Abstract
Obstructive sleep apnea (OSA) is a highly prevalent disease characterized by recurrent episodes of upper airway obstruction that result in recurrent arousals and episodic oxyhemoglobin desaturations during sleep. Significant clinical consequences of the disorder cover a wide spectrum, including daytime hypersomnolence, neurocognitive dysfunction, cardiovascular disease, metabolic dysfunction, and cor pulmonale. The major risk factors for the disorder include obesity, male gender, and age. Current understanding of the pathophysiologic basis of the disorder suggests that a balance of anatomically imposed mechanical loads and compensatory neuromuscular responses are important in maintaining upper airway patency during sleep. OSA develops in the presence of both elevated mechanical loads on the upper airway and defects in compensatory neuromuscular responses. A sleep history and physical examination is important in identification of patients and appropriate referral for polysomnography. Understanding nuances in the spectrum of presenting complaints and polysomnography correlates are important for diagnostic and therapeutic approaches. Knowledge of common patterns of OSA may help to identify patients and guide therapy.
Keywords: critical pressure, diagnosis, obstructive sleep apnea, pathophysiology
Obstructive sleep apnea (OSA) is a highly prevalent disease, affecting 4% of men and 2% of women,1 and strongly linked to the current obesity epidemic. The disorder is characterized by recurrent episodes of upper airway obstruction, and is associated with reductions in ventilation, resulting in recurrent arousals and episodic oxyhemoglobin desaturations during sleep.2 Significant clinical consequences of the disorder cover a wide spectrum including daytime hypersomnolence,3,4 neurocognitive dysfunction,5 cardiovascular disease (hypertension, stroke, myocardial infarction, heart failure),6,7 metabolic dysfunction,8–10 respiratory failure, and cor pulmonale.11 The major risk factors for the disorder include obesity, male gender, postmeno-pausal status, and age, and are discussed in detail elsewhere.12–14 The rising prevalence of obesity in the United States suggests that OSA will represent an escalating public health burden.15 Therefore, knowledge and understanding of the pathogenic basis, clinical presentation, and diagnosis of OSA are essential for the development of preventive, screening, and therapeutic strategies to reduce the public health burden of the disorder. In this review, we will review the current state of knowledge regarding the pathophysiologic basis of OSA and its clinical presentation and diagnosis.
Pathophysiology of Upper Airway Obstruction in OSA
The pharynx is a complex structure that serves several purposes including speech, swallowing, and respiration. The human pharynx is composed of > 20 muscles and divided into four sections that include the nasopharynx (from the nasal turbinates to the start of the soft palate), velopharynx (from the start of the soft palate to the tip of the uvula), oropharynx (from the tip of the uvula to the tip of the epiglottis), and hypopharynx (from the tip of the epiglottis to the level of the vocal cords). The human pharynx can be considered as a collapsible tube that is uniquely susceptible to collapse due to the presence of a floating hyoid bone, a longer airway, and a less direct route for inspired air to travel when compared to other mammals. The presence of soft tissues and bony structures, which increase extraluminal tissue pressures surrounding the upper airway, can predispose the pharynx to collapse. In contrast, the actions of pharyngeal dilator muscles maintain pharyngeal patency due to reflex pathways from the CNS and within the pharynx. The presence of these opposing forces suggest that increased pharyngeal collapsibility is due to alterations in anatomically imposed mechanical loads and/or in dynamic neuromuscular responses to upper airway obstruction during sleep.
Measurements of Pharyngeal Collapsibility
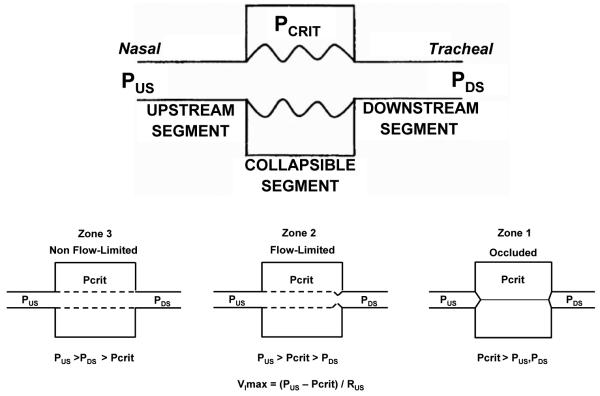
Quantitative measurements of mechanical and neuromuscular contributions to pharyngeal collapsibility have been difficult to derive during sleep. One approach has been to model the upper airway as a collapsible tube (ie, a Starling resistor). The relationship of pressure and flow through collapsible tubes has been well defined in the pulmonary and systemic circulation, the intrathoracic airways, and more recently the upper airway. In the Starling resistor model (Fig 1), the collapsible segment of the tube is bound by an upstream and downstream segment with corresponding upstream pressure (Pus) downstream pressure (Pds) and upstream resistance and downstream resistance. Occlusion occurs when the surrounding pressure (Pcrit), becomes greater than the intraluminal pressure, resulting in a transmural pressure of zero. In this model of the upper airway, Pus is atmospheric at the airway opening and Pds is the tracheal pressure. When the Pcrit is significantly lower than the Pus and Pds (Pus > Pds > Pcrit; analogous to West zone 3 of the lung), flow throughthe tube follows the principles of an Ohmic resistor. When the Pds falls during inspiration below Pcrit (Pus > Pcrit > Pds; analogous to West zone 2 of the lung), inspiratory airflow limitation occurs and is independent of further decreases in Pds. Under this condition, the pharynx is in a state of partial collapse and maximal inspiratory airflow varies linearly as a function of the difference between the Pus and Pcrit. Finally, when the Pus falls below Pcrit (Pcrit > Pus > Pds; analogous to West zone 1 of the lung), the upper airway is occluded.
Figure 1.
In the Starling resistor model, the collapsible segment of the tube is bound by an upstream and downstream segment with corresponding upstream and downstream pressures (Pus and Pds) and resistances (upstream resistance pressure and downstream resistance; data not shown). See text for further explanation (adapted in part from Gleadhill et al18). Vimax = maximal inspiratory flow; Rus = upstream resistance.
Operationally, Pcrit in the human upper airway is determined by lowering the nasal pressure until inspiratory airflow ceases. Measurements of Pcrit have been shown to define a spectrum of upper airway obstruction from normal breathing (Pcrit < − 10 cm H2O), to snoring (Pcrit range, − 10 to − 5 cm H2O), to obstructive hypopneas (Pcrit range, − 5 to 0 cm H2O) and, finally, obstructive apneas (Pcrit > 0 cmH2O) during sleep.16–18 Patients with the upper airway resistance syndrome (UARS), an entity characterized by flow-limited breathing that result in arousals, has been shown to have Pcrit levels that are between snoring and hypopneas.19 Depending on the methodology, measurements of Pcrit reflect either the contributions of anatomically imposed mechanical loads on the upper airway or dynamic neuromuscular responses that maintain upper airway patency. The details regarding the physiologic concept of Pcrit are reviewed in further detail elsewhere.20
Contribution of Anatomic Factors to OSA
OSA is known to be associated with alterations in upper airway anatomy. Structural changes including tonsillar hypertrophy,21 retrognathia,22 and variations in craniofacial structures23,24 have been linked to an increased risk of sleep apnea, presumably by increasing upper airway collapsibility. Ethnic differences in craniofacial features are one potential mechanistic explanation for observed differences in OSA prevalence and severity for a given level of obesity.24,25 During wakefulness, CT and MRI studies26–28 have demonstrated increased fatty tissue deposition and submucosal edema in the lateral walls of the pharynx, both of which narrow the pharyngeal lumen and may predispose to obstruction during sleep.
Based on the presence of upper airway anatomic alterations in OSA patients, investigators29,30 have proposed that structural or mechanical alterations are a primary determinant of upper airway obstruction during sleep. Recent data suggest that structural alterations in the lateral pharyngeal walls and tongue aggregate on a familial basis, suggesting genetic susceptibility to OSA.31 In addition, experimental data in the absence of neuromuscular activity demonstrate a reduction in maximal pharyngeal area and elevated Pcrit in OSA subjects compared with normal subjects.29 Furthermore, obesity, jaw position, acromegaly, tonsillar hypertrophy, and a smaller bony enclosure surrounding the pharynx have been demonstrated to predispose toward pharyngeal collapsibility.23,32–36 These studies imply that upper airway structural differences distinguish OSA patients from normal subjects, and may predispose to upper airway obstruction when protective neuromuscular mechanisms wane at sleep onset.37
Obesity, the major risk factor for OSA, has been linked with elevations in neck circumference and increased amounts of peripharyngeal fat,38,39 which could narrow and compress the upper airway. Furthermore, increased parapharyngeal fat has been correlated with increased sleep apnea severity.27,40 Finally, a study41 of resected uvular tissue revealed greater amounts of submucosal fatty tissue in patients with OSA. The compressive effects of fatty tissue deposited around the pharynx therefore may increase upper airway collapsibility, and possibly offset the effects of dilator muscles that maintain airway patency. Obesity may also increase pharyngeal collapsibility through reductions in lung volumes, particularly decreases in functional residual capacity, which are accentuated with the onset of sleep. Reductions in function residual capacity may increase pharyngeal collapsibility through reductions in tracheal traction on the pharyngeal segment. Conversely, increases in lung volumes result in increased tracheal traction and stabilize the upper airway during inspiration.42–44 In OSA patients, increases in lung volumes have been shown to decrease continuous positive airway pressure (CPAP) requirements and OSA severity, suggesting corresponding improvements in pharyngeal collapsibility.45,46
Contribution of Neuromuscular Factors to OSA
It should be noted, however, that anatomically imposed mechanical loads on the upper airway may not be sufficient to produce pharyngeal collapse during sleep. For example, women have been shown to have a smaller pharynx and oropharyngeal junction than men,47 despite having a lower prevalence of OSA.1 Furthermore, measurements of Pcrit under conditions of low neuromuscular activity, which reflect upper airway mechanical loads, demonstrate significant overlap between OSA and normal subjects.48 Thus, nonstructural (ie, neuromuscular) factors must also play a role in protecting the upper airway. In fact, changes in upper airway neuromuscular activity during sleep were originally described by Remmers et al,2 who demonstrated that genioglossal electromyogram (EMGgg) activity was reduced at apnea onset and increased with arousal when airway patency was restored. Subsequently, it was recognized that upper airway obstruction could trigger a variety of neuromuscular responses that restore upper airway patency by recruiting muscles that dilate and elongate the airway. Various pharyngeal muscle groups are important in stabilizing the upper airway throughout the respiratory cycle (tonic activity, eg, tensor palatini) and in dilating the airway during inspiration (phasic activity, eg, genioglossus). Pharyngeal motor output is modulated by a number of factors that include wake vs sleep state dependent mechanisms, local mechanoreceptor responses to negative pressure, and ventilatory control mechanisms.
In OSA patients during wakefulness, elevated genioglossal and tensor palatini muscle activity have been observed and are significantly lowered with the application of positive nasal pressure.49 In contrast, normal subjects had lower levels of genioglossal and tensor palatini muscle activity that were not further reduced when positive nasal pressure was applied. These observations suggested that increased upper airway dilator muscle activity compensates for a more anatomically narrow upper airway in the OSA patient. Thus, reductions in upper airway muscle activity with sleep onset through serotonergic, cholinergic, noradrenergic, and histaminergic pathways may lead to upper airway obstruction and have been hypothesized to be due to the loss of a “wakefulness stimulus” that may be greater in OSA patients than healthy control subjects.37,49,50
Pressure-sensing mechanisms play a prominent role in modulating upper airway neuromuscular activity during wakefulness and sleep. A negative pressure reflex within the upper airway serves to stabilize the upper airway during inspiration. At least three lines of evidence suggest that the negative pressure reflex is primarily mediated by mechanoreceptors within the pharynx. First, there is a tight relationship between EMGgg and pharyngeal pressure independent of the central respiratory pattern generator within the brainstem.51 Second, topical anesthesia to the pharyngeal mucosa attenuates the relationship between genioglossal muscle activity and pharyngeal pressure with an increased number of obstructive apneas and hypopneas during sleep in normal subjects and loud snorers, and/or increased duration of apneic episodes.52–54 Third, marked decreases in EMGgg activity with corresponding increases in pharyngeal collapsibility have been observed in patients when breathing through a tracheostomy compared to nasal breathing,55,56 suggesting that negative pressure within the pharynx during inspiration stabilizes upper airway patency.
It is possible that OSA results from trauma to the upper airway due to repetitive collapsing and opening of the upper airway over time, resulting in muscle and neuronal fiber injury. Upper airway sensory pathways may be impaired in OSA patients because temperature, two-point discrimination, and vibratory thresholds are disrupted in OSA patients compared to normal individuals.57–59 Sensory receptor dysfunction could attenuate the response of upper airway dilator muscles to the markedly negative airway pressures generated during periods of upper airway obstruction. Further evidence for sensorimotor dysfunction and an upper airway myopathy is provided by graded histopathologic and immunochemical alterations in the palatopharyngeus and muscularis uvulae in OSA patients, relative to asymptomatic snorers and normal subjects.60–63 The extent to which such mechanisms are important in the pathogenesis of OSA, however, remains to be established.
Contribution of Neuroventilatory Factors to OSA
Ventilatory control mechanisms may also play a role in modulating pharyngeal collapsibility during sleep. Preactivation of the pharyngeal dilator muscles stabilizes the upper airway prior to the inflow of air and suggests CNS coordination between the upper airway and diaphragm. The CNS is influenced by central and peripheral chemoreceptors with conditions of hypercapnia and hypoxemia increasing central drive to the upper airway and decreasing pharyngeal collapsibility.64,65 Increased hypercapnic ventilatory responses, prolonged circulatory times, or low oxygen stores within the body can result in ventilatory instability that leads to the development of periodic breathing.66 Sleep also unmasks a highly sensitive apneic threshold (the Paco2 level below which an apnea occurs) that remains within 1 to 2 mm Hg of the normal waking eupnic Paco2 level. Therefore, a brisk ventilatory response as seen during an arousal in the susceptible individual can result in hypocapnia that is near or at the sleeping apneic threshold and result in a hypopnea or apnea with the reinitiation of sleep.67,68 In fact, measurements of loop gain, a measure of ventilatory control instability, have been demonstrated to be high in patients with more severe OSA compared to patients with mild OSA (the details regarding loop gain and its components, plant gain and controller gain are described in detail elsewhere).66,69,70 Furthermore, in OSA patients with moderate mechanical loads on the upper airway, a high loop gain predicted OSA severity.71 In contrast, during periods of ventilatory instability, individuals with low levels of mechanical loads on the upper airway appear to be resistant to the development of upper airway obstruction.68,72
Nevertheless, whether alteration of loop gain is important in the pathogenesis of upper airway obstruction or is a consequence of OSA has not been established. OSA can develop in normal subjects with the application of negative nasal pressure to the upper airway (lowering the Pus near or below the Pcrit level seen in normal subjects), which reduces the transmural pressure across the upper airway to near or below zero, and results in upper airway obstruction with recurrent obstructive hypopneas and/or apneas.48,73 Furthermore, in a study48 of matched normal subjects and OSA patients, recurrent obstructive hypopneas occurred at similar levels of upper airway obstruction. Taken together, these findings suggest that reductions in airflow through the development of upper airway obstruction are a necessary and sufficient condition for the initiation of OSA, and that ventilatory control mechanisms may be relevant to sustaining recurrent OSA.
Interaction of Anatomic and Neuromuscular Factors on Pharyngeal Collapsibility
It is likely that a combination of upper airway mechanical loads and disturbances in neuromuscular mechanisms account for the pathogenesis of OSA. For example, in a group of OSA subjects, one third of the variability in OSA severity was ascribed to mechanical loads, suggesting that neuromuscular mechanisms accounted for the remaining two thirds.74 Using techniques to partition the relative contribution of mechanical and neuromuscular factors toward pharyngeal collapsibility, it has been shown that OSA patients during sleep have both an increased mechanical load on the upper airway (passive Pcrit) and impaired neuromuscular responses to upper airway obstruction (active Pcrit).48 As demonstrated in Figure 2, a Pcrit of approximately − 5 cm H2O represents the disease threshold, above which obstructive hypopneas and apneas occurred. In normal subjects, when mechanical loads on the upper airway lowered the Pcrit below the disease threshold, OSA was not present. In contrast, a subgroup of normal subjects had elevated mechanical loads that raised the Pcrit above the disease threshold and placed them at risk of OSA, but were protected through the recruitment of neuromuscular responses that lowered the Pcrit below the disease threshold. The authors suggested that the development of OSA requires a “two-hit” defect, with defects in both upper airway mechanical and neuromuscular responses.48
Figure 2.
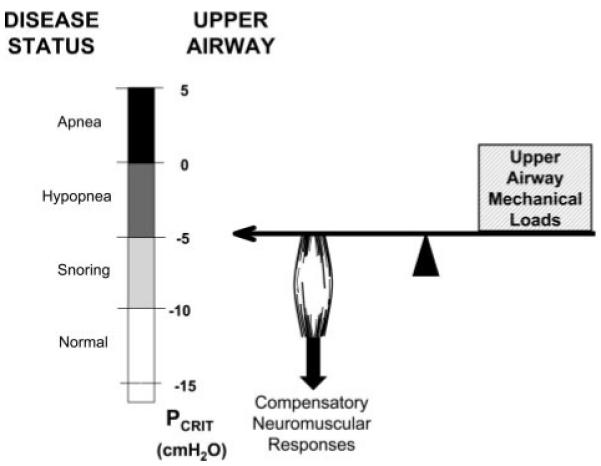
Illustration of the relative role of anatomically imposed mechanical loads and compensatory neuromuscular responses in maintaining upper airway patency. A Pcrit of approximately − 5 cm H2O represents the disease threshold, the level above which obstructive hypopneas and apneas occurred. When mechanical loads on the upper airway are below the disease threshold, OSA is not present, regardless of whether neuromuscular responses are recruited. When mechanical loads on the upper airway are above the disease threshold, recruitment of compensatory neuromuscular responses are necessary to maintain upper airway patency; otherwise, OSA events will occur. Under this paradigm, the development of OSA requires a “two-hit” defect, with defects in both upper airway mechanical and neuromuscular responses (used with permission from Patil et al48).
Clinical Presentation
The classic signs and symptoms for OSA include signs of upper airway obstruction during sleep, insomnia, and daytime hypersomnolence in the setting of obesity; however, a broad range of symptoms can be reported (Table 1). Generally, these symptoms develop over years and progress in association with increases in weight, aging, or transition to menopause. A detailed longitudinal sleep history and physical examination are essential in identifying at- risk individuals because as many as 90% of cases in men and 98% of cases in women may go undiagnosed for many years.75 The prevalence of OSA is higher in patients with certain comorbid conditions including hypertension, stroke, coronary artery disease, congestive heart failure, and diabetes mellitus, suggesting that these patient populations should be screened for symptoms and signs of OSA.76 The typical presentation of obesity, snoring, and witnessed apneas are less predictive of OSA with age, suggesting that the diagnosis may be missed in an “atypical patient.”77 Therefore, screening questions concerning sleep disturbances should be included as part of the physician’s review of systems because OSA is a common disorder and can be treated successfully.
Table 1. Symptoms and Signs of OSA.
| Daytime symptoms |
| Daytime sleepiness or fatigue |
| Difficulties with concentration and short-term memory |
| Depression |
| Nocturnal symptoms |
| Awakenings |
| Insomnia |
| Nocturia |
| Obstructive breathing |
| Loud snoring |
| Choking/gasping |
| Witnessed apneas |
| Conditions with increased risk |
| Menopausal status in women |
| Family history of OSA |
| Hypertension |
| Stroke |
| Diabetes mellitus |
| Alcohol use |
| Pulmonary hypertension |
| Signs |
| Upper body obesity |
| Crowded pharyngeal airspace |
| Retrognathia |
| Reduced cricomental space |
| Macroglossia |
| Lateral peritonsillar narrowing |
| Lower extremity edema |
| Tonsillar hyperplasia |
| Elevated Mallampati score |
Women in particular are often not referred for evaluation of OSA, typically due to lower suspicion for the disorder on the part of physicians or due to underreporting of the classic symptoms by patients. Furthermore, women are less likely to report the classic symptoms of obstructive breathing and daytime hypersomnolence and may report other symptoms including insomnia, heart palpitations, and ankle edema.78 It has been suggested that some of the functional somatic syndromes, including chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome, and migraine headaches, that are seen more commonly in women may be associated with milder forms of OSA such as UARS.79,80 In addition, women report diagnoses of hypothyroidism, asthma/allergies, and depression more often than men, suggesting that symptoms of daytime sleepiness are inappropriately attributed to these medical disorders than consideration for OSA. The relatively low referral of women for evaluation of OSA may account for the large gender disparity in disease prevalence (as much as 10:1) found in clinic-based samples.81 The prevalence of OSA in women that are screened independent of symptoms, however, is half (1:2) that seen in men, suggesting that differences in clinical presentation may partly explain the difference in disease prevalence seen between clinic and population-based samples.
Nocturnal Obstructive Breathing
Obstructive breathing symptoms, which include snoring, snorting, gasping, choking, and witnessed apneic episodes, are perhaps the most common reasons for referral and evaluation of OSA. A history of loud snoring or the bed partner sleeping in a separate room increases the likelihood that OSA will be diagnosed. Patients may report intermittent awakenings when hearing themselves snore, with symptoms of choking and gasping, or for no apparent reason. Eliciting a history of witnessed apneic episodes is a strong predictor for the presence of OSA, although it does not adequately predict the severity of the disorder. Patients are often unaware of nocturnal obstructive breathing symptoms due to their state of consciousness and only learn of the irritating nature of the symptoms from a bed partner. The lack of awareness of obstructive breathing symptoms during the night is often underreported by the patient and may only be obvious if the bed partner is present at the office visit. Furthermore, obstructive breathing symptoms are often under appreciated by the patient and may lead to a delay in diagnosis until other more obvious symptoms (eg, daytime hypersomnolence) are present. When a bed partner is not available to provide objective assessment of nocturnal breathing symptoms in a patient, the physician must increasingly rely on other clinical risk factors, including body-mass index, age, sex, and menopausal status, in determining the patient’s a priori probability of having OSA (see below).
Insomnia and Arousals
Patients with OSA may complain of insomnia.82,83 Insomnia is a symptom complex characterized by difficulties in initiating sleep, intermittent awakenings from sleep, or early morning awakenings with an inability to return to sleep. Patients with symptoms of insomnia documented by reduced total sleep time, fragmented sleep, or early morning awakenings often complain of associated chronic fatigue or lassitude but not hypersomnolence.
An important nocturnal symptom that can lead to the perception of difficulty in maintaining sleep is arousal from sleep. Arousal has been variously defined in the literature but in general can be defined as complete arousal when both EEG evidence and a state of consciousness exist or, as partial arousals, when EEG evidence without consciousness occurs. Normal individuals may have spontaneous partial or complete arousals that are not due to an underlying sleep disorder. Spontaneous complete arousals to levels of consciousness occur approximately one to three times nightly, last 3 to 5 min, but are not associated with symptoms and are terminated with rapid resumption of sleep. Furthermore, 15- to 20-s partial arousals normally occur as often as 5 to 10 times per hour and increase with age.84 Patients may demonstrate arousal with concomitant symptoms such as choking and gasping sensation that help to support a diagnosis of OSA rather than another sleep disorder. OSA patients with complaints of insomnia demonstrate complete arousals more frequently than OSA patients without insomnia symptoms, as suggested by reduced sleep efficiency, total sleep time, and sleep latency.83 Sleep-onset insomnia has also been documented in patients with OSA.85 OSA may promote and exacerbate insomnia symptoms through psychophysiologic conditioning in response to repeated complete arousals.83 Such awakenings may lead to ruminations and a state of hyperarousal that result in difficulties in initiating or maintaining sleep. The role of treatment of OSA in remitting insomnia symptoms independent of cognitive behavioral therapy remains to be established.86
Hypersomnolence
Hypersomnolence is the most common symptom reported by patients with OSA. Pathologic hypersomnolence can be easily determined from the medical interview because patients will complain of intrusion of sleep during normally active situations, such as eating or talking.82 Patients in the early stages of OSA, however, may have subtle and often easily ignored tendencies to sleep, especially during sedentary activities such as reading, watching television, or computer-based activities. Questioning should be directed at whether routine sleep occurs during monotonous or repetitious activities and whether such symptoms occur at work or while driving. Information from family members present at the clinic visit who note a perceptible change in the level of alertness should be carefully considered. Hypersomnolence can be confused with tiredness, fatigue, or lethargy, which can also occur with OSA. Patients may deny clinically apparent hypersomnolence because of social stigma and potential job loss. Moreover, OSA may in fact lead to cognitive impairment, resulting in a decreased ability to perceive sleepiness. Determining the ease with which a person sleeps rather than whether the person is able to stay awake can be helpful in establishing the presence of hypersomnolence. Several standard questionnaires, such as the Epworth sleepiness scale,87 may be useful to administer during the office visit in evaluating the severity of daytime sleepiness symptoms (a score ≥ 10 of 24 is associated with symptoms of daytime sleepiness).
Diagnosis
Risk Stratification for Appropriate Referral for Polysomnography
Appropriate referral by the physician for a sleep study begins with the history and physical examination. Clinical impression using a combination of symptoms, physical examination findings, and other objective data has been used to risk stratify patients for appropriate referral to a sleep laboratory. Obesity, in a population of middle-aged men from the community resulted in an OSA prevalence of > 50%.88 Snoring, while associated with a higher prevalence of OSA, only has a positive predictive value and negative predictive value of 63% and 56%, respectively.89 Witnessed apneas and hypersomnolence separately or together in other studies have positive and negative predictive values that range from 40 to 60%.76 Physical examination findings such as the Mallampati score, tonsillar hyperplasia, and lateral peritonsillar narrowing have been associated with OSA independent of body mass index or neck circumference.90,91 Of note, however, is that these examination findings have no significant predictive abilities in women.91The use of home-based nocturnal oximetry alone as a screening tool for OSA has a sensitivity of only 31% and can lead to an underestimation of OSA severity.92 Combinations of the above factors modestly raise the predictive abilities of various models to levels of 60 to 70%. More recent clinical prediction models that integrate specific clinical examination findings and/or home-based oximetry appear to have improved predictive capabilities and hold promise for risk stratification; however, further validation is required.76 More detailed descriptions of the value of prediction models for OSA can be found elsewhere.76 Clinical impression or symptom-based diagnosis alone, however, lacks the necessary diagnostic accuracy for the disorder; therefore, objective testing through polysomnography is still required.
Polysomnography
Polysomnography is the “gold standard” test for the diagnosis of OSA. The test is an overnight study during which multiple physiologic signals are monitored in the sleeping patient. The signals collected can be classified into three primary groups: those related to recognizing sleep (EEG, electrooculogram, submental electromyogram), those related to cardiac arrhythmia monitoring (ECG), and those related to respiration (airflow, thoracoabdominal effort, and oximetry). Airflow can be monitored in several ways, including an oronasal thermistor, inductive plethysmography, or nasal cannula/pressure transducer system. Monitoring of nasal pressure has become the preferred approach for measuring airflow because the signal is semiquantitative and approximates airflow measurements obtained from a pneumotachograph, the “gold standard” for measurements of airflow.93,94 The role of portable polysomnography in the diagnosis of OSA is currently being debated and will be addressed by a future article in this series.
A trained polysomnogram technologist with over-sight from the sleep physician will analyze the collected study, determine the distribution of sleep states, and characterize the severity of the disordered breathing seen during the night. Standards have been created for the classification of disordered breathing events (Table 2).76 A disordered breathing event is characterized as an apnea (a cessation in airflow of at least 10 s) or a hypopnea (a reduction in airflow of certain magnitude for at least 10 s in duration in association with either an arousal or oxyhemoglobin desaturation. Definitions of hypopneas have generated the most controversy with respect to the appropriate minimum level of oxyhemoglobin desaturation and the reliability of scoring arousals between scorers and centers. However, a oxyhemoglobin desaturation of 3 to 4% and specific EEG criteria for arousals are commonly accepted.76 More recently, some have advocated an additional disordered breathing event named respiratory effort-related arousal (RERA). Definitions of RERAs vary across centers; however, a practice parameter has defined a RERA as a sequence of breaths over a duration of at least 10 s in length with increasing respiratory effort (based on esophageal monitoring) that terminate with an arousal.95-97 RERAs are primarily used to identify patients who may have UARS. However, since RERAs represent a form of upper airway obstruction and have the same underlying pathophysiology as apneas and hypopneas, the current International Classification of Sleep Disorders recommends that UARS be considered a part of OSA and not a separate entity.98 Apneas and hypopneas are classified as obstructive if there is the presence of respiratory effort and central if there is no effort. The number of apneas and hypopneas per hour of sleep (apnea-hypopnea index [AHI]) is the index most commonly used to determine the severity of OSA, with no validated standards regarding severity classification. Current Medicare criteria have adopted an AHI ≥ 15/h (considered to be moderate or severe OSA) or an AHI ≥ 5/h with documented symptoms of excessive daytime sleepiness, impaired cognition, mood disorders or insomnia, or documented hypertension, ischemic heart disease, or stroke to define disease requiring treatment by CPAP. Additional studies, however, are required to determine the adequate AHI thresholds associated with the broad clinical sequelae of OSA.
Table 2. Definitions of Respiratory Events and Indices*.
| Breathing Events | Definition |
|---|---|
| Apnea | A cessation of airflow for at least 10 s. |
| Hypopnea | A reduction in airflow associated with an EEG arousal or oxyhemoglobin desaturation. Several definitions are in use, and there is currently a lack of consensus. The Centers for Medicare and Medicaid Services definition of hypopnea is a respiratory event with a ≥ 30% reduction in thoracoabdominal movement or airflow as compared to baseline lasting at least 10 s, and with a ≥ 4% oxygen desaturation. |
| RERA | Sequence of breaths with increasing respiratory effort leading to an arousal from sleep as shown by progressively more negative esophageal pressure for at least 10 s preceding an arousal with resumption of more normal pressures. A clinical definition has not been agreed upon, and the current research definition is arguably comparable to a hypopnea. |
| Type of breathing event | |
| Obstructive | Continued thoracoabdominal effort in the setting of partial or complete airflow cessation. |
| Central | The lack of thoracoabdominal effort in the setting of partial or complete airflow cessation. |
| Mixed | A respiratory event with both obstructive and central features. Mixed events generally begin without thoracoabdominal effort and end with several thoracoabdominal efforts in breathing. |
| Indices of sleep-disordered breathing | |
| AHI | No. of apneas and hypopnea per hour of total sleep time. |
| Apnea index | No. of apneas per hour of total sleep time. |
| Hypopnea index | No. of hypopneas per hour of total sleep time. |
| RERA index | No. of RERAs per hour of total sleep time. |
| Respiratory disturbance index | No. of apneas, hypopneas, and RERAs per hour of total sleep time. |
| Central apnea index | No. of central apneas per hour of total sleep time. |
| Mixed apnea index | No. of mixed apneas per hour of total sleep time. |
From Kushida et al.76
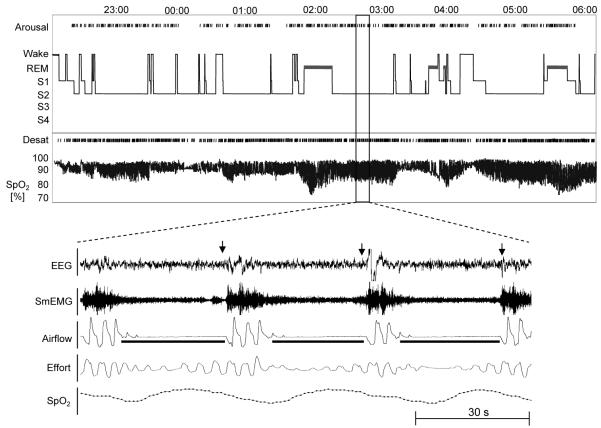
Polysomnography can yield useful correlates of the patient’s clinical presentation. In Figure 3, a summary graph from a sleep study recording in a patient with severe OSA is presented and displays the hypnogram (a summary of the various sleep stages experienced by the patient) and an oximeter tracing. Review of the tracing reveals frequent, severe desaturations associated with each respiratory event but with relatively few transitions from sleep to wake. OSA patients with this pattern often complain of severe daytime sleepiness despite the few transitions to wakefulness. Intermittent arousals from sleep were seen in association with only half of the respiratory events, suggesting that the hypoxemia may also contribute to the daytime sleepiness seen in these patients.3,99 In a study examining OSA severity as a predictor of daytime sleepiness as assessed by multiple sleep latency test (MSLT), the severity of hypoxemia was shown to be an independent predictor of short sleep latency during the MSLT.4 Furthermore, the pattern of severe oxyhemoglobin desaturations is more likely to be seen in obese individuals due to low total body oxygen stores from a reduced functional residual capacity.
Figure 3.
A summary hypnogram and oximetry tracing in a patient with severe OSA (AHI, 84/h). Note the relatively infrequent transitions to lighter stages of sleep in the setting of severe oxyhemoglobin desaturations (Desat) [as low as 70%]. An expanded view of the sleep study is present in the lower panel. In the tracing, three obstructive apneas in association with oxyhemoglobin desaturation (pulse oximetry saturation range [SpO2], 70 to 100%) and arousals (indicated by arrows over the EEG tracing; see text for additional details). SmEMG = submental electromyogram.
In contrast to severe oxyhemoglobin desaturations, patients can present with moderately severe OSA and minimal oxyhemoglobin desaturations (Fig 4). As shown in this figure, the hypnogram reveals frequent sleep state transitions to lighter stages of sleep and brief awakenings with very few episodes of oxyhemoglobin desaturations. OSA patients with frequent transitions to lighter stages of sleep with frequent awakenings, in our clinical experience, can present with complaints of insomnia rather than hypersomnolence due to the sleep disruption, which can resolve with treatment of the OSA.86 Demographic characteristics of patients with this pattern of OSA, in our clinical experience, include individuals of normal weight or who are overweight (but not obese), often young, and women. The fragmented sleep seen in this example emphasizes the need for EEG monitoring because oximetry alone would miss the diagnosis of OSA or underestimate the severity of OSA.
Figure 4.
A summary hypnogram and oximetry tracing in a patient with moderately severe OSA (AHI, 37/h) and sleep fragmentation. Note the frequent transitions from sleep to wakefulness throughout the night. An expanded view of the sleep study is shown in the lower panel. In the tracing, five obstructive hypopneas in association with arousals (indicated by the arrows over the EEG tracing) are shown. Oxyhemoglobin desaturations of ≥ 3 to 4% were not observed in association with these events. Nocturnal oximetry alone would have missed the diagnosis of OSA (see text for further details). See Figure 3 legend for expansion of abbreviations.
Another typical pattern of OSA is seen in Figure 5. As can be seen, the individual has marked oxyhemoglobin desaturation occurring only during periods of rapid eye movement (REM) sleep (thick black bar). In contrast, a pattern of discrete periods of oxyhemoglobin desaturations throughout the night during non-REM sleep suggests the presence of a positional component of OSA. The patient has an AHI of 75/h during REM sleep, 10/h during non-REM sleep, and a total sleep time AHI of 24/h. REM-related patterns of OSA are more frequently seen in women than men.100 REM-related OSA in patients may represent an early sign of impaired neuromuscular responses to upper airway obstruction, suggesting these patients are vulnerable to the effects of CNS-acting agents such as hypnotics or anesthetics. Patients with REM-related OSA may be more prone to adverse clinical events postoperatively after anesthesia, when periods of REM sleep rebound are commonly observed.101 Management of patients with REM-related OSA in the home setting is a matter of some controversy because the clinical sequelae of REM-related OSA has not been well studied. Some studies suggest that REM-related OSA is not associated with hypersomnolence as measured by the MSLT.3 Nevertheless, clinical experience suggests that a subset of REM-related OSA patients respond to therapy with CPAP, suggesting that subtle sleep-disordered breathing events may have been missed during non-REM sleep.
Figure 5.
A summary hypnogram and oximetry tracing in a patient with REM-related OSA, a pattern commonly seen in women (see text for additional details). See Figure 3 legend for expansion of abbreviations.
Summary
OSA is a common sleep disorder that can present in a variety of ways in the pulmonary physician’s office. With a fundamental understanding of the pathophysiology of OSA, the pulmonary physician can routinely integrate questions into their review of system that will assist in appropriate referral for polysomnography and diagnosis of the disorder. Understanding nuances in the spectrum of presenting complaints and polysomnography correlates are important for diagnostic and therapeutic purposes. Knowledge of common patterns of OSA may help to identify patients and guide therapy.
Abbreviations
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- EMGgg
genioglossal electromyogram activity
- MSLT
multiple sleep latency test
- OSA
obstructive sleep apnea
- Pcrit
critical closing pressure
- Pds
downstream pressure
- Pus
upstream pressure
- REM
rapid eye movement
- RERA
respiratory effort-related arousal
- UARS
upper airway resistance syndrome
Footnotes
The manuscript was supported by grants HL50381, HL37379, and HL77137 from the National Heart, Lung, Blood Institute, National Institutes of Health.
No financial or other potential conflicts of interest exist for all the authors.
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM, Bandeen-Roche K, Marx JJ, et al. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–314. [PubMed] [Google Scholar]
- 4.Punjabi NM, O’Hearn DJ, Neubauer DN, et al. Modeling hypersomnolence in sleep-disordered breathing: a novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159:1703–1709. doi: 10.1164/ajrccm.159.6.9808095. [DOI] [PubMed] [Google Scholar]
- 5.Adams N, Strauss M, Schluchter M, et al. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163:1626–1631. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 6.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 7.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 8.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 10.Reichmuth KJ, Austin D, Skatrud JB, et al. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burwell CS, Robin ED, Whaley RD, et al. Extreme obesity associated with alveolar hypoventilation: a Pickwickian syndrome. Am J Med. 1956;21:811–818. doi: 10.1016/0002-9343(56)90094-8. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 13.Ryan CM, Bradley TD. Pathogenesis of obstructive sleep apnea. J Appl Physiol. 2005;99:2440–2450. doi: 10.1152/japplphysiol.00772.2005. [DOI] [PubMed] [Google Scholar]
- 14.Jordan AS, McEvoy RD. Gender differences in sleep apnea: epidemiology, clinical presentation and pathogenic mechanisms. Sleep Med Rev. 2003;7:377–389. doi: 10.1053/smrv.2002.0260. [DOI] [PubMed] [Google Scholar]
- 15.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 16.Smith PL, Wise RA, Gold AR, et al. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 18.Gleadhill IC, Schwartz AR, Schubert N, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 19.Gold AR, Marcus CL, Dipalo F, et al. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–1540. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 20.Gold AR, Schwartz AR. The pharyngeal critical pressure: the whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–1088. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 21.Moser RJ, III, Rajagopal KR. Obstructive sleep apnea in adults with tonsillar hypertrophy. Arch Intern Med. 1987;147:1265–1267. [PubMed] [Google Scholar]
- 22.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome: i. Skeletal morphology. J Laryngol Otol. 1989;103:287–292. doi: 10.1017/s0022215100108734. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Isono S, Tanaka A, et al. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–265. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 24.Cakirer B, Hans MG, Graham G, et al. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 25.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Haponik EF, Smith PL, Bohlman ME, et al. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 27.Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 28.Trudo FJ, Gefter WB, Welch KC, et al. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. Am J Respir Crit Care Med. 1998;158:1259–1270. doi: 10.1164/ajrccm.158.4.9712063. [DOI] [PubMed] [Google Scholar]
- 29.Isono S, Remmers JE, Tanaka A, et al. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 30.Schwab RJ. Pro: sleep apnea is an anatomic disorder. Am J Respir Crit Care Med. 2003;168:270–271. doi: 10.1164/rccm.2305014. [DOI] [PubMed] [Google Scholar]
- 31.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–463. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isono S, Tanaka A, Sho Y, et al. Advancement of the mandible improves velopharyngeal airway patency. J Appl Physiol. 1995;79:2132–2138. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- 33.Launois SH, Feroah TR, Campbell WN, et al. Site of pharyngeal narrowing predicts outcome of surgery for obstructive sleep apnea. Am Rev Respir Dis. 1993;147:182–189. doi: 10.1164/ajrccm/147.1.182. [DOI] [PubMed] [Google Scholar]
- 34.Isono S, Tanaka A, Tagaito Y, et al. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87:1055–1062. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Isono S, Saeki N, Tanaka A, et al. Collapsibility of passive pharynx in patients with acromegaly. Am J Respir Crit Care Med. 1999;160:64–68. doi: 10.1164/ajrccm.160.1.9806054. [DOI] [PubMed] [Google Scholar]
- 36.Kato J, Isono S, Tanaka A, et al. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest. 2000;117:1065–1072. doi: 10.1378/chest.117.4.1065. [DOI] [PubMed] [Google Scholar]
- 37.Fogel RB, Trinder J, White DP, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005;564:549–562. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz I, Stradling J, Slutsky AS, et al. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141:1228–1231. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]
- 39.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–514. [PubMed] [Google Scholar]
- 40.Shelton KE, Woodson H, Gay S, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 41.Stauffer JL, Buick MK, Bixler EO, et al. Morphology of the uvula in obstructive sleep apnea. Am Rev Respir Dis. 1989;140:724–728. doi: 10.1164/ajrccm/140.3.724. [DOI] [PubMed] [Google Scholar]
- 42.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 43.Series F, Marc I. Effects of continuous negative airway pressure-related lung deflation on upper airway collapsibility. J Appl Physiol. 1993;75:1222–1225. doi: 10.1152/jappl.1993.75.3.1222. [DOI] [PubMed] [Google Scholar]
- 44.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 45.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 48.Patil SP, Schneider H, Marx JJ, et al. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 49.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 51.Akahoshi T, White DP, Edwards JK, et al. Phasic mechano-receptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol. 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNicholas WT, Coffey M, McDonnell T, et al. Upper airway obstruction during sleep in normal subjects after selective topical oropharyngeal anesthesia. Am Rev Respir Dis. 1987;135:1316–1319. doi: 10.1164/arrd.1987.135.6.1316. [DOI] [PubMed] [Google Scholar]
- 53.Chadwick GA, Crowley P, Fitzgerald MX, et al. Obstructive sleep apnea following topical oropharyngeal anesthesia in loud snorers. Am Rev Respir Dis. 1991;143:810–813. doi: 10.1164/ajrccm/143.4_Pt_1.810. [DOI] [PubMed] [Google Scholar]
- 54.Berry RB, Kouchi KG, Bower JL, et al. Effect of upper airway anesthesia on obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:1857–1861. doi: 10.1164/ajrccm.151.6.7767531. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra A, Fogel RB, Edwards JK, et al. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 56.Schneider H, Boudewyns A, Smith PL, et al. Modulation of upper airway collapsibility during sleep: influence of respiratory phase and flow regimen. J Appl Physiol. 2002;93:1365–1376. doi: 10.1152/japplphysiol.00942.2001. [DOI] [PubMed] [Google Scholar]
- 57.Larsson H, Carlsson-Nordlander B, Lindblad LE, et al. Temperature thresholds in the oropharynx of patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1992;146:1246–1249. doi: 10.1164/ajrccm/146.5_Pt_1.1246. [DOI] [PubMed] [Google Scholar]
- 58.Kimoff RJ, Sforza E, Champagne V, et al. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 59.Guilleminault C, Li K, Chen NH, et al. Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest. 2002;122:866–870. doi: 10.1378/chest.122.3.866. [DOI] [PubMed] [Google Scholar]
- 60.Woodson BT, Garancis JC, Toohill RJ. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope. 1991;101:1318–1322. doi: 10.1002/lary.5541011211. [DOI] [PubMed] [Google Scholar]
- 61.Edstrom L, Larsson H, Larsson L. Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: a muscle biopsy study. J Neurol Neurosurg Psychiatry. 1992;55:916–920. doi: 10.1136/jnnp.55.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friberg D, Gazelius B, Hokfelt T, et al. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apneics and habitual snorers. Regul Pept. 1997;71:29–36. doi: 10.1016/s0167-0115(97)01016-1. [DOI] [PubMed] [Google Scholar]
- 63.Lindman R, Stal PS. Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J Neurol Sci. 2002;195:11–23. doi: 10.1016/s0022-510x(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 64.Seelagy MM, Schwartz AR, Russ DB, et al. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol. 1994;76:2692–2700. doi: 10.1152/jappl.1994.76.6.2692. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz AR, Thut DC, Brower RG, et al. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J Appl Physiol. 1993;74:1597–1605. doi: 10.1152/jappl.1993.74.4.1597. [DOI] [PubMed] [Google Scholar]
- 66.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 67.Dempsey JA, Smith CA, Przybylowski T, et al. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badr MS, Kawak A, Skatrud JB, et al. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol. 1997;110:33–45. doi: 10.1016/s0034-5687(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 69.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–1370. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 70.Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badr MS, Toiber F, Skatrud JB, et al. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 73.King ED, O’Donnell CP, Smith PL, et al. A model of obstructive sleep apnea in normal humans: role of the upper airway. Am J Respir Crit Care Med. 2000;161:1979–1984. doi: 10.1164/ajrccm.161.6.9904096. [DOI] [PubMed] [Google Scholar]
- 74.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 75.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 76.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 77.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 78.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 79.Gold AR, Dipalo F, Gold MS, et al. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123:87–95. doi: 10.1378/chest.123.1.87. [DOI] [PubMed] [Google Scholar]
- 80.Gold AR, Dipalo F, Gold MS, et al. Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep. 2004;27:459–466. doi: 10.1093/sleep/27.3.459. [DOI] [PubMed] [Google Scholar]
- 81.Redline S, Kump K, Tishler PV, et al. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 82.Smith PL. Evaluation of patients with sleep disorders. In: White DP, editor. Seminars in respiratory medicine. Thieme Stratton; New York, NY: 1988. pp. 534–539. [Google Scholar]
- 83.Krakow B, Melendrez D, Ferreira E, et al. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest. 2001;120:1923–1929. doi: 10.1378/chest.120.6.1923. [DOI] [PubMed] [Google Scholar]
- 84.Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995;18:330–333. doi: 10.1093/sleep/18.5.330. [DOI] [PubMed] [Google Scholar]
- 85.Chung KF. Insomnia subtypes and their relationships to daytime sleepiness in patients with obstructive sleep apnea. Respiration. 2005;72:460–465. doi: 10.1159/000087668. [DOI] [PubMed] [Google Scholar]
- 86.Krakow B, Melendrez D, Lee SA, et al. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8:15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 87.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 88.Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 89.Deegan PC, Nolan P, Carey M, et al. Effects of positive airway pressure on upper airway dilator muscle activity and ventilatory timing. J Appl Physiol. 1996;81:470–479. doi: 10.1152/jappl.1996.81.1.470. [DOI] [PubMed] [Google Scholar]
- 90.Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 91.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea: the importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162:740–748. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 92.Ryan PJ, Hilton MF, Boldy DA, et al. Validation of British Thoracic Society guidelines for the diagnosis of the sleep apnoea/hypopnoea syndrome: can polysomnography be avoided? Thorax. 1995;50:972–975. doi: 10.1136/thx.50.9.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Condos R, Norman RG, Krishnasamy I, et al. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 94.Hosselet JJ, Norman RG, Ayappa I, et al. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med. 1998;157:1461–1467. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 95.Ayappa I, Norman RG, Krieger AC, et al. Non-Invasive detection of respiratory effort-related arousals (RERAs) by a nasal cannula/pressure transducer system. Sleep. 2000;23:763–771. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 96.Guilleminault C, Stoohs R, Clerk A, et al. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;104:781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 97.Johnson PL, Edwards N, Burgess KR, et al. Detection of increased upper airway resistance during overnight polysomnography. Sleep. 2005;28:85–90. doi: 10.1093/sleep/28.1.85. [DOI] [PubMed] [Google Scholar]
- 98.American Academy of Sleep Medicine . The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. American Academy of Sleep Medicine; West Chester, IL: 2005. [Google Scholar]
- 99.Mediano O, Barcelo A, de la Pena M, et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. 2007 doi: 10.1183/09031936.00009506. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 100.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 101.Kaw R, Michota F, Jaffer A, et al. Unrecognized sleep apnea in the surgical patient: implications for the perioperative setting. Chest. 2006;129:198–205. doi: 10.1378/chest.129.1.198. [DOI] [PubMed] [Google Scholar]