Abstract
In mammals, the first step in the perception of form and texture is the activation of trigeminal or dorsal root ganglion (DRG) mechanosensory neurons, which are classified as either rapidly (RA) or slowly adapting (SA) according to their rates of adaptation to sustained stimuli. The molecular identities and mechanisms of development of RA and SA mechanoreceptors are largely unknown. We found that the “early Ret+” DRG neurons are RA mechanoreceptors, which form Meissner corpuscles, Pacinian corpuscles and longitudinal lanceolate endings. The central projections of these RA mechanoreceptors innervate layers III through V of the spinal cord and terminate within discrete subdomains of the dorsal column nuclei. Moreover, mice lacking Ret signaling components are devoid of Pacinian corpuscles and exhibit a dramatic disruption of RA mechanoreceptor projections to both the spinal cord and medulla. Thus, the early Ret+ neurons are RA mechanoreceptors and Ret signaling is required for the assembly of neural circuits underlying touch perception.
Introduction
The perception of form and texture is fundamental and essential for the daily lives of most, if not all animals. The first step in the perception of discriminative touch in mammals is the detection of pressure, vibration or stretch of the skin and deflection of hairs by specialized mechanosensory end-organs in the skin (Zelena, 1994). Low threshold, large-diameter trigeminal and dorsal root ganglion (DRG) neurons (mechanoreceptors) innervate these end-organs and are the primary sensory neurons mediating discriminative touch and tactile perception.
DRG mechanoreceptors can be classified according to the morphologies of their peripheral end organs, which include Merkel discs, Ruffini corpuscles, Meissner corpuscles, Pacinian corpuscles, and longitudinal lanceolate endings (Albrecht, 2008; Iggo and Andres, 1982). Glabrous skin contains Merkel discs and Meissner corpuscles, whereas general hairy skin contains Merkel discs and longitudinal lanceolate endings associated with guard hair follicles. Pacinian corpuscles are found in the dermis of humans, although in mice and rats they are normally restricted to joints and the periostium of bones (Zelena, 1994). Mechanoreceptors are further distinguished as being either rapidly adapting (RA) or slowly adapting (SA) based on their rates of adaptation to sustained mechanical stimuli (Mountcastle, 1957). Meissner corpuscles, Pacinian corpuscles and longitudinal lanceolate endings are RA mechanoreceptors (Iggo and Ogawa, 1977) whereas Merkel discs are the principle SA mechanoreceptors in rodents and monkeys (Iggo and Muir, 1969; Pare et al., 2002).
Despite physiological and morphological characterization of mechanoreceptor subtypes, mechanisms of development and unique functions of RA and SA mechanoreceptors are poorly understood, in part due to a lack of molecular identification of these neurons. Therefore, we have sought identification of candidate DRG mechanoreceptor subtypes based on a few broad criteria. First, since all mechanoreceptors are physiologically-defined “Aβ” fibers, then they are almost certainly large diameter, NF200+ DRG neurons (Lawson et al., 1993). Also, mechanoreceptors, like proprioceptors, are born shortly after coalescence of rudimentary ganglia (Lawson et al., 1974). Furthermore, mechanoreceptors account for only a small percentage of all DRG neurons (Lawson et al., 1993). Therefore, candidate mechanoreceptors must be few in number, born shortly after DRG coalescence, and have large diameter soma sizes.
One approach to identify subtypes of DRG sensory neurons is to characterize them based upon their expression of receptors for neurotrophic factors. In fact, most if not all DRG neurons express receptors for one or more neurotrophic growth factors (Marmigere and Ernfors, 2007), which promote neuronal differentiation, maturation and survival. For example, small diameter, unmyelinated peptidergic nociceptors express the nerve growth factor (NGF) receptor TrkA, and are dependent upon NGF–TrkA signaling for expression of nociceptor-specific genes, innervation of the epidermis and survival (Crowley et al., 1994; Luo et al., 2007; Patel et al., 2000; Smeyne et al., 1994). Similarly, neurotrophins are involved in mechanoreceptor development and function. For example, the number of Merkel cells and their associated nerve terminals are decreased in P14 NT3 mutant mice (Airaksinen et al., 1996), and BDNF is required postnatally for the normal transduction properties of SA mechanoreceptors (Carroll et al., 1998). In addition, overexpression of BDNF in the skin leads to enhanced innervation of hair follicles, large Meissner corpuscles, and an increase in the number of Merkel cells (LeMaster et al., 1999) while, conversely, Meissner corpuscles are absent in both BDNF and TrkB null mice (Gonzalez-Martinez et al., 2005; Gonzalez-Martinez et al., 2004; Perez-Pinera et al., 2008). However, Pacinian corpuscles and longitudinal lanceolate endings are present in normal numbers in all neurotrophin and Trk receptor mutant mice. Thus, the identity of trophic factors and their cognate receptors that support development of most populations of RA mechanoreceptors and their peripheral and central axonal projections is lacking.
The glia derived neurotrophic factor (GDNF) family of ligands (GFLs) contains four members, GDNF, neurturin (NRTN), artemin (ARTN) and persephin (PSPN) (Airaksinen and Saarma, 2002). The receptors for GFLs are comprised of a signaling subunit, the receptor tyrosine kinase Ret (Durbec et al., 1996; Trupp et al., 1996), and a GPI-anchored ligand binding subunit the GFL receptor (GFR) (Airaksinen et al., 1999). There are four GFRs in vertebrates, designated GFRα1 through GFRα4. In vitro binding assays indicate that GDNF binds preferentially to GFRα1, NRTN to GFRα2, ARTN to GFRα3, and PSPN to GFRα4 (Airaksinen et al., 1999). GFRα1, GFRα2, GFRα3, but not functional GFRα4 (Lindahl et al., 2000) are expressed in unique patterns in the DRG during development (Luo et al., 2007), suggesting key roles of GFL–GFR/Ret signaling in development of distinct populations of DRG sensory neurons.
Ret is expressed in approximately 60% of adult mouse DRG neurons, which can be broadly divided into two main groups based on their development history. Most Ret+ DRG neurons are small to medium-diameter non-peptidergic nociceptors and are born around E11.5 or later and subsequently express TrkA. Ret is not highly expressed in these neurons until E15.5 or beyond (Luo et al., 2007; Molliver et al., 1997), and recent work has revealed a central role for Ret in their maturation and epidermal innervation (Luo et al., 2007). A distinct, smaller population consists of Ret+ DRG neurons that are born earlier, express Ret prior to E11.5, do not express TrkA and have large soma diameters. These large diameter Ret+/TrkA− neurons, which are referred to as the early Ret+ neurons (Chen et al., 2006; Kramer et al., 2006; Luo et al., 2007; Marmigere and Ernfors, 2007; Molliver et al., 1997), caught our attention because they exhibit aforementioned features of mechanosensory neurons. Here, we show that the early Ret+ DRG neurons are RA mechanoreceptors, which exhibit a modality-specific pattern of innervation of the brainstem and require Ret signaling for their assembly into neural circuits underlying discriminative touch perception.
Results
Molecular characterization of the early Ret+ DRG neurons
We sought molecular identification and characterization of candidate mechanoreceptors which, like proprioceptors, are predicted to be early-born, large diameter, NF200+ DRG neurons. One such neuronal population, the “early Ret+” neurons, uniquely fits this profile. The early Ret+ neurons are born during the first wave of DRG neurogenesis and begin to express Ret on or before E10.5, which is prior to initiation of expression of TrkA in the DRG (fig. S1A–C). Large diameter, TrkC+ DRG neurons, some of which become proprioceptors (Klein et al., 1994), are born at approximately the same time. Interestingly, the early Ret+ and TrkC+ DRG neurons are distinct populations (fig. S1G–I, and (Kramer et al., 2006)). Moreover, while Ret is expressed exclusively in the early Ret+ neurons (Ret+/TrkA−) in the DRG at E12 (Luo et al., 2007), it is expressed in many TrkA+ DRG neurons (Ret+/TrkA+) beginning at E13.5 (fig. S1D–F). Furthermore, 54.3% ± 13.4% of early Ret+ neurons co-express TrkB at E13.5 (fig. S1J–L), while only 25.1 ± 7.4% of the TrkB+ DRG neurons co-express Ret (fig. S1J–L), indicating that TrkB is expressed in both early Ret+ neurons as well as other DRG neurons at this time.
To characterize the early Ret+ DRG neurons with respect to their expression of GFRα co-receptors, double fluorescent in situ hybridization experiments were performed using cRNA probes for Ret and GFRαs and sections from wild-type and TrkA null mice at different developmental stages. We found that GFRα2 and GFRα3 but not GFRα1 are expressed in DRG neurons at E12 (Luo et al., 2007). At E13.5, GFRα1–3 are expressed (Fig. 1A, D, G); GFRα1 is co-expressed with Ret in many neurons (Ret+ neurons: 17±4; GFRα1+ neurons: 17±5; and GFRα1+/Ret+ neurons: 11±4 neurons/DRG section, Figure 1A–C) whereas all GFR2+ neurons are Ret+ (Ret+ neurons: 18±2, GFRα2+ neurons: 9±2, and GFRα2+/Ret+ neurons: 9±2. Figure 1D–F). In contrast, GFRα3 is mainly expressed in Ret− DRG neurons at this time (Ret+ neurons: 21±6, GFRα3+ neurons: 51±9, and GFRα3+/Ret+ neurons: 4±1. Fig. 1G–I). Therefore, we focused on GFRα1 and GFRα2 by analyzing their patterns of expression in TrkA null DRGs. TrkA null mice were used here because the expression of Ret and GFRs in late-born Ret+/TrkA+ DRG neurons is deficient in mice lacking TrkA signaling (Luo et al., 2007) while their expression in early Ret+/TrkA− neurons should be unaltered. At E13.5, approximately 80% of Ret+ DRG neurons are lost in TrkA null DRGs (37 ± 6 vs. 7 ± 3 neurons/DRG, control vs. mutant, Fig. 1J–K), consistent with our finding that Ret+/TrkA+ DRG neurons are present in E13.5 wild-type (WT) DRGs. In addition, GFRα2 exhibits a normal pattern of expression in TrkA mutant DRGs (Fig. 1 L–O); The number of GFRα2+ DRG neurons in TrkA mutants is similar to that of WT controls (10 ± 3 vs. 9 ± 1, respectively), suggesting that at this time point, GFRα2 is expressed exclusively in Ret+/TrkA− neurons (the early Ret+ DRG neurons). On the other hand, GFRα1 is expressed exclusively in Ret+/TrkA+ DRG neurons at E13.5 since GFRα1+ neurons are completely absent in DRGs of the TrkA mutant (29 ± 3 vs. 0, Fig. 1L–M). Similar results were obtained in experiments using P0 pups (fig. S2). Therefore, the early Ret+ neurons express GFRα2, but not other GFRαs. A second, intermediate population of Ret+ neurons emerges from TrkA+ precursors, beginning ~E13.5, and expresses GFRα1. In addition, the non-peptidergic sensory neurons, which also originate from TrkA+ precursors, express Ret beginning ~E16 and GFRα2 from P0 onward (Luo et al., 2007). Thus, at least three distinct populations of Ret+ DRG sensory neurons can be classified based upon their soma sizes, temporal patterns of Ret expression and co-expression of GFRαs (Fig. 1P).
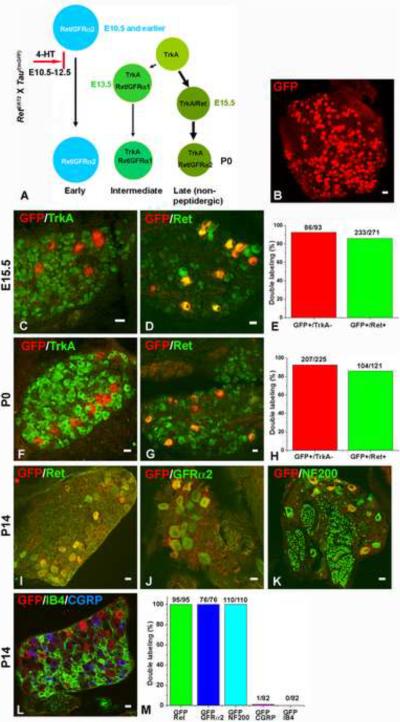
Figure 1. The early Ret+ DRG neurons express GFRα2.
Double fluorescent in situ hybridization of Ret and GFRα1 (A–C), Ret and GFRα2 (D–F), and Ret and GFRα3 (G–I) at E13.5 (n=3, and 6 to 8 sections were examined for each animal, lower lumbar DRGs). J–O: In situ hybridization of Ret (37 ± 6 vs. 7 ± 3), GFRα1 (29 ± 3 vs. 0), and GFRα2 (10 ± 3 vs. 9 ± 1) in control and TrkA null DRGs at E13.5 (control vs. mutant, mean ± SEM positive neurons/section, n=3 animals per genotype, and 6 to 8 sections were examined and quantified for each animal, lower lumbar DRGs). P. Illustration of the three populations of Ret+ DRG neurons based on their developmental origin and temporal patterns of expression of Ret and GFRα co-receptors.
Genetic labeling of the early Ret+ DRG neurons
To test the idea that the early Ret+ neurons are mechanoreceptors, a genetic labeling approach was developed to visualize their central and peripheral axonal projections. Here, we took advantage of the observation that the early Ret+ neurons are the only DRG neurons that express Ret prior to E12.5 We generated a Ret-CreERT2 (RetERT2) knockin mouse line (fig. S3) in which the spatial and temporal patterns of expression of an inducible form of Cre recombinase (CreERT2) (Feil et al., 1997) recapitulate expression of Ret. These RetERT2 mice were crossed to neuron-specific reporter mice (Tauf(mGFP)) (Hippenmeyer et al., 2005), in which myristoylated green fluorescent protein (mGFP) is expressed following Cre-mediated recombination, to generate RetERT2; Tauf(mGFP) mice. We predicted that administration of the ERT2 ligand 4-hydroxytamoxifen (4-HT) to RetERT2; Tauf(mGFP) reporter mice prior to E12.5 would lead to expression of GFP exclusively in early Ret+ DRG neurons (Fig. 2A). In contrast, administration of 4-HT to RetERT2; Tauf(mGFP) mice at E15.5 or later (fig. S4A) is predicted to lead to GFP expression in both the early Ret+ neurons and late-born Ret+ DRG neurons, which emerge from TrkA+ precursors. Using this strategy, administration of 4-HT from E10.5 to E12.5 resulted in 280 ± 25 GFP-labeled neurons in L5 DRGs at P14 (Fig. 2B), which represents ~5% of all L5 DRG neurons at this time (Liu et al., 2007). To address labeling specificity, we co-stained GFP+ neurons with antibodies against TrkA and Ret at both E15.5 and P0. The Early Ret+ neurons should be TrkA− and Ret+ at both ages. At E15.5, 92.5% of GFP+ neurons were TrkA− whereas 86% of them were Ret+ (Fig. 2C–E). Similar findings were obtained with P0 pups (Fig. 2F–H). At P14, 100% of GFP+ neurons were Ret+ (Fig. 2M), suggesting that the few GFP+/Ret− neurons observed at E15.5 and P0 are either lost postnatally or express Ret dynamically. In contrast, 73.7% of GFP-labeled neurons in RetERT2; Tauf(mGFP) mice that received 4-HT at later ages (E15.5–E17.5) are TrkA+ at P0 (fig. S4), indicating that late 4-HT treatment indeed labels both the early Ret+ neurons and late-born populations of Ret+ neurons. We estimate that greater than 60% of early Ret+ neurons are labeled following administration of 4-HT to RetERT2; Tauf(mGFP) mice from E10.5 to E12.5 by comparing the number of GFP+/TrkA− neurons (5 ± 1 neurons/ DRG section Fig. 2F–G) to that of Ret+/TrkA− neurons at P0 (8 ± 3 neurons/DRG (Luo et al., 2007)).
Figure 2. Genetic Labeling of the early Ret+ DRG neurons.
A. Outline of the chemical-genetic strategy for labeling the early Ret+ neurons. RetERT2 and Tauf(mGFP) mice were crossed, and timed pregnant mothers were gavaged with 1.0 to 1.5 mg 4-HT per day from E10.5 to E12.5. B. Whole-mount anti-GFP immunostaining of a labeled L5 DRG. 280 ± 25 GFP+ neurons were labeled. Eight L5 DRGs from four animals of two litters were examined. C–D: Double immunostaining of GFP with TrkA (C) or Ret (D) in E15.5 labeled DRGs. 86/93 (GFP+/TrkA− neurons / total GFP+ neurons) GFP+ neurons are TrkA−, and 233/271 (GFP+/Ret+ neurons / total GFP+ neurons) GFP+ neurons are Ret+. Lumbar DRGs, n=6 from three litters. 4–6 sections from each animal were quantified and shown in E. F–G: Double immunostaining of GFP with TrkA (F) or Ret (G) in P0 labeled DRGs. 207/225 (92%) GFP+ neurons are TrkA−, and 104/121 (86%) GFP+ neurons are Ret+. Lumbar DRGs, n=7 from four litters for GFP/TrkA staining, and n=4 from two litters for GFP/Ret staining. Quantifications are shown in H. I–L: Double immunostaining of GFP and Ret (I), GFP and GFRα2 (J), GFP and NF200 (K), and GFP and CGRP and IB4 in P14 labeled DRGs. All GFP+ neurons (95/95) are Ret+ at this time (Note that, due to the fixation conditions needed for this experiment, Ret immunoreactivity is detected only in those DRG neurons expressing a high level of Ret protein). In addition, GFP+ neurons are GFRα2+ (76/76) and NF200+ (110/110), but not CGRP+ (1/82) or IB4+ (0/82). Quantifications are shown in M. Lumbar DRGs, n=4 from two litters. Scale bar for panel B: 50μm, other panels: 20μm
To further characterize the population of Ret+ neurons labeled by administration of 4-HT from E10.5 to E12.5, we measured their soma sizes and examined their patterns of expression of GFRα2, NF200, CGRP, Parvalbumin and TrkB and binding to the lectin IB4. As expected, the GFP-labeled early Ret+ DRG neurons have large soma sizes (average area: 587±102 μm2, fig. S5A), and 72.9% ± 11% of GFP+ neurons are TrkB+ at P0 (fig. S5B–D). In addition, at P14, 100% of the GFP+ neurons are GFRα2+ and NF200+ (Fig. 2J–K, M). On the other hand, almost none of the GFP+ neurons were found to express CGRP nor did they bind to IB4 (Fig. 2L–M), which are markers for peptidergic (Hokfelt, 1991) and non-peptidergic nociceptors (Lawson, 1992), respectively. Furthermore, GFP+ neurons do not express Parvalbumin (fig. S5E–G), which labels proprioceptors (Carr et al., 1989). Thus, 4-HT treatment of RetERT2; Tauf(mGFP) mice prior to E12.5 specifically labels the early Ret+ neurons, and these large diameter, GFP+ DRG neurons exhibit molecular features of mechanosensory neurons.
The “Early Ret+” neurons innervate RA mechanosensory end organs
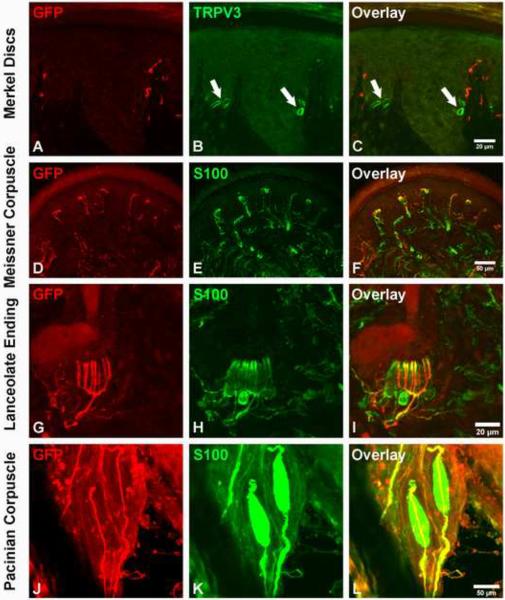
Next, we examined the relationship between GFP+ fibers and the different mechanosensory end organs at P14, an age when they exhibit mature morphologies (Zelena, 1994). We first examined the innervation of Merkel discs using a TRPV3 antibody, which specifically labels Merkel cells (fig. S6A–F). We found that GFP+ fibers are not associated with Merkel cells in the footpad (Fig. 3A–C), touch domes of back hairy skin, guard hair follicles, or regular glabrous skin (fig. S6G–O). Thus, the early Ret+ neurons do not innervate Merkel cells and, therefore, they are not SA mechanosensory neurons. Instead, GFP+ fibers innervate Meissner corpuscles (Fig. 3D–F), which are located in dermal papillae of the footpad. In hairy skin, GFP+ fibers form longitudinal lanceolate endings associated with guard hair follicles (Fig. 3G–I). Furthermore, GFP+ fibers innervate Pacinian corpuscles, which in mice are found in abundance in the periosteum of the fibula (Zelena, 1976) (Fig. 3J–L). Taken together, these findings suggest that the early Ret+ DRG neurons are RA mechanosensory neurons.
Figure 3. The early Ret+ DRG neurons are the RA mechanoreceptors associated with Meissner corpuscles, Pacinian corpuscles and longitudinal lanceolate endings.
A–C: GFP+ fibers do not innervate Merkel cells in the footpad (339/356 {95.2%} of Merkel cells, labeled with the TrpV3 antibody, are not associated with GFP+ axons). The locations of Merkel cells are indicated by white arrows. D–F: GFP+ fibers innervate Meissner corpuscles, visualized by S100 immunostaining, in dermal papillae (114/172 {66.3%} Meissner corpuscles are innervated by GFP+ fibers). G–I: GFP+ fibers form longitudinal lanceolate endings associated with hair follicles, shown by S100 immunostaining (78/184 {42.3%} of hair follicles are associated with GFP+ longitudinal lanceolate endings). J–L: Whole-mount anti-GFP and anti-S100 staining of the periosteum membrane of the fibula. Note that a single GFP+ fiber innervates each Pacinian corpuscle (55/60 {91.7%} of Pacinian corpuscles are innervated by GFP+ fibers). Immunostainings were performed using P14 RetERT2;Tauf(mGFP) mice treated with 4-HT from E10.5 and E12.5. Quantifications were made using four P14 RetERT2;Tauf(mGFP) mice from two litters.
Early Ret+ DRG neurons exhibit a modality-specific pattern of axonal projections to the brainstem
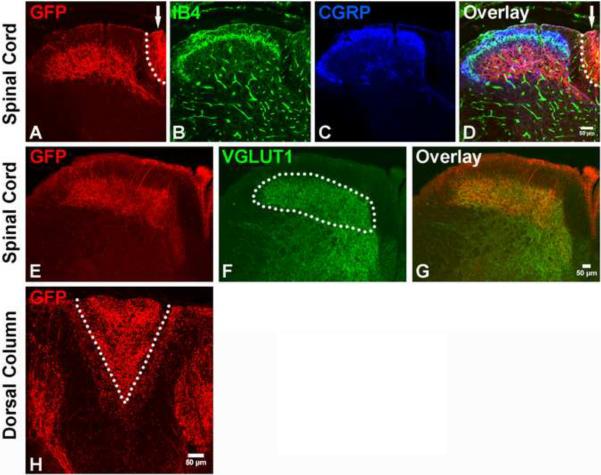
Mechanoreceptors send collateral branches that innervate the dorsal horn of the spinal cord (Brown, 1981) and others that ascend ipsilaterally within the dorsal column (DC) to the gracile and cuneate nuclei (dorsal column nuclei, DCN) of the medulla (Carpenter MB, 1983). As expected, GFP-labeled central axonal projections enter the spinal cord at a dorsal-medial position and innervate layers III through V of the dorsal horn (Fig. 4A–G). GFP+ axons also project within the gracile and cuneate fasciculi of the DC (Fig. 4A, H) and are highly enriched in the gracile fasiculus at cervical levels (Fig. 4H). In addition, GFP+ fibers innervate both the gracile and cuneate nuclei (Fig. 5A, D, G), and remarkably, the termination zones of these axons within the DCN exhibit a unique pattern of zonal segregation. The entire extent of the DCN is clearly visualized and demarcated by the pattern of VGLUT1+ synapses (Fig. 5B, E, H and (Hughes et al., 2007)) as well as the axonal terminations of primary sensory neurons in AdvillinAP mice (data not shown), in which all somatosensory neurons are labeled with human placental alkaline phosphatase (Hasegawa et al., 2007). Within the DCN, GFP+ axons terminate extensively within most of the gracile nucleus (Fig. 5 and fig. S7) except for a small dorsal-rostral zone (Fig. 5D–F and fig. S7), which is rich in VGLUT1 synapses but lacks GFP+ terminations. In the cuneate nucleus, by contrast, many GFP+ fibers terminate within a medial-rostral zone (Fig. 5A–C and fig. S7) while the ventral-caudal region of the cuneate nucleus is virtually devoid of GFP+ endings (Fig. 5D–I and fig. S7). These findings reveal specific termination zones of the early Ret+ DRG neurons within the DCN of the mouse brainstem (Fig 5J), the pattern of which is remarkably similar to those of RA mechanoreceptors revealed by physiological recordings in cats (Dykes et al., 1982). Based on the peripheral and central patterns of GFP+ axonal projections, we conclude that the early Ret+ DRG neurons are RA mechanoreceptors.
Figure 4. Central projections of early Ret+ DRG neurons innervate layers III through V of the spinal cord.
A–D: Central projections of GFP labeled early Ret+ DRG neurons in the upper lumbar level of the spinal cord. Note the presence of GFP+ fibers in both lamina III through V of the spinal cord and the dorsal column (arrow indicates the area which is also outlined by the dotted line). Nociceptor axons innervating spinal cord layers I and II were visualized with CGRP immunostaining (blue) and IB4 binding (green), respectively. E–G: Double staining of GFP and VGLUT1. VGLUT1 labels synapses of the central projections of mechanoreceptors (white dotted line) and proprioceptors (Hughes et al., 2004; Oliveira et al., 2003). H: Dorsal column of the cervical spinal cord. Note that while GFP+ fibers are present in both the gracile (V shape tract, inside the white dotted line) and cuneate fasiculi (area outside the white dotted line), they are greatly enriched in the gracile fasiculus. N=4 from two litters for P14 RetERT2;Tauf(mGFP) mice treated with 4-HT from E10.5 to E12.5.
Figure 5. Modality-specific segregation of mechanosensory axons in dorsal column nuclei.
A, D, G: GFP+ fibers innervating the gracile and cuneate nuclei at different rostral to caudal levels of the medulla. The VGLUT1 staining is used to visualize the gracile and cuneate nuclei (B, E, H). The white dotted line outlines the boundary of the gracile nucleus (Gn), and the yellow dotted line outlines the boundary of the cuneate fasiculus and nucleus (Cn) in C, F, I. Note that GFP+ fibers occupy most of the gracile nucleus (I), but are absent from a dorsal segment at the mid-level of the medulla (F). On the other hand, GFP+ fibers do not innervate the caudal-ventral region of the cuneate nucleus (F and I), but project to the dorsal-rostral region of the cuneate nucleus (C). Arrows indicate the boundaries of the gracile and cuneate nuclei. J. Three-dimensional illustration of the pattern of innervation of the gracile and cuneate nuclei by RA mechanoreceptors. This model is based upon the findings reported in Fig. 5A–I and fig S7. The VGLUT1+ zones occupied by GFP+ fibers are showed in yellow. The green zones are unoccupied VGLUT1+ zones. N=3 for 2 month old RetERT2;Tauf(mGFP) mice treated with 4-HT from E10.5 to E12.5.
Peripheral and central projections of early Ret+ DRG neurons in Retf(CFP); Wnt1Cre mice
To further characterize the pattern of innervation of mechanosensory end organs by Ret+ DRG neurons, and to substantiate our findings using RetERT2; Tauf(mGFP) reporter mice, described above, we made use of a Ret conditional CFP (Retf(CFP)) mouse line in which CFP is expressed in all Ret+ cells following CRE-mediated recombination (Uesaka et al., 2008). For these experiments, Retf(CFP) mice were crossed to a neural crest lineage mouse line, Wnt1Cre mice (Danielian et al., 1998). Thus, in Retf(CFP);Wnt1Cre mice, CFP is expressed in all Ret+ DRG neurons, including the early Ret+ DRG neurons. Consistent with our findings described above, CFP+ fibers do not innervate Merkel cells in glabrous skin or those associated with guard hair follicles in hairy skin (fig. S8A–C, and data not shown). Instead, CFP+ fibers form Meissner corpuscles (fig. S8D–F), longitudinal lanceolate endings (fig. S8G–I), and Pacinian corpuscles (fig. S8J–L). Interestingly, although Wnt1Cre is active in DRG neurons, Schwann cells and their precursors, only axons of DRG neurons are labeled with CFP (fig. S8F,L) indicating that Ret is expressed in RA mechanosensory DRG neurons but not Schwann cells or other neural crest-derived accessory cells. Furthermore, as expected, CFP+ fibers innervate layers I through V in the spinal cord (fig. S8M–P) and are highly enriched in the gracile fasciculus at the cervical level of the spinal cord (data not shown). These peripheral and central patterns of GFP+ axonal innervation indicate that a subpopulation of Ret+ DRG neurons are RA mechanoreceptors, supporting the aforementioned analysis of the early Ret+ DRG neurons using RetERT2; Tauf(mGFP) reporter mice. Hereafter, we refer to the early Ret+ DRG neurons as RA mechanoreceptors.
RA mechanosensory neurons lacking Ret signaling components are present in normal numbers in neonatal mice
The high levels of embryonic expression of Ret and GFRα2, the preferred co-receptor for NRTN (Jing et al., 1997), in RA mechanosensory neurons suggests a role for the NRTN–GFRα2/Ret signaling module in controlling development of these neurons. To test this idea, we first counted the number of RA mechanosensory neurons in NRTN null (Heuckeroth et al., 1999) and GFRα2GFP knockin mice (McDonagh et al., 2007). We measured two parameters to evaluate the number of RA mechanoreceptors at P0: the number of Ret+/TrkA− neurons in NRTN null mice and the number of large-diameter neurons expressing a high level of GFP in GFRα2GFP knockin mice. In both cases, similar numbers of RA mechanosensory neurons were observed in wild-type and mutant animals (Ret+/TrkA− neuron number in control and NRTN null mice: 10 ± 1 vs. 9 ± 1; GFP+ neuron number in GFRα2GFP heterozygous vs. homozygous mutant mice: 5± 1 vs. 4 ± 1) (fig. S8A–H). In addition, we counted TrkB+ DRG neuron number in P0 control (21 ± 3/ DRG section), Ret (21 ± 3/ DRG section), NRTN (21 ± 5/DRG section) and GFRα2 (20 ± 4/DRG section) null mice (fig. S10A–D), and found virtually identical numbers of TrkB+ neurons in these mice, suggesting RA mechanoreceptors are present at P0 in the absence of Ret signaling. Consistent with these findings, our previous work revealed that normal numbers of large-diameter NF200+ neurons are found at P14 in Retf/f;Wnt1Cre mice (Luo et al., 2007). Taken together with the finding of normal numbers of cutaneous RA end organs in Ret mutants at P14, described below, we conclude that NRTN–GFRα2/Ret signaling is not required for survival of most if not all RA mechanosensory neurons, at least up to P14. Interestingly, though not required for survival, Ret and NRTN are required for maximal expression of GFRα2 in RA mechanoreceptors as the level of GFRα2 is diminished in both Retf/f;Wnt1Cre and NRTN null DRGs at P0 (fig. S9A–C).
Since Ret signaling is not required for survival of RA mechanoreceptors and more than half of the early Ret+ neurons express TrkB at E13.5 and P0 (fig. S1L and S5K), we next asked whether TrkB controls their survival. We found similar number of Ret+/TrkA− DRG neurons in control and TrkBf/f;Wnt1Cre DRGs at P0 (6 ± 2 vs 5 ± 2, fig. S10E–J) indicating that TrkB, like Ret, is not required for prenatal survival of most if not all RA mechanoreceptors.
A NRTN-GFRα2/Ret signaling cascade controls development but not maintenance of Pacinian corpuscles
Peripheral endings of RA mechanosensory neurons were next examined for their developmental dependence on Ret signaling. Meissner corpuscles and lanceolate endings are present in normal numbers in both Retf/f;Wnt1Cre and NRTN mutants at P14 (Fig. 6C–D, G–H, fig. S11C, and data not shown) although many of these endings appear morphologically underdeveloped and disorganized (Fig. 6C–D, G–H, and fig. S11A–B). Since Meissner corpuscles and lanceolate endings constitute the majority of RA mechanoreceptors (Johansson and Vallbo, 1979), this result supports our conclusion that Ret is not required for survival of most RA mechanosensory neurons. On the other hand, mice lacking TrkB in all derivatives of the neural crest (TrkBf/f;Wnt1Cre) (fig. S11D–I) or TrkB null mice (Fundin et al., 1997; Gonzalez-Martinez et al., 2005; Gonzalez-Martinez et al., 2004; Perez-Pinera et al., 2008) exhibit a complete absence of Meissner corpuscles and a modest morphological disorganization of lanceolate endings. Interestingly, NF200+ fibers are present in the dermal papillae of the TrkBf/f;Wnt1Cre mice (fig. S11G–H), suggesting that mechansosensory axon terminals reach the dermal papillae but that morphological differentiation of the corpuscle fails in the absence of TrkB signaling.
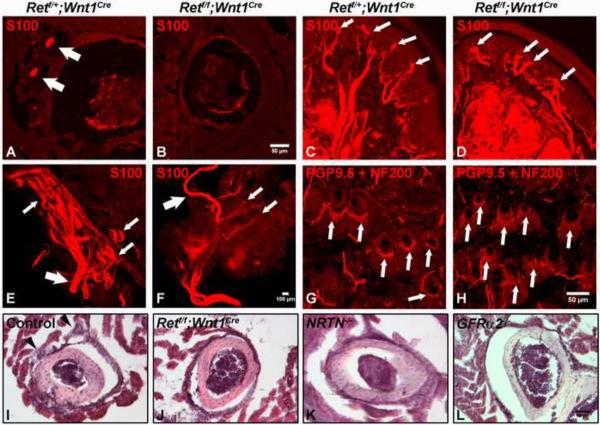
Figure 6. NRTN-GFRα2/Ret signaling is required for development but not maintenance of Pacinian corpuscles.
A–B: Anti-S100 immunostaining of Pacinian corpuscles (white arrows) in the periosteum of the fibula of P14 control and Retf/f;Wnt1Cre mice. Pacinian corpuscles (white arrows) are completely absent (35 ± 5 control vs. 0 mutant, per side) in the Retf/f;Wnt1Cre mice, as visualized using both sections and whole-mount staining (E–F). C–D: Staining of Meissner corpuscles in P14 control and Retf/f;Wnt1Cre mice. Meissner corpuscles (white arrows) appear in normal number in the absence of Ret signaling. However, the morphology of Meissner corpuscles in Retf/f;Wnt1Cre mice is slightly disorganized relative to those found in control mice. E–F: Anti-S100 whole mount immunostaining of Pacinian corpuscles from P14 control and Retf/f;Wnt1Cre mice, Large arrows indicate the interosseous nerve. In the control periosteum membrane, sensory nerves exhibit a tree-like structure on which Pacinian corpuscles are formed (small white arrows indicate a few examples). In contrast, only a few small nerve branches are present (small white arrows), but neither Pacinian corpuscles nor the tree-like nerve branch structures are observed in the Retf/f;Wnt1Cre periosteum membrane, suggesting RA mechanosensory nerves are not present in the absence of Ret. G–H: Staining of longitudinal lanceolate endings (white arrows) in P14 control and Retf/f;Wnt1Cre mice. Longitudinal lanceolate endings are present in Retf/f;Wnt1Cre mice, although they appear morphologically underdeveloped. Higher magnification and quantification of these endings are shown in fig. S11A–C. I–L: H & E staining of Pacinian corpuscles in P14 control (black arrow heads), Retf/f;Wnt1Cre, NRTN null and GFRα2GFP null mice. H & E staining is used here to rule out the potential confounding issue of decreased S100 expression in mutant mice. N≥3 for each mutant genotype.
In striking contrast, we found that the development of Pacinian corpuscles is absolutely dependent upon Ret. These mechanosensory end-organs are completely absent in the periostium of Retf/f;Wnt1Cre mice (Fig. 6A–B, E–F and I–J; 35 ± 5 vs. 0 Pacinian corpuscles/periosteum membrane) as determined by both S100 immunostaining and H & E staining. Moreover, wholemount anti-S100 staining of the periosteum membrane reveals that the nerve branches that normally penetrate Pacinian corpuscles are absent in Retf/f;Wnt1Cre mice (Fig. 6E–F). Since Ret is expressed in RA mechanosensory DRG neurons but not in the accessory cells of the corpuscle, (fig. S8L), Ret is required in RA mechanosensory neurons autonomously for Pacinian corpuscle formation. Remarkably, a similar phenotype is observed in both NRTN and GFRα2GFP null mice (Fig. 6K–L), indicating that a NRTN–GFRα2/Ret signaling cascade controls development of Pacinian corpuscles. Consistent with previous findings (Gonzalez-Martinez et al., 2004; Perez-Pinera et al., 2008), TrkBf/f;Wnt1Cre mice exhibit normal numbers of Pacinian corpuscles (fig. S11 J and M).
While the NRTN–GFRα2/Ret signaling cascade is necessary for development of Pacinian corpuscles, it is not required for the maintenance of these mechanosensory end-organs once they have formed. 4-HT-mediated acute deletion of Ret in three week-old RetERT2/f mice (fig S12A–B) has no effect on the number or morphology of Pacinian corpuscles in mice as old as six months (Fig. S12C–D, G–H). This is in marked contrast to the dramatic loss of epidermal axonal endings of non-peptidergic nociceptors, observed in the same animals (fig. S12E–F, I–J). Thus, NRNT–GFRα2/Ret signaling is absolutely essential for development, but not maintenance of Pacinian corpuscles, while it cooperates with BDNF–TrkB signaling to control development and innervation of both Meissner corpuscles and longitudinal lanceolate endings.
Ret signaling controls central axonal projections of RA mechanosensory neurons
Since Ret is highly expressed in RA mechanosensory neurons when their axons extend into the spinal cord and then into the DCN within the medulla, we next addressed the possibility that Ret signaling controls this process. We first used VGLUT1 staining to visualize the synapses of central projections of all mechanoreceptors in Retf/f;Wnt1Cre mice and their littermate controls. Here, the intensity of VGLUT1 staining within layers III through V of the spinal cord was found to be markedly reduced in Retf/f;Wnt1Cre mice (Fig. 7A, C, D). This deficit is specific for mechanosensory endings because the innervation of both Clarke's nucleus by proprioceptors (Fig. 7A, C, D) and layer II by non-peptidergic nociceptors (Luo et al., 2007) of the mutants is comparable to controls. In addition, the gracile nucleus is markedly smaller in Retf/f;Wnt1Cre mice (Fig. 7B, E, F) compared to littermate controls. Furthermore, the VGLUT1 staining intensity within the gracile nucleus is dramatically reduced in Retf/f;Wnt1Cre mice (Fig. 7B, E, G). In contrast, both the size and VGLUT1 staining intensity of the ventral-caudal region of the cuneate nucleus in Retf/f;Wnt1Cre mice are similar to controls (Fig. 7B, E, F, G), which is consistent with our finding that RA mechanosensory terminations are restricted to the more medialrostral region of the cuneate nucleus. Interestingly, TrkBf/f;Wnt1Cre mice exhibit a normal pattern of VGLUT1 staining in both the spinal cord and medulla (fig. S11K, L, N,O). These results indicate that Ret, but not TrkB, is required for the formation of both branches of the central projections of RA mechanoreceptors.
Figure 7. RA mechanoreceptors require Ret signaling for their assembly into mechanosensory circuits in the spinal cord and medulla.
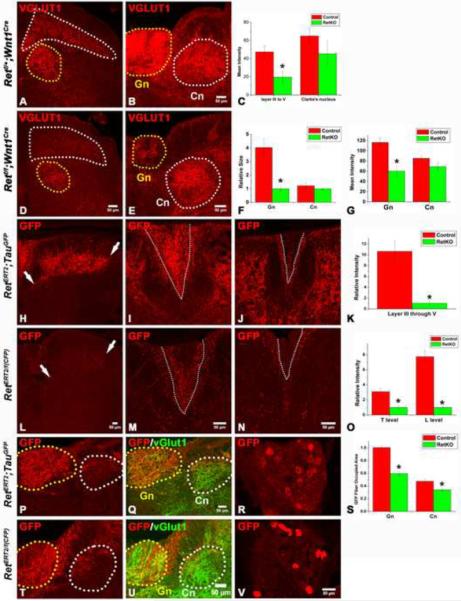
A–B: Synapses of mechanoreceptors, visualized by VGLUT1 staining, in the upper lumbar spinal cord and mid-level medulla of P14 control mice. D–E: Synapses of mechanoreceptors in the upper lumbar spinal cord and mid-level medulla of P14 Retf/f; Wnt1Cre mice. Layers III through V of the spinal cord are outlined by the white dotted line, and Clarke's nucleus is outlined by the yellow dotted line. In the medulla, the gracile nucleus is outlined by the yellow dotted line, and the cuneate nucleus is outlined by the white dotted line. The VGLUT1 staining intensity in layer III through V of spinal cord and gracile nucleus of mutant mice is much lower than that of controls. Quantification of spinal cord staining intensity is shown in panel C (Student's t test, P<0.001 for layers III through V), quantification of the size of the gracile and cuneate nuclei is shown in F (Student's t test, P<0.001 for the gracile nucleus), and quantification of the intensity of the medulla staining is shown in G (Student's t test, P<0.001 for the gracile nucleus). H,L: Anti-GFP staining of RA mechanoreceptor central axonal projections into the spinal cord of P14 RetERT2;Tauf(mGFP) (control) and RetERT/f(CFP) (Ret KO) mice, which were treated with 4-HT at E11.5 and E12.5. Note the reduction in the number of GFP+ axons in RetERT/f(CFP) mice. White arrows indicate the range of projections. Quantification of relative intensity of GFP+ fibers is shown in K. The difference between control and Ret null neurons is statistically significant (Student's t test, P<0.001). I, J: Anti-GFP staining of the dorsal columns of P14 RetERT2;Tauf(mGFP) mice at thoracic (I) and lumbar (J) levels. M,N: Anti-GFP staining of the dorsal columns of P14 RetERT/f(CFP) mice at thoracic (M) and lumbar (N) levels. Many fewer CFP+ fibers are found in the gracile fasciculus of the dorsal column of the mutant (outlined by white dotted lines). Quantification is shown in O. The difference between the control and Ret null neurons is statistically significant (Student's t test, P< 0.001 for the lumbar level, and P<0.01 for the thoracic level). P,Q: Anti-GFP staining of RA mechanosensory fibers innervating the medulla in P14 RetERT2;Tauf(mGFP) mice. T–U: Anti-GFP staining of RA mechanosensory innervation of the medulla in P14 RetERT/f(CFP) mice. The relative area occupied by GFP+ fibers within the gracile (Gn) and cuneat nucleus (Cn) is quantified in S. The difference between the control and Ret null neurons is statistically significant (Student's t test, P<0.01 for the gracile nucleus, and P<0.05 for the cuneate nucleus). R, V: GFP labeled RA mechanosensory DRG neurons in RetERT2;Tauf(mGFP) and RetERT/f(CFP) thoracic DRGs at P14. On average, 8 ± 3 neurons/section are labeled by GFP in RetERT2;Tauf(mGFP) mice while 6 ± 2 neurons/section are labeled by CFP in RetERT/f(CFP) mice. Note that the level of expression of CFP in Ret null neurons is higher than that of control neurons. N=3 for control and Retf/f;Wnt1Cre mice and N=3 for both RetERT2;Tauf(mGFP) and RetERT/f(CFP) mice. 8 to 12 spinal cord sections per animal for each genotype were examined and quantified.
Since VGLUT1 labels synapses of both RA and SA mechanoreceptors, it is possible that the central projection phenotypes observed in Retf/f;Wnt1Cre mice reflect deficits of axonal projections of one or both of these mechanosensory populations. In addition, it is possible that Ret functions in a non-cell autonomous manner to affect RA mechanosensory projections to the spinal cord. Indeed, Ret is expressed in other populations of DRG neurons beginning at E13.5 as well as neurons in the dorsal spinal cord and medulla (Golden et al., 1999), all of which are subjected to Cre-mediated excision in Retf/f;Wnt1Cre mice (data not shown). To distinguish between these possibilities and address the cell autonomy of Ret function, we sought to delete Ret in RA mechanosensory neurons but not other DRG or dorsal horn neurons. Here, we employed RetERT2 mice and the procedure of 4-HT treatment of mice prior to E12.5. Activation of Cre recombinase in spinal interneurons using this strategy is predicted to be minimal because expression of Ret, and hence RetERT2 is expressed in very few neurons in the dorsal spinal cord at E13.5 (data not shown). Indeed, we found that few spinal cord interneurons are labeled in RetERT2;TaumGFP mice following three days of 4-HT treatment prior to E12.5 (Figure 4A, E). Therefore, we generated both RetERT2; Tauf(mGFP) control and RetERT2/f(CFP) conditional mutant mice, which enable selective visualization of the central projections of RA mechanosensory neurons that are either heterozygous or homozygous null for Ret, respectively. The RetERT2/f(CFP) mice harbor one RetERT2 allele, which is null, and one floxed Ret allele f(CFP) which, following Cre-mediated excision is also null for Ret and expresses CFP (Uesaka et al., 2008). Thus, following 4-HT treatment at E11.5–E12.5, CFP+ DRG neurons in RetERT2/f(CFP) mice are Ret null RA mechanosensory neurons while, in contrast, GFP+ neurons in control RetERT2; Tauf(mGFP) mice are RA mechanosensory neurons harboring one functional Ret allele. We used two days instead of three days of 4-HT treatment for these experiments so that relatively few RA mechanosensory neurons would be labeled. The 4-HT-treated RetERT2; Tauf(mGFP) control mice serve as an excellent control for 4-HT-treated RetERT2/f(CFP) mutant mice because: 1) The level of expression of CFP in RetERT2/f(CFP) mutant DRG neurons is comparable to or higher than that of GFP in control RetERT2; Tauf(mGFP) neurons since CFP is directly visible in Retf(CFP) mice following recombination while GFP in TaumGFP mice is not (data not shown); 2) Two days of 4-HT treatment of RetERT2/f(CFP) and control RetERT2; Tauf(mGFP) mice resulted in 6 ± 2 CFP+ and 8 ± 3 GFP+ neurons/DRG section at P14 (Fig. 7R,V), respectively, indicating the labeling efficiency in the two mouse lines is comparable, and; 3) Both GFP and CFP effectively localize to the central axons of mechanoreceptors innervating layers III through V of the spinal cord of control mice (Fig 2A, E, and fig. S8M). As predicted, 4-HT treatment at E11.5 –E12.5 of both control RetERT2; Tauf(mGFP) and mutant RetERT2/f(CFP) mice results in very few GFP+ and CFP+ spinal cord dorsal horn interneurons, respectively (Fig 7H, L), indicating that Ret is deleted in very few spinal cord interneurons in RetERT2/f(CFP) mice. Therefore, any phenotype observed in RA mechanosensory neurons in RetERT2/f(CFP) mice reflects a cell autonomous function of Ret in these neurons. Using this strategy, we observed that while GFP+ neurons of RetERT2; Tauf(mGFP) control mice robustly innervate lamina III through V of the spinal cord, CFP+, Ret null neurons of RetERT2/f(CFP) mice exhibit a striking absence of axonal projections in both the spinal cord dorsal horn (Fig. 7H, L, K) and DC (Fig. 7I, J, M, N, O) of P14 mice. Moreover, there are fewer CFP+ projections in the gracile and cuneate nuclei of RetERT2/f(CFP) mutant mice (Fig. 7P,Q,T,U,S). Therefore, Ret is required cell autonomously for the extension of axonal branches of RA mechanosensory neurons into the spinal cord and the gracile and cuneate nuclei of the medulla.
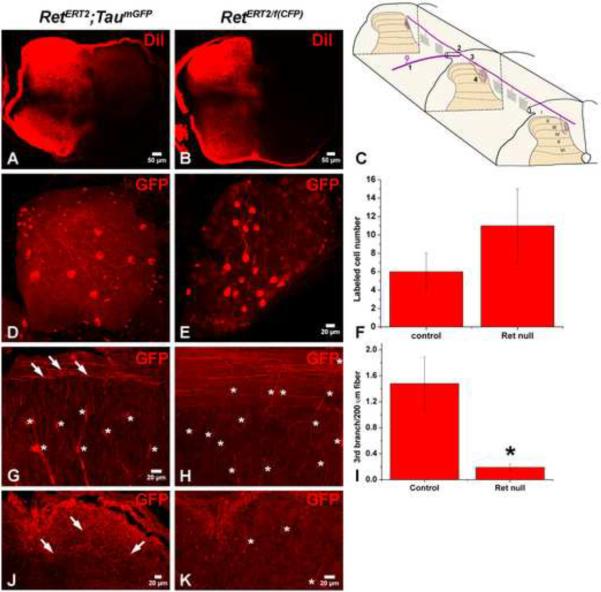
To gain further insight into the nature of the central projection deficit of Ret null RA mechanoreceptors, we analyzed the axons of RetERT2/f(CFP) and RetERT2; Tauf(mGFP) mice at E15.5, just following the time when axons of mechanosensory neurons have sent branches into layers III through V of the spinal cord (Fig. 8A–B and (Ozaki and Snider, 1997)). Mechanoreceptors typically extend a single central branch (step 1) at ~E10.5, which bifurcates upon reaching the spinal cord, projecting in both the rostral and caudal directions (step 2). Interstitial branches then form from these longitudinal projections and penetrate deep into the spinal cord (step 3) beginning ~E13.5. Then, axonal branches in the spinal cord elaborate complicated collaterals within the proper layers of the dorsal horn (step 4) beginning ~E15.5 (Fig. 8C). To visualize individual axons of control and Ret null RA mechanoreceptors, a low dose of 4-HT was used to label 6 ± 2 GFP+ neurons /DRG in control RetERT2; Tauf(mGFP) mice and 11 ± 4 CFP+ neurons/DRG in experimental RetERT2/f(CFP) mice (Figure 8D–F). We found that the initial central and peripheral axonal branches of labeled fibers within the DRGs are comparable in RetERT2; Tauf(mGFP) control (Figure 8D) and RetERT2/f(CFP) mutant mice (Figure 8E). Moreover, many labeled axons projecting rostrally and caudally in the dorsal funiculus, observed using sagittal sections of the thoracic spinal cord, were present in mice of both genotypes (Figure 8G–H). These findings indicate that axonal extension and branching steps 1 and 2 occur normally in Ret null RA mechanoreceptors. However, very few third-order axonal branches emanating from the longitudinal projections of Ret null RA mechanoreceptors were observed in RetERT2/f(CFP) mutant mice (0.19 ± 0.05 third-order branches/200μm rostral-caudal fiber), which is in marked contrast to the large number of these interstitial branches seen in RetERT2; Tauf(mGFP) controls (1.48 ± 0.41 third-order branches/200μm rostral-caudal fiber, Figure 8G–I). This phenotype is also observed when the afferent branches are visualized using horizontal sections through the lumbar spinal cord (Figure 8J–K). Thus, Ret is required at the step 3, for the formation or extension of third-order interstitial branches of RA mechanoreceptors into the spinal cord.
Figure 8. Ret is required autonomously for the formation of the 3rd branch of central RA mechanosensory axons.
A–B: DiI labeling of thoracic DRGs of E15.5 RetERT2;Tauf(mGFP) and RetERT/f(CFP) mice. Consistent with published findings (Ozaki and Snider, 1997), mechanoreceptors have already extended central axonal projections to spinal cord layers III through V at E15.5. C: Illustration of RA mechanoreceptor central projections, which can be subdivided into four steps. This illustration is adapted from (Brown, 1981). D: Whole mount anti-GFP staining of a control RetERT2;Tauf(mGFP) DRG from an animal treated with 0.6mg of 4-HT. Note that the 1st order central projections are visible in the DRG. On average, 6 ± 2 GFP+ neurons are labeled per thoracic DRG (12 DRGs in total, N=3 from two separate litters). E: Whole mount anti-GFP staining of a RetERT/f(CFP) DRG from an animal treated with 1mg of 4-HT. Note that 1st order central projections are visible in the DRG. On average, 11 ± 4 CFP+ neurons are labeled per thoracic DRG (20 DRGs in total, N=4 from three separate litters). F: Quantification of labeled DRG neuron number. G: Anti-GFP staining with sagittal thoracic spinal cord sections of RetERT2;Tauf(mGFP) mice. Note that the 3rd order axonal branches (white arrows) originate from rostral-caudal running fibers and penetrate the spinal cord. “*” indicates the position of blood vessels which are autofluorescent and seen in mice of both genotypes. H: Anti-GFP staining of sagittal thoracic spinal cord sections of RetERT/f(CFP) mice. Note that very few 3rd order axonal branches originating from rostral-caudal running fibers are found in these mice. I: Quantification of the numer of 3rd order axonal branches for the two genotypes. On average, 1.48 ± 0.41 3rd order axonal branches are observed in every 200μm rostral-caudal fiber in RetERT2;Tauf(mGFP) control mice (N=3 from two litters, and more than 100 rostralcaudal fibers were measured) while 0.19 ± 0.05 3rd order axonal branches are observed in every 200μm rostral-caudal fiber in RetERT/f(CFP) mutant mice (N=4 from three litters, and more than 100 rostral-caudal fibers were measured). J–K: Anti-GFP staining using horizontal lumbar spinal cord sections taken from RetERT2;Tauf(mGFP) (J) and RetERT/f(CFP) (K) mice. Similar to that observed using sagittal thoracic sections, there are several GFP+ central projections in RetERT2;Tauf(mGFP) control mice at this age, but almost no CFP+ central projections are found in the RetERT/f(CFP) mice.
Discussion
We have developed a genetic labeling strategy to show that the early-born Ret+ DRG neurons develop into RA mechanoreceptors, which form Meissner corpuscles, Pacinian corpuscles and longitudinal lanceolate endings. Interestingly, these RA mechanoreceptors innervate discrete target zones within the gracile and cuneate nuclei, revealing a modality-specific pattern of mechanosensory neuron axonal projections within the brainstem. In addition, we show that NRTN–GFRα2/Ret signaling is essential for development of Pacinian corpuscles and, importantly, Ret signaling mediates the formation of central connections of most if not all RA mechanosensory neurons in the spinal cord and medulla. Thus, Ret signaling orchestrates the assembly of RA mechanosensory neurons into circuits that underlie tactile discrimination and the perception of touch.
Modality-specific segregation of mechanosensory axons in the dorsal column-medial lemniscal pathway
DRG mechanoreceptors send axonal branches to local circuit neurons located in lamina III through V of the spinal cord (Brown, 1981) as well as on second order projection neurons located in the cuneate and gracile nuclei of the medulla. The DCN, in turn, convey somatosensory information to the thalamus and somatosensory cortex (Carpenter MB, 1983).
Although it is generally appreciated that topographic maps are represented within each of the projections of somatosensory pathways, from the spinal cord to the somatosensory cortex, less is known about modality maps in which RA and SA mechanosensory information is segregated. Interestingly, physiological recordings have pointed to the existence of distinct RA and SA zones along the entire extent of the dorsal-column-medial lemniscal pathway (Dykes, 1983; Dykes et al., 1982; Mountcastle, 1957; Mountcastle, 1984; Rasmusson and Northgrave, 1997). Our findings show that afferents of GFP-labeled early Ret+ neurons almost completely fill the gracile nucleus and this nucleus is remarkably smaller in Ret mutant animals, suggesting that RA mechanoreceptors constitute the majority of fibers that innervate the gracile nucleus. Remarkably, the pattern of Ret+ RA mechanosensory afferent terminations within the mouse DCN described in the present study bears striking resemblance to that found by Dykes and colleagues (Dykes et al., 1982) using physiological recordings in cats. In addition, our results provide an anatomical basis of “modality re-sorting” (Whitsel et al., 1969), a phenomenon in which RA and SA mechanosensory afferents ascending within the gracile and cuneate fasciculi of the DC reorganize such that RA mechanosensory afferents predominate in the gracile fasciculus at cervical levels. Indeed, our labeling experiments show that Ret+ RA mechanoreceptor afferents are greatly enriched in the cervical gracile fasciculus. The observed enrichment of these RA mechanosensory afferents within the gracile fasciculus of the DC, prior to innervation of the DCN, could provide a mechanistic explanation for the formation of modality maps within the DCN. In fact, a similar mechanism of pre-target sorting of axons has been suggested for the establishment of topographic maps in the olfactory system (Imai et al., 2009). Taken together with previous physiological recordings in the cat, raccoon and monkey, our genetic labeling results from mice strongly support the existence of modality maps within the DC and DCN and the idea that such maps are found in many mammalian species.
Pacinian corpuscles require NRTN–GFRα2/Ret signaling during development but not in adulthood
We show that development of Pacinian corpuscles is dependent upon NRTN, GFRα2 and Ret. Indeed, our finding of a common phenotype in NRTN, GFRα2 and Retf/f;Wnt1Cre mutant mice strongly supports the conclusion that GFRα2 is the Ret co-receptor in RA mechanoreceptors, and that a NRTN–GFRα2/Ret signaling cascade in these neurons controls Pacinian corpuscle formation. Our results further show that NRTN-GFRα2/Ret signaling is not required for maintenance of the corpuscle, once it has formed. This conclusion is based on the observation that deletion of Ret in three-week old mice, a time when Pacinian corpuscles are mature (Zelena, 1976), has no obvious effect on corpuscle number or morphology, even in mice up to six months of age. In contrast, Ret is required for both development (Luo et al., 2007) and maintenance (this study) of projections of at least one class of Ret+ DRG sensory neurons, the cutaneous endings of non-peptidergic nociceptors. In all, our findings define the first growth factor signaling module required for development of Pacinian corpuscle and show that Ret is differentially required for the maintenance of axonal projections of select classes of Ret+ DRG neurons.
Distinct roles of Ret and TrkB in the development of axonal projections of cutaneous RA mechanoreceptors
In contrast to the severe deficit of Pacinian corpuscles, the phenotypes associated with cutaneous RA mechanosensory end-organs of Ret mutant mice are relatively modest. Moreover, consistent with previous studies using TrkB null mutants (Gonzalez-Martinez et al., 2004; Perez-Pinera et al., 2008), we found that TrkB functions within cells of neural crest origin to support formation of Meissner corpuscles, pointing to a role in either the axon, Schwann cell or both. Since many early Ret+ neurons express TrkB during development, we favor a model in which TrkB signaling within Ret+ RA mechanoreceptors serves to direct the formation of Meissner corpuscles. Alternatively, since Meissner corpuscles can be innervated by either one or two Aβ fibers (Ide, 1976), and Ret and TrkB expression patterns in the DRG do not completely overlap, it is possible that some Meissner corpuscles are innervated by two molecularly distinct subtypes of Aβ fibers, each expressing either Ret or TrkB, and that the TrkB+ fibers instruct the process of corpuscle formation. Either way, it is clear that Ret plays a relatively minor role in the formation of Meissner corpuscles. In striking contrast, Ret is absolutely essential for extension of interstitial branches of central axons of RA mechanoreceptors in the spinal cord whereas TrkB signaling is dispensable for the formation of these central projections. Therefore, Ret and TrkB signaling differentially support the growth and branching of central axons of RA mechanoreceptors and the formation of cutaneous RA mechanosensory end-organs.
It is also interesting to note that Ret is differentially required for development of the central projections of distinct classes of Ret+ DRG neurons. Although dispensable for the central projections of Ret+ non-peptidergic nociceptors (Luo et al., 2007), we show here that Ret plays a critical role in the formation of central projections of RA mechanoreceptors and hence their synaptic connections in the spinal cord and brain stem. Based on this observation, we propose that NRTN and perhaps other Ret ligands may be effective in supporting regeneration of central axonal projections of RA mechanosensory neurons, which could enable functional recovery of tactile discrimination and the perception of touch following nerve or spinal cord damage.
Method and Materials
Mouse lines and treatments
The details of RetERT2 mouse generation can be found in the supplemental materials. Ret-conditional CFP mice (Retf(CFP)) were generated as described (Uesaka et al., 2008). The Tau-conditional mGFP mouse line (Tauf(mGFP)) (Hippenmeyer et al., 2005) was generously provided by Drs. Gord Fishell (NYU) and Silvia Arber (Biocenter/Friedrich Miescher Institute). The AvilAP mouse line (Hasegawa et al., 2007) was generously provided by Dr. Fan Wang (Duke University). The Retf/f;Wnt1Cre mouse line was described previously (Luo et al., 2007). Descriptions of other mouse lines are found in the supplemental materials.
RetERT2 and Tauf(mGFP) or Retf(CFP) mouse lines were mated for 2 days, and plugs were checked each day. The time when a mouse was found to have plugs is considered as E0.5. In addition, mice without obvious plugs but found pregnant 10 days following the first day of mating were also considered as E10.5. Pregnant females were gavaged with 1.0~1.5mg of 4-HT per day at the indicated times. All animal treatments were done in accordance with the regulations and policies of the Johns Hopkins Animal Care and Use Committee.
In situ hybridization and double fluorescent in situ hybridization
Digoxigenin (DIG)-labeled cRNA probes were used for in situ hybridization. In situ hybridization probes directed against GFRα1, GFRα2, GFRα3, Ret, TrkA, TrkB, and TrkC were described previously (Luo et al., 2007). Details are found in the supplemental data.
Supplementary Material
Acknowledgments
We thanks Drs. Silvia Arber and Gord Fishell for providing Tauf(mGFP) mice, Fan Wang for providing AvilAP mice, and Michael Caterina for the TRPV3 antibody. We thank Xinzhong Dong, Alex Kolodkin, Richard Koerber, Robin Krimm, Derek Molliver, Michael Caterina and members of the Ginty laboratory for helpful discussions and comments on the manuscript. We thank Michael Rutlin, Yin Liu, Amy Strickland, and Huiying Guo for reagents and technical support. This work was supported by NIH grants NS34814 (DDG) and AG13730 (JM). DDG is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional materials and methods can be found in the Supplemental Data.
References
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechanoreceptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Albrecht F. L. R. a. P. J. The Senses: A Comprehensive Reference. Academic Press; San Diego: 2008. Cutaneous Mechanisms of Tactile Perception: Morphological and Chemical Organization of the Innervation to the Skin; pp. 1–32. [Google Scholar]
- Brown AG. Organization in the spinal cord. Heidelberg; Springer-Verlag; Berlin: New York: 1981. [Google Scholar]
- Carpenter MB, S. J. Human Neuroanatomy. 8th edition Williams and Wilkins; Baltimore, MD: 1983. [Google Scholar]
- Carr PA, Yamamoto T, Karmy G, Baimbridge KG, Nagy JI. Analysis of parvalbumin and calbindin D28k-immunoreactive neurons in dorsal root ganglia of rat in relation to their cytochrome oxidase and carbonic anhydrase content. Neuroscience. 1989;33:363–371. doi: 10.1016/0306-4522(89)90216-9. [DOI] [PubMed] [Google Scholar]
- Carroll P, Lewin GR, Koltzenburg M, Toyka KV, Thoenen H. A role for BDNF in mechanosensation. Nat Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- Chen AI, de Nooij JC, Jessell TM. Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron. 2006;49:395–408. doi: 10.1016/j.neuron.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Parallel processing of somatosensory information: a theory. Brain Res. 1983;287:47–115. doi: 10.1016/0165-0173(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Rasmusson DD, Sretavan D, Rehman NB. Submodality segregation and receptive-field sequences in cuneate, gracile, and external cuneate nuclei of the cat. J Neurophysiol. 1982;47:389–416. doi: 10.1152/jn.1982.47.3.389. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL. Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol. 1997;190:94–116. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Farinas I, Del Valle ME, Feito J, Germana G, Cobo J, Vega JA. BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neurosci Lett. 2005;377:12–15. doi: 10.1016/j.neulet.2004.11.078. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Germana GP, Monjil DF, Silos-Santiago I, de Carlos F, Germana G, Cobo J, Vega JA. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res. 2004;1002:120–128. doi: 10.1016/j.brainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han BX, Qi Y, Wang F. Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J Neurosci. 2007;27:14404–14414. doi: 10.1523/JNEUROSCI.4908-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr., Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T. Neuropeptides in perspective: the last ten years. Neuron. 1991;7:867–879. doi: 10.1016/0896-6273(91)90333-u. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Riddell JS, Todd AJ. Upregulation of substance P in low-threshold myelinated afferents is not required for tactile allodynia in the chronic constriction injury and spinal nerve ligation models. J Neurosci. 2007;27:2035–2044. doi: 10.1523/JNEUROSCI.5401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide C. The fine structure of the digital corpuscle of the mouse toe pad, with special reference to nerve fibers. Am J Anat. 1976;147:329–355. doi: 10.1002/aja.1001470307. [DOI] [PubMed] [Google Scholar]
- Iggo A, Andres KH. Morphology of cutaneous receptors. Annu Rev Neurosci. 1982;5:1–31. doi: 10.1146/annurev.ne.05.030182.000245. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat's glabrous skin. J Physiol. 1977;266:275–296. doi: 10.1113/jphysiol.1977.sp011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Yamazaki T, Kobayakawa R, Kobayakawa K, Abe T, Suzuki M, Sakano H. Pre-target axon sorting establishes the neural map topography. Science. 2009;325:585–590. doi: 10.1126/science.1173596. [DOI] [PubMed] [Google Scholar]
- Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney J, Schultz H, Zhou R, Fox GM. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem. 1997;272:33111–33117. doi: 10.1074/jbc.272.52.33111. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurons. Oxford Univeristy Press; Oxford: 1992. [Google Scholar]
- Lawson SN, Caddy KW, Biscoe TJ. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res. 1974;153:399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, Noel T, Forbes ME, Johnson JE, Albers KM. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Timmusk T, Rossi J, Saarma M, Airaksinen MS. Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci. 2000;15:522–533. doi: 10.1006/mcne.2000.0845. [DOI] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron. 2007;54:739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- McDonagh SC, Lee J, Izzo A, Brubaker PL. Role of glial cell-line derived neurotropic factor family receptor alpha2 in the actions of the glucagon-like peptides on the murine intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G461–468. doi: 10.1152/ajpgi.00424.2006. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Central nervous mechanisms in mechanoreceptive sensibility. In: Darian-Smith I, editor. Handbook of Physiology. Bethesda: 1984. pp. 789–879. [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Snider WD. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J Comp Neurol. 1997;380:215–229. [PubMed] [Google Scholar]
- Pare M, Smith AM, Rice FL. Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J Comp Neurol. 2002;445:347–359. doi: 10.1002/cne.10196. [DOI] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Garcia-Suarez O, Germana A, Diaz-Esnal B, de Carlos F, Silos-Santiago I, del Valle ME, Cobo J, Vega JA. Characterization of sensory deficits in TrkB knockout mice. Neurosci Lett. 2008;433:43–47. doi: 10.1016/j.neulet.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Northgrave SA. Reorganization of the raccoon cuneate nucleus after peripheral denervation. J Neurophysiol. 1997;78:2924–2936. doi: 10.1152/jn.1997.78.6.2924. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, Kilkenny C, Salazar-Grueso E, Pachnis V, Arumae U. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Yonemura S, Enomoto H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J Clin Invest. 2008;118:1890–1898. doi: 10.1172/JCI34425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Petrucelli LM, Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969;15:67–78. doi: 10.1016/0006-8993(69)90310-2. [DOI] [PubMed] [Google Scholar]
- Zelena J. The role of sensory innervation in the development of mechanoreceptors. Prog Brain Res. 1976;43:59–64. doi: 10.1016/S0079-6123(08)64338-1. [DOI] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. Chapman & Hall; London: 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.