Abstract
Pemphigus targets desmogleins (Dsgs), which are thought to be synthesized as inactive precursor proteins with prosequences that are cleaved by substilisin-like proprotein convertases, such as furin, to yield mature adhesive molecules. We hypothesized that some pemphigus pathogenic antibodies (Abs), which presumably interfere with adhesion, only bind the mature form. A pathogenic and three non-pathogenic anti-Dsg1 monoclonal Abs (mAbs) isolated from a pemphigus foliaceus (PF) patient, were used for immunoprecipitation and ELISA of recombinant precursor and mature Dsg1. The pathogenic Ab binds mature Dsg1, whereas non-pathogenic Abs bind either only the precursor or both the precursor and mature Dsg1. Competition ELISA showed that the majority of PF sera target the same or nearby epitopes defined by the pathogenic anti-Dsg1 mAb that blocked >20% binding of 29 out of 40 PF sera. Furthermore, the immunoreactivity of 45 PF sera against the mature Dsg1 was 3.2 fold stronger than that against the precursor Dsg1 by ELISA. Similar results were observed in anti-Dsg3 Abs in 47 pemphigus vulgaris sera, suggesting that most pemphigus sera target epitopes that are unmasked by proteolytic processing. These findings support the idea that at least some pathogenic pemphigus autoantibodies induce the loss of cell adhesion by directly binding the trans-interaction site of Dsgs.
INTRODUCTION
Pemphigus is a tissue-specific autoimmune disease characterized by the loss of intercellular adhesion of keratinocytes because of the binding of autoantibodies to the cell surface (Stanley and Amagai, 2006). Pemphigus consists of two major subtypes, pemphigus foliaceus (PF) and pemphigus vulgaris (PV), in which autoantibodies target cadherin-type cell adhesion molecules, desmoglein 1 (Dsg1) and Dsg3, respectively. The autoantibodies are thought to block the cell adhesive function mediated by Dsgs, inducing blister formation in the skin and mucous membranes.
The mechanism by which anti-Dsg autoantibodies induce the loss of keratinocyte cell adhesion is still a matter of discussion. One explanation is that of steric hindrance, in which pathogenic autoantibodies induce the loss of cell adhesion by directly interfering with the trans-interaction of Dsg. Another explanation is that the blister formation requires cellular responses, including internalization and degradation of Dsg, and intercellular signaling, such as p38MAPK, Rho family GTPase, c-myc, protein kinase C, and phospholipase C (Esaki et al., 1995; Berkowitz et al., 2005; Calkins et al., 2006; Waschke et al., 2006; Williamson et al., 2006)(review in (Sharma et al., 2007).
Although it was shown that anti-Dsg antibodies (Abs) are involved in blister formation in pemphigus, not all anti-Dsg Abs are pathogenic. Recent studies using monoclonal Abs (mAbs) from pemphigus mice model or from pemphigus patients’ lymphocytes by phage display showed that there are both pathogenic and non-pathogenic Abs (Tsunoda et al., 2003; Payne et al., 2005; Ishii et al., 2008). For example, we recently isolated a cohort of human anti-Dsg1 mAbs as single-chain variable fragments (scFv) from a PF patient using phage display (Ishii et al., 2008). Out of 67 unique mAbs isolated, only two anti-Dsg1 mAbs showed pathogenic activity, as they induced blister formation in mouse and/or human skin, thereby indicating that anti-Dsg Abs consist of pathogenic and non-pathogenic Abs. Determining factors for influencing Abs’ pathogenicity may be important for developing disease-specific therapy or correct evaluation for disease activity. Epitopes seem to be an important factor for pathogenicity. The pathogenic Abs tend to bind the amino-terminal extracellular domain of Dsgs that is predicted to form the trans-adhesive interface between cells, whereas the non-pathogenic Abs bind more membrane-proximal extracellular domains.
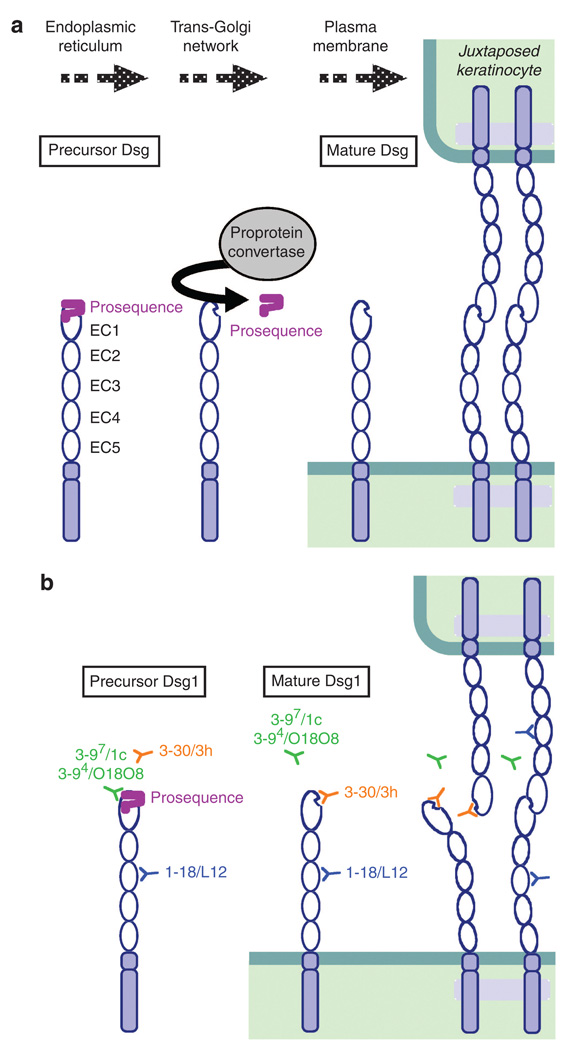
Cadherins, including Dsgs, are thought to be initially synthesized as inactive precursor proteins in the endoplasmic reticulum, and transit through the cis-and trans-Golgi (Ozawa and Kemler, 1990). Subsequently, they are processed by subtilisin-like proprotein convertases, such as furin, to become biologically active mature proteins, and assembled into adherence junctions or desmosomes (Posthaus et al., 1998; Wahl et al., 2003) (Figure 9a). The proteolytic cleavage sites are well conserved among cadherins, although the size of the prosequences is different between classic cadherins and Dsgs, that is, ~130 amino acids and ~25 amino acids, respectively (Koch et al., 2004). Previous studies on E-cadherin showed that, if this proteolytic cleavage is impaired, proE-cadherin integrates into the membrane but is not able to confer adhesiveness, as the prosequence may prevent successful interaction either by steric hindrance or by masking a recognition site (Ozawa and Kemler, 1990). Recent studies using nuclear magnetic resonance revealed that the prosequence of N-cadherin is a folded domain that may prevent the homophilic trans-interactions between the molecules (Koch et al., 2004).
Figure 9. Proteolytic processing of the precursor form of desmogleins (Dsgs) and mAb binding to the precursor and mature form of Dsg1.
(a) Dsgs are thought to be synthesized as inactive precursor Dsgs with prosequences in the endoplasmic reticulum. The prosequences are then removed by subtillisin-like proprotein convertases before transporting them to the plasma membrane and mature Dsgs are assembled into desmosomes. (b) Binding of anti-Dsg1 mAbs to the mature and the precursor form of Dsg1. The pathogenic mAb, 3–30/3h (shown in orange) binds to the mature Dsg1, but not the precursor Dsg1. Some non-pathogenic mAbs, 3–97/1c and 3–94/O18O8 (shown in green), bind to the precursor Dsg1. A non-pathogenic mAb, 1–18/L12 (shown in blue), which recognizes the middle portion of Dsg1, binds to both mature and precursor Dsg1. The pathogenic mAb bind the region of adhesive interface of Dsg1, which are unmasked by proteolytic processing, perhaps blocking trans-interaction of Dsgs.
In this study, we focused on the effect of proteolytic processing on the binding of autoantibodies against Dsgs. We took advantage of the availability of pathogenic and non-pathogenic mAbs. We hypothesized that pathogenic Abs bind the epitopes that are masked by the prosequence and unmasked by proteolytic processing, if pathogenic autoantibodies induce the loss of cell adhesion by directly interfering with the trans-interaction of Dsg. We found that a pathogenic Ab recognized the epitope on Dsg1 that was unmasked by proteolytic processing. Furthermore, the majority of anti-Dsg Abs binding in PV and foliaceus patients target the mature form compared with the precursor form of Dsg. This finding is consistent with the pathogenic Abs binding to adhesive sites on Dsgs, perhaps directly interfering with their function.
RESULTS
Proteolytic cleavage of Dsg1 increases binding of a pathogenic PF scFv on ELISA assay
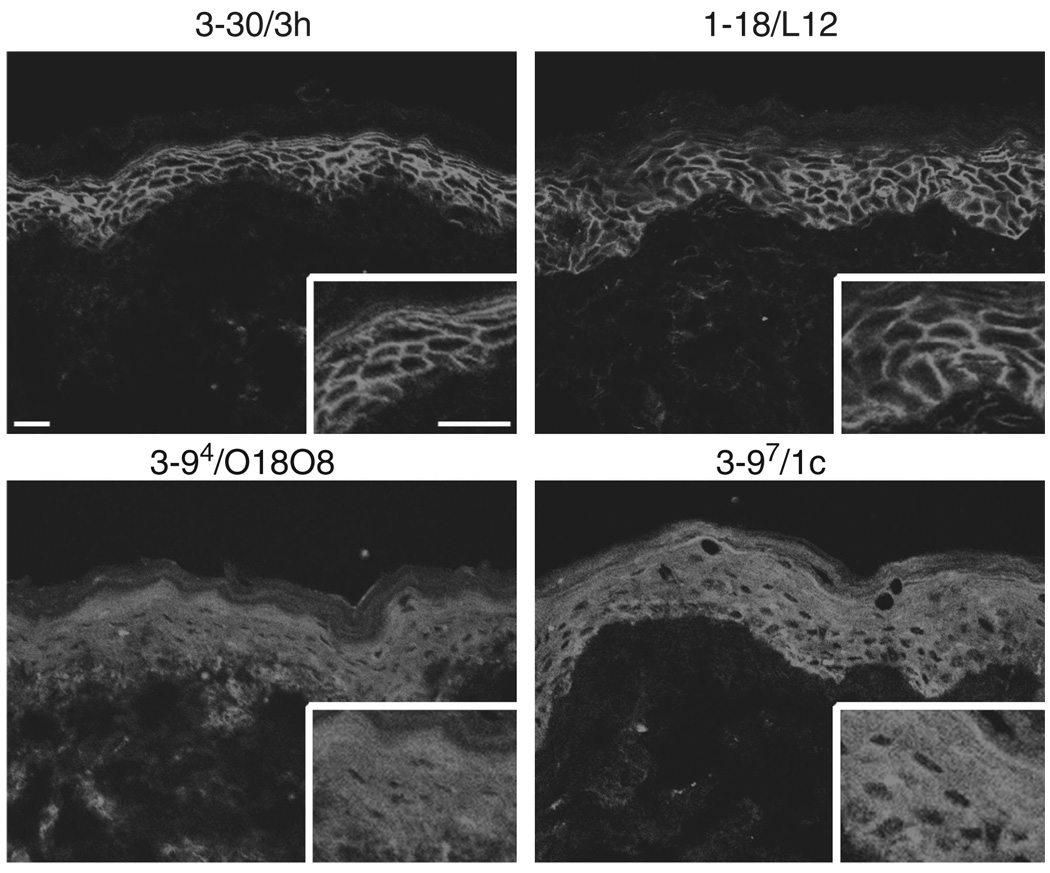
We previously reported the cloning of anti-Dsg1 mAbs from a PF patient (Ishii et al., 2008). Genetic analysis of the PF mAb library showed 67 unique mAbs based on V(D)J gene usage, heavy- and light-chain combinations, and somatic hypermutation. These mAbs were encoded by a total of 12 variable VDJ genes, and also by a total of only five variable heavy-chain (VH) genes. Predictably, all clones were bound to Dsg1, as shown by immunoprecipitation. But intriguingly, some clones immunoprecipitatied a baculovirus-produced recombinant Dsg1 protein with a slightly greater apparent molecular weight on SDS-PAGE than other clones (Ishii et al., 2008). In addition, the Abs that bound only the higher molecular weight Dsg1 polypeptide (3–97/1c, 3–94/O18O8) showed intracellular staining in the superficial layers of the epidermis using immunofluorescence (See Ishii et al., 2008, and Figure 1). As the insect cells transfected with recombinant baculovirus produce both a precursor and a mature protein form of Dsg1 (Hanakawa et al., 2002), and as the precursor form is believed to be localized in the endoplasmic reticulum to early Golgi (Wahl et al., 2003), we hypothesized that some mAbs bound the precursor form of Dsg1.
Figure 1. Confocal microscopy of anti-desmoglein 1 single-chain variable fragment (scFv) mAbs on human skin.
3–30/3h and 1–18/L12 scFv antibodies stained the cell surface of keratinocytes throughout human epidermis. 3–97/1c and 3–94/O18O8 showed cytoplasmic staining in the superficial layers of the epidermis. Scale bars: 50 µm.
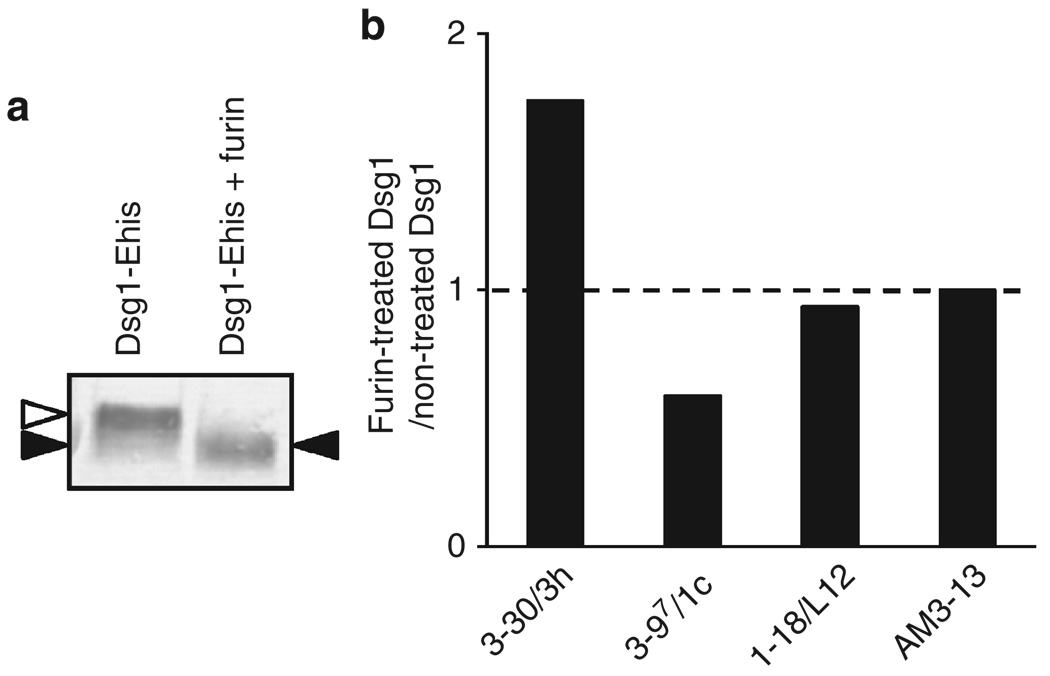
To test the hypothesis, we used furin, a human subtilisin-related proprotein convertase to cleave the precursor form of Dsg1 to obtain a mature form. As previously mentioned, baculovirally produced recombinant Dsg1 contains both precursor form and mature form. To confirm whether furin can cleave the precursor form of recombinant Dsg1 produced by the baculovirus expression system, we directly added furin to the culture supernatant of Dsg1-EHis baculoprotein. As shown in Figure 2a, Dsg1-EHis was detected as double bands, the higher and lower band were most likely the precursor form and mature form, respectively. After furin treatment, Dsg1-EHis was detected as a single band, the molecular weight of which was the same as that of the lower band, indicating that furin cleaved almost all precursor Dsg1-EHis into the mature form. As ELISA plates with baculovirus-produced Dsg1-EHis were expected to contain both mature and precursor forms of Dsg1, we examined the change in reactivity of mAbs against Dsg1 ELISA plates after treating them with furin (Figure 2b). We chose one pathogenic (3–30/3h) and three non-pathogenic PF mAbs (3–97/1c, 3–94/O18O8, 1–18/L12) (detailed in Table 1) as representative Abs. The reactivity of 1–18/L12, which recognizes a linear epitope on the extracellular domain 3 (EC3) of Dsg1, did not show any significant change. However, the reactivity of 3–97/1c was decreased after treatment with furin, consistent with the hypothesis that this Ab recognizes only the precursor form. The reactivity of the pathogenic Ab, 3–30/3h, was significantly increased after furin treatment, indicating that pathogenic 3–30/3h bound to an epitope on Dsg1, which was more accessible or increased in amount after furin treatment.
Figure 2. Binding capacity of anti-desmoglein 1 (Dsg1) single-chain variable fragment (scFv) mAbs to the Dsg1 ELISA plate changes after furin treatment.
(a) Baculovirally produced recombinant Dsg1 (Dsg1-EHis), visualized by immunoblot using anti-E-tag antibody. Dsg1-EHis was detected as double bands, which were considered to be precursor and mature form of Dsg1-EHis (left lane). After treatment with furin protease, only single band was observed (right lane). White arrowhead indicates the precursor form and black arrowhead indicates the mature form. (b) Comparison of anti-Dsg1 scFv mAbs bindings between furin-treated and Tris-buffered saline-calcium (TBS-Ca)-treated (control) Dsg1 ELISA plates. The ratio was calculated according to the following formula: (optical density (OD) values obtained in furin-treated wells)/(OD values obtained in TBS-Ca-treated wells). Control ratio (ratio = 1) is shown by a broken line. The binding of 3–30/3h, a pathogenic mAb, was significantly increased to nearly twofold, whereas that of 3–97/1c, non-pathogenic mAb was decreased. The binding of 1–18/L12 showed no significant change. AM3–13 is an irrelevant scFv and used as a control.
Table 1.
Epitopes and pathogenicity of anti-Dsg1 mAbs
| Name of clones | Epitope type | Deduced epitope | |
|---|---|---|---|
| Pathogenic antibody | 3–30/3h | Conformational | aa 89–101 of Dsg1 |
| Non-pathogenic antibodies | 3–97/1c | Conformational | Precursor form of Dsg1 |
| 3–94/O18O8 | Conformational | Precursor form of Dsg1 | |
| 1–18/L12 | Linear | EC3 of Dsg1 | |
Abbreviations: aa, amino acid; Dsg, desmoglein; EC, extracellular domain.
A pathogenic scFv recognizes the mature, but not precursor, form of Dsg1
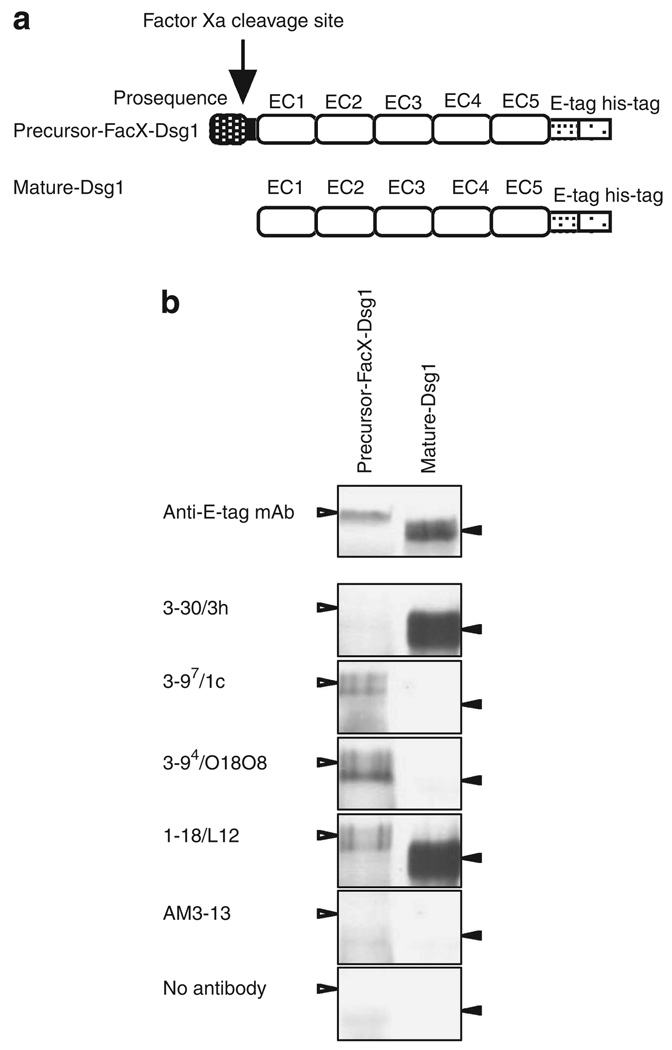
There may be limitations for furin pretreatment of the ELISA plate coated with Dsg, because it may not lead to a complete cleavage of Dsg1 proteins. Therefore, we further confirmed the above observations by analyzing the immunoreactivity of various anti-Dsg1 mAbs against both a precursor and a mature form of a recombinant extracellular region of Dsg1 using immunoprecipitation. To obtain a precursor form of Dsg1, we used a mutated recombinant Dsg1, named precursor-FacX-Dsg1, in which the normal endoproteolytic cleavage site of Dsg1 (R-Q-K-R) was replaced with the recognition site (I-E-G-R) of serum coagulation factor Xa (Figure 3a). Recombinant baculovirus containing the mutant precursor-FacX-Dsg1 complementary DNA was infected into High Five-cultured insect cells, and precursor-FacX-Dsg1 was produced in culture supernatant without cleaving the prosequence. To generate the mature form of Dsg1-EHis, named mature-Dsg1-EHis, we incubated the Dsg1-EHis baculoprotein with furin. As shown in Figure 3b, precursor-FacX-Dsg1 and mature-Dsg1-EHis were produced as a single band on immunoblots, and precursor-FacX-Dsg1 had a slightly higher molecular weight than mature-Dsg1-EHis.
Figure 3. Binding of anti-desmoglein 1 (Dsg1) single-chain variable fragment (scFv) mAbs to precursor-FacX-Dsg1 and mature Dsg1.
(a) Molecular structure of precursor-FacX-Dsg1-EHis and mature-Dsg1-EHis. To obtain precursor form, the recognition sequence in precursor Dsg1 was substituted with that of blood coagulation factor Xa (R-Q-K-R → I-E-G-R) by endogenous proprotein convertases. To obtain mature form of Dsg1, recombinant Dsg1-EHis produced by baculovirus expression system was treated with proprotein convertase furin. (b) Precursor-FacX-Dsg1-EHis and mature-Dsg1-EHis recombinant baculoprotein were immunoprecipitated with representative anti-Dsg1 scFv antibodies or control scFv (AM3–13) and detected on an immunoblot by anti-E-tag antibody.
Precursor-FacX-Dsg1 and mature-Dsg1 were immunoprecipitated with representative anti-Dsg1 mAbs or a control mAb (AM3–13) and detected on western blots by an anti-E-tag Ab. The pathogenic mAb, 3–30/3h, immunoprecipitated only the mature form of Dsg1. Non-pathogenic mAbs, 3–97/1c and 3–94/O18O8, immunoprecipitated only the precursor form of Dsg1. Another non-pathogenic mAb, 1–18/L12, immunoprecipitated both the precursor and mature forms of Dsg1.
Taken together, these data show that a pathogenic anti-Dsg1 mAb recognized the mature form, but not the precursor form of Dsg1, whereas non-pathogenic mAb binds either only the precursor form or both the precursor and mature Dsg1.
The majority of PF sera target the same or nearby epitopes defined by a pathogenic anti-Dsg1 mAb
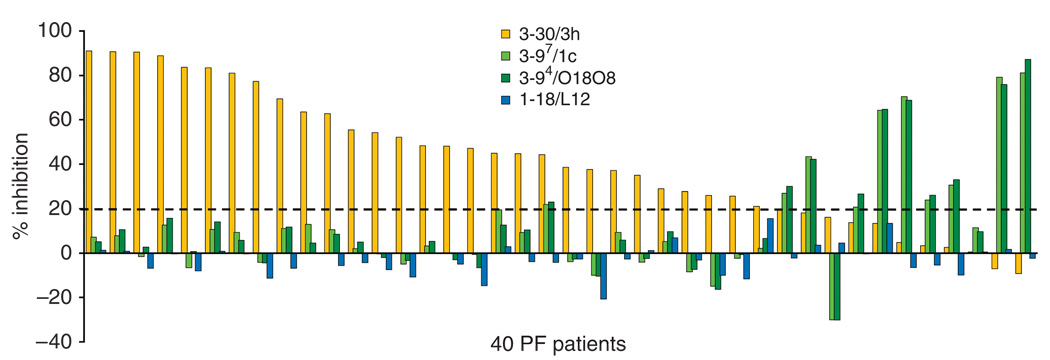
Next, we examined whether other PF sera target epitopes defined by the isolated anti-Dsg1 mAbs. To do so, we carried out competition ELISA to see the inhibition of the binding against Dsg1 of 40 PF sera by adding the mAbs (Figure 4). As competitors, we used the pathogenic mAb, 3–30/3h, and non-pathogenic mAbs, 3–97/1c, 3–94/O18O8, and 1–18/L12. The strongly pathogenic mAb 3–30/3h blocked >20 % binding of 29 out of 40 PF sera (73%). Furthermore, 14 sera were blocked over 50% and seven sera were blocked over 80%. In contrast, non-pathogenic Abs (3–97/1c, 3–94/ O18O8) that recognized precursor Dsg1, blocked >20% binding against Dsg1 in 10 out of 40 (25%) PF patients’ sera. Four sera were blocked over 50% binding by 3–97/1c and 3–94/O18O8. None of the 40 sera were blocked over 20% by 1–18/L12, which is a non-pathogenic Ab recognizing a linear epitope of the middle portion of Dsg1.
Figure 4. Competition ELISA of PF sera binding by anti-desmoglein 1 (Dsg1) single-chain variable fragment (scFv) mAbs.
Competition ELISA of 40 PF sera was carried out to see if the binding against standard Dsg1 ELISA plates of PF sera was blocked by adding anti-Dsg1scFv mAbs. 3–30/3h (shown in orange), 3–97/1c, 3–94/O18O8 (shown in green) and 1–18/L12 (shown in blue) were used as competitors. When >20% inhibition was considered as significant (shown in broken line), the bindings were blocked by pathogenic 3–30/3h mAb in 29 PF sera. 3–97/1c and 3–94/O18O8, which recognized the precursor form, blocked the binding in 10 PF sera.
These data indicate that the pathogenic mAb isolated from one PF patient defines a major pathogenic epitope targeted by most PF sera. On the other hand, the shared immunoreactivity among PF sera against non-pathogenic epitopes defined by several scFv from this PF patient is less, although a certain proportion of anti-Dsg1 reactivity in some PF sera targets the precursor form.
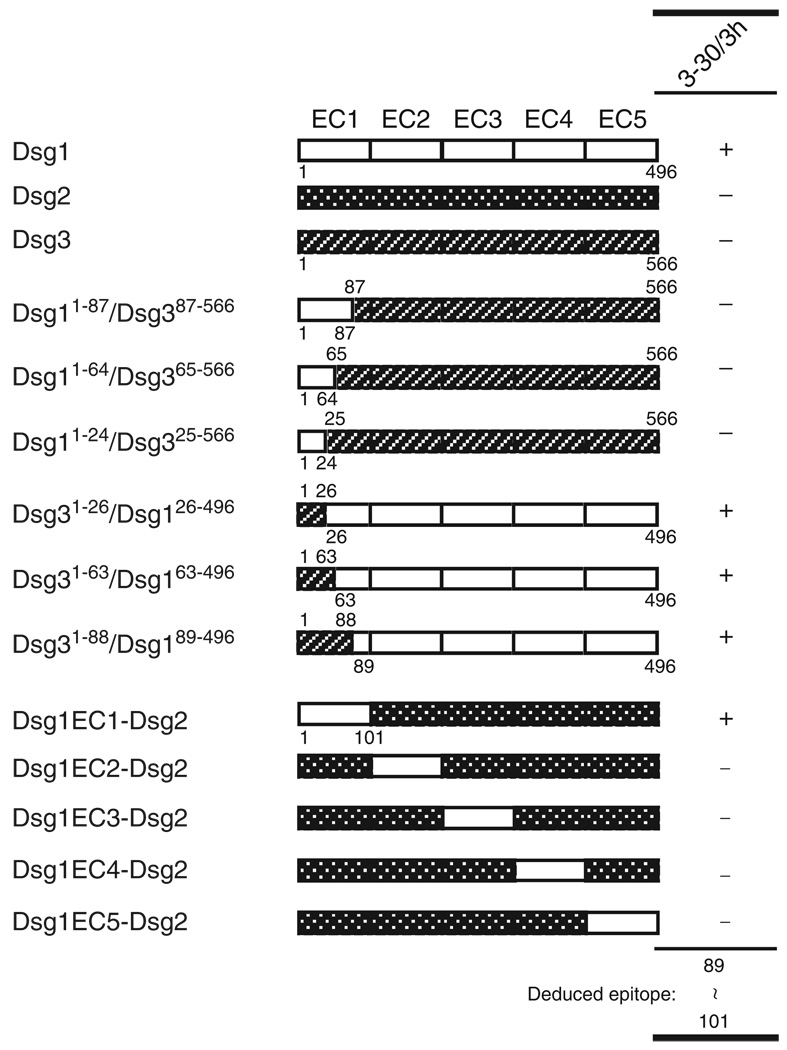
Next, we determined the finer epitope mapping of the pathogenic 3–30/3h using immunoprecipitation–immunoblot assay using a series of six Dsg1/Dsg3 and five Dsg1/Dsg2 chimeric molecules ((Sekiguchi et al., 2001) and Chan et al.(manuscript in preparation)). As shown in Figure 5, immunoprecipitation analysis using a domain-swapped molecule of Dsg1/Dsg2 or Dsg3 revealed that the epitope of 3–30/3h was mapped to amino acid 89–101 of the EC1 region of Dsg1.
Figure 5. Epitope mapping of pathogenic 3–30/3h mAb.
Wild-type and domain-swapped extracellular domains of human desmoglein 1 (Dsg1) and Dsg3, and of Dsg1 and Dsg2 produced by baculovirus expression system were used for immunoprecipitation with 3–30/3h mAb. The molecular structure of domain-swapped molecules is shown. The results of immunoprecipitation assays with 3–30/3h mAb are listed in the right panel.
Immunoreactivity against Dsgs in the majority of pemphigus sera is increased by proteolytic processing of precursor to mature Dsg
As much of the immunoreactivity of PF sera targeted the same or nearby epitopes defined by the pathogenic mAb 3–30/3h, which recognized only the mature form of Dsg1, we hypothesized that most autoantibodies in the PF sera target the mature, compared with the precursor, form of Dsg1. To verify this hypothesis, we generated ELISA plates coated with highly purified mature or precursor Dsg1 to compare the immunoreactivity of multiple pemphigus sera against a precursor and a mature form of Dsg1.
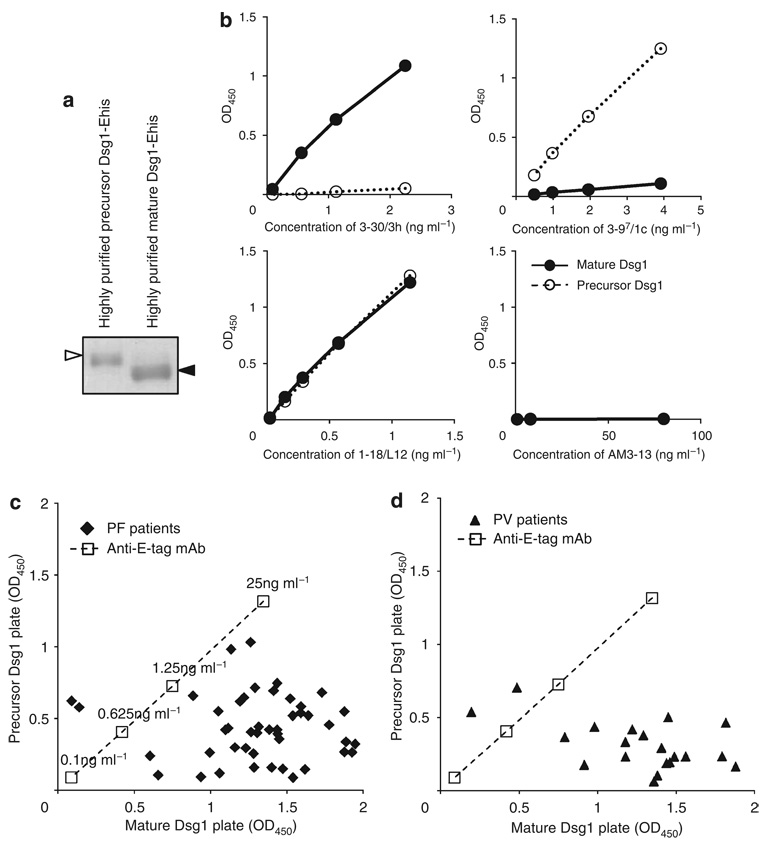
To obtain purified precursor Dsg1, recombinant Dsg1-EHis baculoprotein was passed through an immunoadsorption column containing mAb 3–30/3h to eliminate mature Dsg1. To achieve a purified mature form of Dsg1, recombinant Dsg1-EHis baculoprotein treated with furin was passed through an immunoadsorption column containing mAb 3–97/1c to remove the precursor form (Figure 6a). Those purified recombinant baculoproteins were coated on ELISA plates to generate purified mature Dsg1 and precursor Dsg1 ELISA plates. We confirmed that these ELISA plates contained either mature or precursor forms by immunoreactivities of anti-Dsg1 mAbs (Figure 6b). 3–30/3h reacted against the complete-mature Dsg1 plate in a dose-dependent manner, but did not react to the complete-precursor Dsg1. In contrast, 3–97/1c showed dose-dependent reactivity to the complete-precursor plate, but showed no reactivity to the complete-mature plate. 1–18/L12 exhibited similar reactivity against both plates.
Figure 6. Majority of anti-desmoglein 1 (Dsg1) antibody binding in PF and PV sera targets the mature form more than the precursor form of Dsg1.
(a) Coomassie blue staining of purified recombinant baculoproteins used for precursor and mature Dsg1 ELISA plates. White arrowhead indicates the precursor form and black arrowhead indicates the mature form. (b) Binding curves of representative anti-Dsg1 scFv antibodies (3–30/3h, 3–97/1c, 1–18/L12) and control scFv (AM3–13) against mature and precursor Dsg1 ELISA plate. (c) Dispersion of immunoreactivity of 45 PF sera between the mature and precursor Dsg1 ELISA plate. Each closed square represents single patient. Note that most PF sera had stronger reactivity against mature Dsg1 plate than precursor Dsg1 plate. (d) Dispersion of immunoreactivity of 20 mucocutaneous PV sera between the mature and precursor Dsg1 ELISA plate. Each closed triangle represents single patient. Similar to PF patients, most PV sera had stronger reactivity against mature Dsg1 plate than against precursor Dsg1 plate.
We tested 45 PF sera and 20 mucocutaneous PV sera, which contained an anti-Dsg1 Ab in addition to an anti-Dsg3 Ab. Strikingly, all PF sera, except two, showed stronger reactivity against the mature Dsg1 plate than against the precursor Dsg1 plate (Figure 6c). The average and standard deviation of immunoreactivity, as determined by ELISA optical density (OD), against mature Dsg1 was 1.37 ± 0.32, whereas the average and standard deviation of immunoreactivity against the precursor form was 0.43 ± 0.23. Difference between immunoreactivity against mature and precursor Dsg1 was statistically significant (paired t-test, P < 0.001). For PV sera that contained Dsg1 and Dsg3 Abs, all sera but two also reacted more strongly to the mature Dsg1 ELISA plate than to the precursor ELISA plate (Figure 6d). The average and standard deviation of the ELISA OD of PV sera against mature Dsg1 was 1.26 ± 0.41, whereas the average and standard deviation against precursor Dsg1 was 0.31 ± 0.16, which was a statistically significant difference (P < 0.001). These findings show that the majority of pemphigus sera containing anti-Dsg1 Abs target epitopes that are unmasked by proteolytic processing.
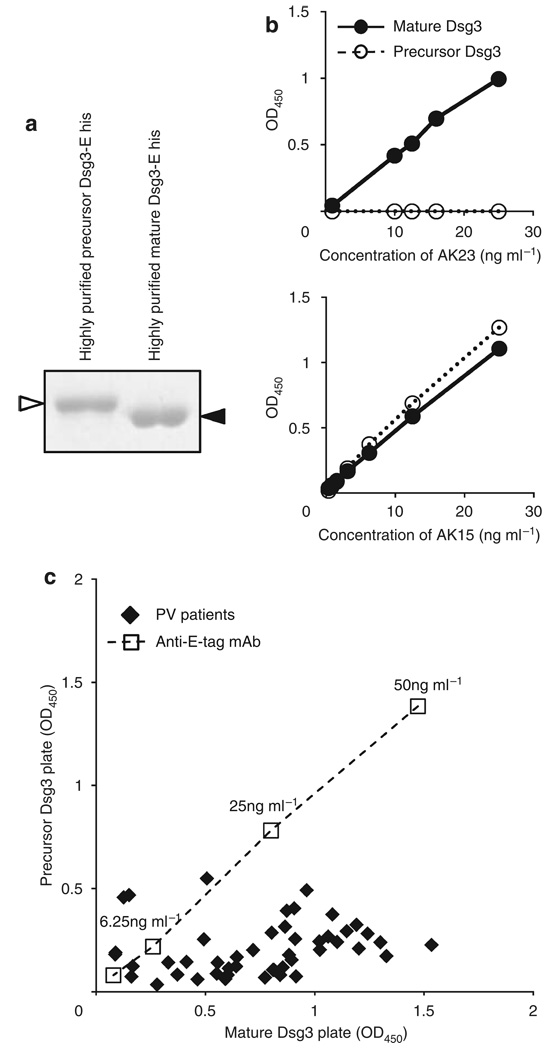
As changes in the reactivity of anti-Dsg3 mAbs have also been observed on Dsg3 ELISA plates with different ratios of precursor and mature Dsg3 (Sharma et al., 2009), we also tested the well-characterized mouse anti-Dsg3 mAb AK series and PV sera on ELISA plates with purified mature or precursor Dsg3. To obtain precursor Dsg3, we used a mouse anti-Dsg3 mAb, AK23, obtained from PV model mice (Tsunoda et al., 2003). AK23, a pathogenic anti-Dsg3 mouse monoclonal Ab, targets an epitope on the adhesive interface of Dsg3 molecules and binds only to the mature form (Tsunoda et al., 2003). Recombinant Dsg3-EHis baculoprotein was passed through an immunoadsorption column containing AK23 mAb to eliminate mature Dsg3. To obtain mature Dsg3, recombinant Dsg3-EHis baculoprotein was treated with furin (Figure 7a). We confirmed the accuracy of ELISA plates coated with the purified mature and precursor Dsg3 by mouse anti-Dsg3 mAbs, AK23, and AK15 (Figure 7b). AK15 is an anti-Dsg3 monoclonal Ab that recognizes a linear epitope in the middle portion of Dsg3, so that the Ab should bind both the mature and precursor forms of Dsg3. AK23 mAb showed dose-dependent reactivity to the complete-mature plate, while showing no reactivity to the complete-precursor plate. AK15 mAb exhibited similar binding capacity to both plates.
Figure 7. Majority of anti-desmoglein 3 (Dsg3) antibody binding in PV sera targets the mature form more than the precursor form of Dsg1.
(a) Coomassie blue staining of purified recombinant baculoproteins used for precursor and mature Dsg1 ELISA plates. White arrowhead indicates the precursor form and black arrow head indicates the mature form. (b) Binding curves of representative anti-Dsg3 mAbs (AK23, AK15) against mature and precursor Dsg3 ELISA plates. (c) Dispersion of immunoreactivity of 47 PV sera between the mature and precursor Dsg3 ELISA plates. Each closed square represents single patient. Most PV sera showed stronger reactivity against mature Dsg3 plate than against precursor Dsg3 plate.
We tested 47 PV sera against these plates. All but five PV sera had stronger reactivity against the complete-mature plate than against the complete-precursor plate (Figure 7c). For PV sera, the average and standard deviation of immunoreactivity against mature Dsg3 was 0.74 ± 0.36, whereas the average and standard deviation of immunoreactivity against the precursor form was 0.20 ± 0.13 (paired t-test, P < 0.001). These data suggest that the majority of anti-Dsg3 immunoreactivity in PV also targets epitopes that are unmasked by proteolytic processing.
Titers measured by mature Dsg ELISA tend to reflect the disease activity more precisely than that by the precursor Dsg ELISA
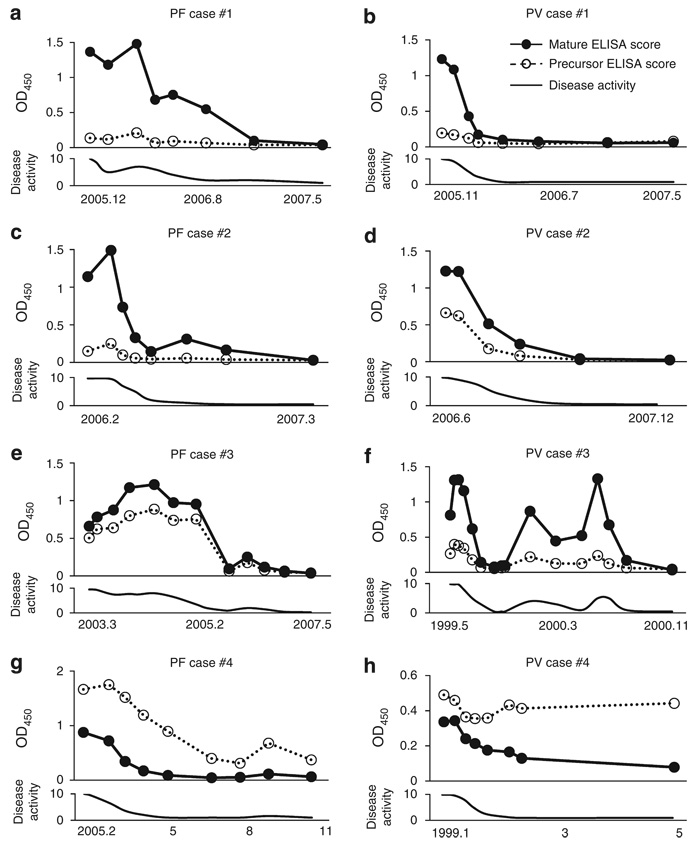
Finally, we analyzed PF and PV cases for immunoreactivity against the mature and precursor forms of Dsg1 and Dsg3 over time, and compared them with clinical disease activity. First, we analyzed three PF and three PV cases in which the immunoreactivity against the mature form was stronger than that against the precursor form of Dsg because we considered them as typical cases. As shown in Figure 8a–f, the immunoreactivity against both the mature and precursor Dsg tended to fluctuate in parallel with disease activity. However, immunoreactivity against the mature form was a more sensitive indicator of disease activity in most patients, because the reactivity against the precursor was often minimal or negative, even at times of disease activity (Figure 8a–d and f). We also analyzed two unusual cases that showed stronger reactivity against the precursor Dsg plate than against the mature form (Figure 8g and h). In one case (Figure 8g), the ELISA reactivity against the precursor Dsg did reflect the disease activity well, but in the other (Figure 8h), the reactivity against the precursor did not fall with decreased disease activity, as did the reactivity against the mature protein. In summary, considering all the patients whom we tested (Figure 8), reactivity against the mature protein correlated well with disease activity, whereas reactivity against the precursor Dsg sometimes did not.
Figure 8. Time-course analysis of immunoreactivity against precursor and mature form of desmoglein (Dsg) in PF and PV patients.
Immunoreactivities against precursor and mature form of Dsg were compared with the disease activity over a time course. four PF patients (a, c, e, and g) and four PV patients (b, d, f, and h) were studied. Each serum was diluted up to 1:100 (a), 1:100 (b), 1:100 (c), 1:200 (d), 1:200 (e), 1:100 (f), 1:6400 (g), and 1:100 (h). Clinical disease activity was subjectively assessed and the disease activity in the first presentation was scored as 10. (0 (normal) to 10 (most severe)). three PF (a, c, and e) and three PV (b, d, and f) were randomly chosen from the PF and PV patients’ sera, which showed stronger reactivity against the mature Dsg plate than against the precursor Dsg plate. As unusual cases, a PF (g) and a PV case (h) who showed stronger reactivity against precursor-Dsg than against the mature-Dsg form were selected.
DISCUSSION
Detailed characterization of monoclonal anti-Dsg1 Abs isolated from a PF patient revealed that the patient had Abs against the mature and precursor forms of Dsg1. We also found that a pathogenic Dsg1 Ab binds only the mature form, whereas non-pathogenic ones bind only the precursor form or bind both mature and precursor forms. These findings led us to explore the immunoreactivity of multiple pemphigus sera against the mature and precursor forms of Dsg1 and 3. Competition ELISA showed that much of the immunoreactivity in multiple PF sera binds to the same, or nearby, epitopes defined by the pathogenic Ab. ELISA using the mature and precursor Dsgs showed that much of the immunoreactivity in the majority of pemphigus sera target the mature form of Dsg than with the precursor form. Furthermore, a longitudinal study of pemphigus patients showed that immunoreactivity against the mature Dsg reflects the disease activity well. All these findings suggest that epitopes that are masked by the prosequence are very important in pathogenesis in pemphigus.
Although the pathogenic PF monoclonal anti-Dsg1 Ab, used in this study, which binds only the mature form of Dsg1, is dependent on the conformation of Dsg1 (Table 1), a pathogenic anti-Dsg Ab has been described that binds a non-conformational epitope (Yeh et al., 2006). In addition, it has been reported that IgG against non-conformational epitopes in pemphigus also correlated with clinical disease activity (Muller et al. 2006, 2008). Although it remains to be determined, it is not unreasonable to assume that the prosequence could block the binding of pathogenic Abs against both conformational and non-conformational pathological epitopes.
High-resolution crystal structure analysis of the entire extracellular domain of C-cadherin provided a mechanistic basis for intermolecular cadherin interactions (Boggon et al., 2002). The trans-adhesive interface is a two-fold symmetrical interaction, as the conserved tryptophan (W2) side chain at the amino-terminal end of the cadherin molecule from one cell inserts into the hydrophobic pocket at the amino-terminal end of a cadherin molecule on an opposing cell. Recent cryo-electron tomography of vitreous sections from human epidermis visualized the three-dimensional molecular architecture of desmosomal cadherins, which resembled the X-ray structure of C-cadherin (Al-Amoudi et al., 2007). Considering the similarity of amino-acid sequences between the ectodomains of classic cadherins and desmosomal cadherins, they probably share similar adhesive domains. Immunoblot analysis using domain-swapped molecule of Dsg1/Dsg2 or Dsg3 revealed that the epitope of 3–30/3h was mapped to amino acid 89–101 of the EC1 region of Dsg1 (Figure 5). When the amino-acid sequences of Dsg1 were superimposed on the predicted structure of C-cadherin, the predicted residues for the adhesive interface of the W2 acceptor sites (hydrophobic pocket) were 24I, 38Y, 78C, 80A, 89E, 91P, and 92L (Boggon et al., 2002). This putative W2-acceptor sites partially overlapped with the presumed epitope of 3–30/3h, indicating that the epitope of 3–30/3h is located in a presumptive hydrophobic pocket of the adhesive interface of Dsg1. Furthermore, previous studies suggested that the prosequence of E-cadherin blocks the N-terminal adhesive region of cadherin to prevent self-aggregation during protein synthesis in the Golgi or endoplasmic structure, and proteolytic processing creates the active form by conformational changes or by exposure of active binding sites (Ozawa and Kemler, 1990). In addition, the three-dimensional structure study of the prosequence of N-cadherin showed that the proregion is a structure similar to the cadherin adhesive domain but lacking features for cadherin–cadherin interactions, suggesting that the prosequence might render interaction sites less accessible and also prevent the propagation of the interactions (Koch et al., 2004). Therefore, by analogy, pathogenic Abs in pemphigus sera target regions of Dsgs that are functionally important and are unmasked by proteolytic processing. This finding is consistent with the hypothesis that the direct inhibition of trans-interaction of Dsg by autoantibodies plays a primary role in inducing blister formation in the pemphigus. However, we cannot rule out an alternative hypothesis that Ab binding to exposed adhesive sites in Dsg somehow causes signaling inside the cells or internalization of Dsg, which leads to acantholysis. In any case, our results showed the importance of pemphigus Ab binding to Dsgs after adhesive domains are exposed by its normal proteolytic processing (Figure 9b).
We should mention that not all pathogenic autoantibodies target the regions that are masked by the prosequence. For example, we have identified another weakly pathogenic anti-Dsg1 monoclonal Ab (3–7/1e) that did not cause blisters in mice, but induced slight blister formation in human skin (Ishii et al., 2008). This Ab bound both the mature and the precursor form of Dsg1. Epitope mapping using a domain-swapped molecule of Dsg1/Dsg2 revealed that the epitope resides in the EC2 domain of Dsg1 (data not shown). It may be possible that such Abs block the cis-interaction of Dsgs. The mechanism of blister formation by such Abs remains to be determined.
Another major finding in this paper is that PF sera have a subset of autoantibodies against only a precursor Dsg1. The frequency of PF sera that contains Abs against the precursor form of Dsg1 may be high because inhibition of ELISA in a previous paper showed that the binding of the non-pathogenic scFvs against precursor Dsg1 was inhibited by four out of the six PF sera tested (Ishii et al., 2008). However, in almost all PF sera, only a small proportion of their reactivity is against the precursor form of Dsg1, as shown by the competition ELISA of the PF sera by anti-Dsg1 scFvs (Figure 4), and by the finding that both PF and PV sera have stronger reactivity against the mature than against the precursor form of Dsg (Figure 6c and Figure 7c). The epitope bound by the scFv mAbs reacting exclusively against the precursor form of Dsg1 could either be the prosequence itself or a conformational epitope on Dsg1 stabilized by the prosequence and lost in the mature protein. Further definition of this exact epitope awaits additional studies.
Cloned scFv that binds exclusively against the precursor Dsg1 shows weak intracellular staining by indirect immunofluorescence, as opposed to the cell surface staining typical of pemphigus. We think this staining is weak because there is probably little intracellular precursor Dsg as most is efficiently processed to the mature cell surface form. We can detect this weak staining because we have potent monoclonal scFv against precursor Dsg1, but such staining would be difficult or impossible to appreciate with polyclonal pemphigus sera that have a predominant Ab response against the mature cell surface Dsg.
We do not know how these Abs against the precursor form are involved in the pathogenesis in PF. It may be possible that PF patients first develop Abs against the intracellular precursor Dsg1, which may not normally be exposed to the immune system but is released at times of keratinocyte injury, and subsequently develop pathogenic Abs against mature Dsg1. Another possibility is that Abs against the unprocessed form of Dsg1 are developed after PF is active as a consequence of the damage to keratinocytes.
The findings we presented here have clinical implications. We showed that the disease activity was more precisely correlated with the level of immunoreactivity against the mature form of Dsg by ELISA than that against the premature Dsg ELISA. There is another therapeutic implication of our findings. We found that much of the immunoreactivity in pemphigus sera target very restricted regions of Dsg that are masked by the prosequece. Therefore, we could develop a new therapeutic strategy by immunoadsorption of Abs against such restricted regions.
In this study, we found that the majority of immunoreactivity in pemphigus sera targets the restricted regions that are unmasked by proteolytic cleavage of prosequence. The finer definition of the pathogenic hot spot on Dsg has provided an important framework to understand the molecular mechanism of blister formation in pemphigus, as well as that of cell–cell adhesion in desmosomes.
MATERIALS AND METHODS
Human sera
Sera were obtained from 45 patients with PF and from 47 patients with PV. The diagnosis of PF and PV was made on the basis of clinical features, histology, and immunofluorescence findings. We chose sera from the clinically active stage. For longitudinal follow-up study, serum samples were collected from three PF and three PV cases, which showed that the immunoreactivity against the mature Dsg form was stronger than that against the precursor form and, as unusual cases, from one PF and one PV case, which showed stronger reactivity against the precursor form than against the mature form. This study was approved by the institution review board of Keio University and was conducted according to the Declaration of Helsinki Principles. All samples were used with informed consent.
Production and purification of anti-Dsg1 scFv mAbs
We used four representative monoclonal anti-Dsg1 scFv Abs isolated from a PF patient by phage display (Table 1). ScFv mAbs were produced in the Top10F’ strain of E coli (Invitrogen, San Diego, CA) and purified by Talon affinity resin (Clontech, Palo Alto, CA) as previously described. (Ishii et al., 2008).
Production of AK series of mouse anti-Dsg3 mAbs
AK series of mouse AK7, AK15, and AK23 mAb against mouse and human Dsg3 were prepared as previously described (Tsunoda et al., 2003).
Plasmid constructs
Construction of pQE-Dsg1-facX
Plasmid pEVmod-Dsg1-EHis with the entire extracellular domain of Dsg1 fused with E-tag and his-tag has been described previously (Ishii et al., 1997). The putative endoproteolytic cleavage site of Dsg1 (RQKR) was substituted with the recognition site of blood coagulation factor Xa (IEGR) using sequential PCR. In brief, the first step PCR was carried out on pEVmod-Dsg1-EHis to generate two fragments (primers A and B for fragment1, and primers C and D for fragment 2) with mutations for the factor Xa recognition site at the 3′end and 5′end, respectively. The two fragments encompassing the mutation were annealed with each other and extended by mutually primed synthesis. Subsequently, this fragment was reamplified by a second PCR step using primers A and D. The following are the primers used in this sequential PCR: 5′-primer A, 5′-GAA-GAT-CTA-CCA-TGG-ACT-GGA-GTT-TCT-TCA-G-3′; 3′-primer B, 5′-CAT-TCA-CGG-CCT-TCG-ATT-GAA-TGC-CAT-TTG-3′; 5′-primer C, 5′-GAA-TCG-AAG-GCC-GTG-AAT-GGA-TCA-AGT-TCG-3′; 3′-primer D, 5′-CCG-GTC-GAC-ATG-TAC-ATT-GTC-TGA-TAA-C. The final PCR product containing the factor Xa recognition site was digested with an NcoI and SalI, and ligated together with NcoI-XhoI-cut pQE-trisystem vector (Invitrogen). This construct was designated as pQE-Dsg1-FacX (p330).
Production of recombinant baculoproteins
Precursor-FacX-Dsg1-EHis
pQE-Dsg1-FacX was cotransfected with the Baculo-sapphire baculovirus DNA (Orbigen, San Diego, CA) into cultured insect Sf9 cells, and recombinant viruses were obtained. For baculoprotein production, High Five cells (Invitrogen) cultured in a serum-free EX cell 405 medium (SAFC biosciences, Lenexa, KS) were infected with high-titer virus stock and incubated at 27°C for 3 days. Precursor-FacX-Dsg1-EHis was secreted in the culture supernatant.
Mature-Dsg1-EHis
Dsg1-EHis baculoprotein was cleaved by incubation with proprotein convertase furin (New England BioLabs, Beverly, MA). Typically, 50 µl of the culture supernatant was incubated with two units of furin at room temperature for 15 hours.
Production of highly purified mature and precursor ELISA plates
Highly purified precursor and mature Dsg1 plates
For obtaining complete-precursor Dsg1-EHis, baculovirus-expressed recombinant Dsg1-Ehis, which contained both the precursor and the mature form, was purified by TALON affinity resin (Clontech) and subsequently adsorbed by a 3–30/3h mAb beads column (Figure 4a) to eliminate the mature form of Dsg1-EHis. For obtaining complete-mature Dsg1-EHis, recombinant Dsg1-EHis baculoprotein was purified by TALON affinity resin, treated with furin (20 unitmg−1) for 16 hours at room temperature in the manufacturer’s recommended buffer (New England Biolabs), and subsequently passed through an affinity column containing 3–97/1C mAb to absorb the precursor form (Figure 4a). The purified complete-precursor Dsg1-EHis and complete-mature Dsg1-EHis were immobilized on 96-well polystyrene plates (Maxisorb Immunoplate; Nunc, Wiesbaden, Germany) by coating each well with 2.5 µgml−1 of recombinant proteins at 4°C for 20 hours.
Highly purified precursor and mature Dsg3 plates
For obtaining complete-precursor Dsg3-EHis, baculovirus-expressed recombinant Dsg3-Ehis, which contained both the precursor and the mature form was purified by TALON affinity resin (Clontech) and subsequently adsorbed by AK23 mAb-bound affinity column (Figure 4a) to eliminate the mature form of Dsg3-EHis (Figure 5a). For obtaining complete-mature Dsg3-EHis, purified recombinant Dsg3-EHis baculoprotein was treated with furin using the above protocol and again purified on TALON affinity resin. Complete-precursor Dsg3-EHis and complete-mature Dsg3-EHis were immobilized on 96-well polystyrene plates (Nunc).
For the longitudinal study to compare reactivity against mature and precursor Dsg ELISA with disease activity, we measured OD of pemphigus sera at an approximate dilution (1:100–1:6,400).
Dsg1 scFv ELISA
The reactivity of scFv against human Dsg1 was measured by Dsg1 ELISA (Medical and Biological Laboratories Co. Ltd, Nagoya, Japan) using a horseradish peroxidase-conjugated anti-HA (Hemagglutinin) monoclonal Ab (clone 3F10, 1:1,000 dilution, Roche Diagnostics Corp., Basel, Switzerland) as a secondary Ab as described (Ishii et al., 2008).
To increase the ratio of the mature form on a standard Dsg1 ELISA plate, the plates were pretreated with 2U/ well of furin (New England Biolabs, Ipswich, MA) in Tris-buffered saline with 1mm CaCl2 at room temperature for 5 hours.
Indirect immunofluorescence
Immunofluorescence for scFvs was carried out on human skin as previously described (Payne et al., 2005). Binding was detected using a rat monoclonal anti-HA Ab (3F10, 1:100 dilution, Roche Diagnostics) and thereafter using a Alexa Fluor 446-conjugated anti-rat IgG (1:200 dilution, Invitrogen). A confocal image was obtained using a Zeiss LSM 510 confocal microscope (Carl Zeiss Meditec, Dublin, CA).
Immunoblotting
The recombinant baculoproteins were size-fractionated by SDS-PAGE and transferred to an Immobilon-P membrane (Millipore, Bedford, MA), and detected using an anti-E-tag Ab (1:2,000 dilution, GE Healthcare Bio-Sciences, Piscataway, NJ), followed by alkaline phosphatase-conjugated goat anti-mouse IgG Abs (1:1,000 dilution, DPRA-Zymax, San Francisco, CA).
Immunoprecipitation–immunoblotting analysis
Baculovirus-infected insect cell culture supernatants containing recombinant proteins were incubated with anti-Dsg1 scFv mAbs for 30 minutes and then immunoprecipitated with anti-HA agarose (Sigma-Aldrich, St Louis, MO) at 4°C for 2 hours with gentle rotation. After washing with Tris-buffered saline-calcium, the immunoprecipitates were resuspended in a Laemmli sample buffer, separated by SDS-PAGE, and transferred to an Immobilon-P membrane (Millipore). The membranes were probed with an anti-E-tag Ab (1:2,000 dilution, GE Healthcare Bio-Sciences) followed by alkaline phosphatase-conjugated goat anti-mouse IgG Abs (1:1,000 dilution, DPRA-Zymax). The recombinant proteins used in the epitope mapping of 3–30/3h mAb have been described previously ((Sekiguchi et al., 2001) and Chan et al. (manuscript in preparation)).
Inhibition ELISA
PF sera were diluted to result in an OD450 reading of ~1.0 in the Dsg1 ELISA without competitors. The diluted PF sera, mixed with scFv mAbs, were analyzed by standard Dsg1 ELISA (Medical and Biological Laboratories) developed using a horseradish peroxidase-conjugated anti-human Fab Ab. Inhibition was calculated according to the following formula: % inhibition = (1 − (OD T/B–OD N/B)/(OD T/Bc − OD N/Bc))*100, where T is the PF sera tested, N is the normal control serum, B is the blocking monoclonal anti-Dsg1 scFvs, and Bc is the control scFv.
ACKNOWLEDGMENTS
We thank Dr Aimee Payne for helpful discussions, Mrs Minae Suzuki for immunofluorescence staining, and Yoshiko Fujii and Sakiko Kobayashi for technical assistance. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases from Ministry of Health, Labor and Welfare of Japan. These studies were also supported, in part, by grants (RO1-AR538807 and RO1-AR546265) from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases.
Abbreviations
- Ab
antibody
- Dsg
desmoglein
- mAb
monoclonal antibody
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
- scFv
single-chain variable fragment
Footnotes
CONFLICT OF INTEREST
Drs Ishii and Stanley have filed for a provisional patent on the Dsg1 mAbs described herein. Ms Kuroda and Dr Hachiya belong to Medical and Biological Laboratories Co. Ltd, who supply Dsg1 and Dsg3 ELISA kits.
REFERENCES
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, et al. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, et al. Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- Esaki C, Seishima M, Yamada T, Osada K, Kitajima Y. Pharmacologic evidence for involvement of phospholipase C in pemphigus IgG-induced inositol 1,4,5-trisphosphate generation, intracellular calcium increase, and plasminogen activator secretion in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. 1995;105:329–333. doi: 10.1111/1523-1747.ep12319948. [DOI] [PubMed] [Google Scholar]
- Hanakawa Y, Schechter NM, Lin C, Garza L, Li H, Yamaguchi T, et al. Molecular mechanisms of blister formation in bullous impetigo and staphylococcal scalded skin syndrome. J Clin Invest. 2002;110:53–60. doi: 10.1172/JCI15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, et al. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128:939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AW, Farooq A, Shan W, Zeng L, Colman DR, Zhou MM. Structure of the neural (N-) cadherin prodomain reveals a cadherin extracellular domain-like fold without adhesive characteristics. Structure. 2004;12:793–805. doi: 10.1016/j.str.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Muller R, Svoboda V, Wenzel E, Gebert S, Hunzelmann N, Muller HH, et al. IgG reactivity against non-conformational NH-terminal epitopes of the desmoglein 3 ectodomain relates to clinical activity and phenotype of pemphigus vulgaris. Exp Dermatol. 2006;15:606–614. doi: 10.1111/j.1600-0625.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- Muller R, Svoboda V, Wenzel E, Muller HH, Hertl M. IgG against extracellular subdomains of desmoglein 3 relates to clinical phenotype of pemphigus vulgaris. Exp Dermatol. 2008;17:35–43. doi: 10.1111/j.1600-0625.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Correct proteolytic cleavage is required for the cell adhesive function of uvomorulin. J Cell Biol. 1990;111:1645–1650. doi: 10.1083/jcb.111.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AS, Ishii K, Kacir S, Lin C, Li H, Hanakawa Y, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115:888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthaus H, Dubois CM, Laprise MH, Grondin F, Suter MM, Muller E. Proprotein cleavage of E-cadherin by furin in baculovirus overexpression system: potential role of other convertases in mammalian cells. FEBS Lett. 1998;438:306–310. doi: 10.1016/s0014-5793(98)01330-1. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J Immunol. 2001;167:5439–5448. doi: 10.4049/jimmunol.167.9.5439. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci. 2007;48:1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sharma P, Choi EJ, Kuroda K, Hachiya T, Ishii K, Payne AS. Pathogenic anti-desmoglein monoclonal antibodies demonstrate variable ELISA activity due to preferential binding of mature versus proprotein isoforms of desmoglein 3. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.41. advance online publication 12 March 2009; doi:10.1038/jid.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JR, Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 2006;355:1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- Tsunoda K, Ota T, Aoki M, Yamada T, Nagai T, Nakagawa T, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J Immunol. 2003;170:2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- Wahl JK, III, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- Waschke J, Spindler V, Bruggeman P, Zillikens D, Schmidt G, Drenckhahn D. Inhibition of Rho A activity causes pemphigus skin blistering. J Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L, Raess NA, Caldelari R, Zakher A, de Bruin A, Posthaus H, et al. Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. Embo J. 2006;25:3298–3309. doi: 10.1038/sj.emboj.7601224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Cavacini LA, Bhol KC, Lin M, Kumar M, Duval M, et al. Pathogenic human monoclonal antibody against desmoglein 3. Clin Immunol. 2006;120:68–75. doi: 10.1016/j.clim.2006.03.006. [DOI] [PubMed] [Google Scholar]