Abstract
Endothelin-1 (ET-1) is a potent mitogen that transmits signals through its cognate G protein-coupled receptors to stimulate extracellular signal-regulated kinase Erk1/2. Endothelin-1 receptors (ET-Rs) are known to interact with caveolin-1 and co-localize in caveolae which integrate different receptor and signaling proteins. We have recently shown that β1Pix binds specifically to ET-Rs. Here, we show that β1Pix binding to caveolin-1 is dependent on heterotrimeric G proteins activation state. β1Pix interaction with different G proteins is increased in the presence of the G protein activator AMF. Moreover, extraction of cholesterol with methyl-β-cyclodextrin disrupts the binding of β1Pix to Gαq, Gα12 and phospho-Erk1/2 but not the binding of β1Pix to Gβ1. The disruption of β1Pix dimerization strongly reduced the binding of caveolin-1, Gαq and Gα12. Constitutively active mutants of Gαq and Gα12 increased Cdc42 activation when co-expressed with β1Pix but not in the presence of β1Pix dimerization deficient mutant β1PixΔ (602–611). ET-1 stimulation increased the binding of phosphorylated Erk1/2 to β1Pix but not to β1PixΔ (602–611). RGS3 decreased ET-1-induced Cdc42 activation. These results strongly suggest that the activation of ET-Rs leads to the compartmentalization and the binding of Gαq to β1Pix in caveolae, where dimeric β1Pix acts as platform to facilitate the binding and the activation of Erk1/2.
Keywords: Caveolin-1, β1Pix, Gαq, Gα12, Endothelin, Cdc42, Caveolae
Introduction
Endothelin-1 (ET-1) is a potent mitogenic peptide that mediates a wide variety of cell signaling including cell proliferation and gene expression through its G protein-coupled receptors, ETAR and ETBR [1] which can activate different G proteins. ETAR acts through Gq and Gs to stimulate phospholipase C (PLC) and adenylate cyclase respectively. ETBR stimulates PLC via Gq, and inhibits adenylate cyclase through Gi [2;3]. ET receptors have also been shown to couple to G12 in NIH3T3 cells where G12 mediates the formation of actin stress fiber formation [4]. ETAR and ETBR are both localized within caveolae [5;6], invaginations of the plasma membrane containing caveolin proteins which act as scaffolding proteins to cluster different signaling molecules such as Gα subunits, Ha-Ras, PKC and Src [7]. We have shown that ET-1 induces cell proliferation through up-regulation of the Cdc42/Rac guanine nucleotide exchange factor β1Pix [8]. β1Pix contains a Src homology 3 (SH3) domain, a Dbl homology (DH) domain, a pleckstrin homology (PH) domain for activation of Rho GTPases and a leucine zipper (LZ) domain responsible for β1Pix dimerization [9]. ET-1 stimulation of mesangial cells induces β1Pix translocation to focal adhesion and Cdc42 activation through PKA-dependent phosphorylation of β1Pix [10]. Moreover, the activation of Gαs signaling pathway results in the binding of 14-3-3β to β1Pix through protein kinase A-dependent phosphorylation leading to the inhibition of Rac1 and membrane ruffle formation [11]. β1Pix regulates mast cell secretion through Gαi-dependent mechanism [12] and β1Pix/Gαi3 interaction mediates p66Shc activation induced by EGF receptor transactivation [13].
Although several studies have shown that Erk1/2 activation by extracellular stimuli requires small GTPases activation, the mechanisms whereby G protein coupled receptors (GPCRs) regulate this signaling pathway are not fully understood. One mechanism of Erk1/2 activation involves Gαi-coupled receptor where ligand stimulation induces the release of Gβγ leading to Ras activation. In addition, ET-1 can also activate Erk1/2 through EGF receptor transactivation [14]. In recent years, there has been increasing evidence that the activation of some small GTPases by GPCRs is mediated by the activation of guanine nucleotide exchange factors (GEFs). For example, a family of RhoAGEFs consisting of p115RhoGEF, PDZ-RhoGEF and leukemia-associated RhoGEF contain a regulator of G protein signaling homology domain that directly binds to Gα12 [15;16]. The p63RhoGEF, however, was found to link RhoA to Gαq/11-coupled receptors by directly interacting with active Gαq/11 [17].
Materials and Methods
Materials
Cell culture media and supplements were obtained from Invitrogen (Carlsbad, CA). Endothelin-1 was purchased from Calbiochem (La Jolla, CA). Anti-ETA-R and anti-ETB-R were from Millipore (Billerica, MD). Anti-Myc, anti-caveolin-1, anti-Gβ1 and anti-Gα subunits antibodies were from Santa Cruz Biotechnologies (Santa Cruz, CA). Antiphospho-Erk1/2 was purchased from Cell Signaling (Danvers, MA). RGS3 antiserum was described previously [18].
Cell culture and transfection
Human mesangial cells were cultured in RPMI 1624 (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 µg/ml) in a 37°C humidified incubator with 5% CO2. Transient transfection of cells with mammalian expression vectors was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Plasmids
The Myc-tagged β1Pix and β1PixΔ (602–611) plasmids have been described [8;11]. Gαq, GαqQ209L, Gα12, Gα12Q231L were obtained from Missouri S&T cDNA Resource Center. RGS3 was inserted into pcDNA3.1 plasmid as described before [18].
Immunoprecipitation and Western blot analysis
Cells were transfected with the appropriate construct for 24 h. Cells were washed twice in phosphate-buffered saline (PBS) and lysed in lysis buffer containing 20 mM Tris, pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1% Triton X-100, 1 mM sodium fluoride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml pepstatin, and 1 µg/ml leupeptin. Equal amounts of proteins were separated by using 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore), immunoblotted with the appropriate antibody, and visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, Inc.). For immunoprecipitation, antibodies against Myc, caveolin-1 or Gα subunits were added to the cell lysate (500 µg) for 2 h of incubation, followed by addition of protein A- or protein G-agarose beads for an additional hour. The beads were washed three times in PBS. The immunoprecipitated proteins were released from the beads by boiling in 1x sample buffer for 5 min and subsequently analyzed by Western blotting. Total cell lysate (TCL) was run to assess the over-expression of the constructs. Expression of recombinant Myc-β1Pix and its mutants was verified by immunoblotting with anti-Myc antibody.
Cdc42 activity assay
Cells were transfected with empty vector, Myc-tagged β1Pix alone or in combination with the indicated Gα subunit for 24 h. Cells were lysed in lysis/wash buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, 1% glycerol, 10 µg/ml leupeptin, and 10 µg/ml aprotinin). To measure the active GTP-bound form of Cdc42 GTPase in the cell lysates, we performed a pull-down assay (Upstate) using recombinant glutathione S-transferase (GST)-p21 binding domain (PBD). Aliquots (500 µg) of the supernatants were incubated with 10 µg of GST-PBD for 1 h and precipitated by centrifugation. Complexes were boiled in a Laemmli sample buffer and then separated on 15% sodium dodecyl sulfate-polyacrylamide gels. The separated proteins were immunoblotted using a monoclonal anti-Cdc42 antibody.
Results
β1Pix interacts with caveolin-1 and G proteins
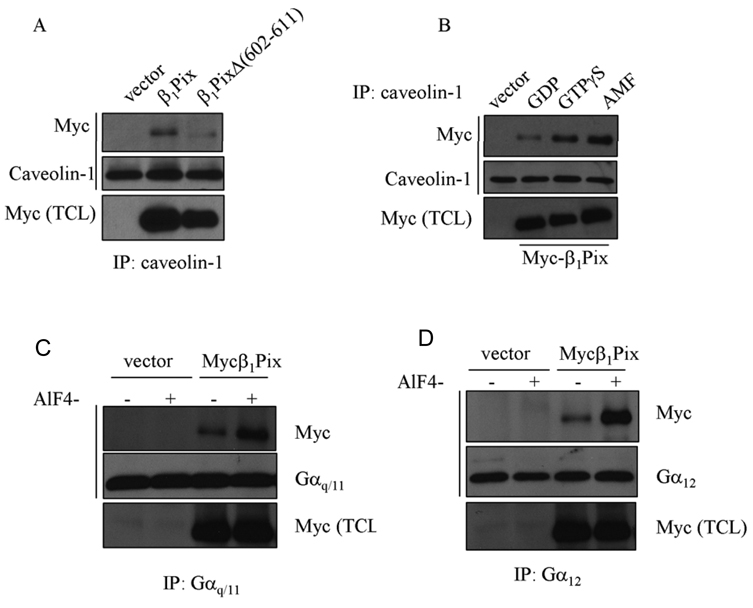
Several studies have implicated caveolin-1 as a scaffolding protein that enables compartmentalization of specific signaling molecules. ET-Rs have been shown to interact with caveolin-1 and to localize to caveolae [5;6]. Recently, we showed that β1Pix binds to ET-Rs [13], therefore we examined whether β1Pix interacts with caveolin-1. To evaluate the potential interaction between β1Pix and caveolin-1 and the role of β1Pix dimerization in this interaction, we expressed wild type β1Pix or β1Pix dimerization deficient mutant, β1PixΔ (602–611). Using anti-caveolin-1 antibody for immunoprecipitation, we found that wild type β1Pix interacts with caveolin-1. This interaction was significantly reduced in cells expressing dimerization deficient β1Pix mutant (Fig. 1A), indicating that dimerization is crucial for caveoiln-1 binding to β1Pix. Caveolin-1 has been shown to interact with different Gα subunits through caveolin-1 scaffolding domain [19;20]. Furthermore, this interaction was regulated by the activation state of G proteins and was significantly decreased by GTPγS [19;21]. Therefore, we set out to determine the effect of G protein activation by AMF on the interaction between β1Pix and caveolin-1. Our result showed that GTPγS induced a strong increase in the interaction between β1Pix and caveolin-1 compared to that obtained with GDP, and this interaction was further increased in the presence of AMF (Fig. 1B).
Figure 1.
The binding of caveolin-1 to β1Pix depends on G protein activation state and β1Pix dimerization. (A) Cells were transfected with Myc-β1Pix or β1PixΔ (602–611) for 24 h. Cell lysates were immunoprecipitated with anti-caveolin-1 antibody and the bound proteins were analyzed by Western Blot using anti-Myc antibody. (B) Cells were transfected with Myc-β1Pix for 24 h and lysed in lysis buffer containing either GDP (10 µM), GTPγS (10 µM) or AMF (AlCl3, 50 µM; MgCl2, 10 mM; NaF, 5 mM). Anti-caveolin-1 was used for immunoprecipitation followed by Western Blot analysis using anti-Myc and anti-caveolin-1 antibodies, respectively. These data are representative of four independent experiments. (C/D) Cells were transfected with empty vector or Myc-β1Pix and lysed in the absence or presence of AMF. Lysates were immunoprecipitated using either anti-Gαq/11 (C) or anti-Gα12 (D) antibodies and the Western Blots were analyzed by anti-Myc antibodies as indicated. Similar results were obtained in three different experiments.
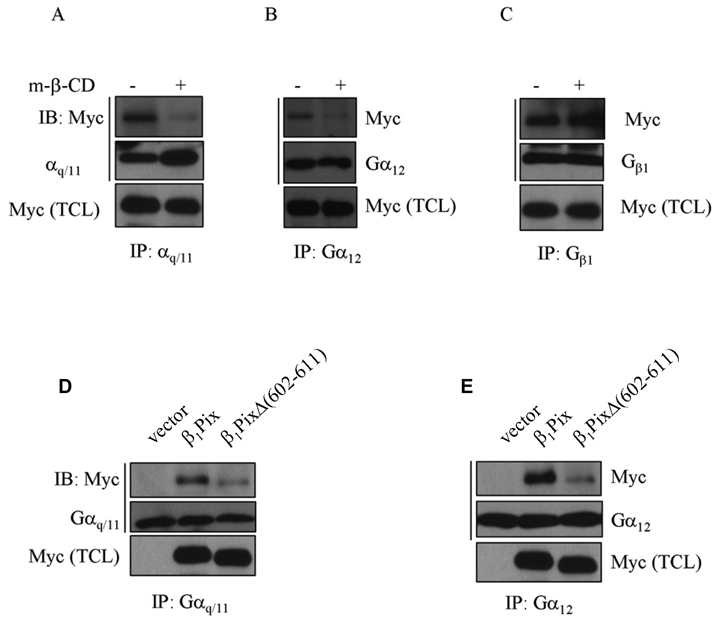
Caveolin-1 is one component of caveolae that functions as scaffolding protein to facilitate signaling between different proteins. G protein α subunits have been identified as caveolin-interacting proteins [19], therefore we were interested to investigate a possible interaction between β1Pix and G proteins. Cells were transfected with empty vector or wild type β1Pix and lysed in the presence or absence of heterotrimeric G protein activator AMF. We found that β1Pix interacts with endogenous Gαq, Gα12 (Fig.1C, D) and Gαs (data not shown) and that this interaction was significantly increased in the presence of AMF. Cells transfected with empty vector served as a negative control. To examine whether the interaction between β1Pix and Gαq or Gα12 depends on the physical integrity of caveolae, cells were treated with methyl-β-cyclodextrin, a cholesterol-depleting agent that disrupts caveolae. Results showed that methyl-β-cyclodextrin blocked the binding of Gαq and Gα12 to β1Pix (Fig. 2A, B), but did not block the interaction between β1Pix and Gβ1 (Fig. 2C).
Figure 2.
The binding of Gαq and Gα12 to β1Pix requires caveolae and β1Pix homodimerization. Cells were transfected with Myc-β1Pix for 24 h and either left untreated or treated with methyl-β-cyclodextrin (5 mM) for 1 h. Cell lysates were immunoprecipitated with anti-Gαq/11 (A), anti-Gα12 (B) or anti-Gβ1 (C) antibodies. Precipitated proteins were separated by SDS-PAGE and immunoblotted with anti-Myc, anti-Gαq/11, anti-Gα12 or anti-Gβ1 antibodies. The content of Gα subunits, Gβ1 and Myc in the total cell lysates indicates the levels of expression of these proteins. These data are representative of similar results obtained in three independent experiments. (D/E) HMCs cells were transfected with Myc-β1Pix or Myc-β1PixΔ (602–611) for 24 h. Lysates were immunoprecipitated using anti-Gαq/11 (D) or anti-Gα12 (E) antibodies and Western blots were analyzed with anti-Myc antibodies. The expression of Myc-β1Pix, Gαq/11 and Gα12 was analyzed by Western Blots performed on the total cell lysates. These data are representative of similar results obtained in three independent experiments.
To determine the role of dimerization on β1Pix binding to G proteins, cells were transfected with β1Pix(wt) or β1PixΔ (602–611). Results showed that the binding of β1Pix to Gαq and Gα12 is strongly reduced in cells expressing β1Pix dimerization deficient mutant β1PixΔ (602–611) (Fig. 2D, E).
Constitutively active Gαq and Gα12 potentiate β1Pix-induced Cdc42 activation
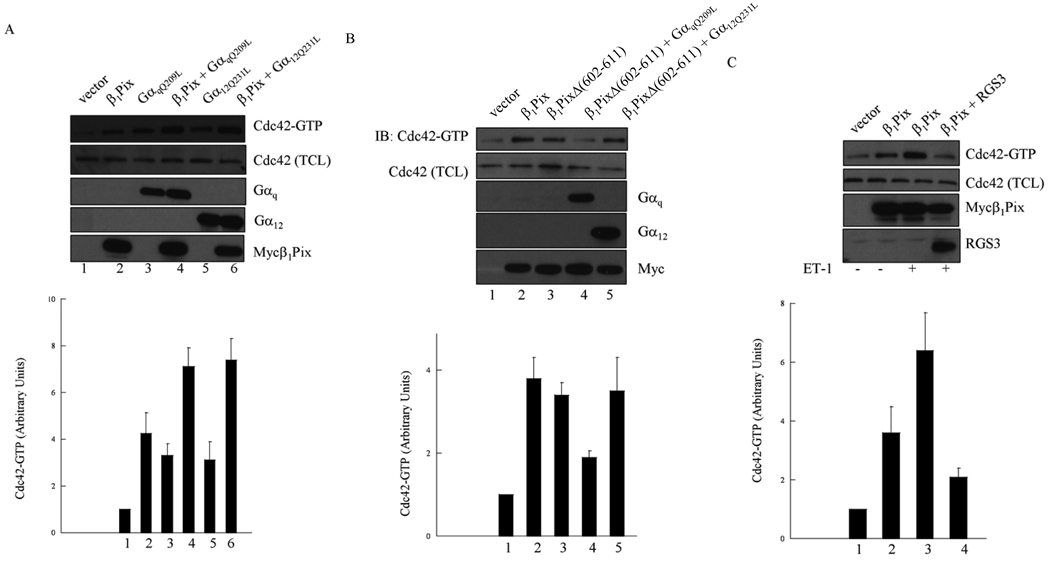
We have demonstrated that the interaction between β1Pix and wild type Gαq or Gα12 is increased in the presence of AMF. In order to investigate the functional significance of these physical interactions, we tested the effect of constitutively active Gαq and Gα12 subunits on Cdc42 activity. To measure active Cdc42 (Cdc42-GTP), we utilized the pull-down assay that consists of measuring Cdc42-GTP bound to GST-Pak1-PBD in lysates of transfected HMCs. Expression of either β1Pix, GαqQ209L or Gα12Q231L alone induced a modest increase in Cdc42 activity (Fig. 3A). The expression of β1Pix with constitutively active Gαq or Gα12 induced a significant increase in Cdc42 activity, indicating that the effect of Gαq or Gα12 and β1Pix on Cdc42 activity were additive (Fig. 3A).
Figure 3.
Gαq and Gα12 increase β1Pix-induced Cdc42 activation. (A) Cells were transfected with empty vector, Myc-β1Pix, GαqQ209L or Gα12Q231L for 24 h as indicated. (B) Cells were transfected with Myc-β1Pix, Myc-β1PixΔ (602–611), Gαq or Gα12 as indicated. (C) Cells were transfected with β1Pix alone or in combination with RGS3 before addition of ET-1 (100 nM) for 5 min. The GTP-bound Cdc42 was measured using pull down assay as described under “Materials and Methods”. The total cell lysates were analyzed for the expression of Myc-β1Pix, Cdc42, Gαq, Gα12 or RGS3. Graphs at the bottom of each panel shows fold-increase in Cdc42-GTP densitometric values from three independent experiments normalized to vector which was assigned a value of 1. Results are expressed as mean ± S.E.M. Data are representative of at least three independent experiments.
We have shown that β1Pix binding to Gαq and Gα12 requires β1Pix dimerization. To investigate whether the interaction of Gαq and Gα12 with β1Pix is functionally relevant to Cdc42 activation, we analyzed the effect of the expression of β1Pix dimerization deficient mutant β1PixΔ (602–611), which binding to Gαq and Gα12 is strongly disrupted, on Cdc42 activity. As shown in Fig. 3B, both β1Pix and β1PixΔ (602–611) alone were able to increase the amount of active Cdc42. However, the co-expression of β1PixΔ (602–611) and GαqQ209L induced a smaller increase in Cdc42 activity compared to cells transfected with β1PixΔ (602–611) alone. The co-expression of β1PixΔ (602–611) and Gα12Q231L induced an activation of Cdc42 that is comparable to that obtained in cells expressing β1Pix (wt) alone (Fig. 3B). These results indicate that the binding of Gαq and Gα12 to β1Pix potentiate Cdc42 activation.
RGS3 inhibits ET-1-induced Cdc42 activation
RGS3 is a member of the regulators of G protein signaling (RGS) family. In vitro experiments with purified glutathione S-transferase-fusion RGS3 showed its ability to bind Gαq [22]. We therefore evaluated the ability of RGS3 to modulate Gαq-mediated signaling. We first showed that β1Pix increased Cdc42 activity, which was further increased by ET-1 stimulation (Fig. 3C). This increase was strongly, although not completely, inhibited in cells expressing RGS3, indicating that ET-1 activation of Cdc42 is mediated at least in part by Gαq.
ET-1 increased phospho-Erk1/2 binding to β1Pix
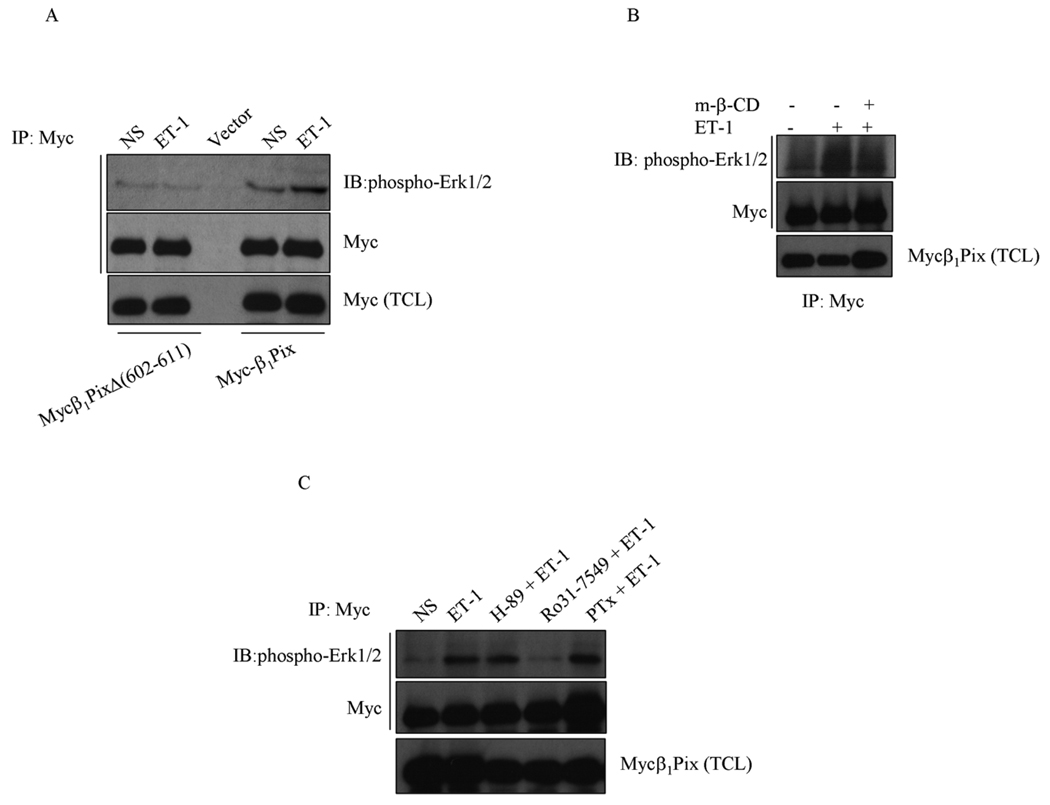
To determine whether β1Pix is involved in Erk1/2 activation, cells were transfected with empty vector, Myc-β1Pix or Myc-β1PixΔ (602–611). Cells were stimulated with ET-1 (100 nM for 5 min) and cell lysates were immunoprecipitated with anti-Myc antibody. We found that in unstimulated cells expressing β1Pix alone phospho-Erk1/2 was bound to β1Pix and ET-1 stimulation further increased the interaction between phospho-Erk1/2 and β1Pix (Fig. 4A). Expression of β1Pix dimerization deficient mutant, β1PixΔ (602–611), significantly decreased ET-1-induced binding of phopsho-Erk1/2 to β1Pix (Fig. 6A). The disruption of caveolae with methyl-β-cyclodextrin strongly inhibited ET-1-induced binding of phospho-Erk1/2 to β1Pix (Fig. 4B).
Figure 4.
ET-1-induced Erk1/2 activation and binding to β1Pix. (A) Cells were transfected with Myc-β1Pix or Myc-β1PixΔ (602–611) as indicated for 24 h before addition of ET-1 (100 nM) for 5 min. (B) Cells were transfected with Myc-β1Pix and treated with methyl-β-cyclodextrin (5 mM) for 1 h before addition of ET-1. (C) Cells were transfected with Myc-β1Pix for 24 h. Cells were incubated with Pertussis Toxin (0.2 µg/ml) overnight or with H-89 (20 µM) or Ro31-7549 (20 µM) for 30 min before stimulation with ET-1 (100 nM) for 5 min. Cell lysates were immunoprecipitated with anti-Myc antibodies and Western blots were analyzed as indicated. These results are representative of three independent experiments.
ET-1-induced binding of phospho-Erk1/2 to β1Pix was strongly inhibited by PKC inhibitor, Ro31-7549 but not by PKA inhibitor, H-89, or pertussis toxin (Fig. 4C). This result further corroborates that Gαq is involved in ET-1-induced activation and binding of Erk1/2 to β1Pix, whereas Gαi/o and Gαs are not. Altogether, these results suggest that dimerization allows β1Pix to bind to caveolin-1 and acts as platform to facilitate the formation of a multi-molecular complex between these proteins which results in Erk1/2 activation and it’s binding to β1Pix.
Discussion
In this study, we have demonstrated that β1Pix interacts with caveolin-1, Gαq and Gα12, and that this interaction was significantly higher in the presence of G protein activator AMF. Co-expression of β1Pix and activated mutants of Gαq or Gα12, induced an additive increase of the amount of active Cdc42. The disruption of β1Pix dimerization severely reduced β1Pix binding to caveolin-1 and G α subunits and prevented the ability of β1Pix to enhance Gαq- and Gα12-induced Cdc42 activity. ET-1-induced Cdc42 activation was reduced by RGS3, which block Gq-mediated signaling. ET-1-induced binding of phospho-Erk1/2 to β1Pix was inhibited by RGS3 and by PKC inhibitor but not by pertussis toxin or PKA inhibitor, H-89. The disruption of caveolae by methyl-β-cyclodextrin completely blocked the binding of β1Pix to Gαq and Gα12, and reduced interaction between β1Pix and phospho-Erk1/2.
Although many members of Rho family small GTPases can be stimulated through G protein-coupled receptors, it remains unclear how the activating signal is transmitted from the receptor to the corresponding GTPase. Active Gα13 has been shown to directly interact and activate p115RhoGEF [16], while another member of p115RhoGEF, LARG, interacts with Gαq and Gα12/13 to activate RhoA [15]. These RhoGEFs interact only with active Gαq and Gα12/13 through their regulator of G protein signaling (RGS) boxes.
We have recently shown for the first time that β1Pix, which does not belong to the family of RGS box-containing RhoA-specific GEFs, interacts specifically with Gαi3 in the presence of AMF [13]. Here, we extend the ability of β1Pix binding to other G proteins, namely Gαq, Gα12 and Gαs (data not shown). Furthermore, we have previously shown that the activation of Gαs signaling pathway induces the phosphorylation of β1Pix by PKA resulting in Cdc42 activation [10]. Here, we show that the co-expression of constitutively active Gαq or Gα12 and β1Pix resulted in an additive increase in Cdc42 activity. Gαq and Gα12 were unable to enhance Cdc42 activity in the presence of β1Pix dimerization deficient mutant indicating that the physical interaction between these Gαq or Gα12 and β1Pix is required for Cdc42 activation. The fact that RGS3 partially blocked Cdc42 activation induced by ET-1, confirms the role of Gq-mediated Cdc42 activation in ET-1 signaling [18] in addition to Gα12 mediated effect. Therefore, it is reasonable to suggest that the activation of β1Pix is induced directly by activated Gαq through stimulation of the ET-Rs.
ET-1-induced binding of activated Erk1/2 to β1Pix was partially inhibited by PKC inhibitor and by RGS3, indicating the involvement of Gαq, but not that of Gαi/o or Gαs. This is in agreement with our previous study showing that RGS3 inhibits ET-1-induced Erk1/2 activation [18]. Gαq-mediated activation of PKC can stimulate Erk1/2 through phosphorylation of C-Raf [23]. Erk1/2 can also be stimulated through Cdc42 activation. The disruption of β1Pix dimerization or the disruption of caveolae by cholesterol depletion strongly inhibited the binding of Gαq and activated Erk1/2 to β1Pix, indicating that this multi-molecular signaling complex requires β1Pix dimerization and intact caveolae.
The downstream signaling responses initiated by ET-Rs through Gα12/13-dependent mechanisms have been less clearly defined than those dependent on activation of Gq, Gs and Gi proteins. Many GPCRs that are able to activate Gαq also couple to Gα12/13. In membranes of aortic smooth muscle cells, ET-Rs have been shown to couple to Gαq as well as Gα12/13. In our study, we show that in the presence of β1Pix, Gα12 increases ET-1-induced Cdc42 activation. Therefore, we cannot exclude the possibility that Gα12 is also involved in ET-1-induced Erk1/2 activation and binding to β1Pix.
Different Gα subunits contain caveolin binding motif and directly bind to caveolin-1 through its caveolin scaffolding domain [24]. Our result does not rule out that β1Pix binds Gαq and Gα12 through caveolin-1. Interestingly, in this case the role of RGS domain in mediating the binding of Gα subunits to p115RhoGEF [16] will be comparable to the role of the scaffolding domain of caveolin-1 in mediating the interaction between Gα subunits and β1Pix. Previous studies have indicated that caveolin-1 preferentially interacts with inactive GDP-bound Gαs and inhibits nucleotide exchange [19]. In our case, β1Pix was able to associate with Gαs (data not shown) and the disruption of caveolae completely blocked the interaction between β1Pix and Gα subunits. Furthermore, activation of heterotrimeric G proteins by AMF showed an increase in the interaction between β1Pix and caveolin-1, consistent with the increased association found between β1Pix and AMF-activated Gαq and Gα12. This strongly suggests that β1Pix can interact with active Gα subunits while still bound to caveolin-1. The results showing that Gαq specifically concentrates in caveolae, whereas Gαs concentrate much more in lipid rafts [20] may explain the sensitivity of inactive Gαs to bind caveolin-1. Tα has been shown to compete with Tβγ to bind caveolin-1 since caveolin binding motif on Tα overlaps with Tβγ binding site [21]. This finding also implies that the trimer (GDP-bound) Tαβγ cannot bind to caveolin-1 since the binding of Tα and Tβγ to caveolin-1 is mutually exclusive. This is in agreement with our finding showing that the binding of Gβ1 to β1Pix was not blocked by cholesterol depletion. Caveolae have been proposed to provide a microenvironment where different signaling molecules (GPCRs, G proteins, Src family tyrosine kinases) are concentrated. Therefore, localization of ET-Rs to caveolae along with Gαq, β1Pix and Erk1/2 may also contribute to allow efficient and specific signaling by ET-R.
Conclusions
Activation of endothelin receptors leads to the compartmentalization and the binding of Gαq to β1Pix in caveolae, where dimeric β1Pix acts as platform to facilitate the binding and the activation of Erk1/2. Our data suggest that the activation of β1Pix is induced directly by activated Gαq, the process triggered by stimulation of endothelin receptors.
Acknowledgment
This work was supported in part by the National Institutes of Health Grants HL22563 and DK41684 (to Sorokin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gomez-Garre D, Ruiz-Ortega M, Ortego M, Largo R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J. Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension. 1996;27:885–892. doi: 10.1161/01.hyp.27.4.885. [DOI] [PubMed] [Google Scholar]
- 2.Aramori I, Nakanishi S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J.Biol.Chem. 1992;267:12468–12474. [PubMed] [Google Scholar]
- 3.Takagi Y, Ninomiya H, Sakamoto A, Miwa S, Masaki T. Structural basis of G protein specificity of human endothelin receptors. A study with endothelinA/B chimeras. J.Biol.Chem. 1995;270:10072–10078. doi: 10.1074/jbc.270.17.10072. [DOI] [PubMed] [Google Scholar]
- 4.Mao J, Yuan H, Xie W, Simon MI, Wu D. Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J.Biol.Chem. 1998;273:27118–27123. doi: 10.1074/jbc.273.42.27118. [DOI] [PubMed] [Google Scholar]
- 5.Chun M, Liyanage UK, Lisanti MP, Lodish HF. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc.Natl.Acad.Sci.U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Murata Y, Fujiyoshi Y, Doi T. Regulated interaction of endothelin B receptor with caveolin-1. Eur.J.Biochem. 2003;270:1816–1827. doi: 10.1046/j.1432-1033.2003.03544.x. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J.Biol.Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 8.Chahdi A, Sorokin A. Endothelin-1 Couples {beta}Pix to p66Shc: Role of {beta}Pix in Cell Proliferation Through FOXO3a Phosphorylation and p27kip1 Down-regulation Independently of Akt. Mol.Biol.Cell. 2008;19:2609–2619. doi: 10.1091/mbc.E07-05-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol.Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 10.Chahdi A, Miller B, Sorokin A. Endothelin 1 induces beta 1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J.Biol.Chem. 2005;280:578–584. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- 11.Chahdi A, Sorokin A. Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol.Cell Biol. 2008;28:1679–1687. doi: 10.1128/MCB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahdi A, Sorokin A, Dunn MJ, Landry Y. The Rac/Cdc42 guanine nucleotide exchange factor beta1Pix enhances mastoparan-activated Gi-dependent pathway in mast cells. Biochem.Biophys.Res.Commun. 2004;317:384–389. doi: 10.1016/j.bbrc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Chahdi A, Sorokin A. Endothelin-1 induces p66Shc activation through EGF receptor transactivation: Role of beta(1)Pix/Galpha(i3) interaction. Cell Signal. 2010;22:325–329. doi: 10.1016/j.cellsig.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 15.Booden MA, Siderovski DP, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol.Cell Biol. 2002;22:4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 17.Lutz S, Freichel-Blomquist A, Rumenapp U, Schmidt M, Jakobs KH, Wieland T. p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedebergs Arch.Pharmacol. 2004;369:540–546. doi: 10.1007/s00210-004-0926-5. [DOI] [PubMed] [Google Scholar]
- 18.Dulin NO, Sorokin A, Reed E, Elliott S, Kehrl JH, Dunn MJ. RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Galpha11. Mol.Cell Biol. 1999;19:714–723. doi: 10.1128/mcb.19.1.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J.Biol.Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 20.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol.Biol.Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott MH, Fliesler SJ, Ghalayini AJ. Cholesterol-dependent association of caveolin-1 with the transducin alpha subunit in bovine photoreceptor rod outer segments: disruption by cyclodextrin and guanosine 5'-O-(3-thiotriphosphate) Biochemistry. 2003;42:7892–7903. doi: 10.1021/bi027162n. [DOI] [PubMed] [Google Scholar]
- 22.Neill JD, Duck LW, Sellers JC, Musgrove LC, Scheschonka A, Druey KM, Kehrl JH. Potential role for a regulator of G protein signaling (RGS3) in gonadotropin-releasing hormone (GnRH) stimulated desensitization. Endocrinology. 1997;138:843–846. doi: 10.1210/endo.138.2.5034. [DOI] [PubMed] [Google Scholar]
- 23.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J.Biol.Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 24.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J.Biol.Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]