and X-irradiated (40 Gy) EB (EB-X) lesions of the VLF and the engrafted animals were sacrificed after 35 days. On average, cells were maintained in culture for 6–8 weeks prior to transplantation. Immunohistochemistry for hPAP was used to identify engrafted cells within the host spinal cord. This method for identification of engrafted cells has been previously used and validated (Han et al., 2004; Hill et al., 2004; Mujtaba et al., 2002). Figure 2A demonstrates that cells derived from adult OPCs survive and integrate into the in vivo environment of EB lesions up to 4 weeks (the latest time point examined) following engraftment. Consistent with a differentiated phenotype, engrafted cells no longer expressed the OPC marker NG2 (Fig. 2C). Further, double-label immunohistochemistry for hPAP and GFAP revealed minimal co-localization, indicating that adult OPCs did not differentiate significantly into astrocytes after transplantation (Fig. 2D).
Fig. 2.
Adult OPCs engrafted into ventrolateral funiculus (VLF) ethidium bromide (EB) lesions of the adult rat thoracic spinal cord survive 4 weeks after transplantation and do not differentiate into astrocytes. (A) Nomarsky image of engrafted EB lesions merged with an immunohistochemical stain for the grafted cell marker human placental alkaline phosphatase (hPAP) demonstrates the survival and integration of transplanted cells within VLF EB lesions 4 weeks following transplantation. (B) Higher magnification view of area boxed in (A). hPAP double-labeling reveals that engrafted adult OPCs no longer express the precursor marker NG2 4 weeks after transplantation (C). hPAP double-labeling shows that engrafted adult OPCs do not differentiate significantly into GFAP+ astrocytes 4 weeks after transplantation (D). Insets (C, D) show the entire region of the VLF and the inner box designates the area shown at higher magnification. Scale bar = 250 μm(A); 125 μm (B); 35 μ(C);25 μm(D).
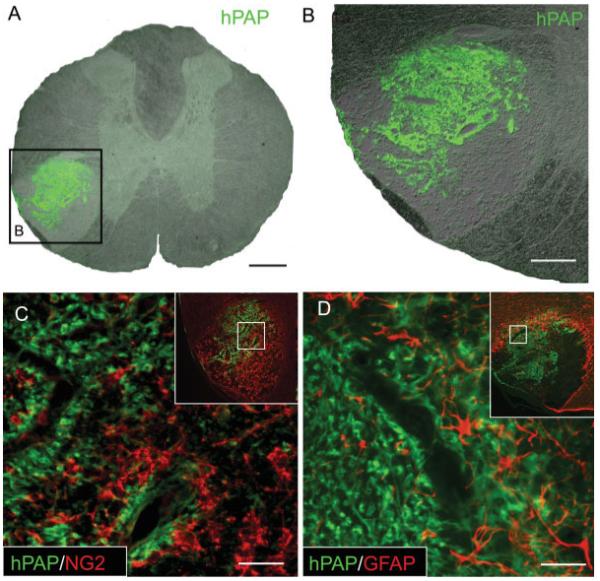
Many engrafted cells exhibited a differentiated phenotype with a characteristic ring-like morphology approximating the shape and size of myelin rings (Fig. 3A). Double-label immunoflourescence for hPAP and the axonal protein NF-M revealed that many hPAP+ rings were indeed ensheathing axons (Fig. 3C). Despite the presence of numerous hPAP+ axon-ensheathing rings within EB-X lesions, CC-1/APC+ oligodendrocytes were largely absent and hPAP co-localization with CC-1 was not observed (Figs. 3D–F) centrally. Histological and ultrastructural analysis confirmed the absence of oligodendrocyte remyelination within central portions of engrafted EB-X lesions. However, occasional APC+/hPAP+ cells were observed in the periphery of lesions (Figs. 3G–I), consistent with previous data (Talbott et al., 2005). As with EB-X lesions, engrafted adult OPCs located within the central, astrocyte-free zone of nonirradiated EB lesions did not express CC-1/APC (data not shown). This suggests that X-irradiation was not responsible for the observed lack of oligodendrocytic differentiation in the center of EB-X lesions.
Fig. 3.
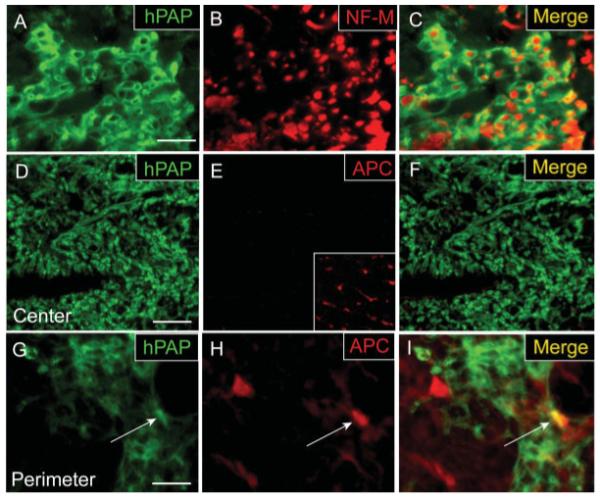
Adult OPCs engrafted into EB-X lesions ensheath axons but do not differentiate into mature oligodendrocytes in the center of lesions. hPAP immunohistochemistry for engrafted cells 4 weeks after transplantation demonstrates that transplanted cells exhibit a characteristic ring-like morphology and many of these rings ensheath NF-M+ axons. (A–C) Despite their myelin-like formations around axons, double-label immunohistochemistry for hPAP and the mature oligodendrocyte marker APC/CC-1 shows that engrafted cells in the center of EB-X lesions do not differentiate into oligodendrocytes (D–F). Inset in (E) shows positive APC/CC-1 staining for oligodendrocytes in the uninjured, contralateral VLF. An occasional hPAP+/APC+ cell (white arrow) was observed in the perimeter of such lesions (G–I). Scale bar = 20 μm (A–C);36 μm in (D–F); 16 μm in (G–I).
Adult OPCs Transplanted into EB Lesions Differentiate into Myelinating Schwann-Like Cells
Control EB-X lesions from nontransplanted animals contained no P0+ SCs (Fig. 4A). In contrast, EB-X lesions from adult OPC engrafted animals demonstrated extensive P0 immunostaining within the lesions (Fig. 4C). Double-label immunohistochemistry for hPAP and P0 revealed extensive co-localization, thereby confirming that P0+ cells within engrafted lesions were indeed transplant-derived (Figs. 4B–G). hPAP+/P0+ transplanted cells were equally observed in both EB and EB-X lesions, indicating that irradiation was not responsible for the observed differentiation.
Fig. 4.
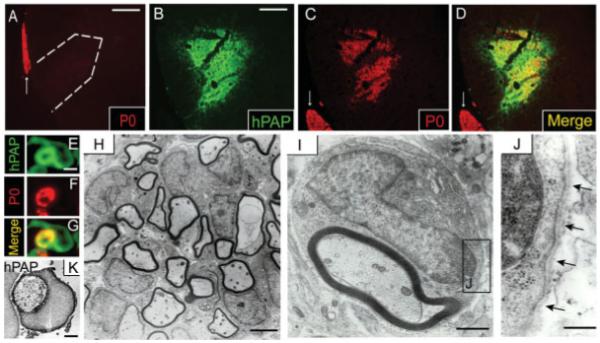
Adult OPCs engrafted into EB-X lesions differentiate into P0-expressing cells ultrastructurally indistinguishable from SCs. The white arrows denote P0+ ventral nerve roots (A,C,D). (A) EB-X lesions do not demonstrate spontaneous SC remyelination 4 weeks postlesion as evidenced by a lack of P0+ cells within lesions. Dashed white line (A) approximates the lesion boundary. Double-label immunohistochemistry shows co-localization between hPAP+ engrafted cells and large patches of P0+ cells throughout VLF EB-X lesions 4 weeks after transplantation (B–D). High magnification double-label confocal microscopy of a single hPAP+ cell within an EB-X lesion reveals a Schwann cell-like morphology with P0 expression in the region approximating a myelin ring (E–G). (H) Electron microscopy confirmed the presence of numerous Schwann-like cells within adult OPC engrafted EB-X lesions. (I) Higher magnification image of a single Schwann-like cell within an engrafted EB-X lesion revealing ultrastructural similarity to a myelinating SC, including a 1:1 cell-to-axon ratio and a basement membrane surrounding the cell membrane (J). Arrows in (J) denote the basement membrane surrounding the Schwann-like cell. (K) Immuno-electron microscopy shows robust plasmalemmal hPAP expression by an engrafted cell ultrastructurally resembling a SC. Scale bar = 120 μm (A); 80 μm (B–D); 3 μm (E–G); 1 μm (K and I); 3 μm (H); 0.3 μm (J).
To better characterize the engrafted cells ultrastructurally, electron microscopy of engrafted EB-X lesions was performed. Numerous Schwann-like cells were present within engrafted EB-X lesions (Fig. 4H). Engrafted cells demonstrated the ultrastructural features that define a myelinating SC, including a 1:1 myelin/axon ratio with a signet ring morphology and a basal lamina surrounding the cells (Figs. 4I,J). EB-X lesions do not spontaneously remyelinate for at least 6 weeks; thus it can be argued that any observed remyelination during that period within EB-X transplanted animals can be attributed to engrafted cells (Akiyama et al., 2001; Chandran et al., 2004; Crang et al., 2004). To further confirm that cells ultrastructurally resembling SCs within engrafted EB-X lesions were transplant-derived, immuno-electron microscopy was performed. Figure 4K shows a Schwann-like cell with robust plasmalemmal staining for hPAP, thus confirming that engrafted adult OPCs differentiate, in vivo, into cells with ultrastructural characteristics of myelinating SCs.
Embryonic GRP Cells Transplanted into EB-X Lesions do not Differentiate into Schwann-Like Cells
To determine whether SC-like differentiation of engrafted cells in EB lesions was a common pathway for other glial-progenitor populations, GRPs from embryonic day 13.5 hPAP expressing rats were similarly transplanted into both irradiated and nonirradiated EB lesions. Prior to transplantation, nearly all GRP cells expressed the glial precursor markers A2B5 and NG2, as determined by FACS (Figs. 5A,B). Four weeks after engraftment, GRPs survive within EB lesions as evidenced by the presence of hPAP+ cells (Fig. 5C). Unlike adult OPCs, however, hPAP+ GRP cells transplanted into EB lesions preferentially differentiate into GFAP+ astrocytes (Figs. 5C–E). Engrafted GRPs did not differentiate into P0+ SCs (Figs. 5F–H) or APC+ oligodendrocytes (data not shown).
Fig. 5.
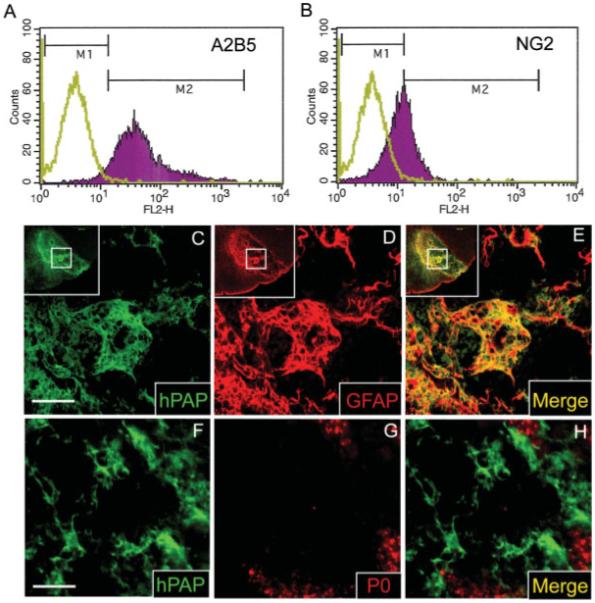
Glial-restricted precursors (GRPs) immunopurified from the E14 rat spinal cord differentiate into astrocytes rather than SCs 4 weeks following transplantation into EB lesions. Embryonic GRPs express the glial precursor antigens A2B5 and NG2 as assessed by FACS (A, B). In (A, B) the green plot represents unlabeled control cells while the purple plot represents the population of cells labeled with fluorescent antibody. Double-label immunohistochemistry for hPAP and GFAP shows that most GRPs differentiate into astrocytes 4 weeks after engraftment into EB lesions of the ventrolateral spinal cord (C–E). (C–E) The insets show the entire region of the VLF and the inner box designates the area shown at higher magnification. Unlike adult OPCs, GRPs do not co-label with the SC myelin protein P0 following engraftment into EB lesions (F–H). As evidenced by the presence of some endogenous SC remyelination (G), this particular animal was not irradiated. Similar results were obtained irrespective of whether or not animals received irradiation, with the exception that endogenous SCs were not observed within EB-X lesions (data not shown). Scale bar = 50 μm in (C–E); 28 μm in (F–H).
Adult OPCs do not Differentiate into P0+ SCs in the Presence of Astrocytes
The EB lesion environment is unique among experimental SCIs in that it is largely devoid of both oligodendrocytes and astrocytes. Previous studies suggest that it is precisely this macroglial-free environment that is responsible for promoting SC-like differentiation of a variety of cell types (Blakemore, 2005). To determine whether the presence of astrocytes alone is sufficient to block SC-like differentiation of adult OPCs in the EB-X lesion, adult OPCs were co-transplanted into EB-X lesions with astrocytes in a 1:1 ratio 4 days after lesion induction. Nontransplanted EB lesions do not contain astroctyes after 4 weeks (Fig. 6A). Four weeks after astrocyte/adult OPC co-transplantion, many GFAP+ astrocytes were present within the lesions (Fig. 6B). Figure 6C demonstrates that in the presence of co-transplanted astrocytes adult OPCs do not differentiate into P0+ SCs. Many adult OPCs engrafted with astrocytes maintained NG2 expression. In the central portion of EB-X lesions, remyelination was absent and adult OPCs engrafted with astrocytes did not express MBP, indicating a lack of mature oligodendrocyte differentiation by engrafted cells (Fig. 6D). Ultrastructural evidence of oligodendrocyte myelin in central portions of EB-X lesions was not observed following adult OPC/astrocyte engraftment (data not shown). MBP and hPAP co-localization was observed in the periphery of engrafted lesions (Fig. 6D). Further, some engrafted cells along the perimeter of EB-X lesions differentiated into cells morphologically resembling mature oligodendrocytes with multiple processes extending to MBP+ myelin rings (Fig. 6E). Immunohistochemical evidence of oligodendrocyte remyelination by engrafted cells in the perimeter of EB-X lesions is supported by ultrastructural evidence of remyelinating oligodendrocytes in this same area (Fig. 6F).
Fig. 6.
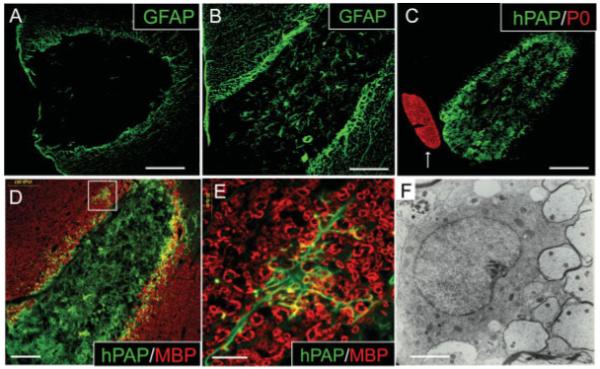
Adult OPCs co-transplanted into EB-X lesions with astrocytes do not differentiate into P0+ SCs. (A) EB is toxic to astrocytes as evidenced by a lack of GFAP+ astrocytes within nontransplanted lesions 4 weeks after EB injection. (B) Many GFAP+ astrocytes are present within astrocyte/adult OPC co-transplanted EB-X lesions. (C) hPAP and P0 double-label immunohistochemistry of astrocyte/adult OPC co-transplanted EB-X lesions reveals a lack of P0+ SC differentiation by hPAP+ adult OPCs. The arrow (C) denotes a P0+ ventral nerve root juxtaposed to the engrafted EB-X lesion. (D) MBP and hPAP co-labeling shows a lack of oligodendrocyte myelin within adult OPC/astrocyte engrafted lesions. Boxed area in (D) is shown at higher magnification in (E). (E) In the perimeter of EB-X lesions occasional adult OPCs appear to differentiate into cells with a mature oligodendrocyte phenotype as evidenced by multiple processes extending out to MBP+ myelin rings. (F) Immunohistochemical evidence of mature oligodendrocyte differentiation by engrafted cells in the perimeter of EB-X lesions is supported by ultrastructural evidence of remyelinating oligodendrocytes in this same area. Thin myelin rings characteristic of remyelinating oligodendrocytes are seen adjacent to a mature oligodendrocyte cell body. Scale bar = 165 μm (A); 83 μm (B); 160 μm (C); 100 μm (D); 20 μm (E); 2 μm (F).
SC-Like Differentiation is BMP-Dependent
RT-PCR analysis of micro-dissected EB-lesioned tissue 5 days after injury revealed that bmp-2 and bmp-4 transcripts are both highly expressed (Fig. 7A). Uninjured white matter from the contralateral VLF similarly expresses both bmp-2 and bmp-4 mRNA. Noggin, a competitive antagonist of BMPs, is present in the uninjured VLF but only weakly detected in the EB-demyelinated VLF, as assessed by RT-PCR of micro-dissected tissue (Fig. 7A). Decreased noggin expression within astrocyte-depleted, EB-lesioned tissue is consistent with previous studies identifying astrocytes as a primary source for noggin in the CNS (Kondo and Raff, 2004b). As shown in Fig. 7B, adult OPCs isolated from the adult spinal cord express bmp receptor Type Ia (bmpr-Ia) and Type II (bmpr-II), but only weakly express bmp receptor Type Ib (bmpr-Ib) mRNA (Fig. 7B). These findings are consistent with those of previous studies examining the bmpr profile of optic nerve OPCs (Kondo and Raff, 2004b). Furthermore, in vitro experiments show that FBS-induced astrocyte differentiation of adult OPCs can be antagonized by noggin, presumably by antagonizing serum-mediated BMP receptor activation (Table 2). The in vitro bioactivity of the Noggin construct used in the present study has been previously demonstrated (Enzmann et al., 2005).
Fig. 7.
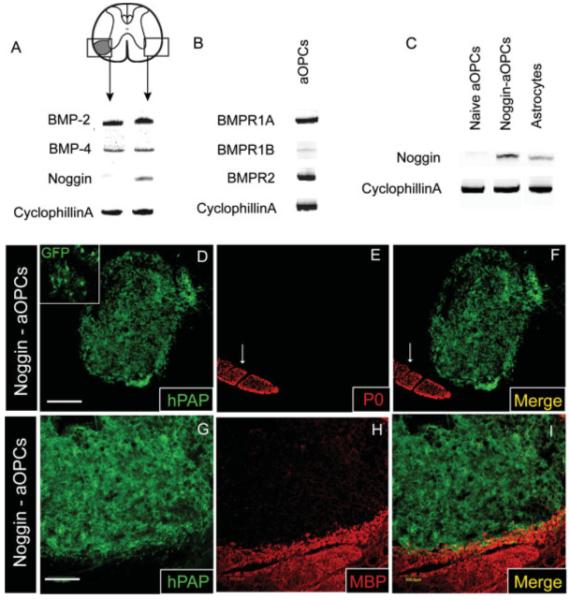
Bmp-2 and bmp-4 transcripts are expressed within EB lesions while noggin mRNA is only weakly expressed. Retroviral transduction of adult OPCs with the BMP antagonist noggin is sufficient to block their differentiation into Schwann-like cells following engraftment into EB lesions. White arrows (E, F) denote P0+ ventral nerve roots. (A) RT-PCR analysis of micro-dissected EB lesioned tissue and contralateral uninjured VLF 6 days after EB injection reveals expression of both bmp-2 and bmp-4 mRNA in both demyelinated and uninjured tissue. Noggin mRNA is clearly present in the uninjured VLF tissue but barely detectable in the demyelinated VLF. (B) Adult OPCs are potentially responsive to BMPs as evidenced by their expression of transcripts for bmpr-Ia and bmpr-II. Adult OPCs only weakly express bmpr-Ib. (C) RT-PCR analysis of astrocytes, naïve adult OPCs, and noggin-transduced adult OPCs reveals expression of noggin mRNA in transduced adult OPCs and astrocytes while naïve adult OPCs only weakly express noggin. (D–F) Adult OPCs transduced with a retrovirus to express green fluorescent protein (GFP) and noggin fail to differentiate into P0+ Schwann-like cells 4 weeks after engraftment. Upper left inset in (D) shows GFP expression by engrafted cells, confirming the presence of noggin-transduced cells within these EB lesions. (G–I) Noggin+ adult OPCs do not differentiate into remyelinating oligodendrocytes as evidenced by a lack of MBP expression within Noggin-adult OPC engrafted EB-X lesions. Scale bar = 250 μm (D–F); 100 μm (G–I).
To examine the role of BMP signaling in mediating SC-like differentiation by adult OPCs in vivo, adult OPCs were transduced to overexpress secreted noggin. RT-PCR confirmed that transduced adult OPCs, as well as astrocytes used for co-grafting experiments (Fig. 6), express noggin mRNA, while naïve adult OPCs do not (Fig. 7C). Unlike naïve adult OPCs (Fig. 4), when noggin-transduced OPCs (Figs. 7D–F) were transplanted into EB and EB-X lesions, differentiation into P0+ SCs was not observed, suggesting that BMPs are at least in part responsible for promoting SC-like differentiation of transplanted adult OPCs in vivo. As with adult OPC/astrocyte grafts, centrally located noggin-expressing adult OPCs did not express the mature oligodendrocyte markers MBP or APC/CC-1 following transplantation into EB-X lesions (Figs. 7G–I). However, occasional transplant-derived APC/CC-1+ oligodendrocytes were observed in the perimeter of such lesions. While some cells maintained NG2 expression, many hPAP+ profiles did not co-localize with APC/CC1, GFAP, NG2, or P0 and therefore their immunophenotype remains indeterminable.
DISCUSSION
In light of the observed SC-like differentiation by adult OPC transplants, it was critical to assess the potential for SC contamination within adult OPC cultures and confirm the transplant origin of remyelinating SCs within engrafted EB lesions. Both FACS and immunohistochemical analysis confirmed the absence of contaminating SCs within adult OPC cultures. The EB-X lesion does not spontaneously remyelinate for at least 6 weeks (Blakemore, 1984). A complete lack of both SC and oligodendrocyte remyelination after 5 weeks in nontransplanted EB-X control animals confirmed the effectiveness of our irradiation protocol. Collectively, these data strongly suggest that the observed Schwann-like cells were derived from engrafted adult OPCs and did not arise from a population of contaminating SCs.
Adult OPC Differentiation in the EB Lesion: The Role of Environmental Signals
Mujtaba et al. (1998) were the first to describe the potential for CNS-derived precursor cells to differentiate along the neural crest lineage. Shortly thereafter, Keirstead et al. (1999) showed that PSA-NCAM+ neural precursors differentiate into SCs in vivo following transplantation into EB-X lesions. A series of subsequent studies from the Kocsis laboratory demonstrated that multipotent progenitors from both CNS and bone marrow lineages likewise preferentially differentiate into SCs following engraftment into EB-X lesions (Akiyama et al., 2001, 2002; Sasaki et al., 2001). Present data further demonstrate that even lineage restricted OPCs isolated from the adult spinal cord maintain the capacity to differentiate into Schwann-like cells within EB lesions. Crang et al. (2004) recently demonstrated that MOG+ OPCs from the adult brainstem differentiate into SCs in the EB-lesioned dorsal columns, consistent with present data.
It is clear that in addition to their intrinsic properties, the environment into which precursor/stem cells are engrafted dictates their differentiation (Cao et al., 2002; Song et al., 2002). Every published study to date demonstrating SC-like remyelination by CNS progenitors has used the EB demyelinating model. Although the EB lesion clearly provides an environment conducive to SC differentiation, specific factors responsible for favoring SC differentiation of CNS precursors in vivo have not yet been conclusively identified. In vitro studies have demonstrated that BMPs can promote neural crest differentiation of embryonic neural stem cells (Gajavelli et al., 2004; Molne et al., 2000; Mujtaba et al., 1998). Crang et al. (2004) recently showed that the neural crest promoting activity of BMPs is not restricted to multipotent stem cells. Surprisingly, lineage committed MOG+ adult OPCs differentiated into p75+/GFAP+ Schwannlike cells expressing P0 mRNA in vitro following sequential exposure to FGF2 and BMPs-2 and 4.
Although it is generally accepted that during development, multipotent cells progressively become more restricted in their differentiation potential, evidence of dedifferentiation by committed precursors has been reported. For example, postnatal OPCs from the optic nerve can be reprogrammed to become CNS stem-like cells following exposure to BMPs and FGF2 (Kondo and Raff, 2000). For such reprogramming to occur, OPCs must first differentiate into Type-2 astrocytes and it is this step that is mediated by BMPs (Kondo and Raff, 2000; Mabie et al., 1997). In the postnatal optic nerve, astrocytes provide a natural source for the BMP antagonist noggin, thus ensuring that OPCs do not normally become Type-2 astrocytes or neural stem cells in the CNS (Kondo and Raff, 2004b). Gilson and Blakemore (2002) first hypothesized that without noggin-producing astrocytes, the macroglial-free environment of EB lesions would be dominated by unopposed signaling of BMPs, which might bias OPC differentiation toward SCs. Present data show that BMP-2 and BMP-4 transcripts are both highly expressed within EB lesions at a time when they could potentially exert a differentiating effect upon engrafted adult OPCs. ED-1+ macrophages and activated endothelial cells both populate EB lesions and represent potential sources of various BMPs (Champagne et al., 2002; Csiszar et al., 2005; Willette et al., 1999). In contrast to BMP-2 and BMP-4, we observed a dramatic decrease in noggin mRNA levels from microdissected EB-lesioned tissue when compared with those from uninjured control. Such an environment favors unopposed BMP signaling. Intriguing results from a recent study reveal that BMP-2 induced differentiation of OPCs into Type-2 astrocytes is associated with chromatin remodeling and upregulation of NSC-related genes (Kondo and Raff, 2004a). These results, coupled with findings from Gajavelli et al. (2004), who recently showed that BMPs can instruct NSCs to differentiate into a variety of neural crest lineages, provide a potential explanation for how adult OPCs within BMP rich/noggin poor EB lesions could be reprogrammed to differentiate into remyelinating SCs. Consistent with this hypothesis, we found that SC differentiation by adult OPCs was not observed when they were engrafted 1:1 with astrocytes which constitutively express noggin. To determine whether noggin expression alone was sufficient to block SC differentiation in vivo, we engrafted noggin-transduced adult OPCs into EB-X lesions. Invariably, noggin overexpression by engrafted adult OPCs was sufficient to block SC-like differentiation. Taken together, these data suggest that the absence of astrocyte-derived noggin within BMP containing EB lesions is, at least in part, responsible for their unexpected differentiation into remyelinating Schwann-like cells.
A CNS progenitor cell’s response to BMPs varies considerably with developmental stage (Grinspan et al., 2000; Mehler et al., 2000). In contrast to OPCs, GRPs are less restricted and maintain the potential to differentiate into Type-1 astrocytes (Rao et al., 1998). Adult OPCs are more mature than GRPs and are considered to be restricted to oligodendrocyte and Type-2 astrocyte phenotypes. These maturity differences likely account for the differential fate of adult OPCs and GRPs following engraftment into EB lesions. Rather than SCs, embryonic GRPs transplanted into EB lesions primarily differentiate into GFAP+ astrocytes. These results are consistent with in vitro studies demonstrating robust astrocytic differentiation by GRPs following exposure to BMPs (Maragakis et al., 2004). At all stages of development, however, BMPs block oligodendrocytic differentiation (Gross et al., 1996; Mabie et al., 1997, 1999; Mehler and Kessler, 1995). Thus, the high BMP and decreased noggin expression levels within EB lesions could explain the observed lack of centrally located oligodendrocyte remyelination following both GRP and adult OPC transplantation. Crang et al. (2004) similarly showed that MOG+ adult OPCs isolated from the rat brainstem and engrafted into dorsal EB-X lesions differentiate into SCs. The nature of remyelination observed by Crang et al. (2004), however, was variable and in some EB-X lesions oligodendrocyte differentiation by MOG+ OPCs was observed. In the present study, oligodendrocyte remyelination by engrafted OPCs was consistently absent in the central, astrocyte-free portions of EB-X lesions. This apparent discrepancy is likely attributable to differences in the cells used for each study. Crang et al. (2004) isolated MOG+ cells from adult rat brainstems that were uniformly MOG+/GalC+. The present study used spinal cord-derived A2B5+/GalC− OPCs. Thus the difference between these two studies likely underscores important differences in the ability of MOG+/GalC+ brainstem-derived OPCs and A2B5+/GalC− spinal cord-derived OPCs to give rise to remyelinating SCs and oligodendrocytes in vivo.
Importantly, neither noggin overexpression by engrafted adult OPCs nor the presence of co-transplanted astrocytes resulted in significant enhancement of oligodendrocyte differentiation by engrafted adult OPCs, suggesting that BMP suppression alone is not sufficient to promote oligodendrocyte remyelination by spinal-cord-derived adult OPCs in EB lesions of the VLF. We have previously shown that endogenous oligodendrocyte remyelination is successful in the perimeter of EB lesions, coinciding with a region of densely populated reactive astrocytes (Talbott et al., 2005). This observation was consistent with previous reports that suggested a pro-remyelinating role for astrocytes (Blakemore and Crang, 1989; Franklin et al., 1991, 1993b; Jasmin and Ohara, 2002). Why co-transplantation of astrocytes with adult OPCs did not induce oligodendrocyte differentiation and remyelination is unclear but can likely be attributed to the nature of the astrocytes used for engraftment. Astrocytes used in the present study were isolated from the postnatal rat brain and maintained in vitro for several passages prior to transplantation and thus do not represent the population of activated endogenous astrocytes present in the perimeter of adult spinal cord EB lesions. Blakemore et al. (2003) found that astrocytes derived from postnatal rat brains and maintained in culture for some time prior to engraftment into EB lesions impaired oligodendrocyte remyelination by transplanted neonatal OPCs. Blanket statements regarding the influence of astrocytes on oligodendrocyte remyelination, therefore, cannot be made, as their role is more likely dependent upon their origin and state of activation.
The robust in vivo differentiation of adult lineage-committed OPCs into remyelinating SCs demonstrated here represents a dramatic example of glial cell plasticity. Because OPCs are an abundant and widespread population of cells endogenous to the adult CNS (Dawson et al., 2003; Levine et al., 2001), elucidation of signals regulating their differentiation into remyelinating SCs has important implications for disorders in which demyelination is a component, including MS and SCI. It remains to be determined whether altering the balance between BMP and noggin activity in these more complex injury environments might similarly influence OPC differentiation into remyelinating SCs. Application of exogenous factors to overcome environmental cues normally restricting OPC differentiation potential will hopefully one day lead to effective remyelinating strategies for clinical application.
ACKNOWLEDGMENTS
We thank Cathie Caple for her technical assistance with electron microscopy, George Harding for his assistance with confocal microscopy, and William DeVries for his assistance with immunohistochemistry. We also thank Dr. Xiao-Ming Xu for providing SC cultures and Dr. R.M. Harland for providing the noggin construct. Finally we thank Darlene A. Burke for her assistance with statistical analyses.
Grant sponsor: NIH/NINDS; Grant number: NS38665; Grant sponsors: NIH/NCRR; Grant number: RR15576; Grant sponsors: Kentucky Spinal Cord and Head Injury Research Trust; Norton Healthcare; Commonwealth of Kentucky Research Challenge for Excellence Trust Fund.
REFERENCES
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF. Remyelination by Schwann cells of axons demye-linated by intraspinal injection of 6-aminonicotinamide in the rat. J Neurocytol. 1975;4:745–757. doi: 10.1007/BF01181634. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. Limited remyelination of CNS axons by Schwann cells transplanted into the sub-arachnoid space. J Neurol Sci. 1984;64:265–276. doi: 10.1016/0022-510x(84)90175-8. [DOI] [PubMed] [Google Scholar]
- Blakemore WF. The case for a central nervous system (CNS) origin for the Schwann cells that remyelinate CNS axons following concurrent loss of oligodendrocytes and astrocytes. Neuropathol Appl Neurobiol. 2005;31:1–10. doi: 10.1111/j.1365-2990.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ. The relationship between type-1 astrocytes, Schwann cells and oligodendrocytes following transplantation of glial cell cultures into demyelinating lesions in the adult rat spinal cord. J Neurocytol. 1989;18:519–528. doi: 10.1007/BF01474547. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Gilson JM, Crang AJ. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Exp Neurol. 2003;184:955–963. doi: 10.1016/S0014-4886(03)00347-9. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–76. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Carroll WM, Jennings AR, Ironside LJ. Identification of the adult resting progenitor cell by autoradiographic tracking of oligodendrocyte precursors in experimental CNS demyelination. Brain. 1998;121(Part 2):293–302. doi: 10.1093/brain/121.2.293. [DOI] [PubMed] [Google Scholar]
- Champagne CM, Takebe J, Offenbacher S, Cooper LF. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30:26–31. doi: 10.1016/s8756-3282(01)00638-x. [DOI] [PubMed] [Google Scholar]
- Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia. 2004;47:314–324. doi: 10.1002/glia.20011. [DOI] [PubMed] [Google Scholar]
- Crang AJ, Gilson JM, Li WW, Blakemore WF. The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. Eur J Neurosci. 2004;20:1445–1460. doi: 10.1111/j.1460-9568.2004.03606.x. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: Role of nuclear factor-κB activation by tumor necrosis factor-α, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Hoffman RL. Schwann cell invasion of the central nervous system of the myelin mutants. J Anat. 1997;190(Part 1):35–49. doi: 10.1046/j.1469-7580.1997.19010035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzmann GU, Benton RL, Woock JP, Howard RM, Tsoulfas P, Whittemore SR. Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord. Exp Neurol. 2005;195:293–304. doi: 10.1016/j.expneurol.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J Neurocytol. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. The reconstruction of an astrocytic environment in glia-deficient areas of white matter. J Neurocytol. 1993a;22:382–396. doi: 10.1007/BF01195559. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. The role of astrocytes in the remyelination of glia-free areas of demyelination. Adv Neurol. 1993b;59:125–133. [PubMed] [Google Scholar]
- Franklin RJ, Hinks GL. Understanding CNS remyelination: Clues from developmental and regeneration biology. J Neurosci Res. 1999;58:207–213. [PubMed] [Google Scholar]
- Gajavelli S, Wood PM, Pennica D, Whittemore SR, Tsoulfas P. BMP signaling initiates a neural crest differentiation program in embryonic rat CNS stem cells. Exp Neurol. 2004;188:205–223. doi: 10.1016/j.expneurol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Gilson JM, Blakemore WF. Schwann cell remyelination is not replaced by oligodendrocyte remyelination following ethidium bromide induced demyelination. Neuroreport. 2002;13:1205–1208. doi: 10.1097/00001756-200207020-00027. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: Survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: Survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Imitola J, Snyder EY, Khoury SJ. Genetic programs and responses of neural stem/progenitor cells during demyelination: Potential insights into repair mechanisms in multiple sclerosis. Physiol Genomics. 2003;14:171–197. doi: 10.1152/physiolgenomics.00021.2002. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Ohara PT. Remyelination within the CNS: Do Schwann cells pave the way for oligodendrocytes? Neuroscientist. 2002;8:198–203. doi: 10.1177/1073858402008003005. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Ben-Hur T, Rogister B, O’Leary MT, Dubois-Dalcq M, Blakemore WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19:7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Kisseberth WC, Brettingen NT, Lohse JK, Sandgren EP. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004a;18:2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff MC. A role for Noggin in the development of oligodendrocyte precursor cells. Dev Biol. 2004b;267:242–251. doi: 10.1016/j.ydbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JR, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Marmur R, Papavasiliou A, Song Q, Kessler JA. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial–astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, Rothstein JD. Glutamate transporter expression and function in human glial progenitors. Glia. 2004;45:133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, De Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Cytokines and neuronal differentiation. Crit Rev Neurobiol. 1995;9:419–446. [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22:74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- Molne M, Studer L, Tabar V, Ting YT, Eiden MV, McKay RD. Early cortical precursors do not undergo LIF-mediated astrocytic differentiation. J Neurosci Res. 2000;59:301–311. doi: 10.1002/(sici)1097-4547(20000201)59:3<301::aid-jnr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Han SS, Fischer I, Sandgren EP, Rao MS. Stable expression of the alkaline phosphatase marker gene by neural cells in culture and after transplantation into the CNS using cells derived from a transgenic rat. Exp Neurol. 2002;174:48–57. doi: 10.1006/exnr.2001.7847. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS, PNS. Dev Biol. 1998;200:1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci USA. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TJ, Durgun MB, Gilmore SA. Schwann cell invasion of ventral spinal cord: The effect of irradiation on astrocyte barriers. J Neuropathol Exp Neurol. 1998;57:866–873. doi: 10.1097/00005072-199809000-00008. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Talbott JF, Loy DN, Liu Y, Qiu MS, Bunge MB, Rao MS, Whittemore SR. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36:120–125. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: A comparative study. Glia. 1999;25:216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Ge B, Duncan ID. Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci USA. 1999;96:4089–4094. doi: 10.1073/pnas.96.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]


