Abstract
An auxotrophic Salmonella dublin (O9,12) strain, SL5631, with a deletion affecting gene aroA, was made into a partial diploid expressing the rfb (O-antigen-repeat-unit-specifying) gene cluster of Salmonella typhimurium (O4,12). By use of O4- and O9-specific antisera in indirect immunofluorescence assays, the resulting hybrid SL7103 was shown to express both the O4- and O9-antigen epitopes in the same bacterium. Qualitative and quantitative sugar analyses by gas-liquid chromatography on peralditol acetates of phenol-water-extracted lipopolysaccharides showed that the S. dublin and S. typhimurium repeating units (estimated on the basis of their tyvelose and abequose contents, respectively) were present in approximately equimolar amounts. The SL7103 hybrid auxotroph was avirulent when given intraperitoneally to NMRI mice in a dose of 10(8) CFU and elicited a protective immunity against intraperitoneal challenge with either virulent S. dublin (50% lethal dose of ca. 1.5 x 10(4) CFU versus < 1 x 10(1) CFU in nonimmunized mice) or virulent S. typhimurium (50% lethal dose of ca. 1 x 10(5) versus < 1 x 10(1) CFU in nonimmunized mice). Compared with the protection elicited in homologous systems (S. dublin SL5631 against S. dublin and S. typhimurium SL1479 against S. typhimurium), the protective efficacy of the hybrid was reduced approximately 70-fold against S. dublin challenge and 100-fold against S. typhimurium challenge. Vaccination with S. typhimurium SL1479 conferred no protection against S. dublin challenge, and vaccination with S. dublin SL5631 conferred no protection against S. typhimurium challenge. The protection elicited by the hybrid strain SL7103 is supposed to be mainly a consequence of serum antibodies directed against the immunodominant O4 and O9 epitopes.
Full text
PDF



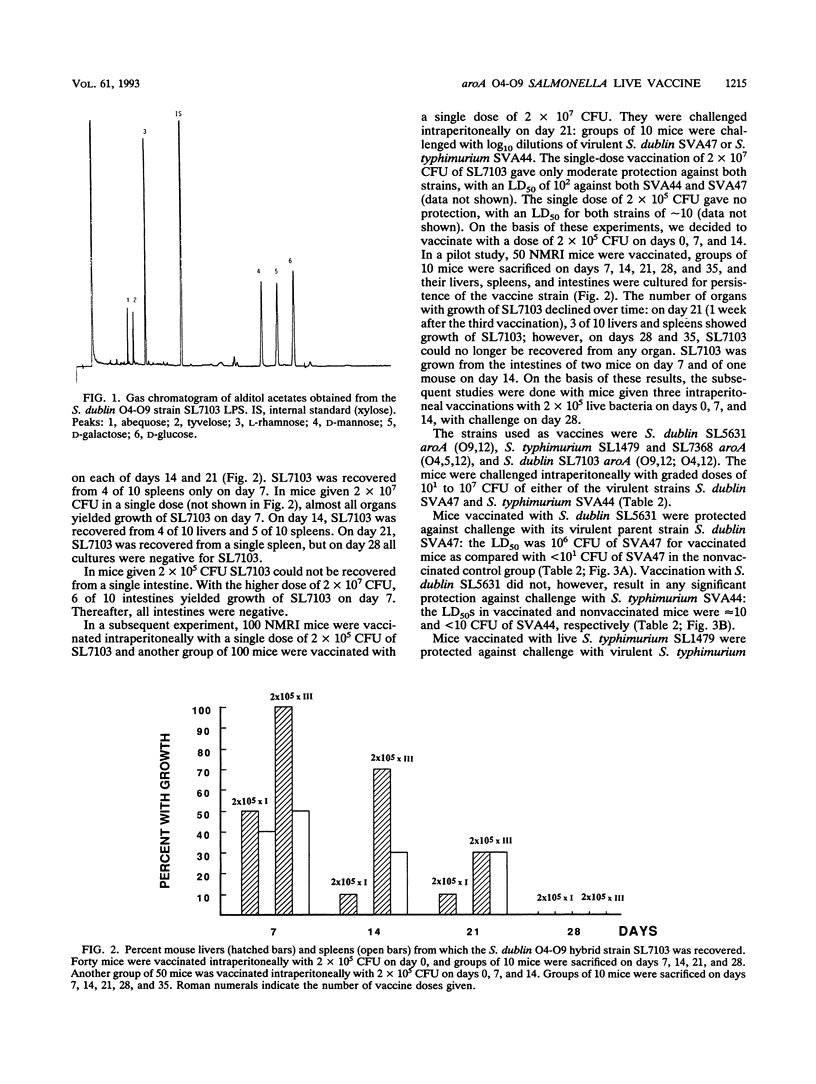
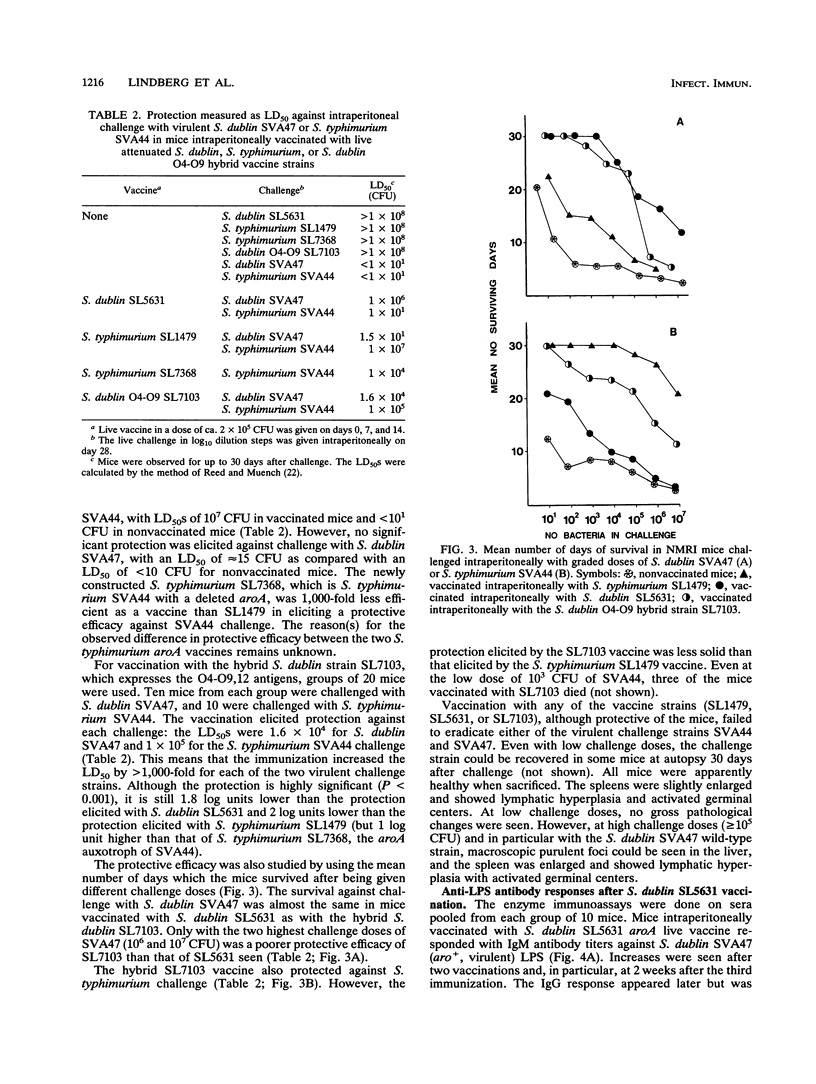
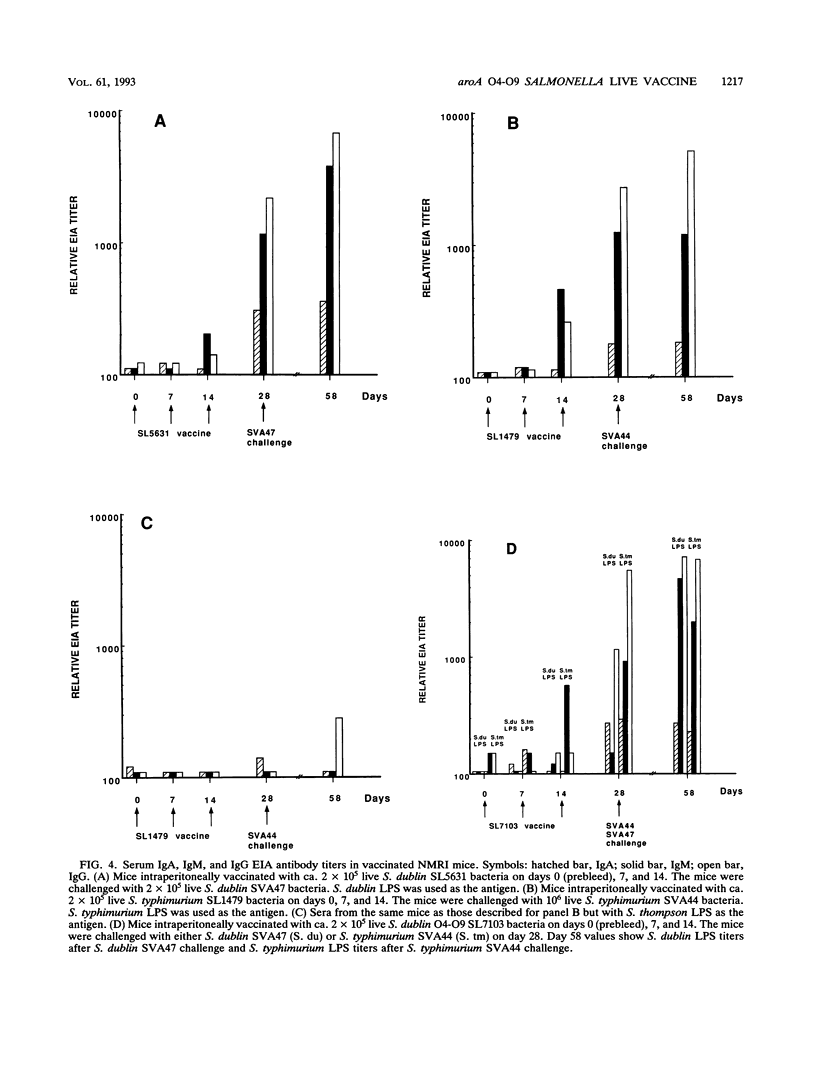



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Angerman C. R., Eisenstein T. K. Correlation of the duration and magnitude of protection against Salmonella infection afforded by various vaccines with antibody titers. Infect Immun. 1980 Feb;27(2):435–443. doi: 10.1128/iai.27.2.435-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Svenson S. B., Lindberg A. A. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987 Mar;2(3):171–183. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Immunity to enteric infection in mice. Infect Immun. 1970 Mar;1(3):243–250. doi: 10.1128/iai.1.3.243-250.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. F., Stocker B. A. Construction of delta aroA his delta pur strains of Salmonella typhi. J Bacteriol. 1988 Sep;170(9):3991–3995. doi: 10.1128/jb.170.9.3991-3995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Sultzer B. M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Genes aroA and serC of Salmonella typhimurium constitute an operon. J Bacteriol. 1985 Jul;163(1):355–361. doi: 10.1128/jb.163.1.355-361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. N., Weintraub A., Lindberg A. A., Stocker B. A. Construction of Salmonella strains with both antigen O4 (of group B) and antigen O9 (of group D). J Bacteriol. 1992 Mar;174(6):1911–1915. doi: 10.1128/jb.174.6.1911-1915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Snellings N. J., Life C. A., Baron L. S. Intraperitoneal mouse virulence of Salmonella typhimurium hybrids expressing somatic antigen 9. Infect Immun. 1974 Sep;10(3):669–671. doi: 10.1128/iai.10.3.669-671.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Evaluation of some extraction methods for the preparation of bacterial lipopolysaccharides for structural analysis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):751–759. doi: 10.1111/j.1699-0463.1972.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Evaluation of the immune response directed against the Salmonella antigenic factors O4,5 and O9. Infect Immun. 1979 Dec;26(3):956–965. doi: 10.1128/iai.26.3.956-965.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen H., Mäkelä O. The protective capacity of immune sera in experimental mouse salmonellosis is mainly due to IgM antibodies. Immunol Lett. 1982 Nov;5(5):267–272. doi: 10.1016/0165-2478(82)90110-9. [DOI] [PubMed] [Google Scholar]
- Saxén H., Mäkelä O., Svenson S. B. Isotype of protective anti-Salmonella antibodies in experimental mouse salmonellosis. Infect Immun. 1984 Jun;44(3):633–636. doi: 10.1128/iai.44.3.633-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigwart D. F., Stocker B. A., Clements J. D. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989 Jun;57(6):1858–1861. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Stocker B. A., Hoiseth S. K., Smith B. P. Aromatic-dependent "Salmonella sp." as live vaccine in mice and calves. Dev Biol Stand. 1983;53:47–54. [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Weintraub A., Johnson B. N., Stocker B. A., Lindberg A. A. Structural and immunochemical studies of the lipopolysaccharides of Salmonella strains with both antigen O4 and antigen O9. J Bacteriol. 1992 Mar;174(6):1916–1922. doi: 10.1128/jb.174.6.1916-1922.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]



