Abstract
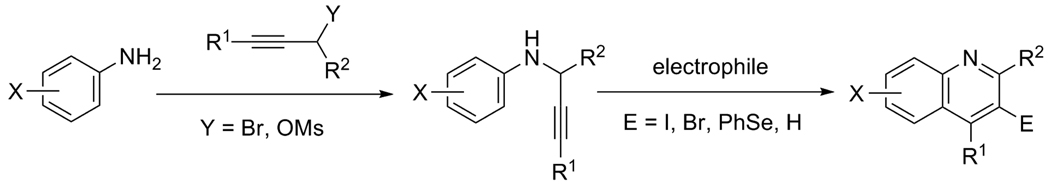
A wide variety of substituted quinolines are readily synthesized under mild reaction conditions by the 6-endo-dig electrophilic cyclization of N-(2-alkynyl)anilines by ICl, I2, Br2, PhSeBr and p-O2NC6H4SCl. The reaction affords 3-halogen-, selenium- and sulfur-containing quinolines in moderate to good yields in the presence of various functional groups. Analogous quinolines bearing a hydrogen in the 3-position have been synthesized by the Hg(OTf)2-catalyzed ring closure of these same alkynylanilines.
Keywords: Substituted Quinolines, Electrophilic Cyclization, Alkynes, Hg-catalyzed Cyclization
Introduction
The quinoline skeleton occurs in numerous natural products, especially in alkaloids.1 Many quinolines display interesting physiological activities and have found applications as pharmaceuticals (e.g., antimalarial drugs, such as quinine or chloroquine) and agrochemicals, as well as being general synthetic building blocks.2 Halogen-containing quinolines are of particular interest, because the halogen atom can play a crucial role in the compound’s bioactivity and provides an avenue for further structure elaboration.3 The isolation and synthesis of naturally-occurring quinoline derivatives have received considerable attention in the literature due to their biological and pharmaceutical importance.4 Although simple 3-bromoquinolines can be obtained by the bromination of quinoline hydrochlorides,5 the site-selective aromatic halogenation of substituted quinolines remains a synthetic challenge.6 3-Haloquinolines have also been synthesized by a photochemical route,7 a modified Skraup quinoline synthesis employing halo-substituted acroleins and anilines,8 and the Friedländer quinoline synthesis.3c Some of these methods suffer relatively low yields, poor regioselectivity, and/or rather lengthy synthetic sequences.
The cyclization of alkynes containing proximate nucleophilic centers promoted by electrophiles is currently of great interest and developing into a very effective strategy for carbo- and heterocyclic ring construction.9 This chemistry provides a convenient approach to the synthesis of functionalized cyclic compounds through the regioselective addition of the nucleophile and the electrophile across the carbon-carbon triple bond. Successful examples of this process have been reported for the synthesis of isoquinolines,10 benzothiophenes,11 polycyclic aromatics,12 indoles,13 furopyridines,14 isoindolin-1-ones,15 isocoumarins,16 isochromenes17 and benzofurans.13d,18 However, no one prior to our recent communication19 had employed this chemistry to synthesize quinolines. Herein, we wish to report the full details of this successful strategy for the synthesis of quinolines using very mild reaction conditions (Scheme 1).
Scheme 1.
Results and Discussion
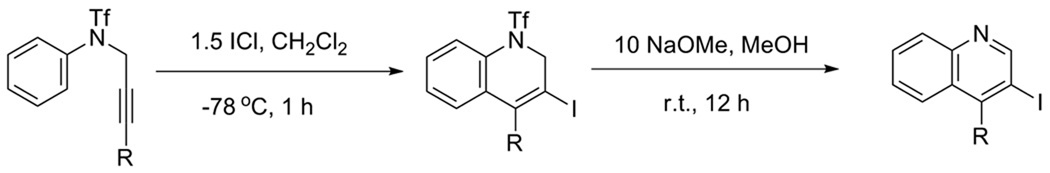
During our early efforts to obtain 3-iodoquinolines, a two-step procedure was first examined (Scheme 2). Triflamide 1 with R = Ph was treated with ICl at −78 °C to afford a dihydroquinoline intermediate, which could be subsequently treated with base to achieve aromaticity.20
Scheme 2.
3-Iodo-4-phenylquinoline (2) was generated in a good yield using this approach (Table 1, entry 1). Thus, we have subsequently employed the reaction conditions developed here on a wide variety of other triflamides and the results are summarized in Table 1, entries 2–10. Good yields have been obtained when the group R on the alkyne is phenyl, as well as relatively electron-rich or electron-deficient aryl groups (entries 1–4). The cyclization of substrates bearing both electron-donating and -withdrawing groups on the aniline moiety proved no difficulty when R is phenyl group (entries 9 and 10). When the 3-methoxyaniline derivative 15 was utilized, two isomeric quinoline products were obtained (entry 9). The product formed from cyclization onto the less sterically hindered position para to the methoxy group is the major isomer.
Table 1.
Synthesis of Quinolines from N-(2-Alkynyl)triflamidesa
| entry | triflamide | electrophile | product | % overall yield |
||
|---|---|---|---|---|---|---|
| 1 | 1 | ICl | 2 | 88 | ||
| 2 | 3 | ICl | 4 | 62 | ||
| 3 | 5 | ICl | 6 | 45 | ||
| 4 | 7 | ICl | 8 | 51 | ||
| 5 | 9 | ICl | 10 | trace | ||
| 6 | 9 | I2/AgClO4 | 10 | trace | ||
| 7 | 11 | ICl | 12 | 25 | ||
| 8 | 13 | ICl | 14 | trace | ||
| 9 | 15 | ICl | 60+25 | |||
| 10 | 18 | ICl | 19 | 56 | ||
| 11 | 20 | ICl | 2 | 74 |
All reactions were run using 0.30 mmol of the N-(2-alkynyl)sulfonamide and 1.5 equiv of ICl in 3 mL of CH2Cl2 at −78 °C for 1 h. After work-up, the solvent was removed, the crude mixture and 10 equiv of NaOMe were directly dissolved in MeOH and stirred overnight.
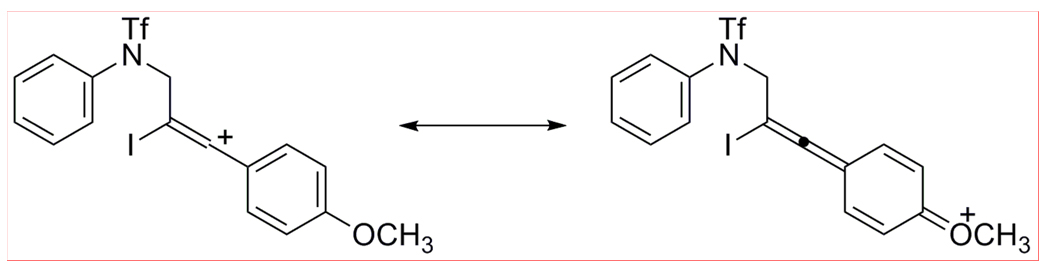
The products of addition of ICl to the triple bond of the triflamides 9 and 13 were the major products observed under the above reaction conditions. These presumably are generated from the relatively stable cations formed by the electron-releasing MeO or vinylic groups, followed by trapping by the chloride anion generated from the ICl (Scheme 3).
Scheme 3.
It appears that the relatively stable iodovinylic cation is reluctant to undergo electrophilic attack on the N-substituted aromatic ring. Therefore, removal of the trifluoromethanesulfonyl group might be expected to increase the nucleophilicity of the aromatic ring and thus improve the yield of the desired cyclization. This has indeed proven to be the case. Furthermore, although this is a convenient and new approach to 3-iodo-1,2-dihydroquinolines, the failure of the reactions with substrates 9 and 13 and the poor yield from the reaction of the alkyl-substituted alkyne 11 encouraged us to search for a more general and straightforward synthetic approach to 3-iodoquinolines. Finally, the cyclization of methanesulfonamide 20 was also successful (entry 11). Obviously, one could obtain dihydroquinolines after the first step without further base-induced deprotection of the nitrogen if one so desired.
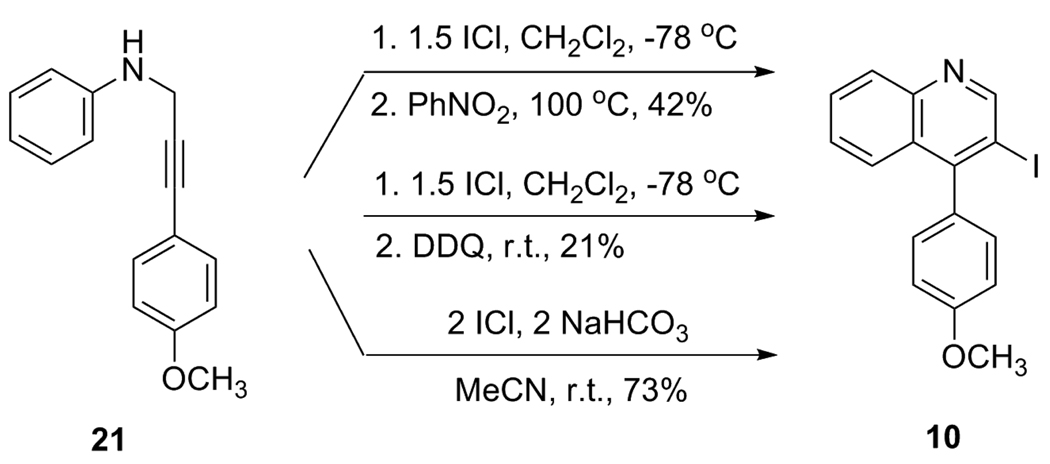
Aniline derivative 21 was next subjected to direct electrophilic cyclization (Scheme 4). A fair amount of the iodoquinoline 10 was generated upon reaction with ICl and subsequent treatment by the oxidizing agents nitrobenzene or DDQ. However, it was later found that the oxidation step was unnecessary, since 10 was formed in a good yield upon iodination with ICl plus NaHCO3 without subsequent addition of an oxidant.
Scheme 4.
Figuring that the conversion of 21 to 10 by ICl might not be the simplest, most convenient and most general of transformations, we choose to optimize the reaction of aniline 22 using I2 in the presence of various bases (eq 1).
 |
(1) |
The results are summarized in Table 2. When a mixture of aniline 22, 3 equiv of I2 and 2 equiv of NaHCO3 in MeCN was stirred at r.t. for 0.5 h, the desired iodoquinoline 2 was obtained cleanly in a 76% yield (Table 2, entry 1). The reaction has also been run on a 2.0 mmol scale and product 2 was generated in a 75% yield, indicating the utility of this procedure on a larger scale. A much longer reaction time was required when decreasing the amount of I2 from 3 to 2 equiv and the yield of 2 dropped substantially (entry 2). Essentially the same yield was obtained when using either 3 or 6 equiv of I2 (compare entries 1 and 3). Thus, we chose to use 3 equiv of I2 in the rest of our optimization work. The reaction was also dramatically slowed down when there was no base employed, even in the presence of 6 equiv of I2 (entry 4). Other carbonate bases, such as Cs2CO3 and NaOCO2CH3, which have been successfully employed in our related isoquinoline synthesis,10 gave a yield comparable to that of NaHCO3 (entries 5 and 6). Organic bases, namely triethylamine and pyridine, actually lowered the yield (entries 7 and 8). The combination of 0.30 mmol of the N-(2-alkynyl)aniline, 3 equiv of I2 and 2 equiv of NaHCO3 in 3 mL of CH3CN at room temperature was therefore chosen as our “optimal” reaction conditions and the scope of this reaction was systematically investigated (Table 3).
Table 2.
Effect of I2 Stoichiometry and Base on the Cyclization of N-(3-Phenyl-2-propynyl)aniline (22) (eq 1)a
| entry | equiv of I2 | base (equiv) | % yield |
|---|---|---|---|
| 1 | 3 | NaHCO3 (2) | 76 |
| 2 | 2 | NaHCO3 (2) | 44b |
| 3 | 6 | NaHCO3 (2) | 75 |
| 4 | 6 | - | 56c |
| 5 | 3 | NaOCO2CH3 (2) | 72 |
| 6 | 3 | Cs2CO3 (2) | 71 |
| 7 | 3 | pyridine (2) | 35 |
| 8 | 3 | Et3N (2) | 45 |
All reactions were run under the following conditions unless otherwise stated: 0.3 mmol of 22, I2 and the base in 3 mL of MeCN were stirred at room temperature for 12 h.
The reaction wasn’t finished after 24 h; 40% of 22 was left.
After 24 h, 25% of 22 was left.
Table 3.
Synthesis of Quinolines by Electrophilic Cyclization of N-(2-Alkynyl)anilines
| entry | propargylic amine | electrophile | product(s) | % yield | |||
|---|---|---|---|---|---|---|---|
| E | |||||||
| 1 | 22 | I2 a | I | 2 | 76 | ||
| 2 | IClb | I | 2 | 83 | |||
| 3 | PhSeBrb | PhSe | 23 | 20 | |||
| 4 | NBSb | Br | 24 | 0c | |||
| 5 | 21 | I2 a | I | 10 | 71 | ||
| 6 | IClb | I | 10 | 73 | |||
| 7 | PhSeBrb | PhSe | 25 | 74 | |||
| 8 | Br2 b | 26 | 51 | ||||
| 9 | 21 | p-O2NC6H4SClb | SC6H4NO2-p | 27 | 12 | ||
| 10 | 28 | I2 a | I | 29 | 78 | ||
| 11 | 30 | I2 a,d | I | 8 | 57 | ||
| 12 | 31 | I2 a | I | 32 | trace | ||
| 13 | 33 | I2 a | I | 34 | 25 | ||
| 14 | 35 | I2 a | 36 + 37 | 55 + 0 | |||
| 15 | 38 | I2 a | I | 12 | 43e | ||
| 16 | IClb | I | 12 | 30 | |||
| 17 | 39 | I2 a | I | 40 | trace | ||
| 18 | 41 | I2 a | I | 42 | 0 | ||
| 19 | 43 | I2 a | 44 | 0 | |||
| 20 | 45 | I2 a | 14 | 80 | |||
| 21 | 46 | I2 a | 47 | 38f | |||
| 22 | 48 | I2 a | 19 | 63 | |||
| 23 | 48 | PhSeBrb | 49 | 75 | |||
| 24 | 50 | I2 a | I | 51 | 88 | ||
| 25 | PhSeBrb | PhSe | 52 | 56 | |||
| 26 | 53 | I2 a | 54 | 79 | |||
| 27 | 55 | I2 a | 56 | 75 | |||
| 28 | 57 | I2 a | 58 | 58d | |||
| 29 | 59 | I2 a | 16 + 17 | 29 + 37 | |||
| 30 | 60 | I2 a | 61 + 62 | 71 + 8 | |||
| 31 | 63 | I2 a | 64 | 75 | |||
| 32 | 65 | I2 a | 66 | trace | |||
| 33 | 67 | I2 a | 68 | 0 | |||
| 34 | 69 | I2 a | 70 | 35g | |||
| 35 | 71 | IClb | 72 | 50 | |||
| 36 | 73 | I2 a | 74 | 62 | |||
| 37 | 75 | I2 a | 76 | 80 | |||
| 38 | 77 | I2 a | 2 | 0 |
All reactions were run under the following conditions unless otherwise stated: 0.3 mol of the propargylic aniline, 3 equiv of I2, and 2 equiv of NaHCO3 in 3 mL of CH3CN were stirred at room temperature for 0.5 h.
Two equiv of ICl/Br2/PhSeBr/p-O2NC6H4SCl/NBS in 1 mL of CH3CN were added dropwise to the solution of 0.3 mmol of propargylic aniline and 2 equiv of NaHCO3 in 2 mL of MeCN over 10 min at r.t. and the solution was stirred for another 10 min.
Only the product formed from bromination of the 4-position of the aniline ring was obtained.
The reaction took 24 h.
The product was obtained together with a 26% yield of 4-n-butyl-3,6-diiodoquinoline.
Compound 2 was also generated in a 25% yield.
7-Amino-3,6-diiodo-8-methyl-4-phenylquinoline was also obtained in a 28% yield.
ICl (4 equiv) in 1 mL of CH2Cl2 was added dropwise to the solution of 0.30 mmol of 71 in 2 mL of CH2Cl2 over 10 min at −78 °C and the solution was stirred for another 10 min.
First, various substituents on the end of the alkyne were examined. In general, electron-rich and electron-deficient aryl groups have generated quinolines in good to excellent yields, presumably because these substituents are capable of stabilizing a vinylic cation intermediate (entries 1, 5, and 10; Table 3). The yield dropped and a much longer reaction time was needed for the p-acetylphenyl-substituted alkyne 30 to react (24 h vs. 0.5 h for alkyne 22) (entry 11). Substrate 31 with a strong electron-withdrawing NO2 group on the para-position of the arene failed to generate any cyclization product (entry 12). The yield of the pyridine derivative 34 also suffers, which might be the result of the coordination of I+ to the nitrogen of the pyridine, further lowering the electron density of the pyridine ring (entry 13).10 Interestingly, an amino group, generally believed to be a stronger nucleophile than a phenyl group, ortho to the alkynyl moiety remains untouched during the cyclization in contrast to our previously reported indole synthesis employing 2-(1-alkynyl)anilines (entry 14).13a This indicates the need for an appropriate leaving group on the nitrogen in order to effect a successful indole synthesis. In fact, cyclization by I2 proceeds in a decent yield even when the terminus of the carbon-carbon triple bond bears an alkyl group. Thus, 4-butyl-3-iodoquinoline (12) can be synthesized in a 43% yield, alongside a 26% yield of 4-butyl-3,6-diiodoquinoline, which is presumably formed by iodocyclization of N-(2-heptynyl)-4-iodoaniline formed by prior iodination of the aniline (entry 15). The desired cyclization did not occur when the acetylene bears a sterically-hindered silyl or t-Bu group, or a simple H atom on the triple bond (entries 17–19). Substitution of a vinylic group on the alkyne presents no problem as the 4-(1-cyclohexenyl)quinoline 14 was formed cleanly in an 80% yield (entry 20). Together with iodoquinoline 2, formed in a 25% yield, the 2,3,4-trisubstituted quinoline 47 was obtained in a moderate yield. This quinoline is most likely generated by elimination of toluene during the aromatization step (entry 21).
An interesting feature of this cyclization is the fact that yields aren’t significantly affected by the substituents on the aniline ring. para-Substituents ranging from a weak electron-withdrawing group, like a Br, to a strong electron-withdrawing group, like a NO2, along with an electron-donating MeO group, all generate the corresponding substituted quinoline derivatives in good yields (entries 22, 24, 26 and 27). A substrate with two methyl groups situated meta to the amino group also afforded a high yield of the corresponding quinoline, although a longer reaction time was needed (entry 28).
The regioselectivity of this cyclization has also been investigated. 3-Methoxyaniline 59 afforded the ortho-cyclization regioisomer 16 and the para-cyclization isomer 17 in 29% and 37% yields respectively (entry 29). On the other hand, very interestingly, the 3-nitroaniline 60 afforded regioisomers 61 and 62 in a 79% combined yield with cyclization occurring primarily ortho to the nitro group (9:1 ratio of ortho to para cyclization) for reasons that are not obvious (entry 30). Excellent regioselectivity was achieved in the cyclization of 2-naphthylamine 63, since only one isomer was observed. The ring closure occurred selectively in the less sterically-hindered 3-position of the naphthalene ring (entry 31). Unfortunately, cyclization onto a pyridine ring proved unsuccessful using our current reaction conditions (entry 32).
Other halogen electrophiles have also been successfully employed in this quinoline synthesis. For example, the stronger electrophile ICl has been utilized in this cyclization. Our “optimal” ICl reaction conditions are the following: 0.30 mmol of the propargylic aniline, 2 equiv of ICl, and 2 equiv of NaHCO3 in 3 mL of MeCN are stirred at room temperature. Three equiv of ICl were not necessary, since all of the alkynes were completely consumed when 2 equiv of ICl were employed. Lowering the temperature to 0 °C or −78 °C did not improve the yields. Yields comparable to those obtained using I2 (compare entry 1 with 2, and entry 5 with 6, Table 3) have been obtained, but the reactions are much faster, being complete in about 5 min. However, using ICl as the electrophile was not helpful in improving the yields of the desired monoiodinated quinolines where I2 only generated a moderate yield, as in entries 15 and 16. When 2 equiv of NBS in MeCN were added dropwise to a mixture of aniline 22 and 2 equiv of NaHCO3 in MeCN, N-(3-phenyl-2-propynyl)-4-bromoaniline was obtained in quantitative yield, and no cyclization to the quinoline was observed after 2 h (entry 4). The reaction of Br2 with alkynylaniline 22 was messy with the formation of mono-, di- and tribromoquinolines evident. In the case of aniline 21, we were able to isolate dibromoquinoline 26 in a 51% yield (entry 8, Table 3).
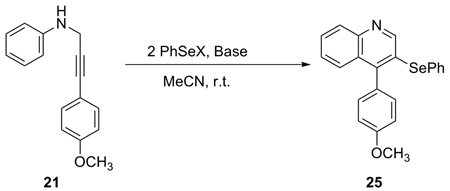
Although PhSeCl and PhSeBr has been found to be effective electrophiles in our electrophilic cyclization of alkynes to isoquinolines,10 isochromenes,17 isocoumarins,16 benzo[b]thiophenes11 and isoindolin-1-ones,15 PhSeCl is not nearly as effective as PhSeBr in this current quinoline process (eq 2 and entries 1 and 3, Table 4). The reaction of PhSeCl plus 2 equivs of NaHCO3 gave only a 38% yield of selenium-substituted quinoline (entry 1, Table 4). This reaction also provided a 19% yield of the 3,6-diselenium-substituted quinoline. No reaction occurred when using PhSeSePh (entry 2, Table 4). Interestingly, when PhSeBr was used as the electrophile, the desired 3-(phenylselenyl)quinoline 25 was obtained in a 74% yield (entry 3). Surprisingly, simply changing the base from NaHCO3 to Na2CO3 resulted in a messy reaction and none of the desired quinoline 25 was observed (entry 4). Organic bases, such as NEt3 and pyridine, provided lower yields (entries 5 and 6). The presence of a base to remove the side product HBr is crucial (entry 7). The reaction of 21 and PhSeBr without any base afforded none of the desired quinoline. Instead, a 42% yield of aniline 21 with a PhSe group para to the aniline nitrogen was obtained. It is important to emphasize that the dropwise addition of ICl and PhSeBr to the reaction vessel is very important in obtaining a clean reaction, but this is unnecessary when using I2. Representative examples of the synthesis of selenium-containing quinolines are included in entries 3, 7, 23 and 25 in Table 3.
Table 4.
| entry | electrophile | base (equiv) | % yield of 25 |
|---|---|---|---|
| 1 | PhSeCl | NaHCO3 (2) | 38b |
| 2 | PhSeSePh | NaHCO3 (2) | 0 |
| 3 | PhSeBr | NaHCO3 (2) | 74 |
| 4 | PhSeBr | Na2CO3 (1) | 0 |
| 5 | PhSeBr | NEt3 (3) | 40 |
| 6 | PhSeBr | pyridine (3) | 48 |
| 7 | PhSeBr | - | 0c |
2 Equiv of PhSeX in 1 mL of MeCN was added dropwise to a solution of 0.3 mmol of propargylic aniline and the base in 2 mL of MeCN at r.t..
A 19% yield of 3,6-di(phenylselenyl)quinoline was also obtained.
Compound 21 bearing a PhSe group para to the amino group was obtained in a 42% yield.
 |
(2) |
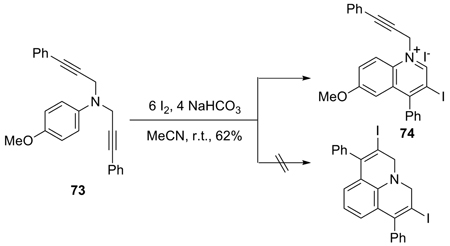
When N,N-disubstituted anilines and derivatives were employed in the reaction with ICl or I2, quinolinium salts can be obtained (entries 35 and 36, Table 3). Thus, the dipropargylic aniline 73 gave a good yield of the isoquinolinium salt 74, when allowed to react with I2 (eq 3). None of the double cyclization product was observed. In a similar fashion, amide 71 reacted with ICl to give isoquinolinium salt 72 (Table 3, entry 35).
 |
(3) |
The highly substituted aniline 75 gave an excellent yield of the highly functionalized quinoline 76 (entry 37). An analogous approach to quinolines might be to cyclize N-(3-phenyl-2-propynylidene)aniline, which can be easily generated by the condensation of aniline with 3-phenylpropynal. However, under our standard I2 reaction conditions, none of the desired iodoquinoline 2 was generated. Instead, the imine starting material decomposed (entry 38, Table 3).
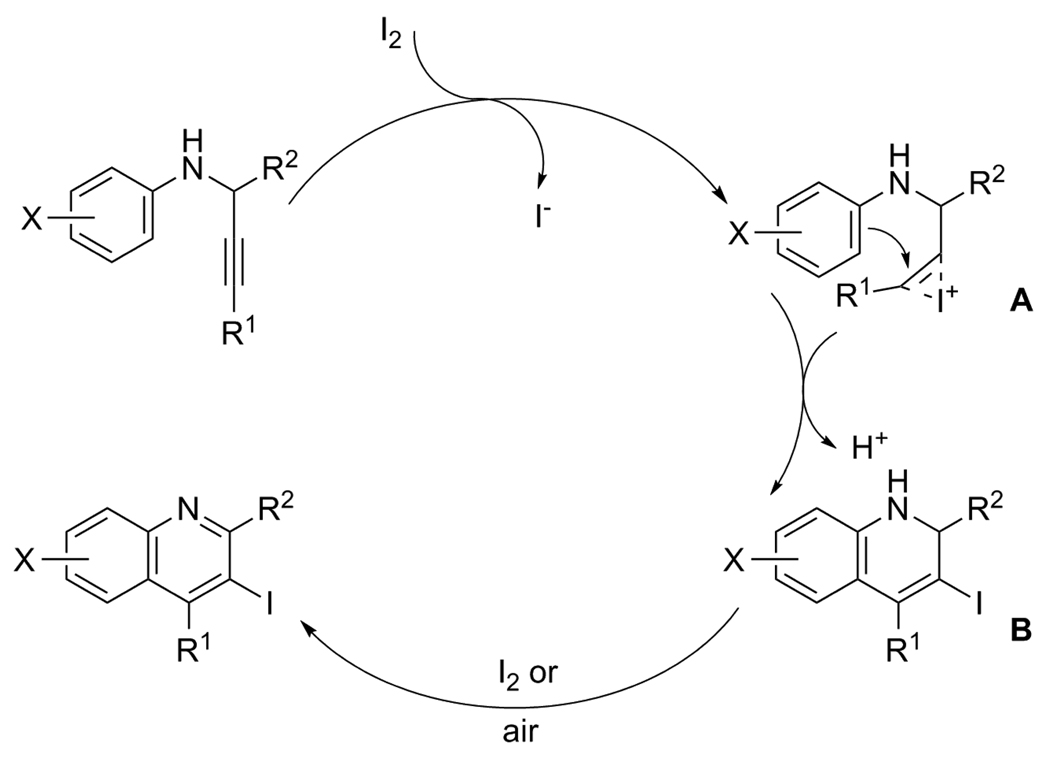
We propose the following mechanism for this iodocyclization process (Scheme 5): (1) the carbon-carbon triple bond of the propargylic aniline coordinates to the iodine cation generating an iodonium intermediate A, (2) intramolecular nucleophilic attack of the aromatic ring of the aniline on the activated triple bond forms dihydroquinoline B, (3) in the presence of I2 or ICl, the dihydroquinoline B is oxidized to the corresponding quinoline.20 It is also possible that the dihydroquinoline is not oxidized to the quinoline until it is exposed to air during the work-up.
Scheme 5.
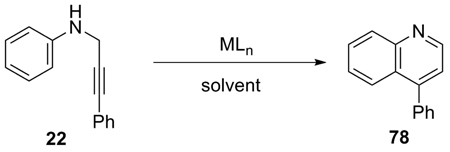
Recently, considerable attention has focused on metal catalysts, which can effectively activate unsaturated bonds towards intramolecular electrophilic attack on arenes. Various heterocycles and carbocycles have been successfully synthesized using this strategy.21–26 However, the direct cyclization of simple propargylic anilines to quinolines has not yet been achieved. Our interest in the synthesis of quinolines bearing a hydrogen in the 3 position encouraged us to first screen those reagents and catalysts previously reported to be active in this kind of chemistry (eq 4). The results are summarized in Table 5.
Table 5.
Metal-Catalyzed Cyclization of Aminoalkyne 22 (eq 4)
| entry | catalyst | solvent | temp (°C) |
time (h) | % yield of 78 (22) |
ref |
|---|---|---|---|---|---|---|
| 1 | 5% AuCl3 | EtOH | 75 | 48 | 10 (75) | 21 |
| 2 | 5% AuCl3/ 15% AgOTf |
MeCN | 70 | 72 | 15 (70) | 22 |
| 3 | 5% RuCl3/ 15% AgOTf |
ClCH2CH2Cl | 65 | 48 | 8 (90) | 23 |
| 4 | 5% PtCl4 | ClCH2CH2Cl | 70 | 48 | 38 (51)a | 24 |
| 5 | 5% PtCl2 | toluene | 80 | 48 | 5 (80) | 25 |
| 6 | 3% Cu(OTf)2 | CH2Cl2 | r.t. | 48 | 0 | – |
| 7 | 3% Hg(O2CCF3)2 |
MeCN | r.t. | 48 | trace | – |
| 8 | 3% Hg(OTf)2/ 15% TMU |
MeCN | r.t. | 24 | 55 (35)a | – |
| 9 | 5% Hg(OTf)2 | MeCN | r.t. | 48 | 50 (45)a | – |
| 10 | 3% Hg(OTf)2 | MeCN | 50 | 168 | 40 (50)a | – |
| 11 | 4% Hg(OTf)2 | DMSO | 100 | 48 | 10 (71) | – |
| 12 | 5% Hg(OTf)2/ 1.2 MnO2 |
MeCN | r.t. | 24 | < 1 | – |
| 13 | 10% Hg(OTf)2 | MeCN | r.t. | 6 | 96 | – |
| 14 | 10% Hg(OTf)2 | CH2Cl2 | r.t. | 24 | 95 | – |
The yield didn’t improve when the reaction was run a longer time.
 |
(4) |
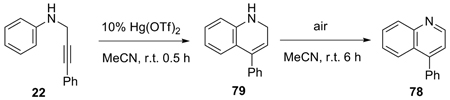
AuCl3 and Au(OTf)3, which have been shown to be effective catalysts for the cyclization of imines derived from carbonyl compounds and propargylic amines,21 and the hydroarylation of alkynes22 were ineffective in this quinoline process (Table 5, entries 1 and 2). The reaction of aniline and 3-phenylpropynal was also messy under the reaction conditions of entry 1. Unsatisfactory results were also obtained from RuCl3/AgOTf, PtCl4, and PtCl2 salts, although they have been shown to affect the cyclization of N-protected anilines to hydroquinolines (entries 3–5).23–25 While both Cu(OTf)2 and Hg(O2CCF3)2 are inactive (entries 6–8), Hg(OTf)2 was found to be the catalyst of choice. Thus, 10% Hg(OTf)2 promotes the cyclization of 22 to quinoline 78 in a 95–96% yield using either MeCN or CH2Cl2 as the solvent at r.t. (entries 13 and 14). Lowering the amount of Hg(OTf)2 or changing the solvent or addition of an oxidant are all detrimental to the reaction (entries 8–12). This reaction is believed to proceed in a stepwise manner, because compound 79 could be separated from the reaction mixture after half an hour and it was gradually converted to compound 78 when stirred in the presence of air, but the absence of mercury salts (eq 5). However, analogous dihydroquinoline intermediates have not been observed in the iodine-promoted ring closure process (Table 3).
 |
(5) |
The scope of this metal-catalyzed process using the above “optimal” reaction conditions was subsequently examined. The compatibility of substrates is very similar to that of the I2 cyclization process. With phenyl and p-MeOC6H4 groups on the end of the alkyne, the desired quinolines were generated in excellent yields (Table 6, entries 1 and 2). An analogous 3,5-dimethylaniline derivative was also cyclized in good yield (entry 4). Only 4% Hg(OTf)2 was needed for the cyclization of substrate 59 with a meta-methoxy substituent (entry 5). Regioisomers 83 and 84 were obtained in a 73% overall yield. The yield of isomer 84 is slightly higher than that of 83. On the other hand, cyclization of the 2-naphthyl derivative 63 afforded a high yield of a single product in which cyclization occurs exclusively in the 1 position of the naphthalene (entry 6). Note that the regiochemistry here is completely the opposite of that of the corresponding iodocyclization (Table 3, entry 31). When an electron-withdrawing ester group was incorporated into the para-position of the aniline, only a 53% yield of quinoline 86 could be obtained employing 20 mol % of Hg(OTf)2 (entry 7). The reaction was not as efficient when the distal end of the triple bond was substituted by either an alkyl or vinylic group (entries 3, 8 and 9). However, we were able to efficiently cyclize substrates bearing electron-rich aromatic rings and either a butyl or cyclohexenyl group on the alkyne, and the amount of catalyst could be reduced to 5 mol % in both cases (entries 8 and 9, Table 6). It is particularly noteworthy that a C-C double bond is readily accommodated in this later process.
Table 6.
Synthesis of Quinolines by the Hg(OTf)2-Catalyzed Cyclization of N-(2-Alkynyl)anilines in MeCNa
| entry | substrate | Hg(OTf)2 | time (h) | product | % | ||
|---|---|---|---|---|---|---|---|
| (mol %) | yield | ||||||
| 1 | 22 | 10 | 6 | 78 | 96 | ||
| 2 | 21 | 10 | 6 | 80 | 88 | ||
| 3 | 38 | 15 | 12 | 81 | 50 | ||
| 4 | 57 | 10 | 6 | 82 | 79 | ||
| 5 | 59 | 4 | 6 | 83 | 35 | ||
| +84 | +38 | ||||||
| 6 | 63 | 10 | 12 | 85 | 65 | ||
| 7 | 50 | 20 | 24 | 86 | 53 | ||
| 8 | 87 | 5 | 3 | 88 | 75 | ||
| 9 | 89 | 5 | 5 | 90 | 73 | ||
| 10 | 45 | 30 | 24 | 91 | 38 | ||
| 11 | 75 | 20 | 12 | 92 | 65 | ||
To a solution of 0.3 mmol of the propargylic aniline in 3 mL of CH3CN was added a catalytic amount of Hg(OTf)2. The reaction mixture was stirred at room temperature for the indicated time.
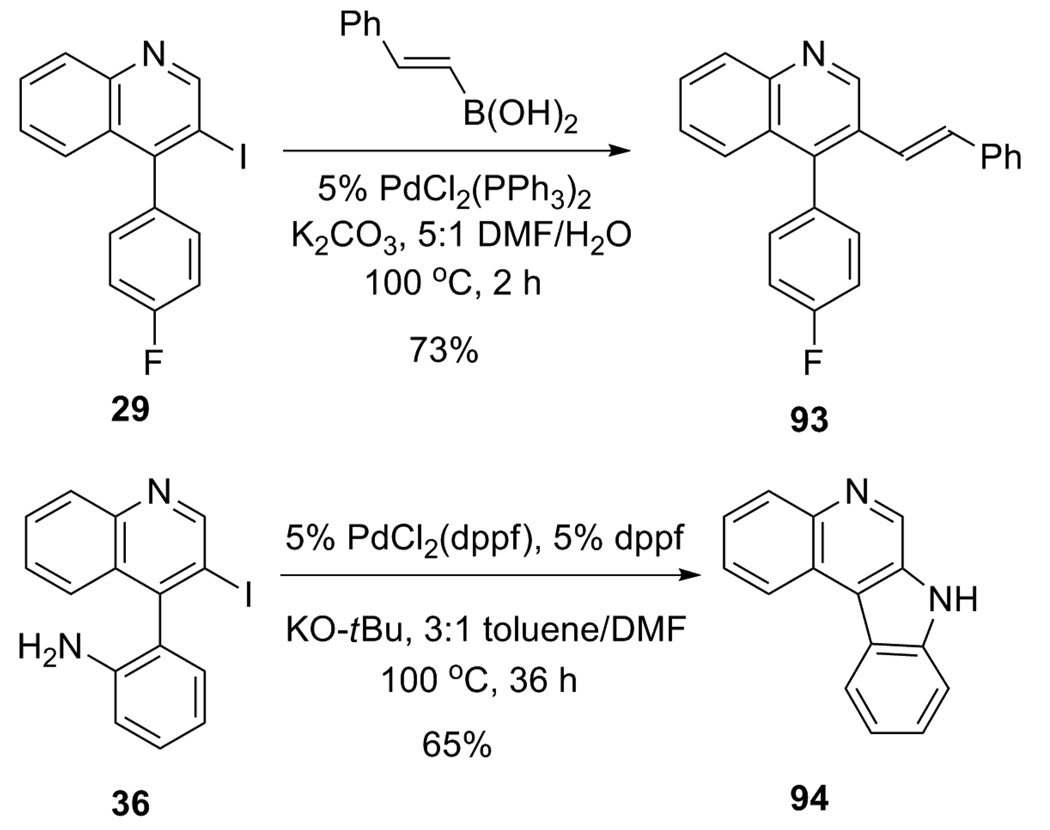
These functionally-substituted quinolines, particularly the iodo derivatives, offer considerable potential for further elaboration. For example, iodoquinoline 29 undergoes a facile Suzuki reaction26 with styrylboronic acid to afford the styryl-substituted quinoline 93 in a 73% yield (Scheme 6). Quinoline 93 is an analog of a CHMG-CoA reductase inhibitor.3c Iodoquinoline 36 readily undergoes an intramolecular palladium-catalyzed Buchwald-Hartwig amination27 to produce the interesting tetracyclic diamine 94 in a 65% yield.
Scheme 6.
Conclusions
A wide variety of 3-halogen-, selenium- and sulfur-containing quinolines have been readily synthesized under mild reaction conditions by the 6-endo-dig electrophilic cyclization of N-(2-alkynyl)anilines by ICl, I2, Br2, PhSeBr and ArSCl as electrophiles. The reaction affords quinolines in moderate to good yields and various functional groups are readily accommodated. Dihydroquinoline derivatives can be obtained when triflamides are used as the starting materials. Quinolinium salts can be obtained when N-alkyl-N-(2-alkynyl)anilines are employed. Quinolines bearing a hydrogen in the 3 position have been synthesized by the Hg(OTf)2-catalyzed ring closure of these same alkynylanilines. Finally, further conversion of the iodine moiety in these iodoquinolines to other functionally-substituted quinolines has been illustrated.
Experimental Section
General
The 1H and 13C NMR spectra were recorded at 300 and 75.5 MHz or 400 and 100 MHz respectively. All melting points are uncorrected. High resolution mass spectra were recorded on a MS50TC double focusing magnetic sector mass spectrometer using EI at 70 eV. All reagents were used directly as obtained commercially unless otherwise noted.
General Procedure for the Electrophilic Cyclization of N-Triflamides by ICl
To a solution of N-phenyl-N-(3-phenylprop-2-ynyl)trifluoromethanesulfonamide (102 mg, 0.30 mmol) in CH2Cl2 (3.0 mL) at −78 °C was added ICl (58.5 mg, 0.36 mmol) in CH2Cl2 (0.5 mL) and the resulting solution was stirred at this temperature for 0.5 h. The reaction mixture was washed with satd aq Na2S2O3 (20 mL) and the organic layer dried over Na2SO4, filtered, and the solvent removed under reduced pressure. To the crude solution of 3-iodo-4-phenyl-1-trifluoromethanesulfonyl-1,2-dihydroquinoline in MeOH (10 mL) was added NaOMe (3.0 mmol, 162 mg) and the mixture was stirred at r.t. in the presence of air for 12 h. The reaction mixture was diluted with diethyl ether (50 mL) and washed with brine (50 mL). The organic layer was dried (Na2SO4), filtered, and the solvent removed under reduced pressure. The residue was purified by column chromatography on a silica gel column.
3-Iodo-4-(3-methoxyphenyl)quinoline (4)
The reaction mixture was chromatographed using 5:1 hexane/EtOAc to afford the product as a white solid in a 62% yield: 117–118 °C; 1H NMR (CDCl3, 300 MHz) δ 3.84 (s, 3H), 6.78 (s, 1H), 6.81–6.83 (m, 1H), 7.03–7.06 (m, 1H), 7.41–7.50 (m, 3H), 7.68–7.72 (m, 1H), 8.08–8.10 (d, J = 6.6 Hz, 1H), 9.22 (s, 1H); 13C NMR (CDCl3, 75 Hz) δ 55.43, 96.11, 114.26, 114.66, 121.40, 126.78, 127.43, 128.90, 129.48, 129.76, 129.86, 141.60, 147.17, 152.24, 156.65, 159.70; IR (CHCl3) 2837, 1613, 1513, 1483, 1246 cm−1; HRMS m/z 360.9972 (calcd for C16H12INO, 360.9964); Anal. Calcd for C16H12INO: C, 53.1; H, 3.3; N 3.9; Found: C, 53.1; H, 3.9; N, 3.5.27
Compounds 38, 39, 41, 43, 46, 48, 53, 55, 59 and 65 were prepared by reaction of the appropriate aniline (2 equiv) and the corresponding propargylic mesylate at room temperature in CH3CN.28
N-(2-Heptynyl)aniline (38)
To a solution of the methanesulfonate29 of 2-heptyn-1-ol (950 mg, 5.0 mmol) in 50 mL of CH3CN was added aniline (930 mg, 10.0 mmol). After being stirred for 20 h under N2, the reaction was quenched by adding brine. The reaction mixture was extracted with Et2O (2 × 30 mL). The extracts were dried over MgSO4 and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (10:1 hexane/EtOAc) on silica gel to afford 589 mg of the product (63% yield) as a light yellow oil. The spectral properties were identical with those previously reported.30
General Procedure for the Electrophilic Cyclization of N-(2-Alkynyl)anilines by I2
In a vial was placed 0.3 mmol of the propargylic aniline, 3 equiv of I2, 2 equiv of NaHCO3, and 3 mL of CH3CN. The reaction mixture was stirred at room temperature, and the reaction was monitored by TLC to establish completion. When finely ground iodine powder was employed, all reactions were complete in 0.5 h. The reaction mixture was then diluted with 25 mL of ether and washed with 20 mL of satd aq Na2S2O3. The organic layer was separated and the aqueous layer was extracted with another 25 mL of ether. The combined organic layers were dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure and the product was isolated by chromatography on a silica gel column.
General Procedure for the Electrophilic Cyclization of N-(2-Alkynyl)anilines by ICl, Br2, PhSeBr or p-O2NC6H4SCl
In a vial was placed 0.3 mmol of the propargylic aniline, 2 equiv of NaHCO3 and 2 mL of CH3CN. 2 Equiv of ICl, Br2, PhSeBr or p-O2NC6H4SCl in 1 mL of CH3CN were added dropwise to the vial. The reaction mixture was stirred at room temperature for 5 min. The reaction mixture was then diluted with 25 mL of ether, and washed with 20 mL of satd aq Na2S2O3 (for the reaction of ICl and Br2) or satd aq NaCl (for the reactions of PhSeBr and p-O2NC6H4SCl). The organic layer was separated and the aqueous layer was extracted with another 25 mL of ether. The combined organic layers were dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure and the product was isolated by chromatography on a silica gel column.
4-(4-Methoxyphenyl)-3-(phenylseleno)quinoline (25)
The reaction mixture was chromatographed using 3:1 hexane/EtOAc to afford 86 mg (74%) of the product as a yellow solid: mp 93–94 °C; 1H NMR (CDCl3, 300 MHz) δ 3.91 (s, 3H), 7.06 (d, J = 6.0 Hz, 2H), 7.26–7.32 (m, 5H), 7.43–7.57 (m, 3H), 7.63–7.68 (m, 1H), 7.68–7.73 (m, 1H), 8.05 (d, J = 8.4 Hz, 1H), 8.67 (s, 1H); 13C NMR (CDCl3, 75 Hz) δ 55.57, 114.25, 126.23, 127.22, 127.34, 128.44, 128.47, 129.16, 129.61, 129.70, 129.74, 129.85, 130.87, 134.81, 147.08, 148.27, 152.43, 159.99; IR (CHCl3) 2923, 2851, 1736, 1250 cm−1; HRMS m/z 391.0482 (calcd for C22H17NOSe, 391.0475).
General Procedure for the Hg(OTf)2-Catalyzed Cyclization of N-(2-Alkynyl)anilines
To a vial containing a solution of 0.3 mmol of the propargylic aniline in 3 mL of CH3CN was added a catalytic amount of Hg(OTf)2. The reaction mixture was stirred at room temperature, and the reaction was monitored by TLC to establish completion. The reaction mixture was then diluted with 25 mL of ether, and washed with 20 mL of satd aq NaI. The organic layer was separated and the aqueous layer was extracted with another 25 mL of ether. The combined organic layers were washed further with 25 mL of satd aq NaCl. The organic layer was dried over MgSO4 and filtered. The solvent was evaporated under reduced pressure and the product was isolated by chromatography on a silica gel column.
4-(4-Methoxyphenyl)quinoline (80)
The reaction mixture was chromatographed using 10:1 hexane/EtOAc to afford 62 mg of the product (88%) as a light yellow oil: 1H NMR (CDCl3, 400 MHz) δ 3.91 (s, 3H), 7.05–7.08 (d, J = 8.7 Hz, 2H), 7.32–7.33 (d, J = 4.2 Hz, 2H), 7.44–7.53 (m, 3H), 7.70–7.75 (m, 1H), 7.96–7.99 (d, J = 8.4 Hz, 1H), 8.15–8.18 (d, J = 8.4 Hz, 1H), 8.92–8.93 (d, J = 4.5 Hz, 1H); 13C NMR (CDCl3, 100 Hz) δ 55.46, 114.11, 121.34, 125.97, 126.54, 127.00, 129.30, 129.89, 130.33, 130.86, 148.26, 148.80, 150.06, 159.91; IR (CHCl3) 3060, 2930, 2849, 1617 cm−1; HRMS m/z 235.1001(calcd for C16H13NO, 235.0997).
Supplementary Material
Acknowledgement
We gratefully acknowledge the National Institute of General Medical Sciences (GM 070620) for support of this research; Kawaken Fine Chemicals Co., Ltd., and Johnson Matthey, Inc. for donations of Pd(OAc)2, and Frontier Scientific and Synthonix for contributions of boronic acids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.(a) Michael JP. Nat. Prod. Rep. 1997;14:605. doi: 10.1039/np9971400011. [DOI] [PubMed] [Google Scholar]; (b) Balasubramanian M, Keay JG. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, editors. Vol. 5. Oxford: Pergamon Press; 1996. pp. 245–266. [Google Scholar]
- 2.(a) Markees DG, Dewey VC, Kidder GW. J. Med. Chem. 1970;13:324. doi: 10.1021/jm00296a048. [DOI] [PubMed] [Google Scholar]; (b) Alhaider AA, Abdelkader MA, Lien EJ. J. Med. Chem. 1985;28:1394. doi: 10.1021/jm00148a004. [DOI] [PubMed] [Google Scholar]; (c) Campbell SF, Hardstone JD, Palmer MJ. J. Med. Chem. 1988;31:1031. doi: 10.1021/jm00400a025. [DOI] [PubMed] [Google Scholar]; (d) De SK, Gibbs RA. Tetrahedron Lett. 2005;46:1647. [Google Scholar]
- 3.(a) Newhouse BJ, Bordner J, Augeri DJ, Litts CS, Kleinman EF. J. Org. Chem. 1992;57:6991. [Google Scholar]; (b) Torii S, Xu LH, Sadakane M, Okumoto H. Synlett. 1992:513. [Google Scholar]; (c) Nobuhide M, Yoshinobu Y, Hiroshi I, Yoshio O, Tamejiro H. Tetrahedron Lett. 1993;24:8263. [Google Scholar]; (d) Croisey-Delcey M, Croisy A, Carrez D, Huel C, Chiaroni A, Ducrot P, Bisagni E, Jin L, Leclercq G. Bioorg. Med. Chem. 2000;8:2629. doi: 10.1016/s0968-0896(00)00194-2. [DOI] [PubMed] [Google Scholar]; (e) Bu XY, Deady LW, Denny WA. Aust. J. Chem. 2000;53:143. [Google Scholar]; (f) Blackburn TP, Cox B, Guildord AJ, LeCount DJ, Middlemiss DN, Pearce RJ, Thornber CW. J. Med. Chem. 1987;30:2252. doi: 10.1021/jm00395a013. [DOI] [PubMed] [Google Scholar]; (g) Amii H, Kishikawa Y, Uneyama K. Org. Lett. 2001;3:1109. doi: 10.1021/ol006936u. [DOI] [PubMed] [Google Scholar]
- 4.O’Dell DK, Nicholas KM. J. Org. Chem. 2003;68:6427. doi: 10.1021/jo034447c. and references therein. [DOI] [PubMed] [Google Scholar]
- 5.(a) Eisch JJ. J. Org. Chem. 1961;27:1318. [Google Scholar]; (b) Kress TJ, Constantino SM. J. Heterocycl. Chem. 1973;10:409. [Google Scholar]
- 6.Trecourt F, Mongin F, Mallet M, Queguiner G. Synth. Commun. 1995;25:4011. [Google Scholar]
- 7.Campos PJ, Tan CQ, Rodriguez MA, Anon E. J. Org. Chem. 1996;61:7195. doi: 10.1021/jo9600215. [DOI] [PubMed] [Google Scholar]
- 8.Baker RH, Tinsley SW, Jr, Butler D, Riegel B. J. Am. Chem. Soc. 1950;72:393. [Google Scholar]
- 9.Larock RC. In: Acetylene Chemistry. Chemistry, Biology, and Material Science. 2. Diederich F, Stang PJ, Tykwinski RR, editors. New York: Wiley-VCH; 2005. pp. 51–99. [Google Scholar]
- 10.Huang Q, Hunter JA, Larock RC. J. Org. Chem. 2002;67:3437. doi: 10.1021/jo020020e. [DOI] [PubMed] [Google Scholar]
- 11.(a) Yue D, Larock RC. J. Org. Chem. 2002;67:1905. doi: 10.1021/jo011016q. [DOI] [PubMed] [Google Scholar]; (b) Hessian K, Flynn B. Org. Lett. 2003;5:4377. doi: 10.1021/ol035663a. [DOI] [PubMed] [Google Scholar]
- 12.(a) Goldfinger MB, Crawford KB, Swager TM. J. Am. Chem. Soc. 1997;119:4578. [Google Scholar]; (b) Goldfinger MB, Swager TM. J. Am. Chem. Soc. 1994;116:7895. [Google Scholar]; (c) Yao T, Campo MA, Larock RC. Org. Lett. 2004;6:2677. doi: 10.1021/ol049161o. [DOI] [PubMed] [Google Scholar]; (d) Barluenga J, Vazquez-Villa H, Ballesteros A, Gonzaléz JM. Org. Lett. 2003;5:4121. doi: 10.1021/ol035691t. [DOI] [PubMed] [Google Scholar]
- 13.(a) Yue D, Larock RC. Org. Lett. 2004;6:1037. doi: 10.1021/ol0498996. [DOI] [PubMed] [Google Scholar]; (b) Barluenga J, Trincado M, Rubio E, Gonzaléz JM. Angew. Chem., Int. Ed. 2003;42:2406. doi: 10.1002/anie.200351303. [DOI] [PubMed] [Google Scholar]; (c) Amjad M, Knight DW. Tetrahedron Lett. 2004;45:539. [Google Scholar]; (d) Yao T, Yue D, Larock RC. J. Comb. Chem. 2005;7:809. doi: 10.1021/cc050062r. [DOI] [PubMed] [Google Scholar]
- 14.Arcadi A, Cacchi S, Giuseppe SD, Fabrizi G, Marinelli F. Org. Lett. 2002;4:2409. doi: 10.1021/ol0261581. and references therein. [DOI] [PubMed] [Google Scholar]
- 15.Yao T, Larock RC. J. Org. Chem. 2005;70:1432. doi: 10.1021/jo048007c. [DOI] [PubMed] [Google Scholar]
- 16.(a) Biagetti M, Bellina F, Carpita A, Stabile P, Rossi R. Tetrahedron. 2002;58:5023. [Google Scholar]; (b) Rossi R, Carpita A, Bellina F, Stabile P, Mannina L. Tetrahedron. 2003;59:2067. [Google Scholar]; (c) Yao T, Larock RC. J. Org. Chem. 2003;68:5936. doi: 10.1021/jo034308v. [DOI] [PubMed] [Google Scholar]
- 17.(a) Barluenga J, Vazquez-Villa H, Ballesteros A, Gonzaléz JM. J. Am. Chem. Soc. 2003;125:9028. doi: 10.1021/ja0355372. [DOI] [PubMed] [Google Scholar]; (b) Yue D, Della Cá N, Larock RC. Org. Lett. 2004;6:1581. doi: 10.1021/ol049690s. [DOI] [PubMed] [Google Scholar]; (c) Barluenga J, Campos-Gomez E, Rodriguez D, Gonzalez-Bobes F, Gonzaléz JM. Angew. Chem., Int. Ed. 2005;44:5851. doi: 10.1002/anie.200501195. [DOI] [PubMed] [Google Scholar]
- 18.(a) Cacchi S, Fabrizi G, Moro L. Synlett. 1998;7:741. [Google Scholar]; (b) Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Moro L. Synlett. 1999;9:1432. [Google Scholar]
- 19.Zhang X, Campo MA, Yao T, Larock RC. Org. Lett. 2005;7:763. doi: 10.1021/ol0476218. [DOI] [PubMed] [Google Scholar]
- 20. Tokuyama H, Sato M, Ueda T, Fukuyama T. Heterocycles. 2001;54:105.. (b) For prior work on the oxidation of dihydroquinolines to quinolines, see: Forrest TP, Dauphinee GA, Deraniyagala SA. Can J. Chem. 1985;63:412..
- 21.Abbiati G, Arcadi A, Bianchi G, Di Giuseppe S, Marinelli F, Rossi E. J. Org. Chem. 2003;68:6959. doi: 10.1021/jo0347260. [DOI] [PubMed] [Google Scholar]
- 22.Reetz MT, Sommer K. Eur. J. Org. Chem. 2003;68:3485. [Google Scholar]
- 23.Youn SW, Pastine SJ, Sames D. Org. Lett. 2004;6:581. doi: 10.1021/ol036385i. [DOI] [PubMed] [Google Scholar]
- 24.Pastine SJ, Youn SW, Sames D. Org. Lett. 2003;5:1055. doi: 10.1021/ol034177k. [DOI] [PubMed] [Google Scholar]
- 25.Nishizawa M, Takao H, Yadav VK, Imagawa H, Sugihara T. Org. Lett. 2003;5:4563. doi: 10.1021/ol035622e. [DOI] [PubMed] [Google Scholar]
- 26. Occhiato EG, Trabocchi A, Guarana A. J. Org. Chem. 2001;66:2459. doi: 10.1021/jo001807c.. (b) For a review, see: Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457..
- 27.The HRMS analysis is not as precise as desired, but the spectral data are entirely consistent for compound 4.
- 28.Marshall JA, Wolf MA. J. Org. Chem. 1996;61:3238. [Google Scholar]
- 29.For the synthesis of propargylic mesylates from propargylic alcohols, see: Tanabe Y, Yamamoto H, Yoshida Y, Miyawaki T, Utsumi N. Bull. Chem. Soc. Jpn. 1995;68:297..
- 30.Inoue J, Cui Y-S, Sakai O, Nakamura Y, Kogiso H, Kador PF. Bioorg. Chem. 2000;8:2167. doi: 10.1016/s0968-0896(00)00158-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.