Abstract
Objective
To explore whether associations of potential risk factors for incidental Lewy Body Disease (iLBD) may be similar to Parkinson Disease (PD).
Design, Setting, and Patients
We identified brain-autopsied residents of Olmsted County, MN and immediate vicinity(1988–2004), age>60, without evidence of neurodegenerative disease or tremor, and evaluated by at least one physician within one year of death. Analysis for “incidental” Lewy pathology was done blinded to clinical abstraction.
Main Outcome Measures
Whether risk factors previously associated with PD in Olmsted County, MN are also associated with iLBD.
Results
Of 235 subjects, 34 had iLBD(14.5%). The overall risk factor profiles for iLBD and PD were fairly similar between the two sets of OR estimates, with 11/16 ORs in the same direction. Prior Olmsted County studies documented 7 risk factors with statistically significant associations with PD; for two of these, the ORs for iLBD were in the same direction and statistically significant (physician, caffeine), whereas for three, they were in the same direction but not significant (education, head injury, number-of-children); they were in the opposite direction but not statistically significant for 2 (depression, anxiety). ILBD was not associated with various end-of-life conditions or causes-of-death, although they were slightly older and more likely cachectic.
Conclusions
Based on this exploratory study, iLBD and PD appear to have similar risk factor profiles. Thus, at least some cases of ILBD might represent preclinical PD, arrested PD or a partial syndrome due to a lesser burden of causative factors. ILBD is not explained by non-specific end-of-life brain insults.
Lewy bodies (LB) and Lewy neurites (LN) are the histopathologic hallmarks of Parkinson disease (PD), clinically characterized by not only the motor disorder, but also cognitive/behavioral, neuropsychiatric features, sleep disorders, and autonomic dysfunction. The lifetime risk of PD has been estimated at 1.6%.1 However, this contrasts with the incidence of Lewy body related pathology. Among neurologically normal people ages 60 years and older, 8% to 17% have Lewy bodies on postmortem examination.2–4 This has been termed incidental Lewy body disease (iLBD).
A major revision in our conceptualization of Parkinson’s disease was heralded by the recent publication of Braak PD staging.5,6 In this scheme, PD does not start in the dopaminergic substantia nigra, but rather in the lower brainstem, olfactory bulb and autonomic system7,8; only later does it involve higher brain levels. Although there are exceptions to the Braak pattern9–13, it is consistent with the majority of cases. Incidental Lewy body disease is crucial to this scheme and has been assumed to represent the earliest, preclinical stages of PD.14,15 Presumably, iLBD patients would have developed PD if they lived sufficiently long or if the PD pathogenic process had not been arrested.
There are, however, alternative explanations for iLBD. Rather than representing the earliest PD state, iLBD might simply reflect low-grade brain insults accumulating over a lifetime, or during agonal pre-mortem states. It might also reflect non-specific brain aging processes, akin to neurofibrillary tangles that accumulate with age.16 The assumptions that iLBD represents preclinical or arrested PD require validation.
A number of risk factors have been associated with PD. Many of these have been assessed in the population of Olmsted County, Minnesota, the location of the Rochester site of the Mayo Clinic; these include estrogen/ovarian status (females),17,18 smoking, caffeine, and alcohol consumption,19 anxiety or depression,20 diabetes mellitus, cancer, stroke,21 education, occupation,22 head injury23 and number of children.24 If iLBD represents preclinical or arrested PD, the epidemiologic risk factor profile should be similar in these two conditions.
In this study, we explored whether PD and iLBD may indeed share epidemiologic risk factors. We utilized the Mayo Clinic Tissue Registry, which serves as a repository of brain autopsy specimens for patients from Olmsted County and the immediate vicinity; and we utilized the Mayo Clinic records linkage system, which compiles medical information for patients from Olmsted County and the immediate vicinity. Thus, we compared risk factors from identified iLBD cases to those previously measured in our Olmsted County PD population. We also assessed whether iLBD segregated with various chronic diseases and agonal conditions, which might suggest that it is caused by non-specific brain insults. Though the number of iLBD cases in this neuropathological series is similar to or exceeds that of most other neuropathological series, the analyses presented herein are intended to be exploratory owing to what is still a relatively small sample size.
METHODS
STUDY SUBJECTS
We employed the Tissue Registry of the Mayo Clinic Rochester Epidemiology Project to identify subjects from Olmsted County, MN and the immediate vicinity that died between 1988–2004 with available brain autopsy tissues plus adequate Mayo medical records documenting no clinical evidence of neurodegenerative conditions. Inclusion criteria: (a) age >60 at death; (b) medical record documentation of at least one medical evaluation during the last year of life. Exclusion criteria: (a) medical record documentation of parkinsonism, tremor, dementia, or other neurodegenerative disorders; (b) medical record documentation of large structural brain lesions that might have interfered with the post mortem assessment for Lewy body pathology (e.g., massive hemorrhage or stroke, large brain tumors).
NEUROPATHOLOGICAL METHODS
For each brain, frontal and temporal cortex, cingulate gyrus, hippocampus, nucleus basalis of Meynert, midbrain, pons, medulla and spinal cord were sectioned. The formalin-fixed and paraffin-embedded sections from cortical and subcortical regions were stained for hematoxylin and eosin (H&E), thioflavin-S, phospho-tau and alpha-synuclein microscopy.4,25–27 The histological evaluation included region-specific, semiquantitative assessments of atrophy, neuronal loss, astrogliosis, LBs, and LNs. All neuropathological assessments were blinded to the medical records abstraction findings.
MEDICAL RECORDS ABSTRACTION
Medical records were obtained using the medical records linkage system of the Rochester Epidemiology Project. These were abstracted focusing on specific exposures that had been previously assessed in published case-control or cohort studies of PD in the Olmsted County, MN population.17–24 We restricted our analyses to risk factors that were defined similarly if not identically between these previous studies of PD and this study of iLBD. Listed alphabetically, these factors included alcohol, anxiety, caffeine, cancer, depression, diabetes mellitus, education, estrogen therapy, head injury, number of children, occupations, oophorectomy, smoking, and stroke.
In addition to cancer and diabetes that have already been mentioned, we assessed various systemic illnesses or agonal states that might non-specifically associate with incidental brain pathology. These included cachexia, chemotherapy, COPD, congestive heart failure, coronary artery disease, hypertension, peripheral vascular disease, sudden unexpected death, and other terminal diseases. The medical records abstraction was blinded to the neuropathological findings.
STATISTICAL ANALYSES
Numerical variables were summarized with the sample mean, standard deviation (SD), minimum, and maximum. Categorical variables were summarized with number and percentage. Associations between patient characteristics and iLBD were investigated using logistic regression models adjusted for age and gender. Odds ratios (ORs) were estimated along with 95% confidence intervals (CIs). Three variables were investigated for association in only men or only women: number of children was considered only in men, in keeping with published findings for PD; oophorectomy and estrogen replacement therapy were considered only in women. P-values ≤ 0.05 were considered statistically significant. No adjustments for multiple comparisons were made in these exploratory analyses. ORs for iLBD were compared with previous estimated ORs in PD primarily via exploratory graphical summaries; no test of agreement was conducted due to the lack of independence of ORs and the inability of any such test to adjust for this. However, as a numerical summary of the degree of agreement between estimated ORs for iLBD and PD, Lin’s concordance correlation coefficient28 was estimated. The concordance correlation coefficient ranges from −1 to 1, with a value of 1 indicating perfect agreement. Statistical analyses were performed using SPLUS (version 8.0.1; Insightful Corporation, Seattle, Washington).
RESULTS
Of the 235 subjects included in this study, 130 were women (55.3%). ILBD was neuropathologically documented in 34 subjects (14.5%), defined by the presence of alpha-synuclein immunoreactive neuronal or neuritic lesions in one or more selected brain regions; the remaining 201 cases without Lewy pathology served as normal controls. Of the iLBD cases, 18 (52.9%) were women, which mirrored the gender distribution among the 235 total subjects. The mean duration of Mayo medical records (duration from first Mayo visit to death) was 48 years in the iLBD subjects (range, 5–74 years), compared to a mean of 41 years (range, 2 months-78 years) in the control group (P=0.015). All but 16 subjects had medical records dating back at least five years pre-mortem. There were frequent physician contacts during the last five years of life, with a median 20 physician visits among the iLBD cases (25th percentile = 12; 75th percentile = 24 visits/5 years); the control subjects had a median 21 physician visits during that time (25th percentile = 11; 75th percentile = 31 visits/5 years).
Table 1 lists the exposures previously studied in published case-control or cohort studies of Parkinson's disease (PD) in Olmsted County, MN.17–24 The largest of the case-control PD studies had 197 PD cases and 197 matched controls. The cohort study involving oophorectomy involved a substantially larger number of patients (2,327 with oophorectomy and 2,280 without). For each exposure, the table provides the available sample size of the iLBD cases and normal brain autopsy controls, plus the estimated ORs (95% CIs) and p-values for this study. The far-right columns of the table provide the estimated ORs (95% CIs) and p-values for the published studies of PD in Olmsted County.17–24
Table 1.
Population-based studies in Olmsted County, Minnesota: risk factors associated with iLBD in this study, as compared to risk factor associations with Parkinson's disease (PD) in previously published Olmsted studies
| Summary of each variable | Associations with iLBD* | Association with PD in prior Olmsted County studies† |
||||
|---|---|---|---|---|---|---|
| Variable | iLBD cases (N=34) |
Controls (N=201) |
Estimated OR (95% CI) | P-value | Estimated OR (95% CI) |
P-value |
| Age at death‡ | 82±10 (75, 90 ) | 78±9 (72, 84) | 1.70 (1.10 – 2.64) | 0.018 | N/A | N/A |
| Gender (Male) | 16 (47%) | 89 (44%) | 1.43 (0.66 – 3.10) | 0.36 | N/A | N/A |
| History of smoking | 20 (59%) | 127 (63%) | 1.08 (0.46 – 2.50) | 0.87 | 0.69 (0.45 – 1.08) | NS |
| History of alcohol use | 23 (70%) | 122 (64%) | 1.61 (0.68 – 3.80) | 0.28 | 1.04 (0.61 – 1.76) | NS |
| History of caffeine use§ | 16 (67%) | 128 (84%) | 0.36 (0.14 – 0.96) | 0.042 | 0.35 (0.16 – 0.78) | 0.01 |
| Years of education (≥ 9) | 29 (88%) | 157 (80%) | 2.65 (0.84 – 8.33) | 0.096 | 2.0 (1.1 – 3.6) | SIGNIF |
| Estrogen therapy, females only |
0 (0%) | 11 (10%) | 0.24 (0.01 – 4.23) | 0.36 | 0.60 (0.22 – 1.65) | NS |
| History of head injury | 1 (3%) | 5 (2%) | 1.44 (0.16 – 13.19) | 0.75 | 4.3 (1.2 – 15.2) | 0.02 |
| Occupation of physician | 4 (12%) | 4 (2%) | 6.84 (1.46 – 32.06) | 0.015 | 3.7 (1.0 – 13.1) | 0.05 |
| Occupation of teacher | 5 (15%) | 20 (10%) | 1.27 (0.42 – 3.89) | 0.67 | 1.1 (0.5 – 2.1) | 0.9 |
| Occupation of farmer | 5 (15%) | 26 (13%) | 0.88 (0.29 – 2.62) | 0.81 | 1.1 (0.6 – 1.9) | 0.8 |
| Number of children (≥ 1), males only |
14 (88%) | 74 (83%) | 1.61 (0.32 – 8.02) | 0.56 | 2.65 (1.15 – 6.10) | 0.02 |
| History of anxiety | 3 (9%) | 41 (20%) | 0.40 (0.11 – 1.39) | 0.15 | 2.2 (1.4 – 3.4) | 0.0003 |
| History of depression | 8 (24%) | 59 (30%) | 0.72 (0.30 – 1.70) | 0.45 | 1.9 (1.1 – 3.2) | 0.02 |
| Cancer | 18 (53%) | 104 (52%) | 1.01 (0.48 – 2.11) | 0.98 | 1.4 (0.9 – 2.0) | NS |
| Stroke | 11 (32%) | 40 (20%) | 1.73 (0.77 – 3.90) | 0.19 | 1.6 (0.8 – 3.2) | NS |
| Diabetes | 5 (15%) | 45 (22%) | 0.74 (0.26 – 2.08) | 0.57 | 0.7 (0.4 – 1.4) | NS |
| Oophorectomy, females only |
0 (0%) | 6 (5%) | 0.43 (0.02 – 8.05) | 0.70 | 1.80 (0.93 – 3.45) | 0.08 |
Odds ratios and p-values result from logistic regression models adjusted for age and gender for all variables except estrogen therapy and oophorectomy, where due to the lack of iLBD cases with the presence of those characteristics, odds ratios result from adding 0.5 to each cell count and p-values result from Fisher’s exact test.
Odds ratios and p-values result from conditional logistic regression models adjusted for various covariates in age and gender matched case-control or cohort studies from the same patient population as the iLBD case-control study. The far-left column does not contain specific p-values for some studies since these were not available in the published manuscripts.
The sample mean ± SD (minimum, maximum) is given for age at death, and odds ratios correspond to a 10-year increase.
Caffeine use was focused on coffee use in the PD case-control study whereas all caffeine use was considered in the iLBD case-control study. Information was unavailable for the following variables: history of alcohol use (N=10), history of caffeine use (N=61), years of education (N=5), and oophorectomy in females (N=2).
N/A = not available; SIGNIF = significant; NS = not significant.
In the current study, there was evidence of an increased risk of iLBD in subjects who were physicians (OR=6.84, 95% CI 1.46–32.06, p = 0.015), which was also observed in the PD study (OR= 3.7, 95% CI: 1.0–13.1). There was a reduced risk of iLBD in subjects who ever drank caffeinated beverages (OR=0.36, 95% CI 0.14–0.96, p = 0.042), which again was observed in the PD study (OR=0.35, 95% CI: 0.15–0.78). There was a non-statistically significant association of iLBD with higher education (9 or more years; OR=2.65, 95% CI 0.84–8.33, p = 0.096), which was similar in magnitude and statistically significant in the PD study (OR=2.0, 95% CI: 1.1–3.6). Of the remaining 4 risk factors with statistically significant associations with PD, ORs for iLBD were in the same direction but not statistically significant for history of head injury (1.44 [iLBD] vs. 4.3 [PD]) and number of children in males (1.61 [iLBD] vs. 2.65 [PD]); they were in the opposite direction but not statistically significant for depression (0.72 [iLBD] vs. 1.9 [PD]) and anxiety (0.40 [iLBD] vs. 2.2 [PD]). Table 2 summarizes ORs estimated for each risk factor in iLBD and PD according to magnitude and statistical significance of each OR. Age was also associated with an increased risk of iLBD (OR=1.70 [10 year increase], 95% CI: 1.10–2.64, P=0.018); we did not have an OR for age and PD in the same population.
Table 2.
Power to detect odds ratios with 34 iLBD cases and 201 controls
| Power to detect given odds ratio (%) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic frequency | OR=1.2 | OR=1.5 | OR=2.0 | OR=2.5 | OR=3.0 | OR=3.5 | OR=4.0 |
| 10% | 3 | 8 | 21 | 37 | 51 | 62 | 71 |
| 20% | 4 | 12 | 33 | 54 | 70 | 82 | 89 |
| 30% | 4 | 14 | 38 | 61 | 78 | 88 | 93 |
| 50% | 4 | 14 | 36 | 59 | 75 | 86 | 92 |
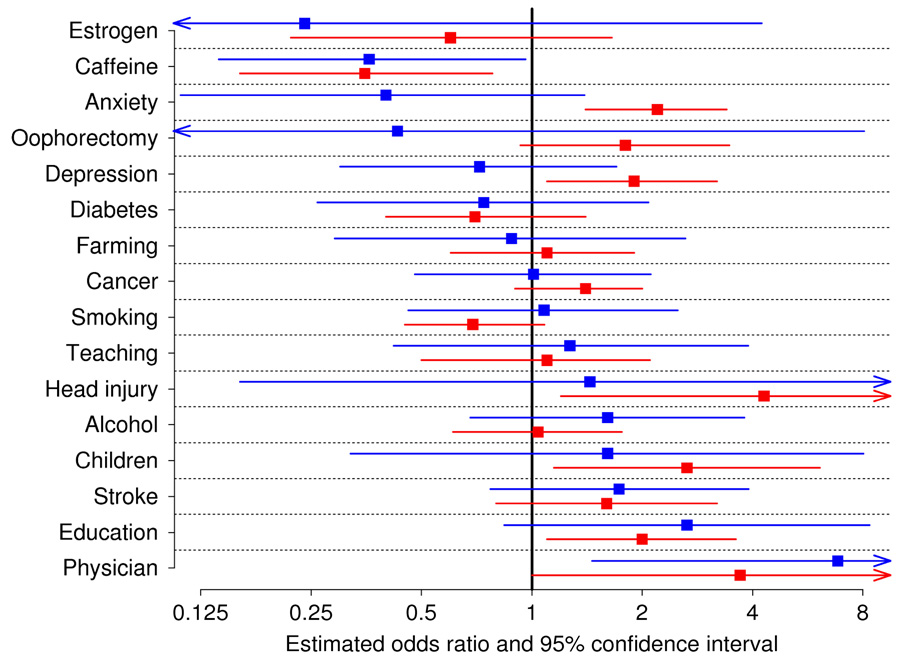
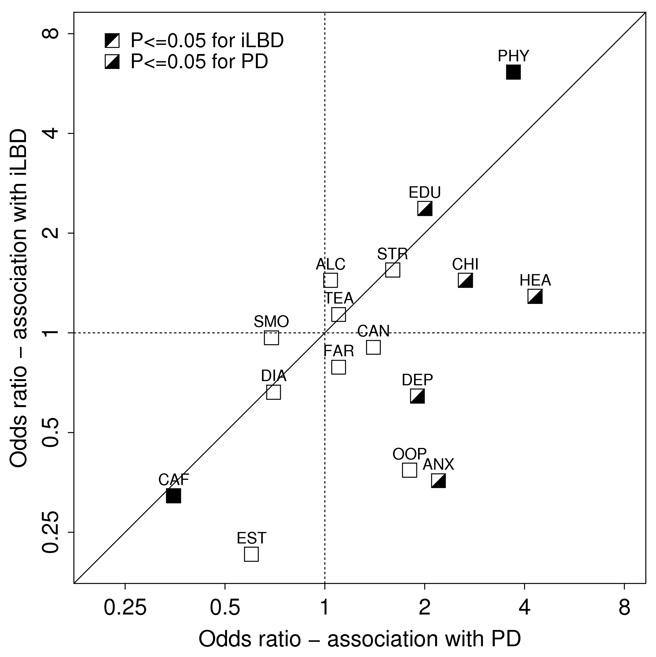
Figure 1a is a graphical representation to compare risk factors in iLBD and PD, displaying estimated ORs and 95% CIs for each risk factor. Figure 1b offers an alternate comparison of risk factors in iLBD and PD, displaying estimated ORs for each, along with a reference line of equality. In comparing ORs of risk factors for iLBD and PD, it is important to take into account the degree of precision of ORs, particularly for several ORs associated with iLBD for which the 95% CIs were particularly wide (estrogen use in women, oophorectomy in women, history of head injury, number of children in men, and occupation of physician). For 11 of 16 risk factors, iLBD and PD had ORs in the same direction. Only anxiety, depression, farming occupation, oophorectomy, and smoking had ORs with different directions. Of those, the odds ratios for farming (0.88 and 1.1) were both relatively close to 1. Lin’s concordance correlation coefficient28 measuring the level of agreement between all iLBD and PD ORs was equal to 0.52, which in the context of this study can reasonably be considered to represent moderate to good agreement.
Figure 1a.
Association of risk factors with iLBD and PD in Olmsted County, MN. Squares represent odds ratios, horizontal lines represent 95% confidence intervals, blue represents values for iLBD, while red represents values for PD.
Figure 1b.
Association of risk factors with iLBD and PD in Olmsted County, MN. Scatterplot of odds ratios for risk factors observed for PD (x-axis) versus iLBD (y-axis). The first three letters are shown for each risk factor (see Figure 1a for full name).
We had only limited statistical power to detect associations of the risk factors with iLBD. A large reason for this was that only 14.5% of neuropathological examinations yielded iLBD cases. The previously published Olmsted County epidemiologic studies of PD patients included much larger N’s. Table 2 provides the statistical power for a range of frequencies and odds ratios with 34 cases and 201 controls. Given this limitation and also the lack of power in the moderately sized PD case-control studies, we examined the overall pattern of the associations in addition to statistical significance of those associations.
We also compared the iLBD group (N=34) to the normal control subjects (N=201) with respect to systemic illnesses or non-specific end-of-life medical states that might suggest a non-specific etiology (Table 3). After adjusting for age and gender, there was a significant association of iLBD with only one of these 11 factors; persons with chronic cachexia had an increased risk of iLBD (OR=3.25, 95% CI 1.10–9.59, p = 0.034).
Table 3.
Population-based studies in Olmsted County, Minnesota: the association of non-specific systemic illnesses and end of life medical states with iLBD
| Summary of each variabl |
Association with iLBD* |
|||
|---|---|---|---|---|
| Variable | iLBD cases (N=34) | Controls (N=201) | Estimated OR (95% CI) | P-value |
| Diabetes | 5 (15%) | 45 (22%) | 0.74 (0.26 – 2.08) | 0.57 |
| Insulin dependent diabetes | 2 (6%) | 18 (9%) | 0.90 (0.19 – 4.26) | 0.90 |
| High blood pressure | 20 (61%) | 142 (71%) | 0.67 (0.31 – 1.45) | 0.31 |
| Terminal disease | 12 (35%) | 71 (35%) | 1.03 (0.48 – 2.23) | 0.94 |
| COPD | 7 (21%) | 61 (30%) | 0.64 (0.26 – 1.56) | 0.32 |
| Cancer | 18 (53%) | 104 (52%) | 1.01 (0.48 – 2.11) | 0.98 |
| Metastatic cancer | 3 (9%) | 26 (13%) | 0.73 (0.20 – 2.60) | 0.63 |
| Chemotherapy | 3 (9%) | 27 (13%) | 0.87 (0.24 – 3.19) | 0.84 |
| CAD | 14 (41%) | 108 (54%) | 0.61 (0.29 – 1.29) | 0.20 |
| PVD | 6 (18%) | 30 (15%) | 1.31 (0.49 – 3.51) | 0.59 |
| Sudden unexpected death | 5 (15%) | 25 (12%) | 1.40 (0.43 – 3.64) | 0.68 |
| Cachexia | 6 (18%) | 14 (7%) | 3.25 (1.10 – 9.59) | 0.034 |
Odds ratios and p-values result from logistic regression models adjusted for age and gender.
COMMENT
The epidemiological risk factor profile for iLBD in our autopsy sample was fairly similar to the risk factor profile for PD previously reported in a similar Olmsted County, MN population. The direction of the odds ratios was the same for 11 of the 16 factors we assessed. Of the 7 risk factors with statistically significant associations with PD, 5 had ORs in the same direction for iLBD with 2 statistically significant associations and 1 borderline significant association. Though a larger sample with more iLBD patients is necessary to make strong conclusions, these findings are consistent with the hypothesis that iLBD is indeed related to PD. This hypothesis is supported by three other clinical observations previously reported in iLBD, documenting REM sleep behavior disorder,15,29 reduced bowel movement frequency30 as well as olfactory dysfunction,31 similar to PD.
We also assessed a battery of medical conditions, which conceivably might result in chronic low-grade brain insults (non-specific to PD). ILBD was only associated with chronic cachexia, which arguably could be an effect of iLBD rather than a cause of iLBD (cause-effect inversion). Although the ILBD cases were slightly older (mean, four years), this small difference seems unlikely to account for the ILBD status. These findings argue against the hypothesis that iLBD is a non-specific finding unrelated to PD.
Non-clinical evidence also suggests that iLBD is closely linked to PD. Two recent studies independently documented reduced striatal dopaminergic terminal markers among iLBD cases, with values intermediate between those of normal control subjects and PD cases.26,32 Moreover, the topography of Lewy pathology in iLBD mirrors PD, affecting not only the same brain regions,5,6 but also the autonomic nervous system.3,4,7,8,27,33,34 Thus, unlike tangles and tau pathology, which may have a more ubiquitous distribution in advanced age,16 iLBD follows the topographic pattern of PD.
One curious finding in this study was the association of physician-occupation with iLBD (OR=6.84; 95% CI 1.46–32.06, p = 0.015). We previously reported a similar association of physician-occupation with PD, but attributed that finding to surveillance bias (i.e., physicians would be more likely to recognize parkinsonism in themselves). This parallel finding in iLBD suggests that the association of physician occupation with PD is not due to surveillance bias.
As with PD, caffeine consumption was associated with a reduced risk for iLBD. Although caffeine might protect against Lewy pathology and PD, it is also possible that caffeine aversion (as well as the choice of physician occupation) is an early behavioral manifestation of Lewy pathology (cause-effect inversion). Premorbid personality differences have been reported in PD.35–37
This study is not without its limitations. Chief among these is the relatively small number of iLBD cases, which results in low power to detect statistically significant associations, and correspondingly wide confidence intervals for estimated odds ratios, indicating a lack of precision in these estimates. A sample with more iLBD patients is needed to better evaluate associations with iLBD. With that said, although this is a small number of cases compared to epidemiologic studies of PD cases, the number of iLBD cases in this neuropathological series is similar to, or exceeds that of most other neuropathological series. The study of iLBD is obviously limited by the fact that approximately 7–10 brains must be microscopically examined across multiple brain regions to detect one case of iLBD.
Since our iLBD cases were not prospectively examined by a neurologist in life, it is possible that PD may have been overlooked. However, the incidence of iLBD in our series (14.5%) is nearly ten times greater than the lifetime risk (1.6%) for PD in the Olmsted County population.1 This suggests that most of the iLBD cases included in our study were not simply undetected cases of PD.
It is unlikely that the risk factors that we observed for either iLBD or PD in the Olmsted County, MN population are due to measurement biases. The medical records abstraction was blinded to the neuropathology and conversely, the neuropathological analyses were blinded to the medical records abstraction. Since our iLBD cases were clinically undetected, there were obviously no recall biases. The medical records provided a rich source of data, with the mean durations of Mayo records spanning over four decades in both the iLBD and control groups. All subjects had been evaluated by a physician at least once in the last year of their life and very frequently during the last half-decade (median 20–21 physician visits during the last 5 years of life).
Finally, it should be noted that among the 34 iLBD cases, 13 were a distinctly poor fit with the Braak ascending scheme; 12 cases had diffuse Lewy pathology consistent with Braak stages 5–6, plus one had Lewy pathology in only the nucleus basalis. Whether these outriders represent a unique iLBD subset is open to speculation. We re-analyzed the data confined to the 21 remaining cases. In this reanalysis, the OR’s for these iLBD cases were in the same direction as PD for 12 of the 16 variables (compared to 11/16 for the entire iLBD cohort of 34 cases). In addition, the Lin correlation coefficient increased from 0.52 to 0.58, consistent with a slightly greater association of these 21 iLBD cases with PD. With this smaller N, however, none of the PD-related variables reached statistical significance.
Overall, these findings are reasonably consistent with the hypothesis that at least some iLBD cases are on a neurodegenerative disease continuum with PD, i.e., represent an early stage in the PD process. However, if this hypothesis is correct, it remains unknown if such iLBD cases might represent preclinical PD cases that died before clinical manifestation, or arrested PD, with some unknown factor terminating the progression to PD. Alternatively, if PD is due to the additive effects of multiple factors, iLBD might reflect an incomplete syndrome, with sub-threshold involvement of the same factors. Further study of iLBD cohorts may provide insights regarding factors that modify the progression of Lewy pathology and hence may suggest primary prevention strategies.
ACKNOWLEDGMENT
The authors thank Mike Oelkers and Connie McDonough for help with the Tissue Registry, and Monica Casey-Castanedes, Virginia Phillips and Linda Rousseau for histological technical support.
Funding/Support: This work was supported by the Morris K. Udall Parkinson's Disease Research Center of Excellence grant (P50 NS40256), by the NIH grant 2R01 ES10751, by the Mayo Alzheimer’s Disease Research Center (P50 AG16754), and by the Mayo Alzheimer’s Disease Patient Registry (U01 AG06786).
Footnotes
Author contributions: Dr. Ahlskog had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Frigerio, Maraganore, DelleDonne, Dickson, Ahlskog; Acquisition of data: Frigerio, Fujishiro, Klos, Parisi, Dickson; Analysis and interpretation of data: Frigerio, Fujishiro, Maraganore, Heckman, Crook, Josephs, Boeve, Dickson, Ahlskog; Drafting of the manuscript: Frigerio, Maraganore, Dickson, Ahlskog; Critical revision of the manuscript for important intellectual content: Fujishiro, Klos, DelleDonne, Heckman, Crook, Josephs, Parisi, Boeve; Statistical analysis: Heckman, Crook; Obtained funding: Dickson; Administrative, technical, and material support: Ahlskog; Study supervision: DelleDonne, Ahlskog.
Financial Disclosure: Dr. Frigerio reports no disclosures; Dr. Fujishiro reports no disclosures; Dr. Ahn reports no disclosures; Dr. Josephs reports no disclosures; Dr. Maraganore reports a provisional application for patent under 37 CFR § 1.53 (c) entitled “Predicting Parkinson’s Disease.” No monies have been awarded to date. Dr. Maraganore also reports a provisional application for patent entitled “Method of Treating Neurodegenerative Disease” that has been licensed to Alnylam Pharmaceuticals, Inc. Less than $10,000 has been awarded to date; Dr. DelleDonne reports no disclosures; Dr. Parisi reports no disclosures; Dr. Klos reports no disclosures; Dr. Boeve reports grant support from Myriad Pharmaceuticals and Honorarium from GE Healthcare; Dr. Dickson reports no disclosures; Dr. Ahlskog reports no disclosures.
REFERENCES
- 1.Elbaz A, Bower JH, Maraganore DM, et al. Risk tables for parkinsonism and Parkinson's disease. Journal of Clinical Epidemiology. 2002;55(1):25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 2.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32(3):284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 4.Klos KJ, Ahlskog JE, Josephs KA, et al. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66(7):1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 2007;113(4):421–429. doi: 10.1007/s00401-007-0193-x. [DOI] [PubMed] [Google Scholar]
- 9.Parkkinen L, Kauppinen T, Pirttila T, et al. Alpha-synuclein pathology does notn predictextrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 10.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol. 2008;115:409–415. doi: 10.1007/s00401-008-0344-8. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 12.Zaccai J, Brayne C, McKeith I, et al. Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology. 2008;70:1042–1048. doi: 10.1212/01.wnl.0000306697.48738.b6. [DOI] [PubMed] [Google Scholar]
- 13.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? Journal of Neuropathology & Experimental Neurology. 2002;61(5):413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 15.Boeve BF, Dickson DW, Olson EJ, et al. Insights into REM sleep behavior disorder pathophysiology in brainstem predominant Lewy body disease. Sleep Med. 2007;8(1):60–64. doi: 10.1016/j.sleep.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiology of aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 17.Benedetti MD, Maraganore DM, Bower JH, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Movement Disorders. 2001;16(5):830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 18.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70(3):200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study.[comment] Neurology. 2000;55(9):1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 20.Shiba M, Bower JH, Maraganore DM, et al. Anxiety disorders and depressive disorders preceding Parkinson's disease: a case-control study. Movement Disorders. 2000;15(4):669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Leibson CL, Maraganore DM, Bower JH, Ransom JE, O'Brien PC, Rocca WA. Comorbid conditions associated with Parkinson's disease: a population-based study. Movement Disorders. 2006;21(4):446–455. doi: 10.1002/mds.20685. [DOI] [PubMed] [Google Scholar]
- 22.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology. 2005;65(10):1575–1583. doi: 10.1212/01.wnl.0000184520.21744.a2. [DOI] [PubMed] [Google Scholar]
- 23.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology. 2003;60(10):1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 24.Frigerio R, Breteler MM, de Lau LM, et al. Number of children and risk of Parkinson's disease. Movement Disorders. 2007;22(5):632–639. doi: 10.1002/mds.21341. [DOI] [PubMed] [Google Scholar]
- 25.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 26.DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65(8):1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 27.Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115(4):437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 28.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989 Mar;45(1):455–268. [PubMed] [Google Scholar]
- 29.Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45(4):709–712. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 30.Abbott RD, Ross GW, Petrovitch H, et al. Bowel movement frequency in late-life and incidental Lewy bodies. Movement Disorders. 2007;22(11):1581–1586. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 31.Ross GW, Abbott RD, Petrovitch H, et al. Association of olfactory dysfunction with incidental Lewy bodies. Movement Disorders. 2006;21(12):2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- 32.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115(4):445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwanaga K, Wakabayashi K, Yoshimoto M, et al. Lewy body-type degeneration in cardiac plexus in Parkinson's and incidental Lewy body diseases. Neurology. 1999;52(6):1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- 34.Orimo S, Uchihara T, Nakamura A, et al. Axonal {alpha}-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain. 2008;131(3):642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 35.Menza MA, Golbe LI, Cody RA, Forman NE. Dopamine-related personality traits in Parkinson's disease. Neurology. 1993;43(3 Pt 1):505–508. doi: 10.1212/wnl.43.3_part_1.505. [DOI] [PubMed] [Google Scholar]
- 36.Tomer R, Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson's disease: effects of asymmetric dopamine deficiency. J Neurol Neurosurg Psychiatry. 2004;75(7):972–975. doi: 10.1136/jnnp.2003.024885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishihara L, Brayne C. What is the evidence for a premorbid parkinsonian personality: a systematic review. Movement Disorders. 2006;21(8):1066–1072. doi: 10.1002/mds.20980. [DOI] [PubMed] [Google Scholar]